ABSTRACT
The pathological spectrum of nonalcoholic fatty liver disease includes simple steatosis and nonalcoholic steatohepatitis (NASH), the latter of which is the leading cause of cirrhosis and hepatocellular carcinoma. The available evidence shows that parenchymal cell injury and death trigger inflammation and tissue fibrosis. During the development of liver fibrosis, stromal cells dramatically changes in their cellular component and activation status responding to hepatocyte injury due to various etiologies. It is important to understand how cell death induces chronic inflammation and fibrosis, and the disease-specific macrophages and fibroblasts responsible for NASH development under metabolic stress. This review discusses recent progress in the understanding the pathogenesis of NASH, focusing on disease-specific macrophages and fibroblasts.
Key Words: chronic inflammation, macrophages, fibroblasts
INTRODUCTION
Chronic inflammation is now recognized as a molecular basis underlying metabolic syndrome, which is a constellation of visceral fat obesity, impaired glucose metabolism, dyslipidemia, and elevated blood pressure.1 Non-alcoholic fatty liver disease (NAFLD) is considered as the hepatic phenotype of metabolic syndrome.2 The pathological spectrum of nonalcoholic fatty liver disease includes simple steatosis and nonalcoholic steatohepatitis (NASH), the latter of which is characterized by chronic inflammation and fibrosis. NASH is a frequently diagnosed chronic liver disease, and is seen as the leading cause of cirrhosis and hepatocellular carcinoma (HCC).3-5 During the course of NASH development, stromal cells dramatically changes in their cellular component and activation status responding to parenchymal cell (hepatocyte) injury or death.6 This review discusses recent progress in the understanding NASH pathogenesis focusing on the disease-specific macrophages and fibroblasts.
CLINICAL CHARACTERISTICS OF NAFLD/NASH
The prevalence of NAFLD has increased worldwide along with sedentary lifestyles and overnutrition. Although the prevalence varies with ethnicity, sex, age, comorbidities, and the diagnostic method, it is estimated that about 20%–30% of world population has NAFLD.7 The natural history of NAFLD is poorly understood because invasive liver biopsy remains the gold-standard for the diagnosis of NASH. About 10–20% of NAFLD patients are supposed to have NASH and approximately 20% of those with NASH will develop advanced liver fibrosis, which increases the risk of extrahepatic complications, cirrhosis, and HCC.8,9 The prevalence and severity of NAFLD is substantially increased in patients with type 2 diabetes, suggesting that insulin resistance is a shared pathophysiological variable and that type 2 diabetes promotes the progression of NAFLD.6,10 Current evidence supports the importance of fibrosis as the strongest determinant of the increased risk of HCC and liver-related mortality.11-13 A better understanding of how hepatic steatosis progresses to NASH and HCC, which will lead to improvement of overall outcomes.
Various serum biomarkers and combined indexes have been validated for the diagnosis of NASH and grading of disease severity, and imaging can be used to estimate liver stiffness. However, the available modalities do not have the sensitivity and specificity to distinguish NASH from hepatic steatosis.12 The first-line clinical recommendation to manage NASH is lifestyle modification for weight loss, but achieving and maintaining weight loss is difficult and has shown limited effects in those with advanced liver fibrosis.8 There are no approved medications for NASH, but clinical trials evaluating the benefits of interventions that target cell death, metabolic pathways, inflammatory mechanisms, the gut-liver axis, and fibrogenic pathways are ongoing.14
ANIMAL MODELS OF NAFLD AND NASH
To understand the molecular mechanisms underlying the development of NASH, many attempts have been made to establish animal models of NASH/HCC.15,16 Steatosis is readily induced by overnutrition with high-fat diet (HFD) feeding, or animal strains that have genetic defects in the regulation of appetite and energy expenditure such as ob/ob or db/db mice.16 However, HFD-induced obesity leads to mild liver fibrosis only after long-term feeding, usually for more than 1 year, and leptin signal-deficient mice are resistant to liver fibrosis.17-19 In contrast, chemically-induced liver fibrosis is not accompanied by obesity, insulin resistance, and hepatic steatosis. Methionine and choline-deficient (MCD) diets can induce hepatic lipid accumulation and fibrosis, but the mice become cachexic and insulin sensitive.16 In this regard, we have reported melanocortin 4 receptor-deficient (MC4R-KO) mice fed an HFD or Western diet (WD) as a novel rodent NASH model.20,21 MC4R is a seven-transmembrane G protein-coupled receptor that is expressed in the hypothalamus and regulates food intake and body weight downstream of the leptin signaling.22 MC4R-KO mice develop hyperphagic obesity and insulin resistance, and sequentially exhibit NASH-like liver phenotypes after 20 weeks of HFD or WD feeding and HCC after 1 year.20 Because MC4R expression is primarily limited to the central nervous system and is not detected in the liver, it is possible that the hepatic phenotype in MC4R-KO mice results from metabolic derangements induced by hyperphagic obesity. A study by Nogueiras et al reported that MC4R signaling in the brain directly controlled lipid metabolism in the liver, and we previously reported that the central melanocortin system promoted peripheral monocyte chemotaxis.23,24 Further investigation of the molecular mechanisms of the induction of NASH phenotypes by an HFD or WD in MC4R-KO mice is needed, but it appears to be a useful model of diet-induced steatosis, liver fibrosis, and HCC.
HEPATOCYTE DEATH–INDUCED INFLAMMATION AND FIBROSIS
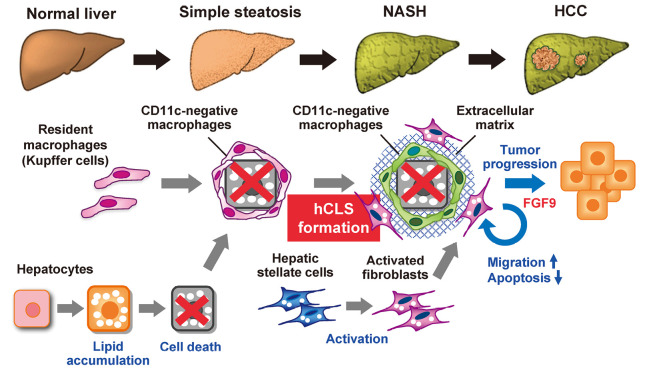
The proposed molecular mechanisms of NASH include the two-hit hypothesis and the more recent multiple parallel-hit hypothesis that include excess lipid accumulation in the liver and inflammation caused by cytokines, oxidative stress, and endotoxin.25,26 In contrast to simple benign hepatic steatosis, metabolic stress (lipotoxicity) that results in hepatocyte injury or death triggers the development of inflammation and fibrosis, and promotes disease progression.27 Inflammation is necessary to clear cell debris and activate tissue repair programs. On the other hand, when cellular stress and injury persist chronically and repair programs are dysregulated, stromal cells including immune cells, fibroblasts, and hepatic sinusoidal cells, dramatically change in their cellular component and activation status, leading to tissue fibrosis and organ dysfunction. Several lines of evidence indicate that inhibition of hepatocyte death results in improvement of liver inflammation and fibrosis.28-30 Using our NASH model, we found hepatic crown-like structures (hCLS) where CD11c-positive macrophages surround dead or dying hepatocytes that contain large lipid-droplets (Fig. 1).31,32 Activated fibroblasts and increased collagen deposition are seen near hCLS, and the number of hCLS is positively correlated with the extent of liver fibrosis.31 hCLS was observed in liver biopsy specimens from patients with NAFLD/NASH, but are rarely observed in patients with chronic viral hepatitis.31 In comparison with NAFLD scoring system, the number of hCLS was positively associated the ballooning degeneration score, which reflects hepatocyte injury.31,32 The clinical findings are consistent with experimental data showing that hepatocyte death triggers hCLS formation, which promotes inflammation and fibrosis.
Fig. 1.
Potential role of CD11c-positive macrophages and activated fibroblasts in the progression from hepatic steatosis to NASH and HCC
During the development of NASH, resident macrophages (Kupffer cells) aggregate around dying or dead hepatocytes to constitute hCLS, and these macrophages become CD11c-positive in response to hepatocyte death, with unique gene expression profiles. Metabolic stress such as saturated fatty acids induces FGF9 expression in activated fibroblasts, thereby increasing cell migration and viability of fibroblasts and hepatoma cells, and thus promoting tumor growth.
hCLS-MEDIATED LIVER FIBROSIS IN NASH
Macrophages are the major phagocytic cells in the liver, with proinflammatory and profibrotic activity, and they contribute to the pathogenesis of liver fibrosis in various etiologies. Recently, evidence has accumulated suggesting that macrophages are highly heterogenous in their origin and polarization, and activation status based on each etiology. For example Satoh et al reported a novel macrophage subset that was responsible for lung fibrosis.33 In experimental models of liver fibrosis, large numbers of bone marrow-derived macrophages are recruited during the development of NASH and infiltrate the liver.34,35 In addition, Kupffer cells, which are tissue-resident macrophages form hCLS, and become CD11c-positive in response to hepatocyte death (Fig. 1).32 CD11c-positive macrophages have gene expression profiles distinct from the CD11c-negative macrophages that remain scattered in the liver. Depletion of CD11c-positive macrophages was found to abolish hCLS formation and fibrogenesis in MC4R-KO mice.32 These findings indicate that CD11c-positive macrophages may be a NASH-specific macrophage subset that drives metabolic stress-induced liver fibrosis.
CHARACTERIZATION OF ACTIVATED FIBROBLASTS IN NASH
During liver tissue remodeling caused by chronic inflammation, hepatic stellate cells (HSCs) transdifferentiate into activated fibroblasts or myofibroblasts with proliferative, migratory, and inflammatory properties. HSCs promote the formation of the extracellular matrix following activation by hepatocytes and other stromal cells.36,37 The fibroblasts may have activation triggers unique to each etiology much like macrophages. The pathogenic role of profibrotic factors has been investigated mainly in liver fibrosis models without obesity and/or HCC development. In the MC4R-KO model, activated fibroblasts from mice fed a WD for 20 weeks had gene expression patterns that differed from those of fibroblasts from mice with carbon tetrachloride-induced fibrosis as a model without obesity or HCC development. Cancer-associated pathways were upregulated in activated fibroblasts even in MC4R-KO mice that had developed NASH.38 Transcription of the fibroblast growth factor 9 (FGF9) gene, the most upregulated gene in cancer-associated pathways, was induced by metabolic stress such as saturated fatty acids.38 FGF9 expression was found to increase fibroblast and hepatoma cell migration and viability in vitro and promote tumor growth in an in vivo xenograft model (Fig. 1).38 It is well known that cancer-associated fibroblasts affect the tumor microenvironment that promote tumor progression and metastasis.39 The evidence indicates that activation of fibroblasts in NASH promotes proinflammatory and profibrogenic activity and creates a microenvironment promoting tumor progression.
CONCLUSION
Considerable evidence shows that parenchymal cell injury or death trigger subsequent inflammation and tissue fibrosis. Review of recent evidence helps to understand how cell death induces chronic inflammation and fibrosis in NASH. The presence of disease-specific macrophages and fibroblasts is responsible for NASH development in response to metabolic stress. hCLS are sites of interaction between dead hepatocytes, macrophages, and fibroblasts that induce chronic inflammation and fibrogenesis. hCLS formation precedes the development of NASH and might be a histological marker of the progression from hepatic steatosis to NASH. hCLS formation may be novel target for preemptive medicine therapy.
ACKNOWLEDGEMENT
This work was supported in part by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (16H05171, 16KT0110, 16K08732, 17H05500, and 19K07475) and AMED-CREST (JP19gm1210009). This work was also supported by research grants from Takeda Science Foundation, The Hori Sciences and Arts Foundation, Suzuken Memorial Foundation, the Japan Diabetes Society, MSD Life Science Foundation and Terumo Life Science Foundation. We thank Dr. Joel K. Elmquist (University of Texas Southwestern Medical Center) for his generous gift of MC4R-KO mice.
Abbreviations
- FGF9
fibroblast growth factor 9
- HCC
hepatocellular carcinoma
- hCLS
hepatic crown-like structure
- HFD
high-fat diet
- MC4R
melanocortin 4 receptor
- MCD
methionine and choline-deficient
- NAFLD
nonalcoholic-alcoholic fatty liver disease
- NASH
nonalcoholic-alcoholic steatohepatitis
- WD
Western diet
REFERENCES
- 1.Matsuzawa Y, Funahashi T, Nakamura T. Molecular mechanism of metabolic syndrome X: contribution of adipocytokines adipocyte-derived bioactive substances. Ann N Y Acad Sci. 1999;892:146–154. doi: 10.1111/j.1749-6632.1999.tb07793.x. [DOI] [PubMed]
- 2.Anstee QM, Targher G, Day CP. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat Rev Gastroenterol Hepatol. 2013;10(6):330–344. doi: 10.1038/nrgastro.2013.41. [DOI] [PubMed]
- 3.Younossi ZM. Non-alcoholic fatty liver disease: a global public health perspective. J Hepatol. 2019;70(3):531–544. doi: 10.1016/j.jhep.2018.10.033. [DOI] [PubMed]
- 4.Mahady SE, George J. Management of nonalcoholic steatohepatitis: an evidence-based approach. Clin Liver Dis. 2012;16(3):631–645. doi: 10.1016/j.cld.2012.05.003. [DOI] [PubMed]
- 5.Wong MCS, Huang JLW, George J, et al. The changing epidemiology of liver diseases in the Asia-Pacific region. Nat Rev Gastroenterol Hepatol. 2019;16(1):57–73. doi: 10.1038/s41575-018-0055-0. [DOI] [PubMed]
- 6.Machado MV, Diehl AM. Pathogenesis of nonalcoholic steatohepatitis. Gastroenterology. 2016;150(8):1769–1777. doi: 10.1053/j.gastro.2016.02.066. [DOI] [PMC free article] [PubMed]
- 7.Younossi Z, Tacke F, Arrese M, et al. Global perspectives on nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Hepatology. 2019;69(6):2672–2682. doi: 10.1002/hep.30251. [DOI] [PubMed]
- 8.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology. 2012;142(7):1592–1609. doi: 10.1053/j.gastro.2012.04.001. [DOI] [PubMed]
- 9.Rinella ME, Sanyal AJ. Management of NAFLD: a stage-based approach. Nat Rev Gastroenterol Hepatol. 2016;13(4):196–205. doi: 10.1038/nrgastro.2016.3. [DOI] [PubMed]
- 10.Younossi ZM, Golabi P, de Avila L, et al. The global epidemiology of NAFLD and NASH in patients with type 2 diabetes: a systematic review and meta-analysis. J Hepatol. 2019;71(4):793–801. doi: 10.1016/j.jhep.2019.06.021. [DOI] [PubMed]
- 11.Ekstedt M, Hagstrom H, Nasr P, et al. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology. 2015;61(5):1547–1554. doi: 10.1002/hep.27368. [DOI] [PubMed]
- 12.Angulo P, Kleiner DE, Dam-Larsen S, et al. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology. 2015;149(2):389–397. doi: 10.1053/j.gastro.2015.04.043. [DOI] [PMC free article] [PubMed]
- 13.Vilar-Gomez E, Calzadilla-Bertot L, Wai-Sun Wong V, et al. Fibrosis severity as a determinant of cause-specific mortality in patients with advanced nonalcoholic fatty liver disease: a multi-national cohort study. Gastroenterology. 2018;155(2):443–457. doi: 10.1053/j.gastro.2018.04.034. [DOI] [PubMed]
- 14.Reimer KC, Wree A, Roderburg C, Tacke F. New drugs for NAFLD: lessons from basic models to the clinic. Hepatol Int. 2020;14(1):8–23. doi: 10.1007/s12072-019-10001-4. [DOI] [PubMed]
- 15.Liu Y, Meyer C, Xu C, et al. Animal models of chronic liver diseases. Am J Physiol Gastrointest Liver Physiol. 2013;304(5):G449–468. doi: 10.1152/ajpgi.00199.2012. [DOI] [PubMed]
- 16.Farrell G, Schattenberg JM, Leclercq I, et al. Mouse models of nonalcoholic steatohepatitis: toward optimization of their relevance to human nonalcoholic steatohepatitis. Hepatology. 2019;69(5):2241–2257. doi: 10.1002/hep.30333. [DOI] [PubMed]
- 17.Ito M, Suzuki J, Tsujioka S, et al. Longitudinal analysis of murine steatohepatitis model induced by chronic exposure to high-fat diet. Hepatol Res. 2007;37(1):50–57. doi: 10.1111/j.1872-034X.2007.00008.x. [DOI] [PubMed]
- 18.DeLeve LD, Wang X, Kanel GC, Atkinson RD, McCuskey RS. Prevention of hepatic fibrosis in a murine model of metabolic syndrome with nonalcoholic steatohepatitis. Am J Pathol. 2008;173(4):993–1001. doi: 10.2353/ajpath.2008.070720. [DOI] [PMC free article] [PubMed]
- 19.Saxena NK, Ikeda K, Rockey DC, Friedman SL, Anania FA. Leptin in hepatic fibrosis: evidence for increased collagen production in stellate cells and lean littermates of ob/ob mice. Hepatology. 2002;35(4):762–771. doi: 10.1053/jhep.2002.32029. [DOI] [PMC free article] [PubMed]
- 20.Itoh M, Suganami T, Nakagawa N, et al. Melanocortin 4 receptor-deficient mice as a novel mouse model of nonalcoholic steatohepatitis. Am J Pathol. 2011;179(5):2454–2463. doi: 10.1016/j.ajpath.2011.07.014. [DOI] [PMC free article] [PubMed]
- 21.Suganami T, Tanaka M, Ogawa Y. Adipose tissue inflammation and ectopic lipid accumulation. Endocr J. 2012;59(10):849–857. doi: 10.1507/endocrj.ej12-0271. [DOI] [PubMed]
- 22.Balthasar N, Dalgaard LT, Lee CE, et al. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell. 2005;123(3):493–505. doi: 10.1016/j.cell.2005.08.035. [DOI] [PubMed]
- 23.Nogueiras R, Wiedmer P, Perez-Tilve D, et al. The central melanocortin system directly controls peripheral lipid metabolism. J Clin Invest. 2007;117(11):3475–3488. doi: 10.1172/JCI31743. [DOI] [PMC free article] [PubMed]
- 24.Tanaka M, Suganami T, Sugita S, et al. Role of central leptin signaling in renal macrophage infiltration. Endocr J. 2010;57(1):61–72. doi: 10.1507/endoerij.k09e-296. [DOI] [PubMed]
- 25.Day CP, James OF. Steatohepatitis: a tale of two “hits”? Gastroenterology. 1998;114(4):842–845. doi: 10.1016/s0016-5085(98)70599-2. [DOI] [PubMed]
- 26.Tilg H, Moschen AR. Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis. Hepatology. 2010;52(5):1836–1846. doi: 10.1002/hep.24001. [DOI] [PubMed]
- 27.Feldstein AE, Canbay A, Angulo P, et al. Hepatocyte apoptosis and fas expression are prominent features of human nonalcoholic steatohepatitis. Gastroenterology. 2003;125(2):437–443. doi: 10.1016/s0016-5085(03)00907-7. [DOI] [PubMed]
- 28.Goto T, Itoh M, Suganami T, et al. Obeticholic acid protects against hepatocyte death and liver fibrosis in a murine model of nonalcoholic steatohepatitis. Sci Rep. 2018;8(1):8157. doi: 10.1038/s41598-018-26383-8. [DOI] [PMC free article] [PubMed]
- 29.Komiya C, Tanaka M, Tsuchiya K, et al. Antifibrotic effect of pirfenidone in a mouse model of human nonalcoholic steatohepatitis. Sci Rep. 2017;7:44754. doi: 10.1038/srep44754. [DOI] [PMC free article] [PubMed]
- 30.Witek RP, Stone WC, Karaca FG, et al. Pan-caspase inhibitor VX-166 reduces fibrosis in an animal model of nonalcoholic steatohepatitis. Hepatology. 2009;50(5):1421–1430. doi: 10.1002/hep.23167. [DOI] [PubMed]
- 31.Itoh M, Kato H, Suganami T, et al. Hepatic crown-like structure: a unique histological feature in non-alcoholic steatohepatitis in mice and humans. PLoS One. 2013;8(12):e82163. doi: 10.1371/journal.pone.0082163. [DOI] [PMC free article] [PubMed]
- 32.Itoh M, Suganami T, Kato H, et al. CD11c+ resident macrophages drive hepatocyte death-triggered liver fibrosis in a murine model of nonalcoholic steatohepatitis. JCI Insight. 2017;2(22):e92902. doi: 10.1172/jci.insight.92902. [DOI] [PMC free article] [PubMed]
- 33.Satoh T, Nakagawa K, Sugihara F, et al. Identification of an atypical monocyte and committed progenitor involved in fibrosis. Nature. 2017;541(7635):96–101. doi: 10.1038/nature20611. [DOI] [PubMed]
- 34.Seki E, de Minicis S, Inokuchi S, et al. CCR2 promotes hepatic fibrosis in mice. Hepatology. 2009;50(1):185–197. doi: 10.1002/hep.22952. [DOI] [PMC free article] [PubMed]
- 35.Miura, K., Yang, L., van Rooijen, N., Ohnishi, H. & Seki, E. Hepatic recruitment of macrophages promotes nonalcoholic steatohepatitis through CCR2. Am J Physiol Gastrointest Liver Physiol. 2012;302(11):G1310–1321. doi: 10.1152/ajpgi.00365.2011. [DOI] [PMC free article] [PubMed]
- 36.Tsuchida T, Friedman SL. Mechanisms of hepatic stellate cell activation. Nat Rev Gastroenterol Hepatol. 2017;14(7):397–411. doi: 10.1038/nrgastro.2017.38. [DOI] [PubMed]
- 37.Burt AD. Pathobiology of hepatic stellate cells. J Gastroenterol. 1999;34(3):299–304. doi: 10.1007/s005350050264 [DOI] [PubMed]
- 38.Asakawa M, Itoh M, Suganami T, et al. Upregulation of cancer-associated gene expression in activated fibroblasts in a mouse model of non-alcoholic steatohepatitis. Sci Rep. 2019;9(1):19601. doi: 10.1038/s41598-019-56039-0. [DOI] [PMC free article] [PubMed]
- 39.Kalluri R. The biology and function of fibroblasts in cancer. Nat Rev Cancer. 2016;16(9):582–598. doi: 10.1038/nrc.2016.73. [DOI] [PubMed]