ABSTRACT
Neoadjuvant chemotherapy (NAC) using the combination of anthracycline and taxanes is the standard regimen for patients with primary breast cancer. Among the taxanes, conventional paclitaxel (PTX) and docetaxel have usually been adopted in the neoadjuvant or adjuvant setting. Nanoparticle albumin-bound paclitaxel (nab-PTX) is a solvent-free formulation that can be delivered to cancer cells at higher doses than conventional PTX. This study is a retrospective observational study in a single institution. We evaluated the efficacy and safety of nab-PTX followed by 5-fluorouracil, epirubicin, and cyclophosphamide (FEC) in the neoadjuvant setting. In this study, 50 patients with primary breast cancer received nab-PTX (q3w, 260 mg/m2 ± trastuzumab 6 mg/kg) followed by FEC (q3w, 5-fluorouracil 500 mg/m2, epirubicin 100 mg/m2, and cyclophosphamide 500 mg/m2) prior to surgery. The efficacy was evaluated using the clinical response rate (CRR), pathological complete response (pCR) rate, and Ki67 labeling index. Safety was evaluated using the frequency of treatment-related adverse events and relative dose intensity (RDI). All patients received at least one course of chemotherapy. The CRR and pCR rate were 88.0% and 40.0%, respectively. The mean Ki67 labeling index was significantly decreased from 47.7% to 24.6% after NAC. The safety profiles were comparable with previously reported regimens, and high RDIs were obtained (97.2% for nab-PTX and 95.5% for FEC). This study illustrated the efficacy and tolerability of a neoadjuvant regimen of nab-PTX followed by FEC.
Key Words: breast cancer, nab-PTX, FEC, neoadjuvant chemotherapy
INTRODUCTION
Neoadjuvant chemotherapy (NAC) including anthracycline and taxanes has been widely used for patients with primary breast cancer, especially those who have locally advanced disease. It has been reported that the response to NAC can predict patient prognosis; namely, patients who achieve pathological complete responses (pCRs) have favorable prognoses.1,2 Although several neoadjuvant and adjuvant chemotherapy regimens, such as anthracyclines, taxanes, capecitabine, and gemcitabine, have been evaluated to date,3,4 which regimen is the most effective and safe remains controversial.
Nanoparticle albumin-bound paclitaxel (nab-PTX) is a unique non-solvent-containing protein formulation. It can obviate the need for prophylactic anti-histamine and steroid treatment because of its much lower risk of hypersensitivity compared with conventional paclitaxel (PTX), although it is prone to cause peripheral neuropathy.5 Nab-PTX allows the accumulation of a higher dose of PTX into cancer cells than conventional PTX, which possibly induces stronger cytotoxic effects.6,7 In a phase 3 trial of patients with metastatic breast cancer, nab-PTX exhibited superior antitumor activity and improved progression-free survival compared with conventional PTX.8 These results suggest the superiority of nab-PTX over conventional PTX for neoadjuvant chemotherapy. In the neoadjuvant setting, previous studies reported that pCR was achieved in 5.7%–38% of patients who received nab-PTX–containing regimens.9-11 In patients with human epidermal growth factor receptor 2 (HER2) positivity, the pCR rate was increased up to 49% following the treatment with 5-fluorouracil, epirubicin, and cyclophosphamide (FEC) or epirubicin and cyclophosphamide (EC) followed by nab-PTX plus trastuzumab.12 Despite these promising effects of nab-PTX–containing NAC regimens, conventional PTX or docetaxel is more likely to be adopted in clinics. Indeed, the guideline of the National Comprehensive Cancer Network does not strongly recommend nab-PTX in NAC, and it is considered an option.13
In this study, we aimed to clarify the efficacy and tolerability of a NAC regimen consisting of nab-PTX and FEC in patients with primary breast cancer.
PATIENTS AND METHODS
Patient population
We performed a retrospective observational study in a single institution. Patients who visited Yokkaichi Municipal Hospital from August 2013 to December 2017 with previously untreated breast cancer were enrolled. All patients were pathologically confirmed to have invasive carcinoma via core needle biopsy or vacuum-assisted biopsy, and they had measurable disease. The patients’ Eastern Cooperative Oncology Group performance status scores were 0 or 1. Estrogen receptor (ER) positivity, progesterone receptor positivity, the HER2 status, and the Ki67 labeling index were evaluated using biopsied tissues. The breast tumor size of each patient was measured via contrast-enhanced magnetic resonance imaging (MRI) before and after NAC. All patients were proven to not have distant metastatic sites using computed tomography prior to chemotherapy. The postoperative pathological classification was evaluated by one pathologist according to the Union for International Cancer Control staging system for breast cancer (7th edition).
Treatment
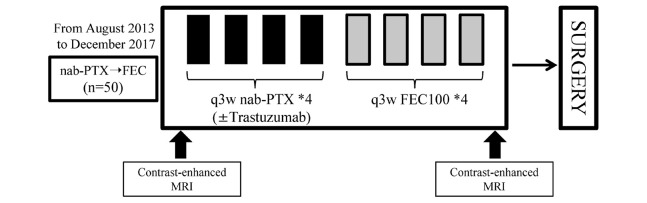
Figure 1 presents the treatment schedule. Four cycles of nab-PTX (q3w, 260 mg/m2) followed by four cycles of FEC (q3w, 5-fluorouracil 500 mg/m2, epirubicin 100 mg/m2, and cyclophosphamide 500 mg/m2) were administered prior to surgery. For patients with HER2 positivity, trastuzumab (8 mg/kg in the first cycle and 6 mg/kg in the remaining cycles) was added to nab-PTX. Pre-medication, such as antiemetic drugs and granulocyte colony-stimulation factor, was administered per each physician’s discretion. The dose of each chemotherapy agent was reduced in the event of febrile neutropenia, grade 3–4 thrombocytopenia, or grade 3–4 non-hematological toxicities (excluding nausea, vomiting, and fatigue). After the completion of NAC, all patients underwent MRI to evaluate the change of tumor size. The operative procedure (i.e., total or partial mastectomy) was decided in consideration of the result of MRI and the patient’s preference. Axillary lymph node dissection was performed when the nodes were pathologically found to be metastatic before NAC; otherwise, sentinel node biopsy was conducted. Postoperative radiation therapy was performed for patients who opted for breast-conserving surgery. Post-surgical endocrine therapy was administered to patients with ER-positive lesions for a minimum of 5 years. For patients with HER2-positive cancer, trastuzumab was administered for 1 year after surgery.
Fig. 1.
A flowchart shows the treatment strategy.
Four cycles of nanoparticle albumin-bound paclitaxel (nab-PTX) followed by four cycles of 5-fluorouracil, epirubicin, and cyclophosphamide (FEC) were administrated prior to surgery. Contra-enhanced magnetic resonance imaging (MRI) was performed before and after chemotherapy.
Clinicopathological response and toxicity assessment
The clinical response rate (CRR) was assessed via MRI to evaluate the change of the primary tumor size before breast surgery based on Response Evaluation Criteria In Solid Tumors version 1.1.14 The pathological response to chemotherapy was assessed by one pathologist as follows: pCR was defined as necrosis and/or the replacement of cancer cells by granulation and/or fibrosis at both primary and lymph node sites. The presence of residual duct components was defined as quasi-pCR. The Ki67 labeling index was evaluated using biopsied tissues resected before and after NAC. In patients with pCR, the Ki67 labeling index was calculated as 0%. Adverse events for laboratory and non-laboratory toxicities were summarized for each chemotherapeutic regimen using the National Cancer Institute Common Toxicity Criteria for Adverse Events, version 4.0.15 If adverse events did not improve during the drug holiday, chemotherapy was discontinued.
The relative dose intensity (RDI) represents the ratio of the amount of actually administered dose (actual dose intensity) to the planned dose (planned dose intensity) for a scheduled period. Indeed, the RDI (%) was defined as follows: (actual dose intensity/planned dose intensity) × 100.16
Statistical methods
All statistical analyses were performed using EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria). This modified version of the R commander comprises additional statistical functions frequently used in biostatistics.17 To compare means between two groups, the Mann-Whitney U-test was employed according to the data distribution. P < 0.05 was considered statistically significant.
Ethical Consideration
This study was conducted in accordance with the Declaration of Helsinki and approved by Yokkaichi Municipal Hospital’s institutional review board (No.2013-34). Prior to NAC, all patients provided written informed consent for the administration of nab-PTX and FEC for individual treatment and the use of their clinical data for retrospective analysis in the future.
RESULTS
Patient characteristics
Fifty patients were evaluated in this study. Their baseline characteristics are summarized in Table 1. The median age was 53.3 years (range, 31–72 years). The mean tumor size was 2.9 ± 1.2 cm (T1, 13 patients; T2, 32 patients; and T3, five patients), and 22 patients (44.0%) were clinical nodal stage N1 or N2. The Union for International Cancer Control stage distribution was as follows: stage I, 11 patients; stage IIA, 17 patients; stage IIB, 19 patients; and stage IIIA, three patients. The ER, progesterone receptor, and HER2 statuses at the primary tumor sites were as follows: ER-positive, n = 13; ER-negative, n = 37; progesterone receptor-positive, n = 10; progesterone receptor-negative, n = 40; HER2-positive, n = 19; and HER2-negative, n = 31. When the ER and HER2 statuses were combined, two patients were ER+/HER2−, 11 patients were ER+/HER2+, nine patients were ER−/HER2+, and 28 patients were ER−/HER2−. Regarding surgery, 30 and 20 patients underwent total and partial mastectomy, respectively. Twenty patients underwent axially lymph node dissection.
Table 1.
Patient characteristics
| Value | |
| Age (median, range), years | 53.3 (31–72) |
| Performance status | |
| 0 | 50 (100 %) |
| 1 | 0 (0 %) |
| Clinical tumor stage | |
| T1 | 13 (26.0 %) |
| T2 | 32 (64.0 %) |
| T3 | 5 (10.0 %) |
| Clinical nodal stage | |
| N0 | 28 (56.0 %) |
| N1 | 21 (42.0 %) |
| N2 | 1 (2.0 %) |
| Clinical stage | |
| I | 11 (22.0 %) |
| IIA | 17 (34.0 %) |
| IIB | 19 (38.0 %) |
| IIIA | 3 (6.0 %) |
| ER status | |
| Positive | 13 (26.0 %) |
| Negative | 37 (74.0 %) |
| PgR status | |
| Positive | 10 (20.0 %) |
| Negative | 40 (80.0 %) |
| HER2 status | |
| Positive | 19 (38.0 %) |
| Negative | 31 (62.0 %) |
| Subtype | |
| ER+/HER2− | 2 (4.0 %) |
| ER+/HER2+ | 11 (22.0 %) |
| ER−/HER2+ | 9 (18.0 %) |
| ER−/HER2− | 28 (56.0 %) |
| Surgery | |
| Total mastectomy | 30 (60.0 %) |
| Partial mastectomy | 20 (40.0 %) |
| Axially lymph node dissection | 20 (40.0 %) |
ER: estrogen receptor, HER2: human epidermal growth factor receptor 2, PgR: progesterone receptor.
Clinical and pathological assessments
The clinical and pathological responses after NAC are summarized in Table 2. The CRR, which includes complete and partial responses, was 88.0%. Specifically, 26 patients (52.0%) achieved complete responses, and 18 patients (36.0%) achieved partial responses. Conversely, three patients (6.0%) had stable disease, and one patient (2.0%) had progressive disease. The therapeutic response could not be evaluated in two patients (4.0%) because they underwent surgery at another hospital.
Table 2.
Clinical and pathological responses
| Variable | n (%) |
| Clinical response | |
| CR | 26 /50 (52.0 %) |
| PR | 18 /50 (36.0 %) |
| SD | 3 /50 (6.0 %) |
| PD | 1 /50 (2.0 %) |
| Unknown | 2 /50 (4.0 %) |
| Pathological response | |
| Overall pCR | 20 /50 (40.0 %) |
| Subtype, pCR | |
| ER+/HER2− | 0 /2 (0.0 %) |
| ER+/HER2+ | 4 /11 (36.4 %) |
| ER−/HER2+ | 3 /9 (33.3 %) |
| ER−/HER2− | 13 /28 (46.4 %) |
| Overall quasi-pCR | 24 /50 (48.0 %) |
| Subtype, quasi-pCR | |
| ER+/HER2− | 1 /2 (50.0 %) |
| ER+/HER2+ | 5 /11 (45.5 %) |
| ER−/HER2+ | 4 /9 (44.4 %) |
| ER−/HER2− | 14 /28 (50.0 %) |
CR: complete response, ER: estrogen receptor, HER2: human epidermal growth factor receptor 2, pCR: pathological complete response, PD: progressive disease, PgR: progesterone receptor, PR: partial response, SD: stable disease.
Regarding the pathological response after NAC, 20 patients (40.0%) achieved pCR as follows: ER+/HER2−, n = 0; ER+/HER2+, n = 4 (36.4%); ER−/HER2+, n = 3 (33.3%); and ER−/HER2−, n = 13 (48.0%). In total, 24 patients (48.0%) achieved quasi-pCR as follows: ER+/HER2−, n = 1 (50.0%); ER+/HER2+, n = 5 (45.5%); ER−/HER2+, n = 4 (44.4%); and ER−/HER2−, n = 14 (50.0%) (Table 2).
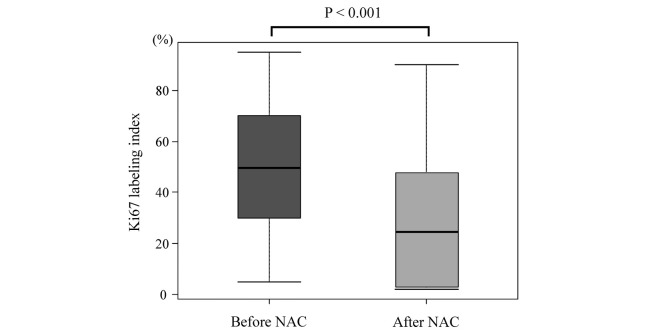
When the mean changes of the Ki67 labeling index in evaluable patients were compared between pre-NAC and post-surgery, the value was significantly decreased from 47.7 to 24.6% after NAC (P < 0.001) (Fig. 2).
Fig. 2.
Box plots showing change of the Ki67 labeling index between before and after neoadjuvant chemotherapy (NAC).
The mean Ki67 labeling index was significantly decreased from 47.7% to 24.6%.
Toxicities and RDI
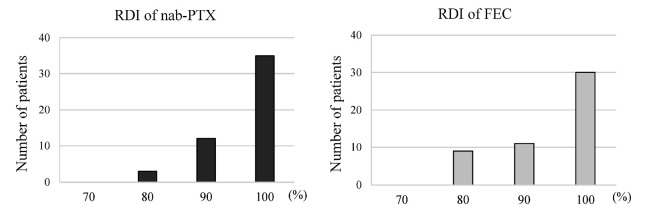
The incidence of treatment-related adverse events for each chemotherapeutic regimen is listed in Table 3. Comparing the adverse events between these two chemotherapies, arthralgia, peripheral neuropathy, and rash were likely to occur during nab-PTX, whereas stomatitis, fatigue, and nausea were likely to occur during FEC. Grade 3 adverse events during nab-PTX included neutropenia in two patients (4.0%), liver dysfunction in one patient (2.0%), and rash in three patients (6.0%). Grade 3 adverse events during FEC included neutropenia in three patients (6.0%), anemia in two patients (4.0%), and stomatitis, fatigue, and nausea in one patient each (2.0%). No patients experienced grade 4 adverse events during either regimen. The mean RDI of nab-PTX was 97.2% (range, 80.0−100%), and that of FEC was 95.5% (range, 80.0−100%) (Fig. 3).
Table 3.
Adverse events of each chemotherapeutic regimen
| nab-PTX followed by FEC (n = 50) | ||||
| Adverse events | nab-PTX | FEC | ||
| All Grade
n (%) |
Grade 3
n (%) |
All Grade
n (%) |
Grade 3
n (%) |
|
| Neutropenia | 3 (6.0) | 2 (4.0) | 6 (12.0) | 3 (6.0) |
| Anemia | 0 | 0 | 2 (4.0) | 2 (4.0) |
| Hepatopathy | 5 (10.0) | 1 (2.0) | 0 | 0 |
| Stomatitis | 2 (4.0) | 0 | 12 (24.0) | 1 (2.0) |
| Fatigue | 4 (8.0) | 0 | 21 (42.0) | 1 (2.0) |
| Constipation | 3 (6.0) | 0 | 8 (16.0) | 0 |
| Nausea | 1 (2.0) | 0 | 13 (26.0) | 1 (2.0) |
| Arthralgia | 26 (52.0) | 0 | 1 (2.0) | 0 |
| Peripheral neuropathy | 48 (96.0) | 0 | 24 (48.0) | 0 |
| Rash | 11 (22.0) | 3 (6.0) | 2 (4.0) | 0 |
| Anaphylactic shock | 0 | 0 | 0 | 0 |
| Keratitis | 0 | 0 | 1 (2.0) | 0 |
| Fever | 4 (8.0) | 0 | 1 (2.0) | 0 |
| Hand foot syndrome | 1 (2.0) | 0 | 2 (4.0) | 0 |
| Diarrhea | 0 | 0 | 3 (6.0) | 0 |
| Edema | 1 (2.0) | 0 | 2 (4.0) | 0 |
| Blurred vision | 1 (2.0) | 0 | 1 (2.0) | 0 |
| Myalgia | 1 (2.0) | 0 | 0 | 0 |
FEC: 5-fluorouracil, epirubicin, and cyclophosphamide, nab-PTX: nanoparticle albumin-bound paclitaxel.
Fig. 3.
Histogram of the relative dose intensity (RDI) for each chemotherapeutic regimen.
The mean RDI for nanoparticle albumin-bound paclitaxel (nab-PTX) was 97.2%, whereas that for 5-fluorouracil, epirubicin, and cyclophosphamide (FEC) was 95.5%.
DISCUSSION
This study demonstrated the clinical outcomes of nab-PTX followed by FEC in the neoadjuvant setting for patients with breast cancer. This regimen provided comparable clinical and pathological effects as the conventional PTX-containing regimens. In addition, this regimen was associated with a significant decrease in the Ki67 labeling index, acceptable safety, and high RDIs.
Taxanes have been widely used for decades in the treatment of breast, lung, and advanced ovarian cancers.18,19 In breast cancer, taxanes have been recognized as key drugs for neoadjuvant and adjuvant chemotherapy, and the pCR rates of PTX- and docetaxel-containing regimens are 30%–40% and 20%–30%, respectively.11,20-22 Recently, nab-PTX has been developed and administered to patients with breast cancer. Because nab-PTX facilitates the accumulation of a higher PTX dose into cancer cells, it has been expected to exert more feasible effects, and several studies have demonstrated its superiority over conventional PTX for patients with breast cancer.8,11 Moreover, some studies reported nab-PTX–including regimens in NAC, and the pCR rates were 22.2 and 30.3% when FEC and EC were combined with nab-PTX, respectively.23,24 In another study, the pCR rate of nab-PTX and cyclophosphamide followed by FEC was 37.3%.25 In this study, the combination of nab-PTX followed by FEC offered CRR and pCR rate of 88.0% and 40.0%, respectively, which are considered equal or superior to the outcomes of conventional taxanes or nab-PTX combined with anthracycline.
Interestingly, this study also found that this regimen significantly decreased the Ki67 labeling index. Ki67, a nuclear protein associated with cellular proliferation, is a well-established marker for predicting the outcomes of patients with breast cancer receiving NAC.26 A recent retrospective study reported that patients who developed metastases exhibited higher Ki67 labeling indices after NAC than those who did not develop metastases.27 Our results suggest that nab-PTX followed by FEC can attenuate cellular proliferation, which possibly leads to better prognoses.
Previous studies reported that nab-PTX is more likely to cause peripheral neuropathy instead of less allergy-related events compared with conventional PTX.24,28 Indeed, peripheral neuropathy during nab-PTX was observed in almost all patients in this study. However, no patients experienced grade 3/4 peripheral neuropathy. As previously reported, despite the high frequency of peripheral neuropathy, nab-PTX hardly causes severe neuropathy.8 Conversely, FEC frequently cause adverse events, such as fatigue, nausea, stomatitis, and neutropenia.29,30 Notably, extremely high RDIs were recorded for both regimens (97.2% for nab-PTX and 95.5% for FEC). Although RDIs exceeding 85% are considered important for maintaining therapeutic effects during breast cancer chemotherapy,31 the FEC regimen tends to lead to low RDIs because of the high frequency of the subjective adverse events (e.g., fatigue and nausea).32 When patients experience these subjective symptoms, they are likely to feel anxiety and refuse further chemotherapy. Administration of taxanes prior to FEC is believed to contribute to maintaining higher RDIs.
This study is a retrospective observational study conducted in a single center without a pre-planned design of patient management and data analysis. The limitations include the small sample size and lack of a randomized controlled design. In addition, long-term outcomes were not evaluated. Because of an observational study, patient selection bias possibly led to increased RDIs without any dropout cases. These points should be reminded to interpret the results.
In conclusion, the neoadjuvant regimen of nab-PTX followed by FEC provides feasible clinical benefits, acceptable safety, and good tolerability. On the basis of this study, further clinical trials comparing the efficacy and safety of nab-PTX and conventional PTX followed by FEC regimen are warranted.
ACKNOWLEDGEMENT
We thank all the patients who participated in our study, as well as their families. We thank Joe Barber Jr., PhD, from Liwen Bianji, Edanz Editing China, for editing the English text of a draft of this manuscript.
DISCLOSURE
All authors declare that we have no conflicts of interest.
Abbreviations
- CRR
clinical response rate
- ER
estrogen receptor
- FEC
5-fluorouracil, epirubicin, and cyclophosphamide
- HER2
human epidermal growth factor receptor 2
- MRI
magnetic resonance imaging
- nab-PTX
nanoparticle albumin-bound paclitaxel
- NAC
neoadjuvant chemotherapy
- pCR
pathological complete response
- PTX
paclitaxel
- RDI
relative dose intensity
REFERENCES
- 1.Gralow JR, Burstein HJ, Wood W, et al. Preoperative therapy in invasive breast cancer: pathologic assessment and systemic therapy issues in operable disease. J Clin Oncol. 2008;26(5):814–819. doi: 10.1200/jco.2007.15.3510. [DOI] [PubMed]
- 2.Kitajima K, Miyoshi Y, Yamano T, Odawara S, Higuchi T, Yamakado K. Assessment of tumor response to neoadjuvant chemotherapy in patients with breast cancer using MRI and FDG-PET/CT-RECIST 1.1 vs. PERCIST 1.0. Nagoya J Med Sci. 2018;80(2):183–197. doi: 10.18999/nagjms.80.2.183. [DOI] [PMC free article] [PubMed]
- 3.Earl HM, Vallier AL, Hiller L, et al. Effects of the addition of gemcitabine, and paclitaxel-first sequencing, in neoadjuvant sequential epirubicin, cyclophosphamide, and paclitaxel for women with high-risk early breast cancer (Neo-tAnGo): an open-label, 2x2 factorial randomised phase 3 trial. Lancet Oncol. 2014;15(2):201–212. doi: 10.1016/s1470-2045(13)70554-0. [DOI] [PubMed]
- 4.von Minckwitz G, Rezai M, Loibl S, et al. Capecitabine in addition to anthracycline- and taxane-based neoadjuvant treatment in patients with primary breast cancer: phase III GeparQuattro Study. J Clin Oncol. 2010;28(12):2015–2023. doi: 10.1200/jco.2009.23.8303. [DOI] [PubMed]
- 5.Ibrahim NK, Desai N, Legha S, et al. Phase I and pharmacokinetic study of ABI-007, a cremophor-free, protein-stabilized, nanoparticle formulation of paclitaxel. Clin Cancer Res. 2002;8(5):1038–1044. [PubMed]
- 6.Gardner ER, Dahut WL, Scripture CD, et al. Randomized crossover pharmacokinetic study of solvent-based paclitaxel and nab-paclitaxel. Clin Cancer Res. 2008;14(13):4200–4205. doi: 10.1158/1078-0432.ccr-07-4592. [DOI] [PMC free article] [PubMed]
- 7.Madappa N Kundranda JN. Albumin-bound paclitaxel in solid tumors: clinical development and future directions. Drug Des Devel Ther. 2015;9:3767–3777. [DOI] [PMC free article] [PubMed]
- 8.Gradishar WJ, Tjulandin S, Davidson N, et al. Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancer. J Clin Oncol. 2005;23(31):7794–7803. doi: 10.1200/jco.2005.04.937. [DOI] [PubMed]
- 9.Robidoux A, Buzdar AU, Quinaux E, et al. A phase II neoadjuvant trial of sequential nanoparticle albumin-bound paclitaxel followed by 5-fluorouracil/epirubicin/cyclophosphamide in locally advanced breast cancer. Clin Breast Cancer. 2010;10(1):81–86. doi: 10.3816/CBC.2010.n.011. [DOI] [PubMed]
- 10.Shimada H, Ueda S, Saeki T, et al. Neoadjuvant triweekly nanoparticle albumin-bound paclitaxel followed by epirubicin and cyclophosphamide for Stage II/III HER2-negative breast cancer: evaluation of efficacy and safety. Jpn J Clin Oncol. 2015;45(7):642–649. doi: 10.1093/jjco/hyv055. [DOI] [PubMed]
- 11.Untch M, Jackisch C, Schneeweiss A. Nab-paclitaxel versus solvent-based paclitaxel in neoadjuvant chemotherapy for early breast cancer (GeparSepto-GBG 69): a randomised, phase 3 trial. Lancet Oncol. 2016;17(7):E270-E270. [DOI] [PubMed]
- 12.Tanaka S, Iwamoto M, Kimura K, et al. Phase II study of neoadjuvant anthracycline-based regimens combined with nanoparticle albumin-bound paclitaxel and trastuzumab for human epidermal growth factor receptor 2-positive operable breast cancer. Clin Breast Cancer. 2015;15(3):191–196. doi: 10.1016/j.clbc.2014.12.003. [DOI] [PubMed]
- 13.Network NCC. Clinical Practice Guidelines in Oncology. Breast Cancer Version 3. 2019. https://www.nccn.org/professionals/physician_gls/default.aspx. Published September 6, 2019. Accessed February 4, 2020.
- 14.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed]
- 15.Cancer Therapy Evaluation Program (CTEP). Common terminology criteria for adverse events (CTCAE) v4.0. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm. Accessed February 4, 2020.
- 16.Morita S, Kikumori T, Tsunoda N, et al. Feasibility of dose-dense epirubicin and cyclophosphamide with subcutaneous pegfilgrastim 3.6 mg support: a single-center prospective study in Japan. Int J Clin Oncol. 2018;23(1):195–200. doi: 10.1007/s10147-017-1177-z. [DOI] [PubMed]
- 17.Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48(3):452–458. doi: 10.1038/bmt.2012.244. [DOI] [PMC free article] [PubMed]
- 18.Rowinsky EK, Donehower RC. Paclitaxel (taxol). N Engl J Med. 1995;332(15):1004–1014. doi: 10.1056/nejm199504133321507. [DOI] [PubMed]
- 19.Wani MC, Taylor HL, Wall ME, Coggon P, McPhail AT. Plant antitumor agents .6. Isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus-Brevifolia. J Am Chem Soc. 1971;93(9):2325–2327. doi: 10.1021/ja00738a045. [DOI] [PubMed]
- 20.Loibl S, de la Pena L, Nekljudova V, et al. Neoadjuvant buparlisib plus trastuzumab and paclitaxel for women with HER2+primary breast cancer: a randomised, double-blind, placebo-controlled phase II trial (NeoPHOEBE). Eur J Cancer. 2017;85:133–145. doi: 10.1016/j.ejca.2017.08.020 [DOI] [PMC free article] [PubMed]
- 21.Untch M, Fasching PA, Konecny GE, et al. Pathologic complete response after neoadjuvant chemotherapy plus trastuzumab predicts favorable survival in human epidermal growth factor receptor 2-overexpressing breast cancer: results from the TECHNO trial of the AGO and GBG study groups. J Clin Oncol. 2011;29(25):3351–3357. doi: 10.1200/jco.2010.31.4930 [DOI] [PubMed]
- 22.Zhang P, Yin Y, Mo HN, et al. Better pathologic complete response and relapse-free survival after carboplatin plus paclitaxel compared with epirubicin plus paclitaxel as neoadjuvant chemotherapy for locally advanced triple-negative breast cancer: a randomized phase 2 trial. Oncotarget. 2016;7(37):60647–60656. doi: 10.18632/oncotarget.10607. [DOI] [PMC free article] [PubMed]
- 23.Futamura M, Nagao Y, Ishihara K, et al. Preoperative neoadjuvant chemotherapy using nanoparticle albumin-bound paclitaxel followed by epirubicin and cyclophosphamide for operable breast cancer: a multicenter phase II trial. Breast Cancer. 2017;24(4):615–623. doi: 10.1007/s12282-016-0748-6. [DOI] [PMC free article] [PubMed]
- 24.Kojima Y, Kawamoto H, Nishikawa T, et al. Feasibility study of weekly nanoparticle albumin-bound paclitaxel (150 mg/m(2)) followed by fluorouracil, epirubicin, and cyclophosphamide therapy as neoadjuvant chemotherapy for HER2-negative breast cancer. Clin Breast Cancer. 2018;18(5):374–379. doi: 10.1016/j.clbc.2018.01.002. [DOI] [PubMed]
- 25.Shigematsu H, Kadoya T, Masumoto N, et al. The efficacy and safety of preoperative chemotherapy with triweekly abraxane and cyclophosphamide followed by 5-fluorouracil, epirubicin, and cyclophosphamide therapy for resectable breast cancer: a multicenter clinical trial. Clin Breast Cancer. 2015;15(2):110–116. doi: 10.1016/j.clbc.2014.09.010. [DOI] [PubMed]
- 26.Cheang MCU, Chia SK, Voduc D, et al. Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst. 2009;101(10):736–750. doi: 10.1093/jnci/djp082. [DOI] [PMC free article] [PubMed]
- 27.Tokuda E, Horimoto Y, Arakawa A, et al. Differences in Ki67 expressions between pre- and post-neoadjuvant chemotherapy specimens might predict early recurrence of breast cancer. Hum Pathol. 2017;63:40–45. doi: 10.1016/j.humpath.2017.02.005. [DOI] [PubMed]
- 28.Untch M, Jackisch C, Schneeweiss A, et al. Nab-paclitaxel versus solvent-based paclitaxel in neoadjuvant chemotherapy for early breast cancer (GeparSepto-GBG 69): a randomised, phase 3 trial. Lancet Oncol. 2016;17(3):345–356. doi: 10.1016/s1470-2045(15)00542-2. [DOI] [PubMed]
- 29.Earl HM, Hiller L, Dunn JA, et al. Efficacy of neoadjuvant bevacizumab added to docetaxel followed by fluorouracil, epirubicin, and cyclophosphamide, for women with HER2-negative early breast cancer (ARTemis): an open-label, randomised, phase 3 trial. Lancet Oncol. 2015;16(6):656–666. doi: 10.1016/s1470-2045(15)70137-3. [DOI] [PubMed]
- 30.Foldi J, Mougalian S, Silber A, et al. Single-arm, neoadjuvant, phase II trial of pertuzumab and trastuzumab administered concomitantly with weekly paclitaxel followed by 5-fluoruracil, epirubicin, and cyclophosphamide (FEC) for stage I-III HER2-positive breast cancer. Breast Cancer Res Treat. 2018;169(2):333–340. doi: 10.1007/s10549-017-4653-2. [DOI] [PubMed]
- 31.Bonadonna G, Valagussa P, Moliterni A, Zambetti M, Brambilla C. Adjuvant cyclophosphamide, methotrexate, and fluorouracil in node-positive breast cancer: the results of 20 years of follow-up. N Engl J Med. 1995;332(14):901–906. doi: 10.1056/nejm199504063321401 [DOI] [PubMed]
- 32.Raza S, Welch S, Younus J. Relative dose intensity delivered to patients with early breast cancer: Canadian experience. Curr Oncol. 2009;16(6):393–397. doi. 10.3747/co.v16i6.311. [DOI] [PMC free article] [PubMed]