ABSTRACT
Sasa veitchii and other Sasa species are traditional medicinal herbs belonging to a group of Japanese bamboos collectively called Kumazasa, and these species possess the potential for a wide variety of uses. The present study aimed to elucidate the anticancer mechanisms exerted by S. veitchii extract (SE) against a human breast cancer cell line, MCF-7 cells. Freeze-dried Sunchlon® was used as the SE, and cell proliferation activity was measured using the [3H]-thymidine incorporation assay. Induction of apoptosis was assessed via Annexin V and caspase-3 fluorescent staining, the induction of necrosis was measured via propidium iodide staining, and cell cycle-related protein expression was determined using western blotting. The IC50 value of the SE was 7.7 μg/mL in MCF-7 cells. Although the primary active ingredient in Sunchlon® is sodium copper chlorophyllin (0.25%), the present results indicated that ingredients other than SCC exert anti-cancer activities (the IC50 value of SCC was 715 μg/mL), and late apoptosis or necrosis was induced in an SE dose-dependent manner. The expression levels of cyclin D1 and Cdk6 were decreased after SE treatment, and there was no change in the Cdk1/2 expression levels. Additionally, the expression of the necrosis-related cell death indicators RIP1 and RIP3 was increased in response to high-dose SE treatments, and this was indicative of cells preparing for programmed cell death. SE induces cell death in MCF-7 cells via the inhibition of cyclin D1 expression at low concentrations, and this extract induces programmed necrosis (necroptosis) by potentiating RIP1/RIP3 expression.
Key Words: Sasa veitchii, cyclin D1, breast cancer, sodium copper chlorophyllin
INTRODUCTION
Within the field of preventative medicine, extracts from natural products are increasingly being considered as a potential source for cancer prophylaxis. Sasa veitchii (S. veitchii) is a traditional medicinal herb that is derived from a genus of Japanese bamboos collectively called Kumazasa. The leaves contain various active components such as polysaccharides, lignin, and chlorophyll.1,2 Since ancient times, Kumazasa has primarily been used for the preserving of food due to its antimicrobial activity. Additionally, Kumazasa leaves have been used as folk medicine to treat stomach aches, diabetes, hypertension, and infectious diseases.3-5 In recent decades, several studies have been conducted to identify the mechanisms underlying the medicinal properties of some Sasa species. Our previous research has been particularly focused on the beneficial effects of S. veitchii, and we have already revealed that Sunchlon®, a S. veitchii extract (SE), contributes to the stability of liver function. For example, the anti-diabetes effects of SE are derived from the prevention of hepatic steatosis through the modulation of adipose tissue differentiation.6 Additionally, SE prevents drug-induced acute hepatitis by exerting hepato-protective effects.7 In a broader sense, the above pharmacological actions are all associated with the inhibition of the inflammatory response, and we therefore expect that SE could prove beneficial for the treatment of cancer, as inflammation is associated with precancerous lesions.
Few studies have been conducted to examine the anticancer effects of S. veitchii. Suzuki et al reported that S. veitchii leaf extracts exert toxic activity on human oral squamous cell lines,8 and this toxicity is more than doubled in tumor cell lines compared to that in normal cell lines. It is unclear, however, how the S. veitchii leaf extract was purified in their study. Additionally, no cytotoxic activity has been reported in other cancer cell lines. In regard to other Sasa species, Ren et al reported that Sasa quelpaertensis (also called Kumazasa) exerts anti-tumorigenic and anti-angiogenic activity in a spontaneous breast cancer mouse model.9 They used S. quelpaertensis extract that was derived from Sasa Health® (commercially marketed by Daiwa Biological Research Institute Co., Ltd.). Sasa Health® is an over-the-counter medicine, but its active ingredient is unknown. Based on this, although an anticancer effect of SE has been reported, the underlying mechanism remains unclear.
As described above, we have reported on several possible therapeutic mechanisms for SE from Sunchlon®. It is known that Sunchlon® contains 0.25% sodium copper chlorophyllin (SCC) as an active ingredient, and several epidemiological studies have demonstrated that a supplementary amount of SCC in natural foods decreases the risk of cancer.10 Additionally, SCC is reported to induce an anti-inflammatory response in lipopolysaccharide-stimulated macrophage cell lines.11 These data suggest that SCC may be involved in the anti-cancer activity of SE.
Based on this, in the present study, we examined the anticancer activity of SE and SCC using the human breast cancer cell line MCF-7 cells.
MATERIAL AND METHODS
Materials
S. veitchii extract was kindly provided by Sunchlon Co. Ltd (Nagano, Japan) in the form of the over-the-counter medicine Sunchlon® (2.82 g of S. veitchii leaf extract is dissolved in 1 mL of Sunchlon®).7 In our experiments, freeze-dried and powdered Sunchlon® (S. veitchii extract, SE) was prepared for use by dissolving it in phosphate buffered saline (PBS). SCC was purchased from Nacalai Tesque, Inc. (Kyoto, Japan). All other chemicals used in this study were of the highest grade of purity commercially available.
Cell culture
Human breast cancer cells (MCF-7) were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA) (Catalog#, ATCC HTB-22). MCF-7 cells were maintained in MEM containing 2 mM L-glutamine (Nacalai Tesque) that was supplemented with 10% FBS and a 1% MEM nonessential amino acid solution (Nakalai Tesque). MCF-7 cells were cultured at 37° C in a humidified atmosphere containing 5% CO2.
Cell proliferation assay
To examine cellular DNA synthesis (cell proliferation), we used the [3H]-thymidine incorporation assay. MCF-7 cells (3 × 103 cells/well) were seeded onto 96-well plates. After 24 h, cells were cultured in the presence of various concentrations of SE (0.1–1000 μg/mL) or SCC (0.25–250 μg/mL) for 24 h. Each culture was pulsed with 1.85 kBq of [3H]-thymidine for 2 h. To measure radioactivity, cell lysates were prepared by the addition of 100 μL of deionized water for 5 min. The lysates were added dropwise onto a glass filter (GA-100, Advantec, Tokyo, Japan) to adsorb cellular DNA, and they were then vacuumed and washed with 5 mL deionized water. Radioactivity of the glass filter was quantified using a liquid scintillation counter.
Detection of apoptotic and necrotic cells
To detect apoptotic and necrotic cells, we used the Apoptotic, Necrotic & Healthy Cells Quantification Kit (Biotium Inc, Fremont, CA, USA) according to the manufacturer’s instructions. MCF-7 cells (1 × 105 cells/well) were seeded onto 24-well plates and pre-cultured at 37° C for 24 h. The cells were then cultured in the presence of various concentrations of SE (10–1000 μg/mL) for an additional 6 h and stained with Annexin V-FITC, propidium iodide (PI), and Hoechst 33342. The presence of apoptotic and necrotic cells was qualitatively analyzed using fluorescence microscopy (EVOS® FLoid® Cell Imaging Station, Thermo Fisher Scientific, Waltham, MA, USA).
Caspase-3 assay
To measure apoptotic cells, we used a Dual Apoptosis Assay Kit with NucView 405 Caspase-3 Substrate (Biotium Inc) and an Annexin V-FITC Detection kit (Nacalai Tesque) according to the manufacturer’s instructions. MCF-7 cells (1 × 105 cells/well) were seeded onto 24-well plates and pre-cultured at 37° C for 24 h. The cells were then cultured with 10 μg/mL of SE for an additional 6 h and stained with Annexin V and NucView 405. The presence of apoptotic cells was qualitatively analyzed using fluorescence microscopy (EVOS® FLoid® Cell Imaging Station, Thermo Fisher Scientific).
Western blotting
MCF-7 cells (5 × 105 cells/well) were seeded onto 6-well plates and pre-cultured at 37º C for 24 h. The cells were then cultured in the presence of various concentrations of SE (10–1000 μg/mL) for another 24 h and harvested using RIPA buffer (Nacalai Tesque) supplemented with protease inhibitor cocktail (Nacalai Tesque). Harvested cells were centrifuged at 18,000 × g for 20 min at 4° C. The resulting supernatants from each sample were collected, and total protein levels were determined using the BCA protein kit (Nacalai Tesque). Protein samples (20 μg) were subjected to sodium dodecyl sulfate–polyacrylamide electrophoresis on a 10% gel and then transferred to a polyvinylidene difluoride (PVDF) membrane. Mouse anti-glycogen synthase kinase-3α/β (GSK-3α/β) monoclonal antibody (Santa Cruz Biotechnology, CA, USA), mouse anti-cyclin D1 monoclonal antibody (Santa Cruz Biotechnology), mouse anti-cyclin dependent kinase 6 (Cdk6) monoclonal antibody (Santa Cruz Biotechnology), mouse anti-Cdk1/2 monoclonal antibody (Santa Cruz Biotechnology), mouse anti-receptor-interacting protein 1 (RIP1) monoclonal antibody (Santa Cruz Biotechnology), mouse anti-RIP3 monoclonal antibody (Santa Cruz Biotechnology), and mouse anti-β-actin monoclonal antibody (MBL, Aichi, Japan) were used as primary antibodies (1:1500 dilution) for immunoblotting. A peroxidase-conjugated anti-mouse IgG (GE Healthcare Japan, Tokyo, Japan) was used as a secondary antibody (1:5000 dilution). Immunoreactive bands were visualized using the ECL system (LAS-1000®, GE Healthcare).
Statistical Analysis
Statistical analysis was performed using one-way analysis of variance (ANOVA) with Tukey-Kramer’s post-hoc test for multiple comparisons. All statistical analyses were performed using SPSS 24.0 software (Chicago, IL, USA), and values of p < 0.05 were considered statistically significant.
RESULTS
Cell proliferation assay
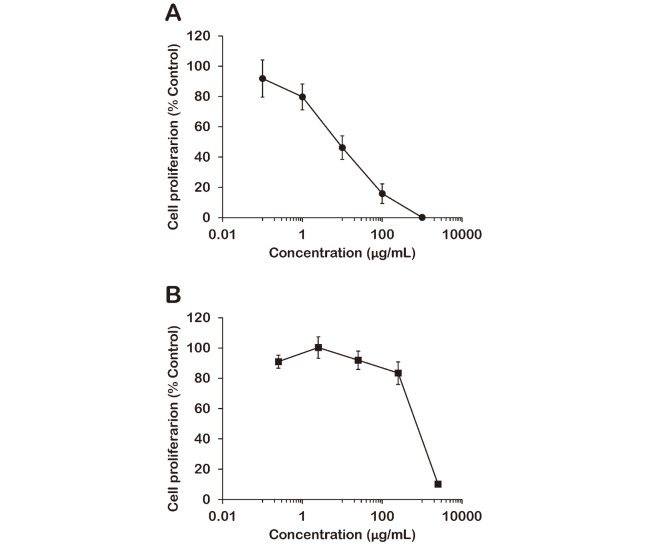
We determined cytotoxicity in MCF-7 cells treated with SE or SCC using a [3H]-thymidine incorporation assay. This assay provides an effective method for detecting proliferative activity by quantifying cellular DNA synthesis. The results are shown in Figure 1. The half-maximal inhibitory concentration (IC50) values of SE and SCC against MCF-7 cells were 7.72 μg/mL and 715 μg/mL, respectively. These values indicate that SE is approximately 100 times more cytotoxic than SCC. The SE we used contains SCC at 0.25%, and based on this, 1000 μg/mL of SE (which killed the vast majority of cells) contains 2.5 μg/mL of SCC. However, 2.5 μg/mL of SCC exerted almost no cytotoxic effect on MCF-7 cells. These results indicated that the cytotoxicity of SE is derived from an active ingredient other than SCC.
Fig. 1.
Cytotoxic activity of Sasa veitchii extract (SE) (A) and sodium copper chlorophyllin (SCC) (B) in a human breast cancer cell line (MCF-7 cells)
MCF-7 cells were treated with various concentrations of SE or SCC for 24 h at 37° C in a humidified atmosphere containing 5% CO2. Each plot indicates the proliferative activity relative to vehicle control (mean ± standard deviation (SD), n = 5).
Identification of apoptotic and necrotic cells
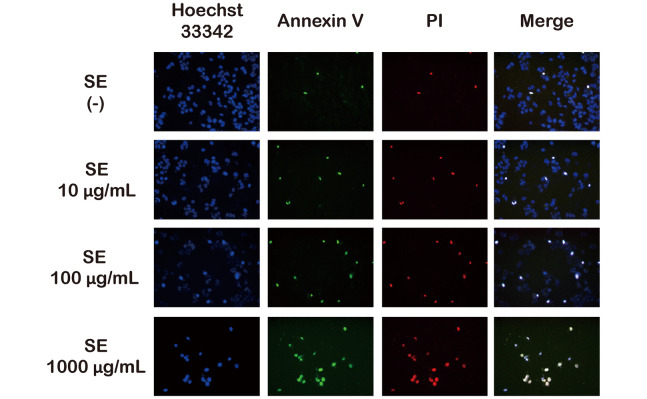
We assayed for living and apoptotic/necrotic cells using Hoechst 33342 (blue), Annexin V (green), and PI (red) fluorescent triple staining. The fluorescence microscopy images are shown in Figure 2. Very few cells in the control group were stained with Annexin V/PI, and treatment with SE increased the number of Annexin V/PI positive cells in a dose-dependent manner. Therefore, the double staining results indicate that SE increased the late apoptotic or necrotic activity of MCF-7 cells in a dose-dependent manner.
Fig. 2.
Identifying the induction of apoptosis and necrosis relative to SE concentration
MCF-7 cells were treated with various concentrations of SE for 6 h. Cells were triple-stained with Hoechst 33342 (blue), Annexin V-FITC (green), and propidium iodide (PI; red) and visualized using fluorescence microscopy.
Effect of SE on RIP1/RIP3 expression and caspase-3 levels
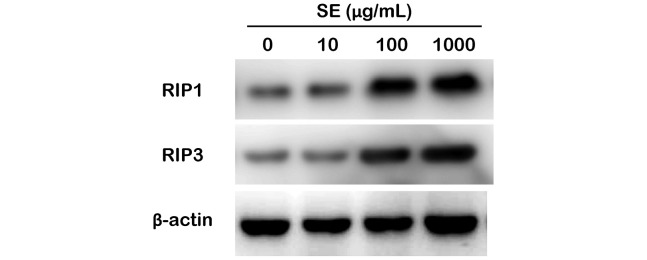
Annexin V and PI double positive staining is known to be present in both apoptotic and necrotic cells.12 To distinguish the necrotic cells, we measured RIP1 and RIP3 via immunoblotting, as RIP1 (also called RIPK1) and RIP3 (also called RIPK3) are key regulators of necrosis-induced cell death.13 As shown in Figure 3, 10 μg/mL of SE had no effect on RIP1 and RIP3 levels. SE strongly induced RIP1 and RIP3 expression at levels greater than 100 μg/mL in MCF-7 cells.
Fig. 3.
Evaluation of RIP1 and RIP3 expression levels relative to SE concentration
Western blot analysis using RIP1 and RIP3 antibodies were performed on total lysates from MCF-7 cells treated with SE for 24 h. Anti-β-actin immunoblotting was used as a loading control.
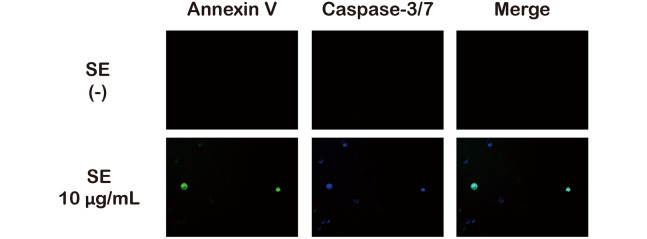
Additionally, we measured caspase-3 activity at 10 μg/mL to more directly detect apoptosis. As shown in Figure 4, cells that were positive for both Annexin V and caspase-3 were detected. These results suggest that SE treatment induced apoptosis at concentrations from 10 μg/mL and induced necroptosis at concentrations greater than 100 μg/mL.
Fig. 4.
Identifying the induction of caspase-3 activity relative to SE concentration
MCF-7 cells were treated with 10 μg/mL of SE for 6 h. Cells were double-stained with caspase-3 (blue) and Annexin V-FITC (green) and visualized using fluorescence microscopy.
Effects on the expression of cell cycle-related proteins
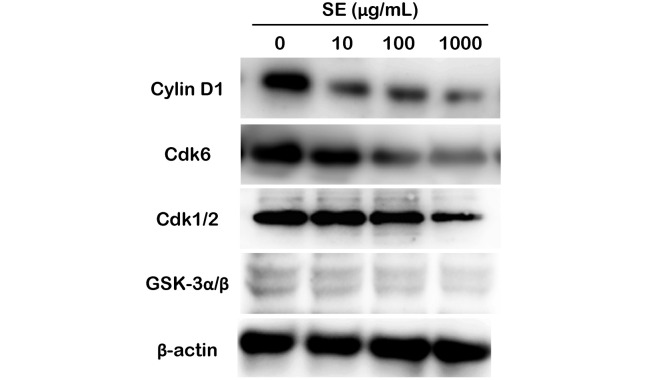
To estimate the expression of cell cycle-related proteins, we performed western blotting analysis as shown in Figure 5. Cyclin D1 and Cdk6 expression levels were decreased depending on SE concentration, while GSK-3α/β and Cdk1/2 expression levels remained constant. Cyclin D1 and Cdk6 are the key proteins that initiate cell cycle progression, and in contrast, Cdk1/2 is required to maintain cell cycle progression. Therefore, our results suggest that SE suppresses the initiation of the cell cycle rather than cell cycle progression.
Fig. 5.
Evaluation of cell cycle-related protein expression relative to SE concentration
Western blot analysis using cyclin D1, Cdk6, Cdk1/2, and GSK-3α/β antibodies were performed on total lysates from MCF-7 cells treated with SE for 24 h. Anti-β-actin immunoblotting was used as a loading control.
DISCUSSION
The present study we determined that SE-induced apoptosis was initiated at low doses of SE (10 μg/mL) in MCF-7 cells. SE treatment also decreased the expression of cyclin D1 and Cdk6. Cyclin D1 expression levels increase in response to mitogen stimulation, and this protein subsequently forms a complex with Cdk6.14 The cyclin-Cdk complex is a primary factor that induces cell-cycle initiation. The cyclin D1-Cdk6 complex inhibits the suppression of cell proliferation and advances the G1 phase of cell cycle.14 However, as SE did not influence the expression level of Cdk1/2, we speculated that its influence on the cell cycle is limited to the G1 phase. The cyclin D1-Cdk6 complex inhibits the induction of cellular apoptosis.15 Therefore, the decrease in the expression of cyclin D1 and Cdk6 may have induced apoptosis in MCF-7 cells. A number of cancer cells overexpress cyclin D, and this overexpression is strongly associated with the proliferation of cancer cells.16 As cyclin D1 is a downstream target of the HER2 signaling pathway,17 the effect of SE on HER2-positive cancers is of particular interest.
Cyclin D is degraded and inactivated by GSK-3β after completing its role within the cell.16 Based on our observation that SE did not affect the expression level of GSK-3α/β in MCF-7 cells, we believe that SE decreased the production of cyclin D1 rather than enhancing its degradation. Furthermore, high doses (greater than 100 μg/mL) of SE increased the expression of RIP1 and RIP3, which are biomarkers for programmed necrosis (necroptosis).13 Although the mechanisms underlying the RIP pathway have not been fully elucidated, this pathway has gained attention as a potential mechanism to promote anticancer effects.
“Kumazasa” is a traditional medicinal herb that is frequently used throughout Japan. In many cases, the name Kumazasa is used not only for S. veitchii, but also for several other variants. In Japan, many Sasa species are commercially produced and are extracted using different methods. In the present study, we used S. veitchii extract obtained from Sunchlon Co. Ltd. The main active ingredient in S. veitchii extract (Sunchlon®) is SCC, which provides nourishment. Min et al reported anticancer activity in the colon by S. quelpaertensis, a distinct species of plant from S. veitchii.18 Additionally, the authors revealed that the active ingredients in S. quelpaertensis were not p-coumaric acid or tricin. Using cancer cell lines other than colon lines, Jang et al also concluded that p-coumaric acid is not the main anti-cancer component of S. quelpaertensis Nakai.19 As mentioned above, the active ingredient in Sasa Health® responsible for its anti-breast cancer activity has not been elucidated.9 Sasa Health® is an alkaline extract of Sasa senanensis rehder or Sasa albo-marginata. The anticancer effects of lignin and various polysaccharides have also been determined20; however, there is a lack of evidence regarding Sasa sp., and the mechanism by which Sasa extracts function has not been extensively investigated. In the present study, we were unable to determine the active ingredient in SE. The SE used in the present study contained SCC in abundance (0.25%), and SCC has been reported to exert anticancer activity by suppressing cyclin D1 expression in MCF-7 cells.21 However, to achieve cyclin D1 suppression by SCC alone, the required SCC concentration was 400 μg/mL. In the present study, we demonstrated an anticancer effect for SE at 10 μg/mL. Additionally, from our previous report, the analysis of SE composition revealed several peaks that were not specific for SCC.22 Therefore, we ruled out the possibility that the anticancer activity of SE was derived solely from SCC. We are currently attempting to isolate the individual components of SE using various organic solvent/water extractions. In the future, as analysis of the components of S. veitchii and other Sasa sp. progresses, our goal is to further elucidate the identity of the anticancer ingredients within these extracts.
In conclusion, we demonstrated that SE treatment induces apoptosis at low doses and necroptosis at high doses. Although the active components of SE have not been fully elucidated, the results of our investigation may be helpful for improving cancer prevention and quality of life for cancer patients.
ACKNOWLEDGEMENT
This research was financially supported by a Kinjo Gakuin University Research Grant.
The authors thank Professor Hideki Mizutani (Kinjo Gakuin University, Japan) for his kind support. We would also like to thank Editage (www.editage.jp) for English language editing.
COMPETING INTERESTS
The authors declare no competing interests.
Abbreviations
- Cdk
cyclin dependent kinase
- GSK-3α/β
glycogen synthase kinase-3α/β
- PBS
phosphate buffered saline
- RIP
receptor interacting protein
- PVDF
polyvinylidene difluoride
- PI
propidium iodide
- SCC
sodium copper chlorophyllin
- SE
Sasa veitchii extract
REFERENCES
- 1.Bai YY, Xiao LP, Shi ZJ, Sun RC. Structural variation of bamboo lignin before and after ethanol organosolv pretreatment. Int J Mol Sci. 2013;14(11):21394–21413. [DOI] [PMC free article] [PubMed]
- 2.Cao X, Zhong L, Peng X, et al. Comparative study of the pyrolysis of lignocellulose and its major components: characterization and overall distribution of their biochars and volatiles. Bioresour Technol. 2014;155:21–27. [DOI] [PubMed]
- 3.Iwata K, Naito E, Yamashita K, et al. Anti pseudorabies virus activity of kumazasa extract. Biocontrol Sci. 2010;15(4):123–128. [DOI] [PubMed]
- 4.Nirmala C, Bisht MS, Bajwa HK, Santosh O. Bamboo: a rich source of natural antioxidants and its applications in the food and pharmaceutical industry. Trends Food Sci. Technol. 2018;77:91–99.
- 5.Park EJ, Jhon DY. The antioxidant, angiotensin converting enzyme inhibition activity, and phenolic compounds of bamboo shoot extracts. Food SciTechn. 2010;43(4):655–659.
- 6.Yoshioka H, Mori M, Fujii H, Nonogaki T. Sasa veitchii extract reduces obesity-induced insulin resistance and hepatic steatosis in obese mice fed a high-fat diet. Nagoya J Med Sci. 2017;79(3):279–290. [DOI] [PMC free article] [PubMed]
- 7.Yoshioka H, Usuda H, Fujii H, Nonogaki T. Sasa veitchii extracts suppress acetaminophen-induced hepatotoxicity in mice. Environ Health Prev Med. 2017;22(1):54. [DOI] [PMC free article] [PubMed]
- 8.Suzuki R, Matsuno S, Sakagami H, Okada Y, Shirataki Y. Search of new cytotoxic crude materials against human oral squamous cell carcinoma using 1H NMR-based metabolomics. Anticancer Res. 2014;34(8):4117–4120. [PubMed]
- 9.Ren M, Reilly RT, Sacchi N. Sasa health exerts a protective effect on Her2/NeuN mammary tumorigenesis. Anticancer Res. 2004;24(5A):2879–2884. [PubMed]
- 10.Nagini S, Palitti F, Natarajan AT. Chemopreventive potential of chlorophyllin: a review of the mechanisms of action and molecular targets. Nutr Cancer. 2015;67(2):203–211. [DOI] [PubMed]
- 11.Yun CH, Jeon YJ, Yang Y, Ju HR, Han SH. Chlorophyllin suppresses interleukin-1 beta expression in lipopolysaccharide-activated RAW 264.7 cells. Int Immunopharmacol. 2006;6(2):252–259. [DOI] [PubMed]
- 12.Crowley LC, Marfell BJ, Scott AP, Waterhouse NJ. Quantitation of apoptosis and necrosis by annexin V binding, propidium iodide uptake, and flow cytometry. Cold Spring Harb Protoc. 2016;2016(11):953–957. [DOI] [PubMed]
- 13.de Almagro MC, Goncharov T, Izrael-Tomasevic A, et al. Coordinated ubiquitination and phosphorylation of RIP1 regulates necroptotic cell death. Cell Death Differ. 2017;24(1):26–37. [DOI] [PMC free article] [PubMed]
- 14.Baker SJ, Reddy EP. CDK4: a key player in the cell cycle, development, and cancer. Genes Cancer. 2012;3(11–12):658–669. [DOI] [PMC free article] [PubMed]
- 15.Alves CL, Elias D, Lyng M, et al. High CDK6 protects cells from fulvestrant-mediated apoptosis and is a predictor of resistance to fulvestrant in estrogen receptor-positive metastatic breast cancer. Clin Cancer Res. 2016;22(22):5514–5526. [DOI] [PubMed]
- 16.Qie S, Diehl JA. Cyclin D1, cancer progression, and opportunities in cancer treatment. J Mol Med (Berl). 2016;94(12):1313–1326. [DOI] [PMC free article] [PubMed]
- 17.Basso AD, Solit DB, Munster PN, Rosen N. Ansamycin antibiotics inhibit Akt activation and cyclin D expression in breast cancer cells that overexpress HER2. Oncogene. 2002;21(8):1159–1166. [DOI] [PMC free article] [PubMed]
- 18.Min SJ, Lim JY, Kim HR, Kim SJ, Kim Y. Sasa quelpaertensis leaf extract inhibits colon cancer by regulating cancer cell stemness in vitro and in vivo. Int J Mol Sci. 2015;16(5):9976–9997. [DOI] [PMC free article] [PubMed]
- 19.Jang M, Ko H, Kim S. Effect of Sasa quelpaertensis Nakai extracts and its constituent p -coumaric acid on the apoptosis of human cancer cell lines. Natural Product Sciences. 2018;24:293.
- 20.Barapatre A, Meena AS, Mekala S, Das A, Jha H. In vitro evaluation of antioxidant and cytotoxic activities of lignin fractions extracted from Acacia nilotica. Int J Biol Macromol. 2016;86:443–453. [DOI] [PubMed]
- 21.Chiu LC, Kong CK, Ooi VE. The chlorophyllin-induced cell cycle arrest and apoptosis in human breast cancer MCF-7 cells is associated with ERK deactivation and Cyclin D1 depletion. Int J Mol Med. 2005;16(4):735–740. [PubMed]
- 22.Yoshioka H, Nonogaki T, Fukaya S, et al. Sasa veitchii extract protects against carbon tetrachloride-induced hepatic fibrosis in mice. Environ Health Prev Med. 2018;23(1):49. [DOI] [PMC free article] [PubMed]