ABSTRACT
Impaired wound healing is one of the most common complications of diabetes, and is known to be caused by multiple complicated factors. For instance, impaired angiogenesis, neuropathy, and hyperglycemia all function to delay subsequent wound closure. Alternatively, moist wound healing, which provides an appropriate environment for wounds, was reported to permit rapid healing by managing wound exudate. Accordingly, wound dressing materials that facilitate moist healing have been developed. The present study sought to clarify the effects of wound dressing material for moist healing of diabetic wounds, in terms of the dynamics of angiogenic factors and macrophages, using a mouse model of naturally occurring diabetes. Wounds with full-thickness skin defects were inflicted on the backs of mice and covered with dressing materials of hydrogel or gauze (control), which were retained for 3, 5, 7, 10, or 14 days following wound generation. During this time, the localization of neutrophils, fibroblasts and macrophages as well as the expression of vascular endothelial growth factor (VEGF) in the wounds and surrounding areas was observed each day. Healing clearly occurred in the hydrogel group with an increase in neutrophils and the angiogenic factor, VEGF. Moreover, the use of hydrogel resulted in a rapid rise in M1 macrophages, which appeared in the early stage of the injury, as well as rapid subsequent appearance of M2 macrophages. Thus, herein, we demonstrate that the formation of a moist environment via wound dressing material effectively improves diabetic wound healing.
Key Words: hydrocolloid dressing, wound healing, M2 macrophage, diabetic mice
INTRODUCTION
Approximately 190 million people worldwide suffer from diabetes with the number expected to double by 2030.1,2 Impaired wound healing, one of the most common complications of diabetes, leads to chronic non-adhesive ulcers that can ultimately result in infections, gangrene, and even amputation.3-5 Although there are various treatment options available for diabetic wounds, their effectiveness remains extremely limited.6,7 Delayed wound healing in diabetes is caused by multiple complicated factors including irregular leukocyte recruitment and phagocytosis, production of cytokines and growth factors, formation of the extracellular matrix as well as migration and proliferation of keratinocytes and fibroblasts.8 In addition, impaired angiogenesis, neuropathy, and hyperglycemia all act to severely delay wound closure in diabetics.9,10 Therefore, wound healing in these patients is an urgent issue that requires further investigation.
Moist wound healing provides an appropriate environment for wounds by managing wound exudate, which leads to rapid healing. Winter et al first reported a study in pigs supporting the theory that moist wounds epithelialized faster than dry wounds.11 They also showed that air-drying wounds delayed epithelialization.12 The advantage of moist wound healing has also been clinically proven by Hinman.13 Further, it has been shown that the essential factors involved in promoting fibroblast proliferation include, formation of the extracellular matrix, such as collagen, and angiogenesis for wound healing, including cell growth factors such as transforming growth factor-β (TGF-β), platelet-derived growth factor (PDGF), fibroblast growth factor (FGF), epidermal growth factor (EGF), vascular endothelial growth factor (VEGF), and hepatocyte growth factor (HGF). Moreover, interleukin (IL)-6 has been reported to be involved in fibroblast proliferation, while IL-22 is important for epidermopoiesis.14 Hence, optimal wound healing is achieved by maintaining a moist environment, allowing for continued release of cellular components and humoral factors.
The process of moist wound healing, is significantly affected by the specific wound dressing materials chosen. An initial characterization performed by Seaman15 reported on the development of various types of wound dressing materials with various features. Specifically, polyurethane films and hydrocolloid composite membranes were the initial products developed for these purposes and as such have been commercially available since 1971. Hydrocolloid composite membranes promote migration of epidermal cells and accelerate healing while preventing the adhesion to wounds and scab formation that is commonly observed in dry wounds.13
Studies have also reported moist healing as a means to reduce the pain unique to superficial wounds, by improving wound closure and preventing nerve endings being exposed to the air.16 Further, Metzeger reported the value provided by moist dressing in reduced labor for medical staff.17 However, the fundamental mechanism by which hydrocolloid dressings promote diabetic wound healing remains unclear. In fact, reports have provided contradictory evidence to that presented above. For instance, Piaggesi reported no significant difference in the healing times of diabetic wounds between conventional gauze and hydrocolloid dressings, as assessed through an analytical epidemiological study.18,19
The aim of this study, therefore, was to clarify the fundamental mechanism of hydrocolloid dressings in promoting diabetic wound healing, while providing further evidence for the effects elicited by moist healing environments. To this end, we used a mouse model of naturally occurring diabetes and prepared full-thickness skin defects to assess wound healing and examine the process of wound closure by topical application of hydrogel wound dressings. Wound repair was assessed using a semi-quantitative analysis of neutrophils, collagen, and angiogenesis; while also evaluating the re-epithelialization of wounds. Furthermore, the correlation of M2 macrophage polarization and proper functioning with healing rate was assessed.
MATERIALS AND METHODS
This animal study was performed according to protocols reviewed and approved by the ethics committee for animal experimentation (201910014, Chubu Univ.).
Animals
Eight-week-old diabetic male mice (a mouse model of diabetes, C57BLKS/J Air − + Leprdb/ + Leprdb, Japan SLC, Inc) (db/db) and 8-week-old male control mice (C57BL/6JJmsSlc) (Control) were used in the study. Mice were provided food and water ad libitum, and were reared under specific-pathogen-free conditions under controlled temperature (22 ± 2°C), humidity (50±10%), and light cycle (12-h light/12-h dark).
Surgical procedure
Mice were divided into four groups: control mice with hydrogel dressing (Control-H), diabetic mice with hydrogel dressing (db/db-H), control mice with gauze dressing (Control-G), and diabetic mice with gauze dressing (db/db-G).
The surgery was performed under general anesthesia with intraperitoneal injection of 10 to 15 mg/kg Somnopentyl (pentobarbital sodium). Two circular (major axis: approximately 1.0 cm, minor axis: approximately 0.6 cm) full-thickness skin defects from the epidermis through the hypodermis were prepared on the back with surgical scissors, following removal of fur, and marked with a dermal curette (Kai Medical Japan). The wounds generated were logged (denoted by day 0), immediately covered with DuoACTIVE®ET (hydrogel; ConvaTec Japan) or gauze, and wrapped with Coban™ to prevent the dressing materials from peeling off naturally. The dressing materials were retained for 0.5, 1, 2, 3, 5, 7, 10, or 14 days after wound generation, after which they were removed to assess the wounds. The healing process from day 14 was tracked after removal of the dressing materials until day 21 after wound generation. Gauze that had become adhered to the wound was removed only if it could be readily peeled away naturally.
Tissue Processing
Mice were euthanized via cardiac blood withdrawal on days 3 (n = 20), 5 (n = 18), 7 (n = 18), 10 (n = 16), 14 (n = 20), and 21 (n = 9) after preparation of full-thickness skin defects. Immediately after euthanasia was administered samples including the wound area as well as the surrounding normal skin, were collected. Following post-fixation with 4% paraformaldehyde at 4°C, samples were embedded in paraffin. Sections were cut from the central region of the wound at a thickness of 4 µm. Before staining, paraffin sections were deparaffinized and rehydrated by successive passages through xylene. Tissue sections were then stained with hematoxylin and eosin (HE) and immunostained.
Approximately ten histological HE images were combined for optical microscopic observations (40×) of wounds and surrounding normal tissue. Specifically, the degree of wound healing was assessed by observing the formation of the epidermis and dermis in each group.
Immunohistochemical Examinations
The presence of multiple factors was assessed in samples from day 0–21. Specifically, the presence of neutrophils was detected on days 3, 5, 7, and 14 after wound generation; VEGF was detected on days 3, 5, 7, 10, and 14; collagen I and collagen III were detected on days 3, 5, 7, 14, and 21; and TGF-β and TNF-α were detected on days 0, 0.5, 1, 2, 3 and 5 to examine the presence of M2 and M1 macrophages, respectively. The following primary antibodies were used for this study: anti-neutrophil (rat-IgG: Cat. No. ab2557, Abcam), anti-VEGF (rabbit-IgG: Cat. No. ab46154, Abcam), anti-collagen III (rabbit-IgG: Cat. No. ab7778, Abcam), anti-collagen I (rabbit-IgG: Cat. No. NB600-408, Novus), anti-TGF-β (rabbit-IgG: Cat. No. ab31013, Abcam), and anti-TNF-α (mouse-IgG: Cat. No. ab1793, Abcam) antibodies. For color development and counterstaining, the 3,3’-diaminobenzidine (DAB) reagent kit (SK-4100 : VECTOR) and hematoxylin were used, respectively, according to manufacturers’ instructions followed by according to a usual method for dehydration, penetration, and mounting.
Analysis
Low magnification images are presented as a means to demonstrate the location of positive-stained cells within the wounds; allowing for both the wound area and the normal range to be visualized in a single field. The degree of positivity detected via immunostaining was semi-quantitatively enumerated. For the sections stained with an anti-neutrophil antibody, the proportions of the positive cells in one field of view (magnification: 200 ×) were classified into – to +++, where – indicated no positivity, + indicated positivity < 20%, ++ indicated 30% ≤ positivity < 50%, and +++ indicated positivity ≥ 50%. The semi-quantified data were then enumerated and presented as percent, which do not represent numerical values but rather indicate evaluation of the area. To classify the proportion of cells positively stained with anti-VEGF, anti-collagen III, or anti-collagen I antibodies, in one field of view (magnification: 200×) were classified into ± to +++ where ± indicated positivity ≤ 10%, + indicated 10% ≤ positivity < 50%, ++ indicated 50% ≤ positivity < 80%, and +++ indicated positivity ≥ 80%, the results of which were then enumerated.
For those stained with an anti-M1 macrophage or anti-M2 macrophage antibody, the proportions of positivity in one field of view (magnification: 200×) were classified into – to ++, where – indicated no positivity, ± indicated positivity < 10%, + indicated 20% ≤ positivity < 50%, and ++ indicated positivity ≥ 50%.
RESULTS
Gross assessment of wounds
As a function of the dressing material, in most cases the wounds in both the Control-G and db/db-G groups became dry and stuck to the gauze, whereas those in both the Control-H and db/db-H groups were maintained in a moist condition throughout the experimental period.
The wounds in the Control-G and db/db-G groups had been stuck to the gauze on after day 5, whereas those on day 14 became dry and allowed the gauze to easily peel away. A slight reduction in the size of the wounds was observed on day 14, as shown in Figure 1, although the surfaces of the wounds were rough. Alternatively, the wounds on day three in both the Control-H and db/db-H groups were moist and their sizes were comparable with those on day 0, immediately after wound generation. Moreover, the wounds gradually reduced in size to form new skin on day 14. A comparison between the db/db-G and db/db-H groups on day 14 clearly showed that the size and surface morphology of the wounds were smaller and smoother in the hydrogel group (Figure 1).
Fig. 1.
Representative images of full-thickness skin defects in db/db and control mice, at 0 and 14 days post-surgery.
upper: hydrogel, bellow: gauze.
Histological assessment of wounds
On day seven after wound generation, full-thickness skin defects remained unchanged in many db/db-G mice as shown in Figure 2. In contrast, formation of the epidermis began in some areas (depending on the mouse) in the Control-G and db/db-H groups, while scars were also observed. Formation of the epidermis was also observed in some wounds in the Control-H group.
Fig. 2.
Transmitted light images of HE stained sections of the defects in db/db and control mice, treated with hydrogel or gauze at 3, 7, and 14 days post-surgery (Scale bar = 500um).
* indicates the area of full-thickness skin defects, arrow is indicating the presence of scars. From day 3 to 14 defective skin was reconstituted.
On day 14, wounds in the db/db-G group had begun to form dermis in some areas and scars were also observed (Figure 2). The formation of the epidermis was observed in the Control-G and the db/db-H groups, although it was incomplete in some mice where the surfaces were rough and scars were observed. In contrast, the epidermis formed with a smooth surface overall in the Control-H group.
Dynamics during the process of wound healing
On days 3 and 5 after wound generation, neutrophils were observed only at the boundary of each wound in all groups, as there was an absence of all tissue layers in the center of the wound, as shown in Figure 3. On day 14, neutrophils were observed not only at the boundaries, but also at the centers of wounds in all groups due to the progression of wound healing. In addition, more neutrophils were observed in the epidermis than in the dermis. The semi-quantified results showed that the amount of neutrophils tended to decrease in all groups on and after day three, as shown in Figure 4. Further, fewer neutrophils appeared in the db/db-G group compared to the db/db-H group on all days examined.
Fig. 3.
Immunohistochemical staining for neutrophils, VEGF, collagen I and collagen III in hydrogel and gauze groups at 3, 5, 7, 14 and 21 days post-surgery (Scale bar = 200um, ND: no data).
Inset: higher magnification image of the square area showing neutrophil-positive cells (arrowhead). Arrows indicate the boundary between defects and normal skin. Numerous immuno-positive cells were observed near the boundary area.
Fig. 4.
Semi-quantification analysis of anti-neutrophil antibody classified into ± to +++; ±: positivity ≤ 10%, +: 10% ≤ positivity <5 0%, ++: 50% ≤ positivity < 80%, +++: positivity ≥ 80%.
Semi-quantification analysis of anti-VEGF antibody, anti-collage I antibody, and anti-collagen III antibody, classified into ± to +++; ±: positivity ≤ 10%, +: 10% ≤ positivity < 50%, ++: 50% ≤ positivity < 80%, +++: positivity ≥ 80%. Neutrophil and VEGF levels were evaluated on days 3, 5, 7, and 14 post-surgery. Collagen I and collagen III were evaluated on days 3, 5, 7, 14, and 21 post-surgery.
On days 3 and 5 after wound generation, VEGF was observed only at the boundary of each wound in all groups, as no tissue existed in the center. On and after day seven, VEGF was observed only in the epidermis in the db/db-G group, whereas it was observed in both the epidermis and dermis in the db/db-H group. The semi-quantified results showed that VEGF tended to gradually increase in a time-dependent manner until day ten, after which it was observed to decrease in the db/db-G group, while its peak shifted to an earlier period of day seven in the db/db-H group (Figure 4).
On days 3 and 5 after wound generation, collagen III was also observed only at the boundary of each wound. On and after day seven, most collagen III was observed in the dermis. The semi-quantified results show that collagen III tended to increase after day 14 in the db/db-G group, whereas it tended to increase after day seven in the db/db-H group (Figures 3 and 4).
On days 3 and 5 after wound generation, collagen I was also observed only at the boundary of each wound. On and after day 7, most collagen I was observed in the dermis. The semi-quantified results showed little change in the amounts of collagen I over time in both db/db-H and db/db-G groups (Figures 3 and 4).
Involvement of polarized macrophages
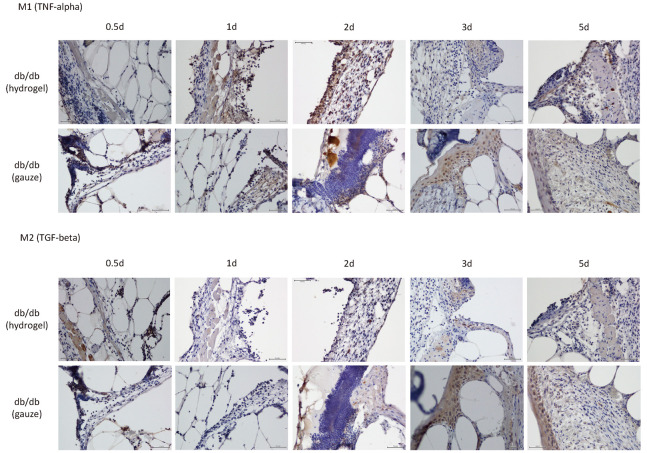
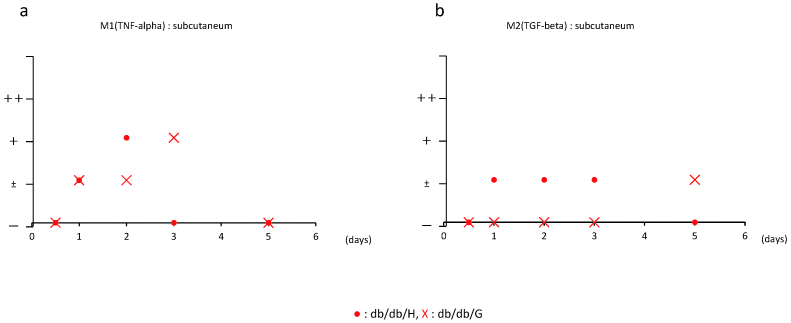
On day one after wound generation, M1 macrophages were observed at the boundary of the wound in both groups, as shown in Figure 5. Their appearance peaked on day three, however, became unobservable by day five in the db/db-G group. The peak appearance of M1 macrophages shifted to an earlier period of day two in the db/db-H group, as shown in Figure 5.
Fig. 5.
Immunohistochemical staining of the boundary area between defective and normal tissue for M1 and M2 at 0.5, 1, 2, 3, and 5 days post-surgery (Scale bar = 100um).
No positive cells were in the defective area at 0.5 day.
M2 macrophages did not appear until day five in the db/db-G group. Conversely, they appeared at the boundary of the wound on day one after wound generation and became undetectable by day five in the db/db-H group (Figures 5 and 6).
Fig. 6.
Semi-quantification analysis of anti-M1 and M2 antibody classified into – to ++; –: no positivity, ±: positivity < 10%, +: 20% ≤ positivity <50%, ++: positivity ≥ 50%, at 0.5, 1, 2, 3, and 5 days post-surgery.
DISCUSSION
Moist wound healing, which provides moisture to skin wounds, has been broadly applied over recent years with hydrocolloid dressings used as the primary material. However, the pathogenicity associated with diabetic wound healing is intricate as the healing process of damaged tissues includes a number of different cell types as well as their individual interactions with extracellular matrix macromolecules.20 Hence, the precise mechanism by which hydrocolloid dressings promote diabetic wound healing has not been fully characterized.
The present study explored the potential role of hydrocolloid dressing in a mouse model of diabetic wound healing. The purpose of this study was to investigate the repair mechanism of hydrocolloid wounds using dry gauze as a control in a diabetic model mice. Our results showed that re-epithelialization and angiogenesis, which are indications of wound healing, are clearly accelerated in diabetic mice treated with hydrogel, as shown in Figures 1 and 2. In these wounds, leukocyte infiltration clearly increased, particularly in the early stages of healing, while inflammatory M1-polarized macrophages decreased rapidly and anti-inflammatory M2-polarized macrophages were detectable (shown in Figures 5 and 6). These data demonstrate that hydrogel plays a critical role in regulating diabetic wound healing by suppressing inflammation. In addition, given the increasing number of vascular cells together with increased expression of VEGF (seen in Figures 3 and 4), our data also demonstrates that new blood vessel formation is evidently promoted in diabetic wounds treated with hydrogel. The promotion of blood vessel growth is expected to facilitate the emergence of fibroblasts and vascular endothelial cells, as well as inflammatory cells (due to topical inflammation caused by the wound), while also enhancing production of the extracellular matrix, including collagen and regenerated granulation tissues. Indeed, an increase in collagen deposition was seen in Figures 3 and 4. The delayed skin regeneration during the later stages of the wound healing process in diabetic mice not treated with hydrogel may be caused by a delay in the formation of extracellular matrix components, allowing for the adhesion of collagen and fibroblasts, and insufficient formation of basement membrane components, despite collagen deposition.
The present study also shows that the application of hydrogel promotes polarization of wound macrophages to the alternately activated phenotypes or M2 phenotype. Our results strongly support the following previously reported conclusions. Macrophages, which are involved in all stages of wound healing, promote inflammation in the early stages and regulate the process of wound repair by activating the proliferation of fibroblasts, keratinocytes, and endothelial cells.21,22 Alternatively, studies have shown that macrophage depletion during the proliferation stage significantly impedes transition to the intermediate stage of repair.23 It should be noted that the macrophage function/phenotype switch does not readily occur in diabetic wounds and that macrophages are maintained primarily in an activated pro-inflammatory M1 state, causing prolonged chronic inflammation.24
In conclusion, the present study demonstrates that hydrogel application clearly contributes to diabetic wound healing and can be associated with collagen accumulation, promotion of angiogenesis, and induction of M2 macrophage production, particularly in wounds. However, the present study is limited, as control of blood glucose levels (which is indispensable in the actual therapeutic settings) was not performed. Moreover, we aseptically prepared wounds and excluded infected wounds in the present study. This was in an effort to prevent the complex biological activities of the bacteria from overcomplicating the results and their subsequent interpretations. Future research will address the control of blood glucose levels and utilize an approach that allows for the assessment of infected wounds. Taken together, these results suggest that the signal dynamics caused by hydrogel treatment could be therapeutically useful for the treatment of uninfected diabetic skin wounds in hyperglycemia.
CONCLUSIONS
We examined the effect of hydrocolloid dressing with hydrogel on the healing of wounds generated in diabetic mice. The hydrogel treatment promoted angiogenesis in wounds and increased macrophage polarization to the M2 phenotype. The elevated M2 macrophages resulting from the hydrogel treatment in turn promoted proliferation of fibroblasts. Thus, our study has demonstrated that treatment with hydrocolloid dressings contributes to diabetic wound healing (uninfected) in hyperglycemia.
ACKNOWLEDGMENTS
We would like to thank Editage (www.editage.jp) for English language editing.
CONFLICT OF INTERESTS
The authors have no conflicts of interest to declare.
Abbreviations
- db/db
a mouse model of diabetes, C57BLKS/J Air − + Leprdb/ + Leprdb,
- EGF
Epidermal growth factor
- FGF
fibroblast growth factor
- HGF
hepatocyte growth factor
- IL
Interleukin
- PDGF
platelet-derived growth factor
- TGF-β
transforming growth factor-β
- VEGF
vascular endothelial growth facto
REFERENCES
- 1.Whiting DR, Guariguata L, Weil C, Shaw J. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract. 2011;94(3):311–321. [DOI] [PubMed]
- 2.Sudharsanan N, Ali MK, Mehta NK, Narayan KM. Population aging, macroeconomic changes, and global diabetes prevalence, 1990–2008. Popul Health Metr. 2015;13:33. [DOI] [PMC free article] [PubMed]
- 3.Greenhalgh DG. Wound healing and diabetes mellitus. Clin Plast Surg. 2003;30(1):37–45. [DOI] [PubMed]
- 4.Peters EJ, Lipsky BA. Diagnosis and management of infection in the diabetic foot. Med Clin North Am. 2013;97(5):911–946. [DOI] [PubMed]
- 5.Noor S, Khan RU, Ahmad J. Understanding Diabetic Foot Infection and its Management. Diabetes Metab Syndr. 2017;11(2):149–156. [DOI] [PubMed]
- 6.Clokie M, Greenway AL, Harding K, et al. New horizons in the understanding of the causes and management of diabetic foot disease: report from the 2017 Diabetes UK Annual Professional Conference Symposium. Diabet Med. 2017;34(3):305–315. [DOI] [PubMed]
- 7.Dinh T, Tecilazich F, Kafanas A, et al. Mechanisms involved in the development and healing of diabetic foot ulceration. Diabetes. 2012;61(11):2937–2947. [DOI] [PMC free article] [PubMed]
- 8.Ahmed AS, Antonsen EL. Immune and vascular dysfunction in diabetic wound healing. J Wound Care. 2016;25(suppl 7):S35–S46. [DOI] [PubMed]
- 9.Yang P, Pei Q, Yu T, et al. Compromised Wound Healing in Ischemic Type 2 Diabetic Rats. PLoS One. 2016;11(3):e0152068. [DOI] [PMC free article] [PubMed]
- 10.Pradhan L, Nabzdyk C, Andersen ND, LoGerfo FW, Veves A. Inflammation and neuropeptides: the connection in diabetic wound healing. Expert Rev Mol Med. 2009;11:e2. [DOI] [PMC free article] [PubMed]
- 11.Winter GD. Formation of the scab and the rate of epithelization of superficial wounds in the skin of the young domestic pig. Nature. 1962;193:293–294. [DOI] [PubMed]
- 12.Winter GD, Scales JT. Effect of air drying and dressings on the surface of a wound. Nature. 1963;197:91–92. [DOI] [PubMed]
- 13.Hinman CD, Maibach H. Effect of Air Exposure and Occlusion on Experimental Human Skin Wounds. Nature. 1963;200:377–378. [DOI] [PubMed]
- 14.McGee HM, Schmidt BA, Booth CJ, et al. IL-22 promotes fibroblast-mediated wound repair in the skin. J Invest Dermatol. 2013;133(5):1321–1329. [DOI] [PMC free article] [PubMed]
- 15.Seaman S. Dressing selection in chronic wound management. J Am Podiatr Med Assoc. 2002;92(1):24–33. [DOI] [PubMed]
- 16.Friedman SJ, Su WP. Management of leg ulcers with hydrocolloid occlusive dressing. Arch Dermatol. 1984;120(10):1329–1336. [PubMed]
- 17.Metzger S. Clinical and financial advantages of moist wound management. Home Healthc Nurse. 2004;22(9):586–590. [DOI] [PubMed]
- 18.Jude EB, Apelqvist J, Spraul M, Martini J; Silver Dressing Study G. Prospective randomized controlled study of Hydrofiber dressing containing ionic silver or calcium alginate dressings in non-ischaemic diabetic foot ulcers. Diabet Med. 2007;24(3):280–288. [DOI] [PubMed]
- 19.Piaggesi A, Baccetti F, Rizzo L, Romanelli M, Navalesi R, Benzi L. Sodium carboxyl-methyl-cellulose dressings in the management of deep ulcerations of diabetic foot. Diabet Med. 2001;18(4):320–324. [DOI] [PubMed]
- 20.Ochoa O, Torres FM, Shireman PK. Chemokines and diabetic wound healing. Vascular. 2007;15(6):350–355. [DOI] [PubMed]
- 21.Brancato SK, Albina JE. Wound macrophages as key regulators of repair: origin, phenotype, and function. Am J Pathol. 2011;178(1):19–25. [DOI] [PMC free article] [PubMed]
- 22.Novak ML, Koh TJ. Macrophage phenotypes during tissue repair. J Leukoc Biol. 2013;93(6):875–881. [DOI] [PMC free article] [PubMed]
- 23.Lucas T, Waisman A, Ranjan R, et al. Differential roles of macrophages in diverse phases of skin repair. J Immunol. 2010;184(7):3964–3977. [DOI] [PubMed]
- 24.Leal EC, Carvalho E, Tellechea A, et al. Substance P promotes wound healing in diabetes by modulating inflammation and macrophage phenotype. Am J Pathol. 2015;185(6):1638–1648. [DOI] [PMC free article] [PubMed]