Abstract
Chronic exposure to manganese (Mn) is associated with neuroinflammation and extrapyramidal motor deficits resembling features of Parkinson’s disease. Activation of astrocytes and microglia is implicated in neuronal injury from Mn but it is not known whether early life exposure to Mn may predispose glia to more severe inflammatory responses during aging. We therefore examined astrocyte nuclear factor kappa B (NF-κB) signaling in mediating innate immune inflammatory responses during multiple neurotoxic exposures spanning juvenile development into adulthood. MnCl2 was given in drinking water for 30-day postweaning to both wildtype mice and astrocyte-specific knockout (KO) mice lacking I kappa B kinase 2, the central upstream activator of NF-κB. Following juvenile exposure to Mn, mice were subsequently administered 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) at 4 months of age. Animals were evaluated for behavioral alterations and brain tissue was analyzed for catecholamine neurotransmitters. Stereological analysis of neuronal and glial cell counts from multiple brain regions indicated that juvenile exposure to Mn amplified glial activation and neuronal loss from MPTP exposure in the caudate-putamen and globus pallidus, as well as increased the severity of neurobehavioral deficits in open field activity assays. These alterations were prevented in astrocyte-specific I kappa B kinase 2 KO mice. Juvenile exposure to Mn increased the number of neurotoxic A1 astrocytes expressing C3 as well as the number of activated microglia in adult mice following MPTP challenge, both of which were inhibited in KO mice. These results demonstrate that exposure to Mn during juvenile development heightens the innate immune inflammatory response in glia during a subsequent neurotoxic challenge through NF-κB signaling in astrocytes.
Keywords: astrocyte, microglia, manganese, MPTP, inflammation, neurodegeneration
Manganese (Mn) is an essential trace element necessary for multiple enzymatic processes in the central nervous system (CNS), where it is a required cofactor for enzymes important for neuronal health such as glutamine synthetase (GS), the key enzyme regulating glutamate-glutamine metabolic shuttling between astrocytes and neurons (Aschner et al., 2009) and superoxide dismutase (Mn-SOD), which protects neurons from the damaging effects of oxidative stress (Zidenberg-Cherr et al., 1983). Mn is highly concentrated in astrocytes, with cellular uptake mediated by divalent metal transporters such as DMT1, ZIP, and SLC39A3 (Aschner et al., 2009). Elevated concentrations of Mn within astrocytes promote a neuroinflammatory phenotype, characterized by expression of numerous genes that cause neuronal injury, including inducible nitric oxide synthase (NOS2), C-C motif chemokine ligand 2 (Ccl2), tumor necrosis factor (Tnf), interleukin 1β (Il1b), and interleukin 6 (Il6) (Kirkley et al., 2017; Popichak et al., 2018).
Neurotoxic levels of Mn correlate with cognitive and behavioral impairment in both adults and children (He et al., 1994; Rugless et al., 2014; Takser et al., 2003), with motor deficits occurring at higher exposures in occupational settings such as mining and welding that can cause Mn-induced parkinsonism or “manganism” (Racette et al., 2017; Rodier, 1955; Wang et al., 1989). Neurological symptoms of manganism include bradykinesia, dystonia, rigidity, and depression (Mergler and Baldwin, 1997; Racette et al., 2017; Rodier, 1955; Wang et al., 1989), although patients with manganism do not display severe nigrostriatal dysfunction or resting tremors and are typically nonresponsive to levodopa (L-DOPA) therapy. These differences are likely due to distinct patterns of neuropathology compared with idiopathic Parkinson’s disease (PD), causing toxicity primarily in more glial rich regions, such as the globus pallidus (Gp), subthalamic nucleus (Sth), and substantia nigra pars reticulata (SNpr) (Guilarte et al., 2006; Peres et al., 2016). In contrast, PD is characterized by preferential loss of dopamine (DA) neurons in the substantia nigra pars compacta (SNpc) and long axonal projections to the striatum (ST). Despite these findings, mechanisms by which Mn exposure could accelerate PD-like neuropathology remain elusive.
We previously demonstrated that Mn-induced neurotoxicity results in marked activation in astrocytes and microglia and expression of numerous neuroinflammatory genes that potentiate neuronal injury (Kirkley et al., 2017; Moreno et al., 2011). Furthermore, we reported that mice pre-exposed to Mn during juvenile development had higher levels of gliosis and neuronal dysfunction than mice exposed to Mn only as adults (Moreno et al., 2009b). Neurotoxic levels of glial activation and neuroinflammation resulting from Mn exposure are modulated by the transcription factor nuclear factor kappa B (NF-κB), a central regulator of innate immune inflammatory responses in lymphoid and myeloid cells, as well as in microglia and astrocytes (Glass et al., 2010; Kirkley et al., 2017; Popichak et al., 2018). NF-κB has distinct functions within different cell types of the CNS, including regulation of inflammatory gene expression in glia and induction of prosurvival genes in neurons including IAP’s, Bcl2, Bcl-XL, and survivin (Glass et al., 2010). In vitro, Mn directly activates microglia, stimulating NF-κB-dependent release of cytokines such as TNFα that stimulate a reactive neurotoxic phenotype in astrocytes (Kirkley et al., 2017). NF-κB is activated by numerous intra- and intercellular stressors, including reactive oxygen species (ROS), inflammatory cytokines, and chemokines, as well as Mn accumulation (Moreno et al., 2011; Popichak et al., 2018).
To determine the role of NF-κB in regulating the neuroinflammatory effects of Mn in reactive astrocytes, we recently developed a novel transgenic mouse with astrocyte-specific deletion of the NF-κB signaling pathway (Kirkley et al., 2019). Mice expressing Cre recombinase under control of the human glial fibrillary acidic protein promoter (hGFAP) were crossed with I kappa B kinase 2 (Ikk2)-loxP mice to ablate inflammatory NF-κB signaling in astrocytes. Selective deletion of IKK2 in astrocytes protected against loss of DA neurons caused by the neurotoxin, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) (Kirkley et al., 2019). Lack of functional NF-κB in astrocytes decreased reactive gliosis in this model and decreased expression of NOS2 and TNFα, thereby reducing neuronal apoptosis. These studies demonstrated that inflammatory activation of glial cells is an important determinant of MPTP neurotoxicity (Kirkley et al., 2019). However, it is unknown whether exposure to Mn can exacerbate the effects of other dopaminergic neurotoxins such as MPTP by modulating glial reactivity and subsequent neuroinflammatory injury. Exposure to MPTP as model neurotoxicant was used to test the potential combinatorial effects of Mn with other mitochondrial inhibitors such as pesticides (Brown et al., 2006) and drugs of abuse (Peres et al., 2016) that could aggravate pathology within the nigrostriatal dopamine system. In this study, we postulated that Mn exposure during juvenile development would stimulate NF-κB-dependent activation of microglia and astrocytes, resulting in chronic neuroinflammation that increases susceptibility to neuronal injury from a secondary exposure to a dopaminergic neurotoxin during aging.
MATERIALS AND METHODS
Animals and treatment regimen
All animals were housed on a 12-h light/dark cycle in a temperature-controlled room (maintained at 22–24°C) and had access to standard chow/water ad libitum. Procedures were approved by Colorado State University Institutional Animal Care and Use Committee (IACUC) and were conducted in compliance of National Institute of Health guidelines. Astrocyte-specific knockout (KO) mice for IKK2 were generated as previously described (Kirkley et al., 2019). Briefly, hGfap-cre+/− (Cat No. 004600; Jackson Laboratories) mice were backcrossed on a C57/BlJ6 background for 12 generations before crossbreeding with Ikk2-loxP+/+ mice (donated by Professor Michael Karin, University of California, San Diego). Four generations of crossbreeding were conducted to acquire hGfap-cre+/−/Ikk2-loxP+/+ (KO). Littermates lacking Cre recombinase (hGfap-cre−/−/Ikk2-loxP+/+) were used as genotype controls for the study. At day P21, mice were administered MnCl2 (50 mg/kg/day) by monitoring water intake and weight gain for 30 days. After P51, mice were placed back on normal drinking water for a period of 2 months. This dose of Mn recapitulates higher levels of intake during the postnatal period from contaminated drinking water and from soy-based formulas, as noted in recent studies (Beaudin et al., 2017) and as we previously published (Moreno et al., 2009b). MPTP-HCl (MedchemExpress; Monmouth Junction, New Jersey) was dissolved in sterile saline (0.9% NaCl2) and administered by sc injection at 20 mg/kg for 4 doses over 1 week. Probenecid was administered by ip injection (100 mg/kg; Sigma) every other day for 1 week (4 dosages total), per our previously published protocol (Hammond et al., 2018). Mice were aged an additional week after treatment with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine and probenecid (MPTPp) before tissue collection.
Behavioral analysis
Two weeks before initial behavioral testing, all mice were acclimated to stress handling by methods closely adapted to previously established protocols (Gouveia and Hurst, 2013; Stuart and Robinson, 2015). Open field activity parameters were assessed using the Versamax behavior chambers with an infrared beam grid detection array (Accuscan Instruments, Inc, Columbus, Ohio) as previously reported (Hammond et al., 2018; Kirkley et al., 2019). Mice were monitored for 10 min under low ambient light in the presence of white noise. Animals were preconditioned 1 day prior to their first treatment and then assessed at day 0 (1st day of treatment) to establish a baseline, day 7, and day 14. Several behavioral parameters were collected and analyzed using Versadat Software (Accuscan Instruments, Inc) including total distance traveled, number of movements, time spent moving, time spent in the margin, and the number of rearing movements. Baseline values were subtracted from each measurement to calculate the change in performance over time relative to baseline at the start of the study period. Stride length was assessed via video recording mice freely walking a glass trackway (5 cm × 1 m) with 3 recordings obtained per mouse per assessment, as previously reported (Hammond et al., 2018; Kirkley et al., 2019). Gait parameters were analyzed using software written in Mathworks/MatLab Software (in collaboration with Dr Edwin Chong, Professor of Electrical and Computer Engineering, Colorado State University). The light/dark was switched 2 weeks prior to the onset of studies so that behavioral measurements were performed during the wake cycle.
Tissue processing
For immunohistochemical analysis: mice were anesthetized under deep isoflurane and transcardially perfused with 0.1-M PBS-cacodylate/heparin (10 U/ml) and 3% paraformaldehyde/PBS. Following decapitation, whole brains were dissected and stored in 3% paraformaldehyde/PBS overnight at 4°C. The next day, samples were transferred to a gradient of 15%–30% sucrose/PBS prior to cryosectioning and storage in cryoprotectant at −20°C until processed for tissue staining. For neurochemical detection of Mn2+ and catecholamines: mice were similarly anesthetized with isoflurane and then rapidly decapitated. Whole brains were dissected and placed on a 1-mm brain block for separation of ST, SN, hypothalamus (Hyp), and cortex (Cx). Specific brain regions were frozen in liquid nitrogen and then stored at −80°C until processed for high-performance liquid chromatography (HPLC) and inductively coupled plasma-mass spectrometry (ICP-MS). Blood was also collected and centrifuged at (1500 rpm at 4°C/15 min) for plasma fractionation and stored at −80°C.
Immunostaining and automated stereological cell counting for fixed mouse brain tissue
For stereological determination of TH+ neurons within the SN, the entire SNpc was serially cryosectioned from the Sth (rostral) to the retrorubal field (caudal) region. Every 6th free-floating section (8 total) was selected from each animal and immunostained for anti-TH (1:500; Millipore AB152) and anti-Neuronal Nuclei (NeuN; 1:250, Millipore; MAB377) by our previously reported protocol (Hammond et al., 2018; Miller et al., 2011). SNpc neurons were automatically quantitated from 10×-objective montage images of each immunostained section using a Hamamatsu ORCA-Flash4.0 digital CMOS camera, ProScan III stage controller (Prior, Rockland, Massachusetts) and CellSens Dimension Software (version 1.12, Olympus, Center Valley, Pennsylvania). For automated relative counts of GFAP+, IBA-1+, and NeuN+ cells, 2 sections/animal were selected from the same anatomical regions of SN and ST (4 animals were analyzed per group). Primary antibodies for glia labeling were anti-GFAP (1:500; DAKO Z0334), anti-IBA1 (1:250; WAKO 016-20001), and anti-TH (Abcam 76442) to demarcate the SNpc and SNpr nuclei. Region of interest was highlighted based on Allen Brain Atlas for reference, following application of an adaptive threshold with shape factor and area (m2) object filters for automatic cell detection, based on previously published algorithms (Tapias et al., 2013). Detected cell number was divided over the area (µm2) of region. The investigator was blinded from all experimental groups during imaging and cell quantification.
Image analysis of microglia morphology and reactive astrocytes
For detection of microglia morphology, the same sections immunostained for anti-IBA1/TH for automated counting were reimaged on a Zeiss Axiovert 200-M inverted wide-field fluorescence microscope equipped and a Hamamatsu ORCA-ER-cooled charge coupled device camera using a 10× and 40× Planapochromat air objectives with Slidebook imaging software (version 5.0, Intelligent Imaging Innovations, Denver Colorado). Optical fractionator method employed counting frame size (150 × 150 μm) and frame spacing (for SN; 250 × 250 μm, for ST; 550 ×550 μm); 40× z-stack images of IBA+ cells from the SNpc, and Gp were acquired and converted to maximum projection, following a binary transformation and then rendered to a skeletonized image in ImageJ, as previously described (Morrison and Filosa, 2013; Schneider et al., 2012). To detect expression of C3 in S100β+ astrocytes, the same optical fractionator method was applied for imaging. Cells were manually quantified based on z-stack images. The investigator was blinded from all experimental groups during cell counting.
Analytical determination of catecholamines, monoamine, and manganese
Flash-frozen samples of brain tissue were processed for HPLC coupled with electrochemical detection to quantitate levels of dopamine (DA), 3,4-dihydroxyphenylacetic acid (DOPAC), homovanillic acid (HVA), serotonin (5-HT), and metabolite 5-hydroxindoleacetic acid (5-HIAA). The Neurochemistry Core Laboratory at Vanderbilt University’s Center for Molecular Neuroscience Research (Nashville, Tennessee) processed all tissue samples from each experimental group with coded labeling for unbiased analysis. Levels of Mn in serum and brain were determined by ICP-MS through the metals analytical core facility at Vanderbilt University School of Medicine, as previously reported (Moreno et al., 2009b).
Evaluation of mRNA expression by qPCR
RNA was isolated from brain tissue flash-frozen in liquid nitrogen and stored at −80°C utilizing the RNeasy Mini Kit (QIAGEN, Valencia, California). Purity and concentration were determined using a NanoDrop ND-1000 spectrophotometer. Five hundred ng of RNA was used as a template for reverse transcriptase reactions using the iScript RT kit (Bio-Rad, Hercules, California). cDNA was mixed with SYBR Green (Bio-Rad) and analyzed as previously reported (Kirkley et al., 2017), utilizing primer pairs for Slc11a2 (Dmt1), Slc39a8 (Zip8), Slc39a10, and Slc39a14. Primer sequences were as follows: Dmt1 (Forward: 5′ CAG CGA GAC TTG GAG TGG TC 3′, Reverse: 5′ CAC AGG ATG ATC CGT GGG A 3′), Zip8 (Forward: 5′ ATC TGC CCC GCG ATC TTA C 3′, Reverse: 5′ CCC CAG ACT TCG GAA AGA CT 3′), Slc39a10 (Forward: 5′ GGC CGT TAC TCA GGC AAG AC 3′, Reverse: GCA TGT TGA ACG AGT CCG AGA), and Slc39a14 (Forward: 5′ CGC CAT TGA AGT ATG GGG GTA 3′, Reverse: 5′ GCA GGA GCC TCT TGT AAA AAG T 3′).
Statistical analysis
All data were presented as mean ± SEM, unless otherwise noted. Experimental values from each mean were analyzed with a Grubb’s (α = .05) test for exclusion of significant outliers. Differences between genotype and treatment condition in each experimental group were analyzed by 2-way ANOVA with Tukey’s post hoc multiple comparisons test. Significance was identified as *p < .05, **p < .01, ***p < .001, ****p < .0001. All statistical analysis was conducted using Prism (version 6.0; GraphPad Software, San Diego, California).
RESULTS
Developmental Weights and Water Consumption During Treatment With MnCl2
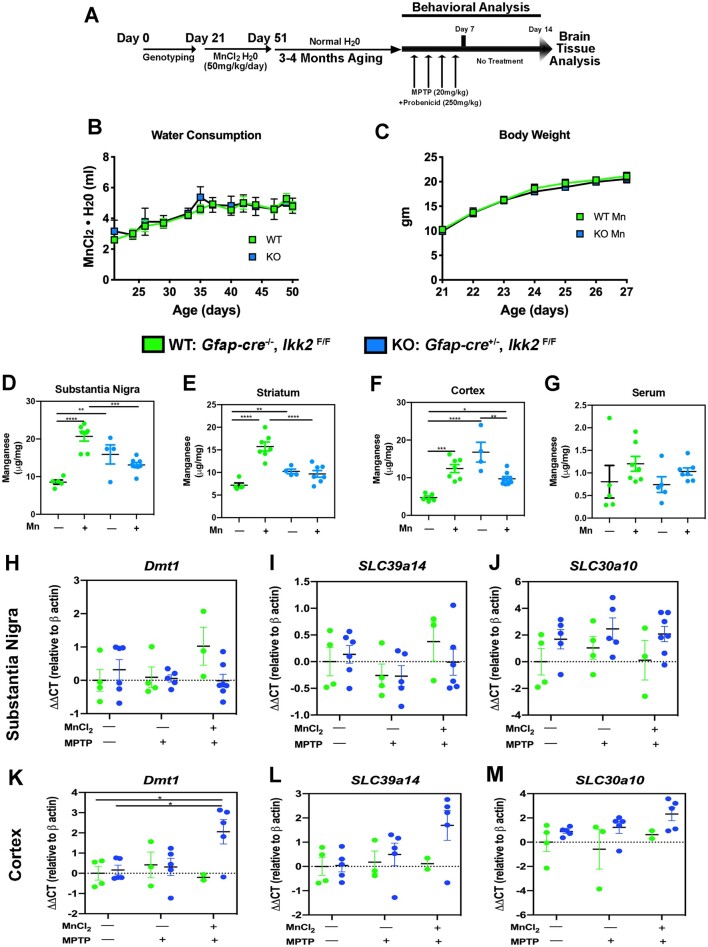
At postnatal day (PND) 7 mice were tail clipped for DNA purification and identification of KO and wildtype (WT) progeny per our previously published PCR genotyping protocol (Kirkley et al., 2019). From PN 21 to 51, encompassing the period of juvenile development to early adulthood, mice were placed on drinking water containing MnCl2 (50 mg/kg/day) prior to later exposure to MPTPp, as depicted in Figure 1A. Based on statistical analysis of MnCl2 water consumption, WT consumption increased 2.19 ± 0.44 (ml) and KO consumption increased 2.63 ± 0.78 (ml) from 21 to 51 PND (n = 4–5; no significance difference between groups) (Figure 1B). For accurate dosing of MnCl2 and detection in change of body weight, mice were weighed every day for the 1st week of treatment. From 21 to 27 PND, WT and KO animals significantly increased in weight by 10.65 ± 0.97 and 10.86 ± 0.83 (g), respectively (n = 8/group; ****p < .0001). No significant difference in body weight was detected between WT and KO groups treated with MnCl2 (Figure 1C). Mn levels in serum and brain were determined at the conclusion of Mn treatment at PN 51, as well as after treatment with MPTP in adult mice. At PN 51, there were increased levels of Mn in WT mice in the substantia nigra (SN), striatum (caudate-putamen, Cp) and cortex, with no increase in Mn evident in KO mice (Figs. 1D–F). There were no differences in Mn in serum between treatment groups in WT or KO mice (Figure 1G). In addition, Mn levels in brain and serum were not different from control following treatment with MPTPp alone in either genotype (data not shown). To understand potential differences in expression of metal transporters between WT and KO mice, we also measured mRNA expression of Slc11a2 (Dmt1), Slc39a8 (Zip8), Slc39a10, and Slc39a14 (Figs. 1H–M). No changes in expression of Dmt1, Slc30a10, or Slc39a14 were detected in the SN of mice between genotypes, either in control mice or in those treated with Mn or Mn + MPTPp (Figs. 1H–J). Levels of these transporters were likewise not different in the cortex between WT and KO mice, with the exception that Dmt1 was somewhat increased in the cortex of KO mice treated with Mn + MPTPp (Figure 1K). Expression of mRNA for Slca8 was not detected in any brain region evaluated.
Figure 1.
Treatment strategy and metal transporter expression for the 2-hit neurodegenerative model with manganese and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). A, Experimental design for 2-hit neurodegenerative model. Wildtype (WT) (Gfap-cre−/−, Ikk2F/F) and astrocyte-specific knockout (KO) (Gfap-cre−/−, Ikk2F/F) mice were given either normal drinking water (18 Ω Milli-Q) or MnCl2·4H2O in drinking water (50 mg/kg/day Mn) from PN 21 to 51, followed by treatment with either vehicle or MPTP 4 months later. Behavioral analysis was conducted throughout the period of treatment with MPTP after collecting baseline data prior to dosing. All mice were terminated 1 week after the final dose of MPTP for analysis of neurochemical and immunohistochemical endpoints. B, Consumption of water and (C) body weight was not different between WT and KO mice exposed to MnCl2·4H2O in drinking water. Levels of Mn were measured at PN 51 in the substantia nigra (SN) (D), striatum (E), Cortex (F), and in serum (G). Expression of mRNA for metal transporters was determined in the SN (H–J) and cortex (K–M) of mice exposed to Mn or to Mn + MPTP at 6 months of age, relative to untreated control animals. *p < .05, **p < .01.
Inhibition of NF-κB in Astrocytes Protects DA Neurons From MPTP-induced Toxicity in the SNpc
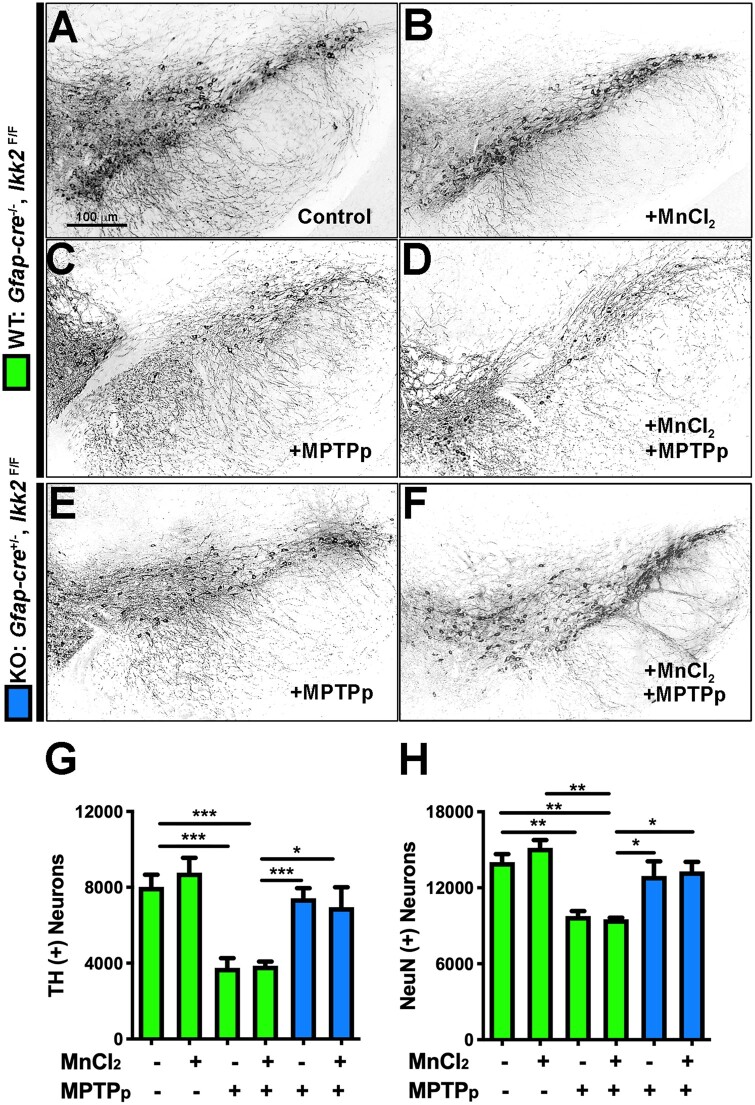
To determine the extent of neuronal loss in WT and KO mice following exposure to Mn and MPTP, the SN of each animal was serially cryosectioned and immunolabeled with anti-TH and anti-NeuN antibodies to label dopaminergic and total neurons, respectively (Figs. 2A–F). The total number of TH+ neurons within the SNpc was quantitated for each experimental animal group as follows: WT control (8024 ± 637.1), WT with MnCl2 (8779 ± 777.0), WT with MPTPp (3759 ± 503.7), WT with MnCl2 + MPTPp (3867 ± 222.1), KO with MPTPp (7421 ± 537.2), KO with MnCl2 + MPTPp (6957 ± 1049). Both WT with MPTPp and with MnCl2 + MPTP displayed 51%–53% loss of TH+ neurons compared with control. KO with MPTPp and MnCl2 + MPTPp had only 7.5%–13.3% loss of TH+ neurons (Figure 2G). The mean estimate of total NeuN+ neurons within the SNpc was quantitated as follows: WT control (14 034 ± 631.2), WT with MnCl2 (15 162 ± 609.8), WT with MPTPp (9778 ± 379.3), WT with MnCl2 + MPTPp (9636 ± 159.0), KO with MPTPp (12 932 ± 1167), KO with MnCl2 + MPTPp (11 695 ± 1152). Comparably, WT with MPTPp and MnCl2 + MPTPp each had a similar 30%–31% loss of total neurons compared with control. KO with MPTPp and MnCl2 + MPTPp only sustained a 7.8%–16.7% loss of NeuN+ cells which was not significantly different from control (Figure 2H) (n = 6 animals/per group; **p < .01, ***p < .001). MnCl2 did not potentiate loss of dopaminergic neuronal soma following later exposure to MPTPp.
Figure 2.
Astrocyte-specific knockout (KO) of I kappa B kinase 2 (IKK2) prevents loss of dopaminergic neurons following combined treatment with manganese and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). The number of NeuN+ (total) and tyrosine hydroxylase + (dopaminergic) neurons in the substantia nigra was determined by 3D designed-based stereology in frozen serial sections from wildtype (WT) (Gfap-cre−/−/Ikk2F/F) and KO (Gfap-cre+/−/Ikk2F/F) mice. Mice received either regular drinking water or drinking water containing 50 mg/kg/day MnCl2 from PN 21 to 51 and were then treated with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine and probenecid (MPTPp) or vehicle at 6 months of age. Dual-labeled immunofluorescence montage images were collected using a 10× air Plan Apochromat objective, counterstained DAPI to identify cell nuclei. Grayscale images of TH labeling are presented for each treatment group as follows: (A) WT—Control, (B) WT—MnCl2, (C) WT—MPTPp, (D) WT—MnCl2/MPTPp, (E) KO—MPTPp, (F) KO—MnCl2/MPTPp. (G) Quantitative assessment of TH+ neurons in the SNpc. (H) Quantitative assessment of total NeuN+ neurons in the SNpc. **p < .01, ***p < .001; N = 6 animals/group.
MnCl2 Potentiates MPTPp-induced Neuronal Cell Death in the Gp and Striatum Activation of NF-κB in Astrocytes
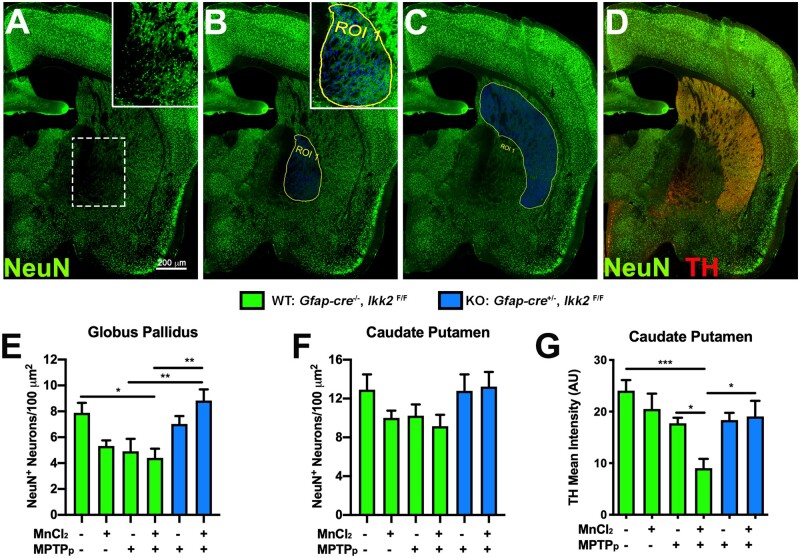
To evaluate level of neuronal dysfunction in other basal ganglia nuclei affected by Mn, anatomically registered cryosections of the basal ganglia were selected for quantitation of total NeuN+ neurons in the Gp and striatum (Cp). Representative montage images of sections immunolabeled with anti-NeuN (green) are depicted in Figures 3A–D. The Gp (Figure 3B) and Cp (Figure 3C) were highlighted as regions of interest (ROIs, highlighted in blue) for automated quantitative detection of the total number of NeuN+ neurons per area (µm2), with individual cell bodies visible in high magnification insets within each ROI. Based on quantitation of total NeuN+/µm2, there was an apparent decrease the number of neurons in WT animals treated with MPTPp or MnCl2 individually, but this trend was not statistically different from control. In contrast, WT animals treated with MnCl2 + MPTPp had a 44.1% loss of NeuN+ neurons within the Gp and KO animals with dual treatment were protected from loss of neurons, with no differences in neuronal numbers detected compared with WT control animals (Figure 3E). Quantitation of NeuN+ cells/µm2 within the Cp showed a similar trend as the Gp, however no groups were statistically different (Figure 3F). To measure the integrity of TH+ presynaptic terminals in the Cp, sections were also coimmunolabeled with anti-TH (red) as depicted in Figure 3D. An apparent decrease in TH+ terminal density in WT animals treated with MnCl2 or MPTPp individually was noted but was not significantly different from control. Dual treatment with both MnCl2 and MPTPp in WT mice caused a decrease in DA terminal density to 62.5% of control animals, whereas KO animals with dual treatment only had a 20.7% loss of terminals compared with control WT mice (Figure 3G) (n = 6–7 animals/group; *p < .05, **p < .01, ***p < .001).
Figure 3.
Sequential exposure to MnCl2 and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) causes neuronal loss in the globus pallidus (Gp) and enhances loss of dopaminergic fibers in the striatum through an astrocyte-dependent mechanism. Image analysis of NeuN+ neurons the Gp and caudate-putamen (Cp) in wildtype (WT) and astrocyte-specific I kappa B kinase 2 (IKK2) knockout (KO) mice. A–D, Total neurons were immunolabeled with NeuN+ (green) and sections were counterstained with TH (Red) to highlight dopaminergic terminals in the Cp (D). Regions of interest (ROIs) were drawn around each anatomically distinct nucleus for quantification of the total number of neurons. The Gp (B) and Cp (C) were identified as ROIs for automated cell detection (blue). Colabeling with anti-TH (red) depicts DA presynaptic terminals in the Cp (D). The total number of NeuN+ cells/100 µm2 was quantitated for the Gp (E) and Cp (F). G, Mean intensity of TH+ immunoreactivity was measured for detection of DA innervation in the Cp. Juvenile exposure to Mn did not potentiate MPTP-induced neuronal loss in the Gp but did enhance MPTP-mediated loss of TH fibers in the Cp. *p < .05, **p < .01, ***p < .001; N = 6–7 animals/group. Abbreviation: MPTPp, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine and probenecid.
IKK2 KO Mice Are Protected Against Behavioral and Neurochemical Deficits Caused by Exposure to Mn and MPTPp
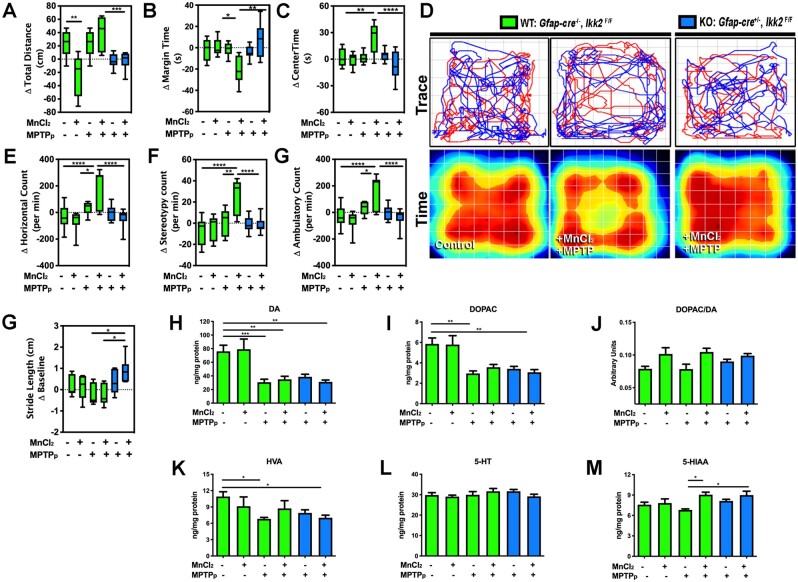
Spontaneous locomotor activity was detected by open field test (OFT) for each experimental group. In adult WT mice treated only with MnCl2 as juveniles, there was a decrease in the total distance traveled but other behavioral parameters were unchanged from baseline (Figure 4A). In contrast, dual-treated WT mice (MnCl2/MPTPp) displayed hyperactive behavior in multiple parameters of OFT analysis. Dual treatment significantly increased levels of margin time (Figure 4B), center time (Figure 4C), horizontal movement (Figure 4E), stereotypy movement (Figure 4F), and ambulatory movement (Figure 4G) compared with MPTPp-only treatment. Interestingly, treated KO animals did not display hyperactive behavior and were not different from WT control animals, as depicted by the graphs in Figures 4A–C and 4E–G, as well as in representative traces and pseudocolored time plots of activity over a 5 min interval (Figure 4D) (n = 7–10 animals/group; *p < .05, **p < .01, ***p < .001, ****p < .0001). Quantitative analysis of stride length from each experimental animal was conducted using a real-time video gait analysis system. WT animals treated with MPTPp and MnCl2/MPTPp exhibited an apparent decrease in stride length compared with WT control that was not statistically significant. However, dual-treated KO animals had a significantly longer stride length compared with treated WT animals during the course of the study (Figure 4H) (n = 5–6 animals/group; *p < .05).
Figure 4.
Juvenile exposure to manganese exacerbates 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced behavioral dysfunction through NF-κB signaling in astrocytes. Open field behavioral testing for locomotor function was conducted for total distance (A), margin time (B), and center time (C), compared with baseline values prior to dosing with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine and probenecid (MPTPp). D, Representative trace plots (top) and pseudocolored heat maps of total time spent (bottom) in the position of the chamber for 5 min intervals are depicted for (wildtype) WT—control, WT—MnCl2/MPTPp, and (knockout) KO—MnCl2/MPTPp animals. (E) Change in horizontal count, (F) stereotypy count, and (G) ambulatory count values were normalized to day 0 baseline values (*p < .05, **p < .01, ***p < .001, ****p < .0001; N = 7–10 animals/group). H, Real-time video gait analysis was utilized to quantitate change of stride length from day 0 (*p < .05; N = 5–6 animals/group). High-performance liquid chromatography analysis was used for detection of neurotransmitter content from striatal tissue for (H) dopamine (DA), (I) 3,4-dihydroxyphenylacetic acid (DOPAC), (J) DOPAC/DA, (K) homovanillic acid (HVA), (L) serotonin (5-HT), and (M) 5-hydroxindoleacetic acid (5-HIAA) (*p < .05, **p < .01, ***p < .001; N = 5–6 animals/group).
Levels of striatal catecholamines and metabolites were detected by HPLC analysis with electrochemical detection. All MPTPp-treated animals with or without MnCl2 exhibited a drastic loss of DA and DOPAC. In addition, a slight increase in DA levels was apparent in MnCl2-only treated WT animals but was not statistically different from control (Figs. 4I and 4J). Dual-treated WT animals also displayed a significantly higher ratio of DOPAC/DA compared with control (Figure 4K). The DA metabolite, HVA, was also decreased with MPTPp treatment, independent of MnCl2 treatment and genotype (Figure 4L). Serotonin (5H-T) content showed no statistical difference between experimental groups (Figure 4M). However, analysis of the serotonin metabolite (5-HIAA) showed a significant increase in both WT and KO dual-treated animals compared with MPTPp-only treatment (Figure 4N) (n = 5–9 animals/group; *p < .05, **p < .01, ***p < .001).
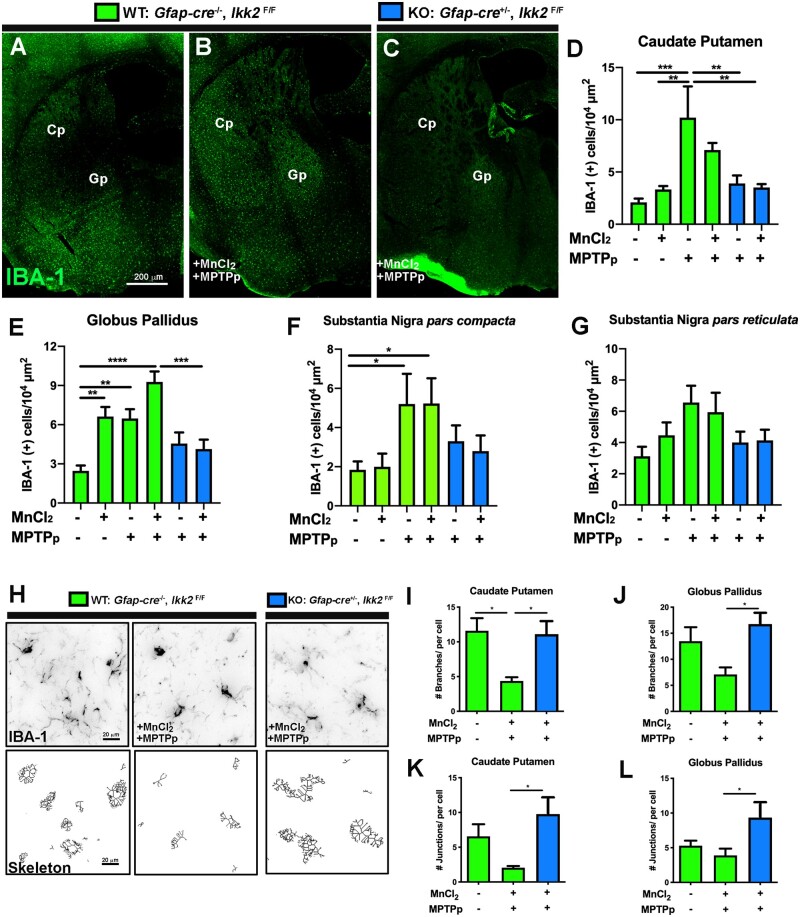
Juvenile Exposure to MnCl2 Intensifies Activation of Microglia in the Gp After Treatment With MPTPp and Is Suppressed in Astrocyte-specific IKK2 KO Mice
The relative number of microglia within basal ganglia was detected by immunolabeling with anti-IBA1 as depicted in representative images of the Gp and Cp (Figs. 5A–C). Both MPTPp and MPTPp/MnCl2 treatments in WT animals increased the number of IBA-1+ cells/µm2 within the Cp and treated KO animals displayed similar numbers as the control group, shown in Figure 5D. Dual-treated WT animals also exhibited the most IBA-1+ cells/µm2 within the Gp, which was reduced in KO animals (Figure 5E) (n = 5–6 animals/group; **p < .01, ***p < .001, ****p < .0001). There was a significant increase in IBA-1+ cells/µm2 in the SNpc of MPTPp-treated WT animals and slightly less in KO animals, which was not statically different from WT (Figure 5F). A similar trend of IBA-1+ cells/µm2 in the SNpr was noted, although not statically different between groups (Figure 5F). For assessment of microglia morphology, 40×-objective IBA-1 (grayscale) images of the Cp and Gp were skeletonized to detect for number of branches as depicted in representative images in Figure 5H. WT dual-treated animals exhibited significantly less branches/cell in the Cp (Figure 5I), Gp (Figure 5J), and less junctions/cell in the Cp (Figure 5K), and Gp (Figure 5K) compared with dual-treated KO animals (n = 7 animals/group; *p < .05).
Figure 5.
Astrocyte-specific deletion of I kappa B kinase 2 (IKK2) suppresses a reactive phenotype in microglia following sequential exposure to MnCl2 and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). The number of microglia in mice sequentially exposed to MnCl2 during juvenile development and to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine and probenecid (MPTPp) as adults was analyzed by automated quantitation of tissue sections immunolabeled with anti-IBA1 (green), as depicted in montage images of (A) (wildtype) WT—control, (B) WT—MnCl2/MPTPp, and (C) (knockdown) KO—MnCl2/MPTPp treatment groups. The total number of IBA1+ cells/104 µm2 was quantified in the caudate-putamen (Cp) (D), globus pallidus (Gp) (E), substantia nigra pars compacta (F) and substantia nigra pars reticulata (G) for all experimental groups (*p < .05; **p < .01, ***p < .001, ****p < .0001; N = 5–6 animals/group). H, Morphological changes in microglia between treatment groups were analyzed by image skeletonization of IBA1+ cells using Image J, as depicted in representative images of WT—Control, WT—MnCl2/MPTPp, and KO—WT + MnCl2/MPTPp. Parameters quantified in each experimental group included the number of branches/cell in Cp/Gp (I, J), and the number of junctions/cell in Cp/Gp (K, L) (*p < .05; N = 7 animals/group).
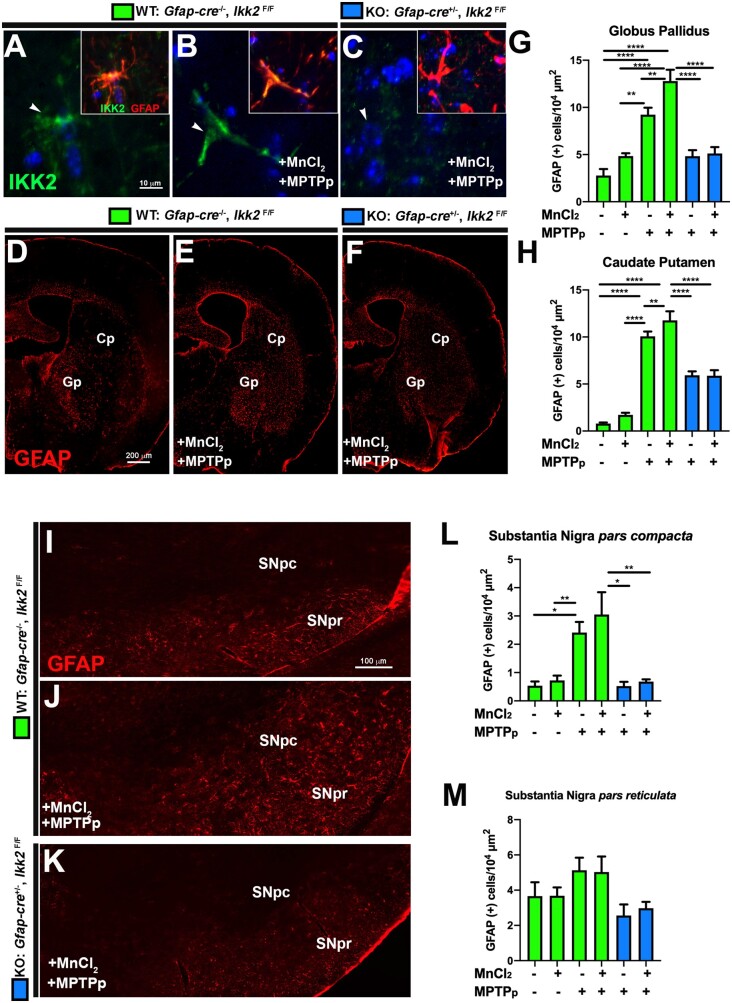
Activation of Astrocytes in the Basal Ganglia Following Dual Treatment With MnCl2 and MPTPp Is Regulated by NF-κB
Expression of IKK2 was determined in hGfap-cre+/−/Ikk2fl/fl mice by coimmunolabeling with anti-GFAP (red) and anti-IKK2 (green), as depicted in representative 100×-objective images from the Gp in WT control (Figure 6A), WT dual-treated (Figure 6B), and KO dual-treated (Figure 6C) mice. Expression of IKK2 protein was present in both control- and dual-treated WT mice but not detected in any experimental group of KO mice, similar to what we previously reported in astrocytes with these transgenic mice (Kirkley et al., 2019). To assess the relative number of GFAP+ cells in multiple nuclei of each experimental group, anatomically consistent cryosections of ST and SN were selected for automated counting of GFAP+ cells/µm2. As depicted in representative 10× montage images of the Gp and Cp, dual-treated WT animals exhibited increased proliferation of GFAP+ astrocytes in both nuclei (Figure 6F). Dual treatment significantly increased the number of GFAP+ cells in the Gp over MPTPp-only treatment in WT animals, which was markedly reduced in KO animals (Figs. 6D–G). Similarly, an increased number of GFAP+ cells was evident in dual-treated WT animals in the Cp but was not statistically different from WT animals treated only with MPTPp in this region (Figure 6H). Comparably, dual-treated WT animals exhibited the greatest amount of GFAP+ immunoreactivity in the SNpc, whereas KO animals had similar numbers of GFAP+ cells as control mice (Figs. 6I–L) (n = 6; *p < .05, **p < .01, ***p < .001, ****p < .001). We also observed an apparent trend of GFAP+ cells in the SNpr of each experimental group that was not statistically significant (Figure 6M).
Figure 6.
Sequential exposure to MnCl2 and -methyl-4-phenyl-1,2,3,6-tetrahydropyridine and probenecid (MPTPp) during development and adulthood increases astrocyte activation through NF-κB signaling. A–C, High-resolution immunofluorescence images of astrocytes in wildtype (WT) (Gfap-cre−/−/Ikk2F/F) and KO (Gfap-cre+/−/Ikk2F/F) mice demonstrate expression of I kappa B kinase 2 (IKK2) (green) and glial fibrillary acidic protein (GFAP) (red) that colocalize in WT mice in Control (A) and MnCl2/MPTPp (B) groups, with no detectable expression of IKK in knockout (KO) mice exposed to MnCl2/MPTPp (C). The total number of GFAP+/104 µm2 was quantified in the globus pallidus (Gp) (D) and caudate-putamen (Cp) (H) for each experimental group, as depicted in 10× montage images of the striatum and Gp immunolabeled with anti-GFAP (red) (D–F). Substantia nigra pars compacta (SNpc) and substantia nigra pars reticulata (SNpr) were also immunostained for GFAP as depicted in representative 10× montage images in (I–K). Analysis of GFAP+/µm2 in SNpc (L) and SNpr (M) (*p < .05, **p < .01, ***p < .001, ****p < .0001; N = 6 animals per group).
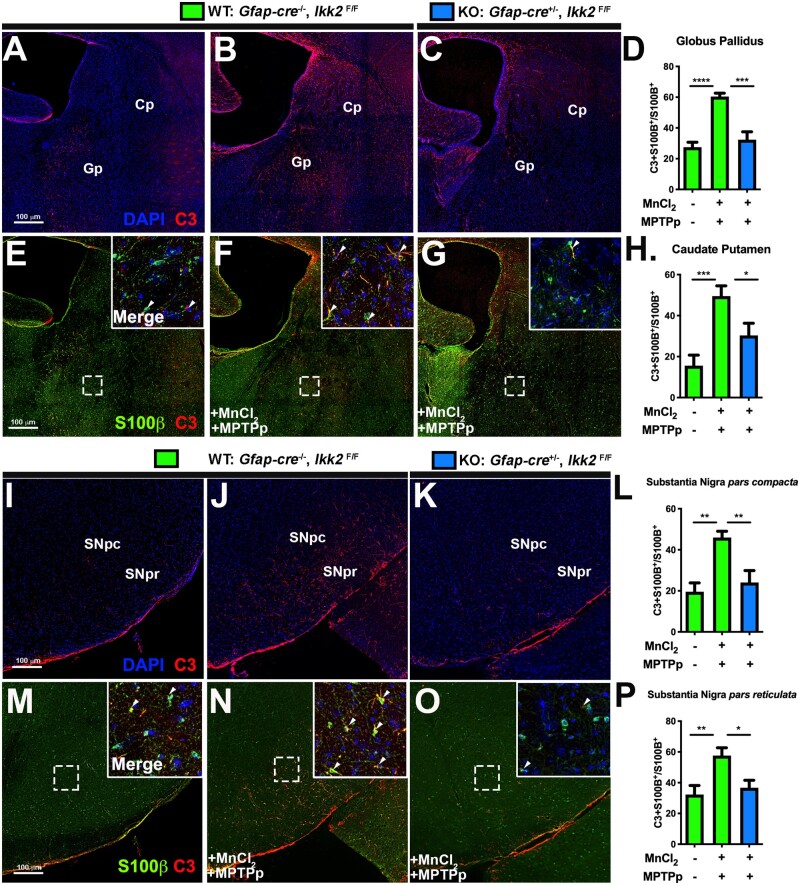
Complement Protein-C3 Is Highly Expressed in Reactive Astrocytes Following Treatment With MnCl2/MPTPp and Downregulated in IKK2 KO Mice
To quantitate number of neurotoxic astrocytes present throughout the basal ganglia, brain sections were stained for expression of the complement protein, C3 (Figure 7), which is uniquely expressed in reactive astrocytes of the CNS (Liddelow et al., 2017). S100β was used to determine the total number of astrocytes. Each brain region was imaged using a motorized stage and montage imaging to generate ROIs representing nuclei of interest, including the Cp, Gp, SNpc, and SNpr. The number of astrocytes coexpressing C3+ and S100β+ was compared with the total number of S100β+ cells. WT animals treated with MnCl2/MPTPp exhibited significantly higher numbers of neurotoxic astrocytes in the Gp (Figs. 7B, 7D, and 7F), Cp (Figs. 7B, 7F, and 7H), SNpc (Figs. 7J, 7L, and 7N), and SNpr (Figs. 7J, 7N, and 7P) than control mice. Inhibition of NF-κB signaling in astrocytes in KO mice suppressed the increase in C3+ astrocytes in each region following treatment with MnCl2/MPTPp, as depicted in the representative images in Figures 7C, 7G, 7K, and 7O (n = 7 animals/group; *p < .05, **p < .01, ***p < .001).
Figure 7.
NF-κB signaling regulates the increase in A1 neurotoxic astrocytes in mice sequentially exposed to MnCl2 and methyl-4-phenyl-1,2,3,6-tetrahydropyridine and probenecid (MPTPp). The number of astrocytes coexpressing C3 and S100β was compared with the total number of S100β+ cells in the caudate-putamen (Cp)/globus pallidus (Gp) (A–H) and the substantia nigra (I–P) by immunofluorescence labeling of serial sections through each brain region in wildtype (WT) and knockout (KO) mice. Expression of C3 (red) and S100β (green) was examined in the Cp/Gp in (A) WT—control, (B) WT—MnCl2/MPTPp, and (C) KO—MnCl2/MPTPp treatment groups. Quantification of the number of C3+ + S100β+ cells in each brain region is shown for the Gp (D), Cp (H), substantia nigra pars compacta (SNpc) (L), and substantia nigra pars reticulata (SNpr) (P) (*p < .05, **p < .01, ***p < .001; N = 7 animals/group).
DISCUSSION
The pathological mechanisms underlying irreversible neurological damage from Mn exposure are not completely understood. However, neuroinflammatory activation of glial cells is a common mechanism underpinning the neurotoxicity of Mn in both humans and animal models of the disease (Criswell et al., 2018; Kirkley et al., 2017; Moreno et al., 2011). We previously demonstrated that juvenile mice were particularly susceptible to Mn-induced neuroinflammation during the early postweaning period until puberty (PN 21–34) (Moreno et al., 2009a), potentially due to a critical period of striatal development in rodents (Soiza-Reilly and Azcurra, 2009). More recently, we characterized a novel astrocyte-specific transgenic mouse deficient in IKK2, the upstream activating kinase for NF-κB, that prevented inflammatory activation of glial cells loss of dopaminergic neurons in the MPTPp animal model of PD (Kirkley et al., 2019). This study expanded on this work by examining IKK2/NF-κB activation and its role in modulation of neuronal loss and glial reactivity within murine astrocytes following juvenile Mn exposure and secondary exposure to the dopaminergic neurotoxicant MPTP.
Combined developmental/adult exposure to Mn and MPTPp caused considerably different OFT performance compared with the other experimental groups, representing an overall hyperactive phenotype (Figure 4). Behavioral alterations, such as abnormal neuromuscular function and fine motor deficits, have been documented in rat models ranging from low (4.8 mg/kg) subchronic to high (50 mg/kg) chronic MnCl2 administration (Beaudin et al., 2017; Witholt et al., 2000). Dual treatment with MnCl2/MPTPp did not exacerbate DA neuronal loss in the SNpc (Figure 2G) or striatal dopamine production (Figure 4H) over MPTPp alone, suggesting that presynaptic DA neurotransmission is not the major system effected by Mn, in contrast to MPTP. However, Mn increased loss of TH+ terminals in the Cp in WT mice following dual treatment with MPTPp compared with control and MPTPp-only treatment (Figure 3G). A low, subchronic dose of MnCl2 for 5 weeks also reported little depletion of nigrostriatal DA depletion (Gwiazda et al., 2002). In contrast, Beaudin et al. (2017) showed chronic administration of 50 mg/kg/day from PND 20 to 460 significantly depleted DA. Extending MnCl2 treatment in our model longer than 30 days would likely have produced more severe effects on DA levels and DA neuron counts upon exposure to MPTPp.
In the Gp, total NeuN+ neurons in the Gp revealed a trend toward decreased numbers of NeuN+ neurons in mice treated with MnCl2 or MPTPp individually but significant loss of NeuN+ neurons was only detected in MnCl2/MPTPp-treated WT animals compared with controls (Figure 3E). Astrocyte-specific deletion of IKK2 prevented neuronal loss in the Gp, indicating that prior exposure to Mn enhances neuronal injury after a secondary exposure to MPTP through a mechanism involving NF-κB in astrocytes. The extent of Mn-induced glial reactivity may influence the dysregulation of neurotransmitters in the Gp, based in part on the model and dosing regimen. For example, chronic exposure to Mn (10 mg/kg) in nonhuman primates depleted expression of GS in the Gp but had no effect on GABAergic or glutamatergic systems (Burton et al., 2009). Other studies in rats treated with 6 mg/kg/day reported a significant increase in brain Mn levels and a decrease in GABA (Erikson and Aschner, 2003).
The hyperactive locomotor responses observed in Mn-treated mice could also be associated with loss of neurons in the Gp, which would decrease GABAergic output to the Sth and therefore increased Glu excitation to the SNpc and increased firing of DA neurons to the ST through the direct pathway (Erikson and Aschner, 2003). An increase in DA firing could explain modest increases in the number of TH+ neurons observed in the SNpc in mice treated only with Mn (Figs. 2G and 2H), as well as increases in DA content in the same group (Figure 4H). Serotonergic neurotransmission is also a possible target of Mn toxicity. Alterations in serotonin (5-HT) can cause abnormalities in motor activity and sleep, both of which are symptoms of manganism (Lesch et al., 1996). There was significant increase in the 5-HT metabolite (5-HIAA) of the ST from both dual-treated animal groups, although no differences in levels of 5-HT were detected between groups (Figs. 4L and 4M). This finding is consistent with previous data from our laboratory where we observed that juvenile mice treated with MnCl2 exhibited high levels of 5-HIAA compared with mice treated with Mn only as adults (Moreno et al., 2009b).
Neuronal cell death in response to MnCl2/MPTPp treatment was significantly modulated by IKK2/NF-κB in astrocytes. Inhibition of astrocyte NF-κB signaling in IKK2 KO mice protected DA neurons from both MPTPp and MnCl2/MPTPp-induced toxicity by approximately 90% compared with control within the SNpc (Figure 2G). KO animals also did not sustain any loss of neurons in the Gp and DAergic terminals of the Cp and were similarly preserved (Figs. 3E–G). We previously reported that Mn caused selective apoptosis of interneurons in the striatum and Gp expressing neuronal nitric oxide synthase, enkephalin, and choline acetyl transferase (Liu et al., 2006). Protection against neuronal loss in these brain regions in IKK2 KO mice is consistent with studies reporting that mice with constitutively active astrocyte-NF-κB had increased production of cytokines in the MPTP model of PD, as well as increased amyloid burden and gliosis in a mouse model of AD (Lian et al., 2016; Oeckl et al., 2012). Similarly, KO of IKK2 in all CNS cells demonstrated protection in a mouse model of autoimmune encephalitis (van Loo et al., 2006). The data presented here are the first to report that astrocyte-specific inhibition of IKK2 protects against reactive gliosis and neuronal death induced by combined treatment with MnCl2 and MPTPp.
Astrocyte-specific deletion of IKK2 also reduced microglial activation in the Gp (Figs. 5E, 5J, and 5L) and Cp (Figs. 5D, 5I, and 5K), suggesting that cell-cell signaling in glia is critical to achieving a neurotoxic inflammatory response. Inhibition of microglial activation in the Gp of KO animals directly correlated with the preservation of NeuN+ cells with dual treatment (Figs. 3E and 5E). However, no significant suppression of microgliosis was observed in the SNpc or SNpr, consistent with the initial characterization of hGFAP-cre+/−/IKK2fl/fl treated with MPTPp (Figs. 5F and 5G) (Kirkley et al., 2019). Microglia stimulate phenotypic activation of neurotoxic A1 astrocytes through release of factors such as TNFα, C1q, and IL1α (Liddelow et al., 2017) that in turn further magnify microglial reactivity through NF-κB-dependent production of neuroinflammatory signaling molecules such as CCL2 (Popichak et al., 2018). Thus, dampening innate immune inflammatory signaling in astrocytes through gene deletion of IKK2 likely prevented more severe neuroinflammatory activation of microglia in this model. We previously reported that immunopurified primary astrocytes isolated from IKK2 KO mice exhibited 70% knockdown of IKK2 (Kirkley et al., 2019), which we also observed here, as depicted in representative images of IKK2/GFAP colocalization in Figures 6A–C. Quantitation of the number of GFAP+ cells/µm2 confirmed that the Gp was most effected nucleus in WT mice exposed to Mn/MPTPp (Figs. 6D and 6F). KO animals exhibited significantly fewer GFAP+ cells/µm2 in the Gp, Cp, and SNpc, although no groups were different in the SNpr, potentially due to constitutively high basal levels of astrocytes present in this brain region (Figs. 6D–M).
Notably, no increases in Mn were observed in astrocyte-specific IKK2 KO mice following Mn treatment at PN 51 in the SN, striatum or in the cortex, although basal levels were somewhat higher in these brain regions (Figs. 1D–F). This suggested that decreased neurotoxicity in KO mice following MPTP treatment in Mn-exposed mice could be due in part to less accumulation of Mn in the brain. We therefore measured mRNA levels of several metal transporters known to regulate Mn and uptake and neurotoxicity, including Slc11a2 (Dmt1), Slc39a8 (Zip8), Slc39a10, and Slc39a14 (Figs. 1H–M). There were no differences amongst these metal transporters in the SN between WT and KO mice, although levels of Dmt1 were increased in the cortex following dual treatment with Mn + MPTP. Expression of Slc39a8 was very low in all brain regions and did not change between genotype and treatment condition, consistent with patterns of expression previously reported (Yue et al., 2014). Both DMT1 and SLC39A10 are important transporters for Mn in the periphery and in brain (Illing et al., 2012; Taylor et al., 2019) and SLC39A10 is protective against Mn neurotoxicity (Illing et al., 2012). DMT1 is at least partly regulated by NF-κB (Paradkar and Roth, 2006) and induced in neurons during exposure to MPTP (Xu et al., 2010). An increase in DMT1 could therefore modulate neurotoxicity but despite higher baseline levels of Mn in KO mice compared with WT in each brain region, deletion of IKK2 in astrocytes still protected against neuronal injury from dual treatment with Mn + MPTP. These data suggest that altered accumulation of Mn in KO mice is not primarily responsible for the observed degree of neuroprotection. Treatment with Mn up to 100 mg/kg results in an apparent saturation point in the brain following oral exposure in mice (Liu et al., 2006; Moreno et al., 2009b), so it is likely that IKK2 inhibition would suppress innate immune inflammatory responses across a range of Mn concentrations.
However, astrocyte-specific deletion of IKK2 prevented MPTP-induced neuronal loss and glial activation even in the absence of Mn exposure, suggesting that attenuating innate immune inflammatory responses in this cell type was directly neuroprotective. Moreover, levels of Mn were similar between genotypes in all groups at the time of treatment with MPTP, indicating that differences in metal transporters were likely not the primary drivers of later neuronal loss in dual-treated animals. However, even slight increases in Mn can have lasting effects on levels of biogenic amines in the striatum (Lasley et al., 2020), suggesting that altered states of glial activation could persist even after Mn was excreted, perhaps due to epigenetic imprinting during the juvenile exposure period. Such mechanisms remained to be elucidated in subsequent studies.
Studies of MPTP neurotoxicity demonstrate that microglia quickly activate following MPTP exposure and trigger a sustained inflammatory phenotype in astrocytes (Hirsch and Hunot, 2009; Huang et al., 2018). To detect neurotoxic A1 astrocytes, we immunolabeled for complement protein-C3 (Figure 7), a component of the classical complement pathway mediating innate immune responses to pathogens (Ricklin et al., 2016) that is uniquely expressed in reactive A1 astrocytes, not in resting A2 astrocytes (Liddelow et al., 2017; Nitkiewicz et al., 2017). C3 is an NF-κB-regulated gene in astrocytes (Lian et al., 2016; Nitkiewicz et al., 2017) that is increased in mouse models of AD (Lian et al., 2016). We observed that IKK2 KO mice had fewer C3+ A1 astrocytes after dual exposure to MnCl2/MPTPp treatment compared with the WT animals (Figs. 7D, 7H, 7L, and 7P) suggesting that astrocyte-NF-κB regulated C3 expression may be an important mechanism to stimulate neuroinflammation and neurodegeneration following 2-hit exposure to Mn and MPTP.
In conclusion, we report a neuroinflammatory mechanism in astrocytes associated with juvenile MnCl2 exposure that exacerbated neurodegeneration from later adult exposure to MPTPp. These data demonstrate that astrocyte-specific gene deletion of NF-κB/IKK2 mitigates the numbers of reactive astrocytes and microglia and protects against neuronal loss in the basal ganglia induced by sequential exposure to Mn and MPTPp.
FUNDING
National Institutes of Health (R01ES021656, R01ES030937 to R.B.T., F31ES026860 to K.A.P., F31NS096841 to S.L.H.).
DECLARATION OF CONFLICTING INTERESTS
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
REFERENCES
- Aschner M., Erikson K. M., Herrero Hernandez E., Tjalkens R. (2009). Manganese and its role in Parkinson's disease: From transport to neuropathology. Neuromol. Med. 11, 252–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaudin S. A., Strupp B. J., Strawderman M., Smith D. R. (2017). Early postnatal manganese exposure causes lasting impairment of selective and focused attention and arousal regulation in adult rats. Environ. Health Perspect. 125, 230–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown T. P., Rumsby P. C., Capleton A. C., Rushton L., Levy L. S. (2006). Pesticides and Parkinson's disease—Is there a link? Environ. Health Perspect. 114, 156–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton N. C., Schneider J. S., Syversen T., Guilarte T. R. (2009). Effects of chronic manganese exposure on glutamatergic and GABAergic neurotransmitter markers in the nonhuman primate brain. Toxicol. Sci. 111, 131–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criswell S. R., Warden M. N., Searles Nielsen S., Perlmutter J. S., Moerlein S. M., Sheppard L., Lenox-Krug J., Checkoway H., Racette B. A. (2018). Selective D2 receptor PET in manganese-exposed workers. Neurology 91, e1022–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erikson K. M., Aschner M. (2003). Manganese neurotoxicity and glutamate-GABA interaction. Neurochem. Int. 43, 475–480. [DOI] [PubMed] [Google Scholar]
- Glass C. K., Saijo K., Winner B., Marchetto M. C., Gage F. H. (2010). Mechanisms underlying inflammation in neurodegeneration. Cell 140, 918–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouveia K., Hurst J. L. (2013). Reducing mouse anxiety during handling: Effect of experience with handling tunnels. PLoS One 8, e66401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilarte T. R., Chen M. K., McGlothan J. L., Verina T., Wong D. F., Zhou Y., Alexander M., Rohde C. A., Syversen T., Decamp E., et al. (2006). Nigrostriatal dopamine system dysfunction and subtle motor deficits in manganese-exposed non-human primates. Exp. Neurol. 202, 381–390. [DOI] [PubMed] [Google Scholar]
- Gwiazda R. H., Lee D., Sheridan J., Smith D. R. (2002). Low cumulative manganese exposure affects striatal GABA but not dopamine. Neurotoxicology 23, 69–76. [DOI] [PubMed] [Google Scholar]
- Hammond S. L., Popichak K. A., Li X., Hunt L. G., Richman E. H., Damale P. U., Chong E. K. P., Backos D. S., Safe S., Tjalkens R. B. (2018). The Nurr1 ligand, 1,1-bis(3′-indolyl)-1-(p-chlorophenyl)methane, modulates glial reactivity and is neuroprotective in MPTP-induced parkinsonism. J. Pharmacol. Exp. Ther. 365, 636–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He P., Liu D. H., Zhang G. Q. (1994). [Effects of high-level-manganese sewage irrigation on children's neurobehavior]. Zhonghua Yu Fang Yi Xue Za Zhi 28, 216–218. [PubMed] [Google Scholar]
- Hirsch E. C., Hunot S. P. (2009). Neuroinflammation in Parkinson’s disease: A target for neuroprotection? Lancet Neurol. 8, 382–397. [DOI] [PubMed] [Google Scholar]
- Huang D., Wang Z., Tong J., Wang M., Wang J., Xu J., Bai X., Li H., Huang Y., Wu Y., et al. (2018). Long-term changes in the nigrostriatal pathway in the MPTP mouse model of Parkinson’s disease. Neuroscience 369, 303–313. [DOI] [PubMed] [Google Scholar]
- Illing A. C., Shawki A., Cunningham C. L., Mackenzie B. (2012). Substrate profile and metal-ion selectivity of human divalent metal-ion transporter-1. J. Biol. Chem. 287, 30485–30496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkley K. S., Popichak K. A., Afzali M. F., Legare M. E., Tjalkens R. B. (2017). Microglia amplify inflammatory activation of astrocytes in manganese neurotoxicity. Neuroinflammation 14, 99. [DOI] [PMC free article] [PubMed]
- Kirkley K. S., Popichak K. A., Hammond S. L., Davies C., Hunt L., Tjalkens R. B. (2019). Genetic suppression of IKK2/NF-κB in astrocytes inhibits neuroinflammation and reduces neuronal loss in the MPTP-probenecid model of Parkinson’s disease. Neurobiol. Dis. 127, 193–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasley S. M., Fornal C. A., Mandal S., Strupp B. J., Beaudin S. A., Smith D. R. (2020). Early postnatal manganese exposure reduces rat cortical and striatal biogenic amine activity in adulthood. Toxicol. Sci. 173, 144–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesch K. P., Bengel D., Heils A., Sabol S. Z., Greenberg B. D., Petri S., Benjamin J., Müller C. R., Hamer D. H., Murphy D. L. (1996). Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science 274, 1527–1531. [DOI] [PubMed] [Google Scholar]
- Lian H., Litvinchuk A., Chiang A. C. A., Aithmitti N., Jankowsky J. L., Zheng H. (2016). Astrocyte-microglia cross talk through complement activation modulates amyloid pathology in mouse models of Alzheimer’s disease. J. Neurosci. 36, 577–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddelow S. A., Guttenplan K. A., Clarke L. E., Bennett F. C., Bohlen C. J., Schirmer L., Bennett M. L., Münch A. E., Chung W.-S., Peterson T. C., et al. (2017). Neurotoxic reactive astrocytes are induced by activated microglia. Nat. Publishing Group 541, 481–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Sullivan K. A., Madl J. E., Legare M., Tjalkens R. B. (2006). Manganese-induced neurotoxicity: The role of astroglial-derived nitric oxide in striatal interneuron degeneration. Toxicol. Sci. 91, 521–531. [DOI] [PubMed] [Google Scholar]
- Mergler D., Baldwin M. (1997). Early manifestations of manganese neurotoxicity in humans: An update. Environ. Res. 73, 92–100. [DOI] [PubMed] [Google Scholar]
- Miller J. A., Runkle S. A., Tjalkens R. B., Philbert M. A. (2011). 1,3-Dinitrobenzene-induced metabolic impairment through selective inactivation of the pyruvate dehydrogenase complex. Toxicol. Sci. 122, 502–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno J. A., Streifel K. M., Sullivan K. A., Hanneman W. H., Tjalkens R. B. (2011). Manganese-induced NF-kappaB activation and nitrosative stress is decreased by estrogen in juvenile mice. Toxicol. Sci. 122, 121–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno J. A., Streifel K. M., Sullivan K. A., Legare M. E., Tjalkens R. B. (2009. a). Developmental exposure to manganese increases adult susceptibility to inflammatory activation of glia and neuronal protein nitration. Toxicol. Sci. 112, 405–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno J. A., Yeomans E. C., Streifel K. M., Brattin B. L., Taylor R. J., Tjalkens R. B. (2009. b). Age-dependent susceptibility to manganese-induced neurological dysfunction. Toxicol. Sci. 112, 394–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison H. W., Filosa J. A. (2013). A quantitative spatiotemporal analysis of microglia morphology during ischemic stroke and reperfusion. J. Neuroinflamm. 10, 1–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitkiewicz J., Borjabad A., Morgello S., Murray J., Chao W., Emdad L., Fisher P. B., Potash M. J., Volsky D. J. (2017). HIV induces expression of complement component C3 in astrocytes by NF-κB-dependent activation of interleukin-6 synthesis. J. Neuroinflamm. 14, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oeckl P., Lattke M., Wirth T., Baumann B., Ferger B. (2012). Astrocyte-specific IKK2 activation in mice is sufficient to induce neuroinflammation but does not increase susceptibility to MPTP. Neurobiol. Dis. 48, 481–487. [DOI] [PubMed] [Google Scholar]
- Paradkar P. N., Roth J. A. (2006). Nitric oxide transcriptionally down-regulates specific isoforms of divalent metal transporter (DMT1) via NF-kappaB. J. Neurochem. 96, 1768–1777. [DOI] [PubMed] [Google Scholar]
- Peres T. V., Schettinger M. R., Chen P., Carvalho F., Avila D. S., Bowman A. B., Aschner M. (2016). “Manganese-induced neurotoxicity: A review of its behavioral consequences and neuroprotective strategies”. BMC Pharmacol. Toxicol. 17, 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popichak K. A., Afzali M. F., Kirkley K. S., Tjalkens R. B. (2018). Glial-neuronal signaling mechanisms underlying the neuroinflammatory effects of manganese. J. Neuroinflamm. 15, 324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racette B. A., Searles Nielsen S., Criswell S. R., Sheppard L., Seixas N., Warden M. N., Checkoway H. (2017). Dose-dependent progression of parkinsonism in manganese-exposed welders. Neurology 88, 344–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricklin D., Reis E. S., Mastellos D. C., Gros P., Lambris J. D. (2016). Complement component C3—The ‘Swiss Army Knife’ of innate immunity and host defense. Immunol. Rev. 274, 33–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodier J. (1955). Manganese poisoning in Moroccan miners. Br. J. Ind. Med. 12, 21–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rugless F., Bhattacharya A., Succop P., Dietrich K. N., Cox C., Alden J., Kuhnell P., Barnas M., Wright R., Parsons P. J., et al. (2014). Childhood exposure to manganese and postural instability in children living near a ferromanganese refinery in Southeastern Ohio. Neurotoxicol. Teratol. 41, 71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider C. A., Rasband W. S., Eliceiri K. W. (2012). NIH image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soiza-Reilly M., Azcurra J. M. (2009). Developmental striatal critical period of activity-dependent plasticity is also a window of susceptibility for haloperidol induced adult motor alterations. Neurotoxicol. Teratol. 31, 191–197. [DOI] [PubMed] [Google Scholar]
- Stuart S. A., Robinson E. S. (2015). Reducing the stress of drug administration: Implications for the 3Rs. Sci. Rep. 5, 14288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takser L., Mergler D., Hellier G., Sahuquillo J., Huel G. (2003). Manganese, monoamine metabolite levels at birth, and child psychomotor development. Neurotoxicology 24, 667–674. [DOI] [PubMed] [Google Scholar]
- Tapias V., Greenamyre J. T., Watkins S. C. (2013). Automated imaging system for fast quantitation of neurons, cell morphology and neurite morphometry in vivo and in vitro. Neurobiol. Dis. 54, 158–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor C. A., Hutchens S., Liu C., Jursa T., Shawlot W., Aschner M., Smith D. R., Mukhopadhyay S. (2019). SLC30A10 transporter in the digestive system regulates brain manganese under basal conditions while brain SLC30A10 protects against neurotoxicity. J. Biol. Chem. 294, 1860–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Loo G., De Lorenzi R., Schmidt H., Huth M., Mildner A., Schmidt-Supprian M., Lassmann H., Prinz M. R., Pasparakis M. (2006). Inhibition of transcription factor NF-κB in the central nervous system ameliorates autoimmune encephalomyelitis in mice. Nat. Immunol. 7, 954–961. [DOI] [PubMed] [Google Scholar]
- Wang J. D., Huang C. C., Hwang Y. H., Chiang J. R., Lin J. M., Chen J. S. (1989). Manganese induced parkinsonism: An outbreak due to an unrepaired ventilation control system in a ferromanganese smelter. Br. J. Ind. Med. 46, 856–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witholt R., Gwiazda R. H., Smith D. R. (2000). The neurobehavioral effects of subchronic manganese exposure in the presence and absence of pre-parkinsonism. Neurotoxicol. Teratol. 22, 851–861. [DOI] [PubMed] [Google Scholar]
- Xu H., Jiang H., Wang J., Xie J. (2010). Rg1 protects the MPP+-treated MES23.5 cells via attenuating DMT1 up-regulation and cellular iron uptake. Neuropharmacology 58, 488–494. [DOI] [PubMed] [Google Scholar]
- Yue F., Cheng Y., Breschi A., Vierstra J., Wu W., Ryba T., Sandstrom R., Ma Z., Davis C., Pope B. D., et al. (2014). A comparative encyclopedia of DNA elements in the mouse genome. Nature 515, 355–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zidenberg-Cherr S., Keen C. L., Lonnerdal B., Hurley L. S. (1983). Superoxide dismutase activity and lipid peroxidation in the rat: Developmental correlations affected by manganese deficiency. J. Nutr. 113, 2498–2504. [DOI] [PubMed] [Google Scholar]