Abstract
Natural rubber is one of the most important polymers produced by plants, which contains cis-1,4-polyisoprene, protein and fatty acids. It has unique properties compared to synthetic rubber and has many different uses in industry. Here, natural rubber of Euphorbia macroclada was characterized due to its abundance in arid areas. Isolation of rubber was done using both acetone and hexane solvents. FT-IR and NMR further characterized and confirmed the structure of rubber as cis-1,4 polyisoprene. GPC analyses showed a molecular weight of 8.180E+2 with polydispersity of 1.287. These data is useful for better understanding of latex composition in family of Euphorbiaceae.
Keywords: Latex, Natural rubber, Spurge, E. macroclada
Introduction
Euphorbia macroclada belongs to family of Euphorbiaceae. This family includes over 2000 species distributed around the world as herbs, shrubs and trees. E. macroclada usually grows in different habitats such as damaged areas, arable lands, dry meadows, steppes, open oak forests, along roads, rocks, etc. and SW Asia is one of the most important centers of its diversity (Yang et al. 2012; Dorsey et al. 2013; Peirson et al. 2013; Riina et al. 2013; Pahlevani et al. 2015). The genus Euphorbia is known by exudes of latex when injured (Biesboer and Koukkari 1992; Mabberley 1997; Barla et al. 2006). One of the important plants in this family is Hevea brasiliensis which is well known as the natural source of industrial rubber production (Dehgan and Schutzman 1994; Mooibroek and Cornish 2000). Natural rubber is a bio-polymer composed of isoprene units. The length of the biopolymers defines the importance molecular mass of rubber (Bouton 1992). Most of our knowledge of latex biochemistry and laticifers is derived from studies on H. brasiliensis (Arif et al. 2004; Takahashi and Koyama 2006; Wagner et al. 2007). This natural raw material is used in medical instruments, surgical gloves, and various engineering and consumable products (Mooibroek and Cornish 2000). Most developed countries are entirely dependent on the introduction of natural rubber for applications where synthetic rubber cannot be replaced. Natural rubber and latex prices continue to rise as demand grows, and global shortages are projected (Bowers 1990). As Hevea has a very narrow genetic base and well establishes only in tropical regions efforts have been made to develop alternative natural rubber resources (Metcalfe 1967; Bowers 1990). Recently two rubber bearing plants, Taraxacum kok-saghyz and Parthenium argentatum, introduced as alternatives (Buranov and Elmuradov 2010; Cornish 2017) and many works are done to improve their processing capacity. In family of Euphorbiaceae other species like E. characias and E. lactiflua are also have been studied for rubber production (Mooibroek and Cornish 2000; Spanò et al. 2012).
To analyze tires and plastics the Fourier-transform infrared spectroscopy (FT-IR) is used which provides information about the chemical structure of a molecule (Amand and Tullin 1999). Nuclear magnetic resonance spectroscopy (NMR), is used for both quantitative and qualitative identification of complex organic and biological compounds (Vandersypen et al. 2001). To detect and evaluate the extracted rubber based on the molecular mass (total mass of the forming atoms) also gel permeation chromatography (GPC) is carried out (Moore 1964).
As there is no information on rubber production in E. macroclada, the aim of this study was to investigate E. macroclada latex for rubber production. Due to its broad diversity in SW Asia and growing in different habitats, this investigation adds new information on E. macroclada latex composition.
Materials and methods
Plant material and rubber extraction
E. macroclada wild plants were collected during autumn from Hamedan plain, located in west part of Iran and stored in Bu-Ali Sina Herbarium with a voucher specimen number EM15. Drops of latex were collected by diagonal cuts on stem using a razor into preweighed micro-centrifuge tubes. Eight samples were collected and stored at 4 °C before rubber extraction. The tubes were re-weighed to obtain the initial weight of the latex. To remove the water and dry latex completely, tubes were kept at 35 °C for 10 days. The tubes were re-weighed to obtain the total amount of water. The dried samples were extracted three times with acetone by addition of one ml acetone then vortex and centrifuge for 5 min at 6822 g, and collection of supernatant into a re-weighted clean tube. After acetone evaporation the tubes were re-weighed to measure the resin content based on the initial latex weight. In the next stage, hexane was used to extract rubber (Bell et al. 2015). Samples were centrifuged and supernatant collected into a re-weighted clean tube. Rubber was extracted three times with hexane. Hexane was dried up under the air and tubes were weighted to obtain the contained rubber. Original collection tubes containing insoluble and other material were dried and weighted to find out the percentage of other contents in initial latex (Bell et al. 2015). The extracted rubber was used for FT-IR, NMR and GPC analysis. The percentage of water, resin, rubber and other insoluble materials of latex were determined (% w/w) as the means of eight measurements.
Rubber analysis
To do the FT-IR, the Bruker Vector 22 spectrophotometer was used. Samples were dissolved in a benzene solution and poured onto a KBr disk to form a thin film. A resolution of 2 cm−1 in the range of 4000–400 cm−1 was considered as described by Spanò et al. (2012) and Rolere et al. (2015). NMR spectroscopy was done by the Bruker AVANCE Aqf-300 MHz device. The rubber extracted in the chlorophorm was dissolved in CDCl3 and measured at 25 °C and chemical shifts were reported in ppm (Mooibroek and Cornish 2000). The molecular weight was measured by GPC. Chromatogram monitor with refractive index (RI) was recorded at 30 °C. The samples were dissolved in tetrahydrofuran (THF) and stored at room temperature overnight. The clear solution was filtered from 1 micron filters and 50 μl of solution was injected by automatic sampler (GPC Agilent 1100) (Cornish et al. 2000). The flow rate was 1 ml/min for the sample. Polystyrene was used as reference material and the Agilent column, PLgel Mixed-C, 5 μm; 300 * 7.5 mm was used.
Results
Natural rubber of E. macroclada latex was extracted by acetone and hexane. Figure 1 shows the rubber extracted from E. macroclada after extraction from one milliliter of latex.
Fig. 1.
Rubber extraction from E. macroclada latex. a Dualization of samples after adding solvent (acetone), b the extracted resin after evaporation of acetone. c The extracted rubber after drying hexane extract
Table 1 shows the different compounds of E. macroclada latex. Water, resin and rubber content of 1 ml of latex were measured using eight samples.
Table 1.
The amount of water, resin and rubber determined in the latex of E. macroclada (%W/W)
| Compounds | % |
|---|---|
| Water | 25.1 |
| Resin | 9.5 |
| Rubber | 8.1 |
| Insoluble materials | 57 |
Fourier transform-infrared spectroscopy provided bands characteristic of cis-1,4-polyisoprene at 886.05, 1374.84, 1458.23, 1712.07, 2867.50 and 2927.33 cm−1 which are due to C–H out-of-plane bending, CH3 deformation, CH2 deformation, C=C stretching, CH2 stretching and CH2 stretching respectively (Fig. 2; Table 2). The spectrum data were assigned according to the literature (Rolere et al. 2015; Ramirez-Cadavid et al. 2017). Also there were other bands not characteristic of cis-1,4-polyisoprene.
Fig. 2.

Infrared spectroscopy of E. macroclada extracted rubber
Table 2.
The FT-IR absorbtion bands of E. macroclada extracted rubber
| Wavenumber (cm−1) | Functional group modes |
|---|---|
| 886.05 | C–H out-of-plane bending |
| 1374.84 | –CH3 deformation |
| 1458.23 | –CH2 deformation |
| 1712.07 | C=C stretching |
| 2867.50 | –CH2 stretching |
| 2927.33 | –CH2 stretching |
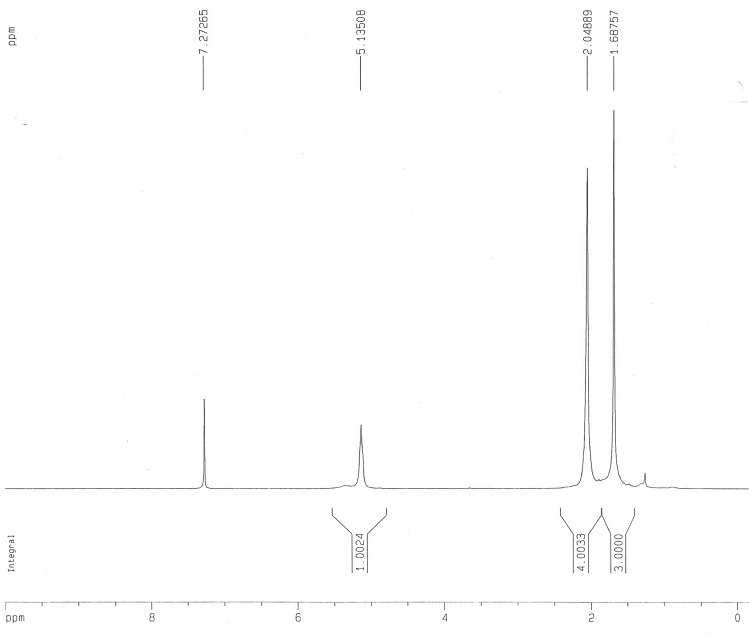
The 1HNMR spectrum of E. macroclada extracted rubber revealed signals at 1–8. There are 8 hydrogens in polyisoprene formula (Fig. 3; Table 3) which is in accordance with the peaks in image below. The peak area of 1.68757 is related to CH3 with three hydrogens, the peak area of 2.04889 is related to 2CH2 with four hydrogens and peak area of 5.13508 is related to CH with one hydrogen. The other signal belongs to the solvent (Fig. 3; Table 3). In the other hand the 1HNMR spectrum of the rubber compound showed the presence of vinyli protons (H) in the 4.5–6.5 regions. The aliphatic protons CH2, CH appeared in the 1–2.5 region. This information in HNMR indicates the presence of polyisoprene in E. macroclada (Fig. 3; Table 3).
Fig. 3.
1HNMR analysis of E. macroclada extracted rubber (left), cis-1,4-polyisoprene formula (right)
Table 3.
The 1HNMR signals of E. macroclada extracted rubber
| Peak ppm | Peak area | Related signal |
|---|---|---|
| 1.68757 | 3.0000 | CH3 |
| 2.04889 | 4.0033 | CH2 |
| 5.13508 | 1.0024 | CH |
| 7.27265 | – | Solvent |
The 13CNMR spectrum of E. macroclada extracted rubber showed different signals in the region between 20 and 150 ppm (Fig. 4; Table 4). The solvent (CDCl3) peaks revealed at 76–78 ppm. Two close peaks at 125.048 and 135.230 ppm represent the core of molecule with a double bond (C=C). Three peaks in the beginnings 23.439, 26.415 and 32.223 are related to 2CH2 and CH3 in the polyisoprene formula respectively (Fig. 4; Table 4).
Fig. 4.

13CNMR analysis of E. macroclada extracted rubber
Table 4.
The 13CNMR signals of E. macroclada extracted rubber
| Peak ppm | Related signal |
|---|---|
| 23.439 | CH2 |
| 26.415 | CH2 |
| 32.223 | CH3 |
| 76.601 | Solvent |
| 77.024 | Solvent |
| 77.447 | Solvent |
| 125.048 | C=C |
| 135.230 | C=C |
According to GPC analysis (Fig. 5), polydispersity index (Mw/Mn) of E. macroclada is 1.287 (Table 5) that is similar to this index from H. brasiliensis and P. argentatum, 1.251 and 1.240 respectively (Pearson et al. 2010).
Fig. 5.

Molecular weight distribution of E. macroclada extracted rubber by GPC
Table 5.
Gel permeation chromatography data for rubber extract of E. macroclada
| Molecular weight | g/mol |
|---|---|
| Mn | 8.180E+2 |
| Mw | 1.053E+3 |
| Mz | 1.884E+3 |
| D (Mw/Mn) | 1.287E+0 |
The average number of molecular weight (Mn), Weighted average molecular weight (Mw), z-average molecular weight (MZ), polydispersity(Mw/Mn)
Discussion
Natural rubber has many uses and unique properties due to proteins, lipids, carbohydrates and minerals that are present in the latex. Hevea in family of Euphorbiaceae is the only tree for rubber production in tropical regions. There is at least 60 Euphorbia species just in Europe which is needed to be explored for rubber production (Mooibroek and Cornish 2000). In addition to Hevea E. characias and E. lactiflua also have been studied for rubber production in this family (Mooibroek and Cornish 2000; Spanò et al. 2012). But there is no report on rubber content of E. macroclada which is growing in broad area of Asia (Pahlevani et al. 2015). The FT-IR data of E. macroclada rubber indicated the connection of carbon to carbon (C=C) and carbon to hydrogen (C–H). The HNMR and C13NMR results represented vinyli and aliphatic protons, and confirmed cis-1,4 polyisoprene connections in E. macroclada rubber. This study showed the amount of rubber and resin in the latex of this plant, 8 and 9.5 percentage, is almost comparable to E. characias amounts, 9.1 and 14 percentage, respectively (Spanò et al. 2012). Although E. macroclada has less rubber amount than Hevea (44%) but it has more comparing with E. larica and E. tirucalli (data are not published yet). Molecular weight of natural rubber is very important for the processing and manufacturing of rubber products. Molecular weight of E. macroclada rubber was Mn = 8.180E+2 and its polydispersity was Mw/Mn = 1.287E+0. In E. characias rubber, the molecular weight of Mn = 93,000 and Mw/Mn = 2.9 has been reported (Spanò et al. 2012). In general, longer polymer chains with narrow distribution have the best performance (Bell 2013). But low molecular weight rubbers can also have different uses in industry as dispersing agents or processing aids (Spanò et al. 2012). The molecular weight of the rubber is largely influenced by the concentration and characteristics of the substrate, especially with the concentration of allylic-PP initiator (Cornish and Siler 1995; Castillon and Cornish 1999; Cornish et al. 2000). H. brasiliensis and P. argentatum synthesize a high molecular weight rubber (Mn = 105–106 g/mol) with wide molecular weight distribution (Mw/Mn = 2–10) (Swanson et al. 1979; Cornish et al. 2000; Puskas et al. 2011). However, the amount of rubber and possibly its molecular characteristics depend on the culture method, plant age, climate, and plant growth conditions (Buranov and Elmuradov 2010).
In conclusion, the current study concentrated on the identification and characterization of cis-1,4 polyisoperene in E. macroclada, an herbaceous and drought resistant plant, by FT-IR, NMR and GPC analysis. Further study on rubber particles and rubber structure of this plant will provide more understanding of its latex composition.
Acknowledgements
This work was supported by Bu-Ali Sina University, Malayer University, Boom Exir Pars Biotech. Co. Hamedan, and Biotechnology Development Council, Iran.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Hedayat Bagheri, Email: hbagheri@basu.ac.ir.
Behrooz Mohammad Parast, Email: bmp2013@yahoo.com.
References
- Amand LE, Tullin CJ. The theory behind FTIR analysis. Gothenburg: Department of Energy Conversion, Chalmers University of Technology; 1999. [Google Scholar]
- Arif SAM, Hamilton RG, Yusof F, Chew NP, Loke YH, Nimkar S, Beintema JJ, Yeang HY. Isolation and characterization of the early nodule-specific protein homologue (Hev b 13), an allergenic lipolytic esterase from Hevea brasiliensis latex. J Biol Chem. 2004;279(23):23933–23941. doi: 10.1074/jbc.M309800200. [DOI] [PubMed] [Google Scholar]
- Barla A, Bİrman H, KÜLTÜR Ş, Öksüz S. Secondary metabolites from Euphorbia helioscopia and their vasodepressor activity. Turk J Chem. 2006;30(3):325–332. [Google Scholar]
- Bell JL (2013) Biochemical and genetic characterization of rubber production in prickly lettuce (Lactuca serriola L.). Washington State University Ph.D Theses and Dissertations. Paper AAC3587052. 2013
- Bell JL, Burke IC, Neff MM. Genetic and biochemical evaluation of natural rubber from eastern Washington prickly lettuce (Lactuca serriola L.) J Agric Food Chem. 2015;63(2):593–602. doi: 10.1021/jf503934v. [DOI] [PubMed] [Google Scholar]
- Biesboer DD, Koukkari WL (1992) The taxonomy and biology of leafy spurge. IN: Leafy spurge symposium and proceedings, vol 4. pp 1–6
- Bouton TC. Rubber. In: Kent JA, editor. Riegel’s handbook of industrial chemistry. 9. New York: Van Nostrand Reinhold; 1992. pp. 598–622. [Google Scholar]
- Bowers JE. Natural rubber-producing plants for the United States. Beltsville: National Agricultural Library; 1990. [Google Scholar]
- Buranov AU, Elmuradov BJ. Extraction and characterization of latex and natural rubber from rubber-bearing plants. J Agric Food Chem. 2010;58(2):734–743. doi: 10.1021/jf903096z. [DOI] [PubMed] [Google Scholar]
- Castillon J, Cornish K. Regulation of initiation and polymer molecular weight of cis-1,4-polyisoprene synthesized in vitro by particles isolated from Parthenium argentatum (Gray) Phytochemistry. 1999;51(1):43–51. doi: 10.1016/S0031-9422(98)00719-5. [DOI] [Google Scholar]
- Cornish K. Alternative natural rubber crops: why should we care? Technol Innov. 2017;18:245–256. doi: 10.21300/18.4.2017.245. [DOI] [Google Scholar]
- Cornish K, Siler DJ. Effect of different allylic diphosphates on the initiation of new rubber molecules and on cis-1,4-polyisoprene biosynthesis in guayule (Parthenium argentatum Gray) J Plant Physiol. 1995;147(3–4):301–305. doi: 10.1016/S0176-1617(11)82157-7. [DOI] [Google Scholar]
- Cornish K, Castillón J, Scott DJ. Rubber molecular weight regulation, in vitro, in plant species that produce high and low molecular weights in vivo. Biomacromolecules. 2000;1(4):632–641. doi: 10.1021/bm000034z. [DOI] [PubMed] [Google Scholar]
- Dehgan B, Schutzman B. Contributions toward a monograph of neotropical Jatropha: phenetic and phylogenetic analyses. Ann Missouri Bot Gard. 1994;81:349–367. doi: 10.2307/2992102. [DOI] [Google Scholar]
- Dorsey BL, Haevermans T, Aubriot X, Morawetz JJ, Riina R, Steinmann VW, Berry PE. Phylogenetics, morphological evolution, and classification of Euphorbia subgenus Euphorbia. Taxon. 2013;62(2):291–315. doi: 10.12705/622.1. [DOI] [Google Scholar]
- Mabberley DJ. The plant–book: a portable dictionary of the vascular plants. Cambridge: Cambridge University Press; 1997. [Google Scholar]
- Metcalfe CR. Distribution of latex in the plant kingdom. Econ Bot. 1967;21(2):115–127. doi: 10.1007/BF02897859. [DOI] [Google Scholar]
- Mooibroek H, Cornish K. Alternative sources of natural rubber. Appl Microbiol Biotechnol. 2000;53(4):355–365. doi: 10.1007/s002530051627. [DOI] [PubMed] [Google Scholar]
- Moore JC. Gel permeation chromatography. I. A new method for molecular weight distribution of high polymers. J Polym Sci Part A Gen Pap. 1964;2(2):835–843. doi: 10.1002/pol.1964.100020220. [DOI] [Google Scholar]
- Pahlevani AH, Liede-Schumann S, Akhani H. Seed and capsule morphology of Iranian perennial species of Euphorbia (Euphorbiaceae) and its phylogenetic application. Bot J Linn Soc. 2015;177(3):335–377. doi: 10.1111/boj.12245. [DOI] [Google Scholar]
- Pearson CH, Cornish K, McMahan CM, Rath DJ, Brichta JL, Van Fleet JE. Agronomic and natural rubber characteristics of sunflower as a rubber-producing plant. Ind Crops Prod. 2010;31(3):481–491. doi: 10.1016/j.indcrop.2010.01.010. [DOI] [Google Scholar]
- Peirson JA, Bruyns PV, Riina R, Morawetz JJ, Berry PE. A molecular phylogeny and classification of the largely succulent and mainly African Euphorbia subg. Athymalus (Euphorbiaceae) Taxon. 2013;62(6):1178–1199. doi: 10.12705/626.12. [DOI] [Google Scholar]
- Puskas JE, Mcmaham C, Deffieux AM, Kennedy JP (2011) Department of Agriculture, University of Akron and US. Biosynthesis of polyisoprenoids. U.S. Patent Application 12/998,516
- Ramirez-Cadavid DA, Cornish K, Michel FC., Jr Taraxacum kok-saghyz (TK): compositional analysis of a feedstock for natural rubber and other bioproducts. Ind Crops Prod. 2017;107:624–640. doi: 10.1016/j.indcrop.2017.05.043. [DOI] [Google Scholar]
- Riina R, Peirson JA, Geltman DV, Molero J, Frajman B, Pahlevani A, Barres L, Morawetz JJ, Salmaki Y, Zarre S, Kryukov A. A worldwide molecular phylogeny and classification of the leafy spurges, Euphorbia subgenus Esula (Euphorbiaceae) Taxon. 2013;62(2):316–342. doi: 10.12705/622.3. [DOI] [Google Scholar]
- Rolere S, Liengprayoon S, Vaysse L, Sainte-Beuve J, Bonfils F. Investigating natural rubber composition with Fourier transform infrared (FT-IR) spectroscopy: a rapid and non-destructive method to determine both protein and lipid contents simultaneously. Polym Test. 2015;43:83–93. doi: 10.1016/j.polymertesting.2015.02.011. [DOI] [Google Scholar]
- Spanò D, Pintus F, Mascia C, Scorciapino MA, Casu M, Floris G, Medda R. Extraction and characterization of a natural rubber from Euphorbia characias latex. Biopolymers. 2012;97(8):589–594. doi: 10.1002/bip.22044. [DOI] [PubMed] [Google Scholar]
- Swanson CL, Buchanan RA, Otey FH. Molecular weights of natural rubbers from selected temperate zone plants. J Appl Polym Sci. 1979;23(3):743–748. doi: 10.1002/app.1979.070230309. [DOI] [Google Scholar]
- Takahashi S, Koyama T. Structure and function of cis-prenyl chain elongating enzymes. Chem Rec. 2006;6(4):194–205. doi: 10.1002/tcr.20083. [DOI] [PubMed] [Google Scholar]
- Vandersypen LM, Steffen M, Breyta G, Yannoni CS, Sherwood MH, Chuang IL. Experimental realization of Shor’s quantum factoring algorithm using nuclear magnetic resonance. Nature. 2001;414(6866):883. doi: 10.1038/414883a. [DOI] [PubMed] [Google Scholar]
- Wagner S, Bublin M, Hafner C, Kopp T, Allwardt D, Seifert U, Arif SA, Scheiner O, Breiteneder H. Generation of allergen-enriched protein fractions of Hevea brasiliensis latex for in vitro and in vivo diagnosis. Int Arch Allergy Immunol. 2007;143(4):246–254. doi: 10.1159/000100569. [DOI] [PubMed] [Google Scholar]
- Yang Y, Riina R, Morawetz JJ, Haevermans T, Aubriot X, Berry PE. Molecular phylogenetics and classification of Euphorbia subgenus Chamaesyce (Euphorbiaceae) Taxon. 2012;61(4):764–789. doi: 10.1002/tax.614005. [DOI] [Google Scholar]