Abstract
Background
SARS-CoV-2 infection activates coagulation and stimulates innate immune system. Little is known about coagulopathy and response of inflammation and infection in ICU patients with COVID-19. Derangement of coagulation and markers of infection and inflammation induced by SARS-CoV-2 infection, as well as their correlations were elucidated.
Methods
One hundred eight ICU patients with COVID-19 (28 survivors and 80 non-survivors) in Tongji hospital and Wuhan Jinyintan hospital, in Wuhan, China were included. Coagulation parameters, infectious and inflammatory markers were dynamically analysed. The correlation between coagulopathy of patients and infectious and inflammatory markers was verified.
Results
SARS-CoV-2-associated coagulopathy occurred in most cases of critical illness. Raised values of d-dimer and FDP were measured in all patients, especially in non-survivors, who had longer PT, APTT, INR, as well as TT, and lower PTA and AT compared to survivors. SIC and DIC mostly occurred in non-survivors. CRP, ESR, serum ferritin, IL-8, and IL-2R increased in all patients, and were much higher in non-survivors who had significantly higher levels of IL-6 and IL-10. D-dimer was positively associated with CRP, serum ferritin (p = 0.02), PCT (p < 0.001), and IL-2R (p = 0.007). SIC scores were positively correlated with CRP (p = 0.006), PCT (p = 0.0007), IL-1β (p = 0.048), and IL-6 (p = 0.009). DIC scores were positively associated with CRP (p < 0.0001), ESR (p = 0.02), PCT (p < 0.0001), serum ferritin (p < 0.0001), IL-10 (p = 0.02), and IL-2R (p = 0.0005).
Conclusion
Prothrombotic state, SIC, and DIC are the characteristics of coagulation in ICU patients with COVID-19. CRP, ESR, serum ferritin, IL-8, IL-2R, IL-6, and PCT were stimulated by SARS-CoV-2 infection. CRP, PCT, serum ferritin, and IL-2R indicate the coagulopathy severity of patients with COVID-19.
Keywords: SARS-CoV-2, COVID-19, coagulopathy, markers of infection and inflammation, correlation
Introduction
SARS-CoV-2, a newly RNA beta coronavirus, first confirmed in Wuhan, China in December 2019, causing COVID-19, has become globally pandemic, has currently infected more than 8 million people, and led to more than 400,000 deaths worldwide, totals that are increasing every day.1 SARS-CoV-2 infects humans by respiratory droplet and close contact.2 The respiratory system is the most susceptible to infringement during infection, leading to the most frequent symptoms: fever and cough.2 Acute respiratory distress syndrome is the leading cause of death among COVID-19 patients, especially for those critically ill.3 The primary therapeutic measurements for patients with COVID-19 are supportive care and symptomatic treatments. One prominent clinical feature of COVID-19 is the activation of coagulation.4,5 The parameters of coagulation in patients with COVID-19 are found to be abnormal, which indicate the severity of COVID-19.4,5 Another paramount characteristic of COVID-19 is the stimulation of the innate immune system to produce large amounts of pathogenic cytokines, which involve the progression of the disease.6–8 Ample evidence suggests that crosstalk between coagulation and inflammation, interacting mutually, aggravates the conditions in sepsis.9 In addition, the infectious response adds a bridge between derangement of coagulation and sepsis,10,11 however, little is known about the relationship between coagulation abnormality with markers of infection and inflammation in ICU patients with COVID-19.
Therefore, the aim of this study is to reveal the derangement of coagulation and markers of infection and inflammation induced by SARS-CoV-2 infection as well as the correlation of coagulopathy of ICU patients with infectious and inflammatory markers.
Patients and Methods
Setting and Study Participants
This retrospectively observational study included 28 ICU survivors discharged and 80 ICU non-survivors with SARS-CoV-2 infection, confirmed by RT-PCR or NGS between 31 December 2019 and 1 March 2020, in Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, and Wuhan Jinyintan Hospital in Wuhan, China, which were designated as treatment centres for severe and critically ill COVID-19 patients transferred from other Wuhan hospitals. All patients from the researchers’ medical unit were diagnosed with COVID-19 based on WHO interim guidance in our study.
The study was approved by the Ethics Committees and Institutional Review Boards of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, and Wuhan Jinyintan Hospital, Wuhan, China (TJ-IRB20200225), who approved a waiver of written informed consent from patients with COVID-19 by reason of emerging infectious disease. Collection and analysis of data were anonymous.
Data Collection and Definition
Basic information including age, sex, and hospital duration, history of smoking, malignancy and history, comorbidities consisting of hypertension, diabetes, COPD, CHD, CKD, CLD, and symptoms, such as fever, cough, sputum, fatigue or myalgia, nausea or vomiting, as well as diarrhoea, was collected from electronic medical records of ICU patients with COVID-19. Coagulation variables covering PT, APTT, d-dimer, INR, PTA, Fbg, FDP, TT, AT, and inflammatory indicators containing CRP, PCT, serum ferritin, ESR, IL-1β, IL-2R, IL-6, IL-8, IL-10, and TNF-α were also extracted on day 1, day 4, day 7, and the last day in hospital including the date of non-survivor death and the date of survivor discharge from hospital. Besides, data pertaining to DVT, GI bleeding, cerebrovascular accident during hospitalisation, and anti-coagulation therapy were gathered for analysis.
The definition of fever was that the axillary temperature exceeded 37·3 °C as per a previous report.12 DIC and SIC were diagnosed based on the ISTH scoring system (Tables S1 and S2).4,5,13 The SOFA score was calculated according to the scoring system described by the European Society of Intensive Care Medicine.14
Statistical Analysis
Age, duration of hospitalisation, and SOFA score were shown as median (IQR). Coagulation variables and infectious indicators were express as mean ± SEM. Statistical methods including Fisher’s exact test, chi-squared test, and Mann Whitney test were used depending on data type. Correlations were assessed by Spearman rank test. All data were analysed using GraphPad Prism 5 (GraphPad software, CA, USA). P < 0.05 was considered statistically significant.
Results
Clinical Features of Patients with COVID-19
One hundred and eight ICU patients infected with SARS-CoV-2 were recruited in our study. All of them were critically ill cases, 28 of whom survived and 80 died. The median age thereof was 66 y (IQR 57.0–73.8 y). The mean average age of those in the non-survivor group exceeded that of those in the survivor group (67.5 versus 59.0 y, p = 0.0024). Males accounted for 73% of all participants, besides, 75% of non-survivors and 46% of survivors were males, which were comparable (p = 0.0054). There was no significant difference in history of smoking between survivors (4%) and non-survivors (11%, p = 0.45). More patients had hypertension in the non-survivor group (53%) than in the survivor group (18%, p = 0.0015). No statistical significance was found between survivors and non-survivors for other comorbidities including diabetes, COPD, CHD, CKD, and CLD. Five percent of non-survivors had a history of malignancy whereas none had so among survivors, which was statistically insignificant. The median duration of hospitalisation was 12.5 d (8.0–15.8) for survivors and 9.0 d (6.0–13.8, p = 0.13) for non-survivors; however, more patients had a history of surgery in the non-survivor group (28%) than in the survivor group (7%, p = 0.033).
The most common symptom was fever (84%), followed by cough (77%), fatigue or myalgia (50%), sputum (45%), diarrhoea (16%), then nausea or vomiting (9%), all of which were insignificant in survivor and non-survivor groups.
During hospitalisation, DVT occurred in 28% of non-survivors but did not occur among survivors (p = 0.0008). Although 4% of the non-survivor group had cerebrovascular accident and none in the survivor group, there was no meaningful difference between the two groups (p = 0.57), besides, both of which had GIB (6% versus 4%, p = 1). On admission, non-survivors had a higher SOFA score (p < 0.0001) and a greater probability of progressing into SIC (p < 0.0001). Moreover, more than half of the non-survivors had developed into overt DIC, which was never seen among survivors (p < 0.0001) by the end of hospitalisation. Furthermore, a higher proportion of patients in the non-survivor group (51%) received anti-coagulation therapy compared to those in the survivor group (4%, p < 0.0001).
The aforementioned data are listed in Table 1.
Table 1.
Demographic and Clinical Characteristics of Patients with COVID-19
| Variables | Total (n = 108) |
Survivors (n = 28) |
Non-Survivors (n = 80) |
P value |
|---|---|---|---|---|
| Age, years | 66.0 (57.0–73.8) | 59.0 (32.3–69.5) | 67.5 (59.3–75.0) | 0.0024 |
| Sex | 0.0054 | |||
| Female | 35 (32%) | 15 (54%) | 20 (25%) | |
| Male | 73 (68%) | 13 (46%) | 60 (75%) | |
| Current smoker | 10 (9%) | 1 (4%) | 9 (11%) | 0.45 |
| Comorbidity | ||||
| Hypertension | 47 (44%) | 5 (18%) | 42 (53%) | 0.0015 |
| Diabetes | 20 (19%) | 4 (14%) | 16 (20%) | 0.58 |
| COPD | 5 (5%) | 1 (4%) | 4 (5%) | 1 |
| CHD | 16 (15%) | 4 (14%) | 12 (15%) | 1 |
| CKD | 6 (6%) | 3 (11%) | 3 (4%) | 0.18 |
| CLD | 0 | 0 | 0 | |
| Malignancy | 4 (4%) | 0 | 4 (5%) | 0.57 |
| Surgery history | 24 (22%) | 2 (7%) | 22 (28%) | 0.033 |
| Hospital stay, days | 10.0 (6.0–15.0) | 12.5 (8.0–15.8) | 9.0 (6.0–13.8) | 0.13 |
| Time from symptom onset to admission, days | 9.0 (6.0–12.0). | 4.0 (3.0–9.0) | 10.0 (7.3–13.0) | 0.0004 |
| Time from admission to ICU, days | 4.0 (2.0–6.0) | 3.0 (2.0–4.0) | 4.0 (2.0–6.0) | 0.26 |
| Symptom | ||||
| Fever | 91 (84%) | 24 (86%) | 67 (84%) | 1 |
| Cough | 83 (77%) | 21 (75%) | 62 (78%) | 0.78 |
| Sputum | 49 (45%) | 9 (32%) | 40 (50%) | 0.102 |
| Fatigue or myalgia | 54 (50%) | 12 (43%) | 42 (53%) | 0.38 |
| Nausea or vomiting | 10 (9%) | 3 (11%) | 7 (9%) | 0.71 |
| Diarrhoea | 17 (16%) | 3 (11%) | 14 (18%) | 0.55 |
| DVT | 22 (20%) | 0 | 22 (28%) | 0.0008 |
| GIB | 6 (6%) | 1 (4%) | 5 (6%) | 1 |
| Cerebrovascular accident | 3 (3%) | 0 | 3 (4%) | 0.57 |
| SIC score (ISTH ≥ 4) | 34 (31%) | 1 (4%) | 33 (41%) | < 0.0001 |
| DIC score (ISTH ≥ 5) | 42 (39%) | 0 | 42 (53%) | < 0.0001 |
| SOFA score | 3.0 (2.0–4.0) | 1.0 (1.0–2.0) | 3.0 (2.0–4.0) | < 0.0001 |
| Anti-coagulation therapy | 42 (39%) | 1 (4%) | 41 (51%) | < 0.0001 |
Abbreviations: COVID-19, Coronavirus disease 2019; COPD, chronic obstructive pulmonary disease; CHD, coronary heart disease; CKD, chronic kidney disease; CLD, chronic liver disease; ICU, intensive care unit; DVT, deep vein thrombus; GIB, gastrointestinal bleeding; SIC, sepsis-induced coagulopathy; DIC, disseminated intravascular coagulation; SOFA, sequential or sepsis-related organ failure assessment.
Coagulation Changes of Critically Ill Patients with COVID-19
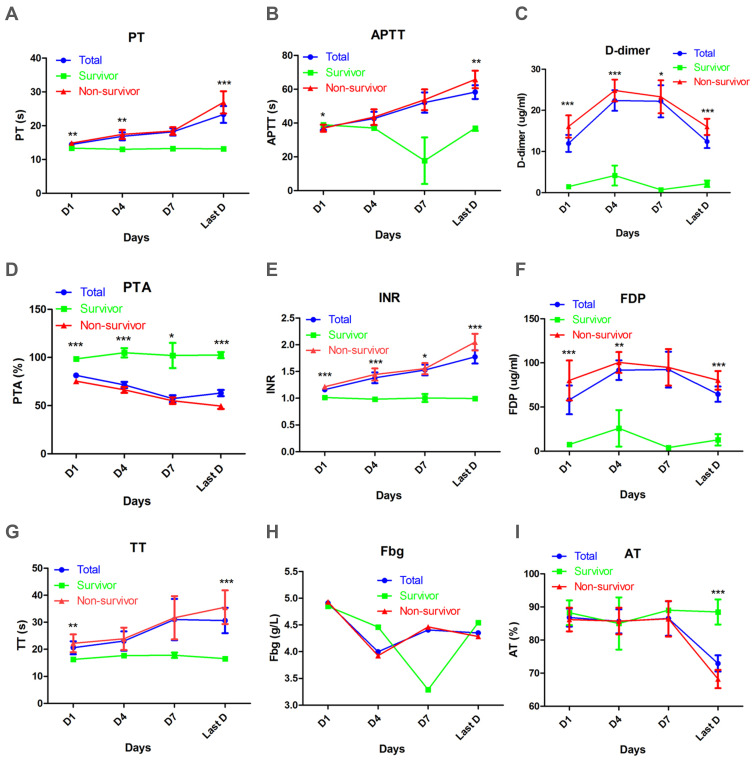
Coagulation information was extracted from electronic medical records, and indicators reflecting the coagulation state of COVID-19 were compared between survivor and non-survivor groups at day 1, day 4, day 7, and the last day in hospital (Figure 1). PT was prolonged in the non-survivor group at day 1 (14.9 ± 0.3 s), day 4 (17.4 ± 1.3 s), and the last day (26.9 ± 3.3 s), comparable to the survivor group at day 1 (13.4 ± 0.1 s, p = 0.0059), day 4 (13.0 ± 0.3 s, p = 0.0077), and last day (13.2 ± 0.2 s, p < 0.0001), all of which were within the normal range (11.5–14.5 s) (Figure 1A). APTT was normal (normal range 29.0–42.0 s) for the survivor group during hospitalisation, but for the non-survivor group, APTT increased continually, to its longest by the last day (65.8 ± 5.2 s) which was much longer than that in the survivor group (36.9 ± 1.6 s, p = 0.0034) (Figure 1B). D-dimer levels elevated all the patients (normal range <0.5 μg/mL). The increase of d-dimer was even greater in the non-survivor group at day 1 (16.1 ± 2.7 μg/mL), day 4 (24.8 ± 2.7 μg/mL), day 7 (23.3 ± 4.0 μg/mL), and the last day (16.0 ± 2.0 μg/mL) than those of the survivor group (1.5 ± 0.5 μg/mL, p < 0.0001), (4.2 ± 2.4 μg/mL, p = 0.0003), (0.7 ± 0.3 μg/mL, p = 0.0198), and (2.2 ± 0.8 μg/mL, p < 0.0001) (Figure 1C).
Figure 1.
Dynamic analysis of coagulation parameters in survivors, non-survivors, and all patients with COVID-19 in ICU. Absolute values of PT (A), APTT (B), d-dimer (C), PTA (D), INR (E), FDP (F), TT (G), Fbg (H), AT (I) of survivors (green), non-survivors (red) and all patients (blue) were analysed at different times after admission. Error bars, mean ± SEM, *p < 0.05, **p < 0.01, ***p < 0.001.
PTA was within normal range (75.0–125.0%) for the survivor group at day 1 (98.6 ± 2.6%), day 4 (104.9 ± 4.8%), day 7 (102.0 ± 13.0%), and the last day (102.5 ± 3.2%). For the non-survivor group, PTA decreased over time and was much lower than that of the survivor group at the corresponding time (75.5 ± 2.4%, p < 0.0001), (66.4 ± 3.1%, p < 0.0001), (55.1 ± 3.3%, p = 0.0227), and (49.5 ± 2.9%, p < 0.0001) (Figure 1D). Conversely, INR was elevated continually for the non-survivor group at day 1 (1.2 ± 0.0), day 4 (1.4 ± 0.1), day 7 (1.5 ± 0.1), and the last day (2.0 ± 0.2) and was higher than that of the survivor group at day 1 (1.0 ± 0.0, p < 0.0001), day 4 (1.0 ± 0.0, p < 0.0001), day 7 (1.0 ± 0.0, p = 0.0353), and the last day (1.0 ± 0.0, p < 0.0001), all of which were not beyond normal limits (0.8–1.2) (Figure 1E). In contrast to d-dimer, levels of FDP were elevated in both groups (normal range <5.0 μg/mL), but those in the non-survivor group had higher levels than those in the survivor group, especially at day 1 (80.1 ± 22.7 versus 7.6 ± 1.9 μg/mL, p < 0.0001), day 4 (100.5 ± 11.8 versus 25.9 ± 20.7 μg/mL, p = 0.0022), and the last day (80.3 ± 10.6 versus 12.9 ± 6.4 μg/mL, p < 0.0001) (Figure 1F).
TT was beyond the normal range (14.0–19.0 s) and had a prolonged trend in the non-survival group. TT was normal in the survivor group, and was thus shorter than that in the non-survivor group, particularly at day 1 (16.3 ± 0.2 versus 22.2 ± 3.3 s, p = 0.0045) and the last day (16.6 ± 0.3 versus 35.6 ± 6.2 s, p = 0.0088) (Figure 1G). No significant difference in Fbg was found (most values were within a normal range) between non-survivors and survivors (Figure 1H). AT was not comparable for non-survivors and survivors at day 1, day 4, and day 7, and values were within reference limits (80.0–120.0%): however, AT in the non-survivor group at the last day decreased to 68.2 ± 2.8%, which was lower than that of the survivor group (88.5 ± 3.8%, p = 0.0001) (Figure 1I).
Changes of Infectious Indicators for Patients Infected with SARS-CoV-2
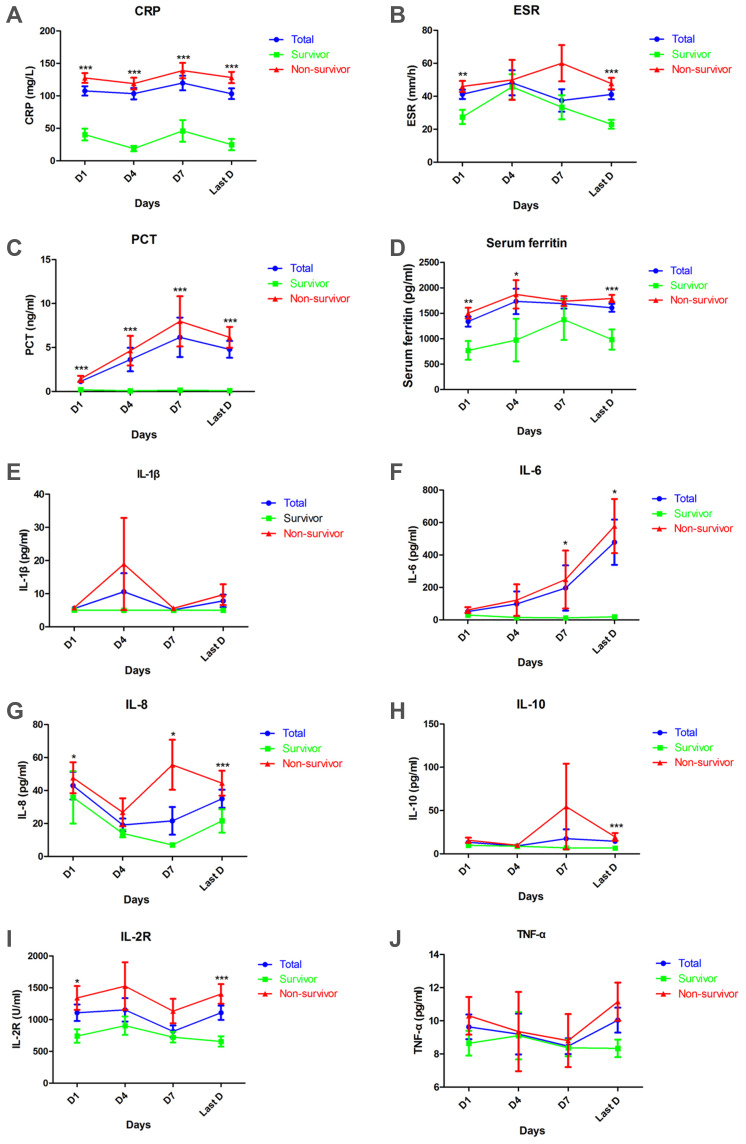
Coagulopathy of COVID-19 was inevitably correlated with inflammatory reaction, which was initiated by SARS-CoV-2 infection, revealed through inflammatory indicators, cytokines, and cytokine receptor (Figure 2). Levels of CRP in survivor and non-survivor groups were higher than 10.0 mg/L at day 1, day 4, day 7, and the last day. Meanwhile, the non-survivor group showed significantly higher levels of CRP at all times (127.6 ± 7.5 versus 40.3 ± 9.1 mg/L, p < 0.0001) (118.9 ± 9.0 versus 18.8 ± 4.2 mg/L, p < 0.0001) (138.9 ± 11.8 versus 46.0 ± 16.8 mg/L, p = 0.0003) (128.3 ± 8.7 versus 24.9 ± 8.7 mg/L, p < 0.0001) (Figure 2A). Meanwhile, ESR exceeded 15.0 mm/h in both groups at all times. ESR was faster in the non-survivor group than in the survivor group at day 1 (46.0 ± 3.3 versus 27.4 ± 4.3 mm/h, p = 0.0046) and the last day (147.7 ± 3.5 versus 23 ± 2.7 mm/h, p < 0.0001) (Figure 2B). As COVID-19 progressed in the non-survivor group, values of PCT increased from 1.4 ng/mL to 8.0 ng/mL, much higher than that of survivor group at all times (p < 0.0001), whose PCT levels were less than 0.5 ng/mL (Figure 2C). Furthermore, serum ferritin levels, in both groups, exceeded 500 μg/L at all times, but the non-survivor group had higher levels of serum ferritin at day 1 (p = 0.0019), day 4 (p = 0.04), and the last day (p = 0.0002) (Figure 2D).
Figure 2.
Dynamic analysis of infectious and inflammatory indicators in survivors, non-survivors, and all patients with COVID-19 in ICU. Concentrations of CRP (A), ESR (B), PCT (C), serum ferritin (D), IL-1β, (E), IL-6 (F), IL-8 (G), IL-10 (H), IL-2R (I), TNF-α (J) in serum of survivors (green), non-survivors (red), and all patients (blue) were analysed at different times after admission. Error bars, mean ± SEM, *p < 0.05, **p < 0.01, ***p < 0.001.
Interleukins including IL-1β, IL-6, IL-8, and IL-10 were measured in our study. Serum levels of IL-1β were less than 5.0 pg/mL for most patients at all times (Figure 2E), however, levels of IL-6 in all the patients exceeded the normal range, which was less 7.0 pg/mL. Especially among non-survivors, levels of IL-6, increased as the disease progressed and were much higher than those of survivors at day 7 (p = 0.03) and the last day (p = 0.01) (Figure 2F). On the contrary, IL-8, unstimulated by viral infection here, was below the upper limit of 62.0 pg/mL, for most patients in both groups, although non-survivors had higher levels of IL-8 at day 1 (p = 0.02), day 7 (p = 0.02), and the last day (p = 0.0008), in comparison to survivors (Figure 2G). Differently, levels of IL-10 fluctuated around the upper limit, 9.1 pg/mL, for survivors, but were elevated for non-survivors. On the last day, levels of IL-10 were much higher in the non-survivor group compared to the survivor group (p = 0.0006) (Figure 2H).
Interleukin receptor, IL-2R, levels increased in the survivor group, but were elevated to a much higher level in the non-survivor group, particularly at day 1 (p = 0.02) and the last day (p = 0.0002) (Figure 2I). Another cytokine, TNF-α, was observed to have been unstimulated in both groups during hospitalisation and no significant difference was found between the two groups (Figure 2J).
Correlation of Coagulopathy with Infectious and Inflammatory Indicators for COVID-19 Patients
SARS-CoV-2 infection caused an inflammatory response, which induced prominent change in coagulation function. The relationship of coagulation function with infectious and inflammatory indicators of COVID-19 patients was analysed (Table 2). D-dimer was positively associated with CRP (r = 0.36, p = 0.0007), serum ferritin (r = 0.29, p = 0.02), PCT (r = 0.45, p < 0.001), and IL-2R (r = 0.45, p = 0.007) for COVID-19 patients on admission (Table 1). Meanwhile, SIC scores were positively correlated with CRP (r = 0.28, p = 0.006), PCT (r = 0.35, p = 0.0007), IL-1β (r = 0.33, p = 0.048), and IL-6 (r = 0.37, p = 0.009) for COVID-19 patients on admission. At the end of hospitalisation, DIC scores were positively related to CRP (r = 0.45, p < 0.0001), ESR (r = 0.25, p = 0.02), PCT (r = 0.46, p < 0.0001), serum ferritin (r = 0.43, p < 0.0001), IL-10 (r = 0.33, p = 0.02), and IL-2R (r = 0.48, p = 0.0005) in COVID-19 patients.
Table 2.
Correlations Between Coagulopathy of COVID-19 Patients and Infectious and Inflammatory Markers
| CRP | ESR | PCT | Serum Ferritin | IL-1β | IL-6 | IL-8 | IL-10 | IL-2R | TNF-α | |
|---|---|---|---|---|---|---|---|---|---|---|
| r (p) | r (p) | r (p) | r (p) | r (p) | r (p) | r (p) | r (p) | r (p) | r (p) | |
| D-dimer | 0.36 | 0.18 | 0.45 | 0.29 | −0.07 | 0.16 | 0.22 | 0.07 | 0.45 | 0.06 |
| (0.0007) | (0.13) | (< 0.0001) | (0.02) | (0.72) | (0.29) | (0.21) | (0.71) | (0.007) | (0.72) | |
| SIC score | 0.28 | −0.10 | 0.35 | 0.23 | 0.33 | 0.37 | 0.31 | 0.10 | 0.28 | −0.11 |
| (0.006) | (0.39) | (0.0007) | (0.052) | (0.048) | (0.009) | (0.07) | (0.60) | (0.10) | (0.52) | |
| DIC score | 0.45 | 0.25 | 0.46 | 0.43 | −0.06 | 0.20 | 0.27 | 0.33 | 0.48 | −0.01 |
| (<0.0001) | (0.02) | (<0.0001) | (<0.0001) | (0.69) | (0.09) | (0.06) | (0.02) | (0.0005) | (0.94) |
Abbreviations: COVID-19, Coronavirus disease 2019; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; PCT, procalcitonin; IL-1β, interleukin-1β; IL-6, interleukin-6; IL-8, interleukin-8; IL-10, interleukin-10; IL-2R, interleukin-2 receptor; TNF-α, tumor necrosis factor-α; SIC, sepsis-induced coagulopathy; DIC, disseminated intravascular coagulation.
Discussion
During the process of fighting against viral or bacterial invasion, immune systems are inevitably activated, thus stimulating the coagulation system.9,15 Thus, SARS-CoV-2 infection was also observed to cause disturbances of coagulation and immune systems, which are non-negligible issues during the treatment of COVID-19 patients. This study reveals that SARS-CoV-2-associated coagulopathy exists in almost all critically ill COVID-19 patients. Values of d-dimer and FDP were elevated in all patients, especially for non-survivors, who also had longer PT, APTT, INR as well as TT, and lower PTA and AT compared to survivors. Besides, more patients in the non-survivor group progressed into SIC and DIC. Moreover, infectious and inflammatory markers including CRP, ESR, serum ferritin, IL-8, and IL-2R increased in all patients, and more obviously so in those in the non-survivor group who also had higher levels of IL-6 and IL-10 in comparison with the survivor group. In the relationship of coagulation with inflammation, we found that D-dimer, SIC score, and DIC score were positively associated with infectious and inflammatory markers.
One prominent clinical feature of coagulation function of patients with COVID-19 is hypercoagulability, which is a risk factor for mortality. Hyaline thrombus or microthrombus was found in vessels of the lung, kidney, heart, brain, and liver by autopsy of COVID-19 patients according to the report of the Seventh Revised Trial Version of the Novel Coronavirus Pneumonia Diagnosis and Treatment Guidance of National Health Commission of China.16 Besides, 28% of non-survivors were detected with deep vein thrombosis using ultrasonography, which explained the sudden incidence of respiratory insufficiency and death of some COVID-19 patients as observed during our clinical work, indicating detachment of deep vein thrombus and pulmonary embolism. In laboratory tests, levels of d-dimer and FDP, reflecting blood hypercoagulable status, increase for COVID-19 patients and markedly for cases of critical illness or death,2,5,12 which was confirmed by our findings. High values of d-dimer are strongly correlated with hospital mortality in patients with sepsis.17 For COVID-19 patients, a high level of d-dimer is an independently risky factor of in-hospital death.4,12
Another clinical characteristic of COVID-19 patients is DIC, which mirrors the critical condition of patients and predicts poor prognosis. Sepsis-induced DIC develops from sequential stages of sepsis with reduced platelet and sepsis-induced coagulopathy.13 Thrombocytopenia is rarely seen in mild, moderate, and severe COVID-19 patients, but is common in cases of critical illness,2,4,18,19 which is consistent with our data suggesting that absolute counts of platelets increased in survivors and decreased in deaths (Figure S1). A previous report shows that 41% of non-survivors and 13.3% of survivors meet SIC criteria associated with 28-day mortality.4 Our data also suggested that 41% of non-survivors and only 4% of survivors underwent SIC, which further supported the fact that sepsis-induced coagulopathy indicates severity of COVID-19. As the conditions of patients worsen, SIC sequentially transforms into overt DIC which is highly related with mortality.20 Most of the deaths with COVID-19 experienced overt DIC,5 which is in line with our results revealing that 53% of non-survivors and none of the survivors underwent overt DIC. Unlike non-sepsis DIC, fibrinolysis is greatly suppressed by PAI-1 in sepsis-associated DIC, which leads to organ failures by decreasing tissue perfusion and in which systematic bleeding rarely occurs.4,21 In our cohort, several patients were observed with gastrointestinal bleeding and cerebrovascular accident, which were mainly attributed to stress reaction or peptic ulcers and hypertension, respectively.
The above traits of coagulopathy of COVID-19 patients are slightly distinguished from SARS and MERS. Thrombocytopenia is common in SARS but rare in COVID. Prolonged APTT is also usual, even in non-ICU patients with SARS. Raised d-dimer was not documented in patients with SARS-CoV infection, which is very different from SARS-CoV-2 infection. DIC is found in few SARS patients, in only 11.4% of deaths, which is much lower than that of COVID-19 patients.22,23 Like ICU COVID-19 patients, around 21% of SARS patients have DVT. Eleven percent of SARS patients have evidence of pulmonary embolism,24 which is unknown in COVID-19 patients for now. Thrombocytopenia in MERS is also common, but slightly lower than in SARS.25 DIC occurs in 14% of patients with MERS,26 which is higher compared to SARS (2.5%),24 but much lower than our data (39% in Table 1).
Sepsis-associated coagulopathy involves various mechanisms, undoubtedly, among which, cytokines are paramount in contributing to the derangement of the coagulation system. Elevated values of pro-inflammatory cytokines (such as TNF, IL-6, and IL-1) and anti-inflammatory cytokines including IL-10 were measured in activation of coagulation.9 IL-6 plays a central role in activating coagulation by crosstalk with protein C, protein S, and antithrombin systems and is a poor prognostic factor for sepsis.27–29 For SARS-CoV-2 infection, the virus itself is able to activate coagulation through binding to angiotensin converting enzyme 2 (ACE2) to damage vascular endothelial cells in addition to stimulating the innate immune system to induce a cytokine storm associated with disease severity.30,31 In previous studies, raised levels of pro-inflammatory cytokines, such as IL-1, IL-2, IL-2R, IL-6, IL-8, IL-10, TNF-α, and IFN-γ, and simultaneously increased levels of anti-inflammatory cytokines, IL-4 and IL-10, were well documented in patients with COVID-19, especially for critical cases.7,8,31 IL-6 is recognised as the key pathogenic cytokine causing a cytokine storm.31 IL-2R, IL-6, IL-8, and IL-10 described in our study, cause similar changes to those mentioned in previous reports, which confirm the existence of the cytokine storm induced by SARS-CoV-2 infection. Besides, this phenomenon also occurs in SARS and MERS such that serum concentrations of pro-inflammatory cytokines (IL-1, IL-6, IL-8, TNF-α, and IFN-γ) increased.32,33
In addition, infectious response was initiated during sepsis. Infectious markers, including CRP and PCT, increased during infection, participate in the abnormality of coagulation, and predict disease severity.10,11 Increased values of CRP, PCT, ESR, and serum ferritin are depicted in COVID-19 patients, and CRP, PCT, and serum ferritin have significantly higher values in severe cases7,12 as supported by our findings.
Inflammation and coagulation interact during infection. Markers of infection and inflammation, including PCT, IL-6, IL-8, and IL-10 are positively correlated with DIC in patients with sepsis.10 For SARS-CoV-2 infection, we found that D-dimer was positively associated with CRP, serum ferritin, PCT, and IL-2R: the SIC score was positively correlated with CRP, PCT, IL-1β, and IL-6, and the DIC score was positively correlated with CRP, ESR, PCT, serum ferritin, IL-10, and IL-2R in COVID-19 patients. Up to now, no evidence has been found to allow investigation of the relationship between coagulation and inflammation in SARS-COV-2 infection. Therefore, our data were the first to allow description of the correlation of coagulopathy of patients with COVID-19 and markers of inflammation and infection.
The primary management of patients with COVID-19 is symptomatic therapy and supportive care as there is no effective drug to treat SARS-CoV-2 infection at present. Prothrombotic state, DVT, and DIC of patients with COVID-19 are managed by use of anticoagulants. Although routine use of anticoagulants, such as LMWH, antithrombin, or thrombomodulin in sepsis-induced DIC patients is controversial: LMWH is able to benefit a group of selected patients with COVID-19, who meet SIC diagnostic criteria or whose d-dimer values are more than six times the upper limit of the normal range.4,34 Thirty-nine percent of our patients received anticoagulant therapy, and most of them were non-survivors with severe coagulation disturbance. IL-6 is the key factor inducing a cytokine storm and is associated with mortality caused by sepsis or sepsis shock. Thus, targeting L-6 receptor therapy for patients with COVID-19 is now on clinical trial (ChiCTR2000029765). All these measurements aim to slow and even stop the deterioration associated with the disease and improve the prognosis of patients with COVID-19.
In summary, prothrombotic state, SIC, and DIC are the characteristics of coagulation in ICU patients with COVID-19. Raised values of CRP, ESR, serum ferritin, IL-8, and IL-2R were detected in most patients with COVID-19, especially among non-survivors. Levels of IL-6 and PCT were significantly higher in non-survivors. Coagulopathy of patients with COVID-19 was significantly positively associated with markers of inflammation and infection.
Acknowledgment
We deeply express our gratitude to the medical staff, patients, and their family members involved in this study.
Funding Statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Abbreviations
APTT, activated partial thromboplastin time; AT, antithrombin; CHD, coronary heart disease; CKD, chronic kidney disease; CLD, chronic liver disease; COPD, chronic obstructive pulmonary disease; COVID-19, Coronavirus disease 2019; CRP, C-reactive protein; DIC, disseminated intravascular coagulation; DVT, deep vein thrombus; ESR, erythrocyte sedimentation rate; Fbg, fibrinogen; FDP, fibrin or fibrinogen degradation product; GIB, gastrointestinal bleeding; ICU, intensive care unit; IFN-γ, interferon-γ; IL-10, interleukin-10; IL-1β, interleukin-1β; IL-2R, interleukin-2 receptor; IL-6, interleukin-6; IL-8, interleukin-8; PAI-1, plasminogen activator inhibitor type 1; INR, International normalised ratio; IQR, inter quartile range; ISTH, International Society on Thrombosis and Haemostasis; LMWH, low-molecular-weight heparin; MERS, Middle East respiratory syndrome; NGS, next-generation sequencing; PCT, procalcitonin; PT, prothrombin time; PTA, prothrombin activity; RT-PCR, real-time reverse transcriptase polymerase chain reaction; SARS, severe acute respiratory syndrome; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SEM, standard error of mean; SIC, sepsis-induced coagulopathy; SOFA, sequential or sepsis-related organ failure assessment; TNF-α, tumour necrosis factor-α; TT, thrombin time; WHO, World Health Organization.
Ethical Approval
The study was approved by the Ethics Committees and Institutional Review Boards of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, and Wuhan Jinyintan Hospital, Wuhan, China (TJ-IRB20200225), who approved a waiver of written informed consent from patients with COVID-19 by reason of emerging infectious diseases. Collection and analysis of data were anonymous.
Disclosure
The authors report no conflicts of interest for this work.
References
- 1.Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382(13):1199–1207. doi: 10.1056/NEJMoa2001316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–481. doi: 10.1016/S2213-2600(20)30079-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang N, Bai H, Chen X, Gong J, Li D, Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thrombosis Haemostasis. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thrombosis Haemostasis. 2020;18:844–847. doi: 10.1111/jth.14768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herold T, Jurinovic V, Arnreich C, et al. Level of IL-6 predicts respiratory failure in hospitalized symptomatic COVID-19 patients. medRxiv. 2020;2020.04.01.20047381. [Google Scholar]
- 7.Qin C, Zhou L, Hu Z, et al. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin Infect Dis. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu J, Li S, Liu J, et al. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. medRxiv. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levi M, van der Poll T. Coagulation and sepsis. Thromb Res. 2017;149:38–44. doi: 10.1016/j.thromres.2016.11.007 [DOI] [PubMed] [Google Scholar]
- 10.Patel P, Walborn A, Rondina M, Fareed J, Hoppensteadt D. Markers of inflammation and infection in sepsis and disseminated intravascular coagulation. Clin Appl Thrombosis Hemostasis. 2019;25:1076029619843338. doi: 10.1177/1076029619843338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lippi G. Sepsis biomarkers: past, present and future. Clin Chem Lab Med. 2019;57:1281–1283. doi: 10.1515/cclm-2018-1347 [DOI] [PubMed] [Google Scholar]
- 12.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iba T, Levy JH, Warkentin TE, et al. Diagnosis and management of sepsis-induced coagulopathy and disseminated intravascular coagulation. J Thrombosis Haemostasis. 2019;17(11):1989–1994. doi: 10.1111/jth.14578 [DOI] [PubMed] [Google Scholar]
- 14.Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related organ failure assessment) score to describe organ dysfunction/failure. Sepsis-Rel Prob Eur Soc Intensive Care Med Intensive Care Med . 1996;22:707–710. [DOI] [PubMed] [Google Scholar]
- 15.Walborn A, Rondina M, Fareed J, Hoppensteadt D. Development of an algorithm to predict mortality in patients with sepsis and coagulopathy. Clin Appl Thrombosis Hemostasis. 2020;26:1076029620902849. doi: 10.1177/1076029620902849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.The Seventh Revised Trial Version of the Novel Coronavirus Pneumonia Diagnosis and Treatment Guidance. 2020. Available from: http://www.nhc.gov.cn/yzygj/s7653p/202003/46c9294a7dfe4cef80dc7f5912eb1989.shtml.
- 17.Rodelo JR, De la Rosa G, Valencia ML, et al. D-dimer is a significant prognostic factor in patients with suspected infection and sepsis. Am J Emerg Med. 2012;30:1991–1999. doi: 10.1016/j.ajem.2012.04.033 [DOI] [PubMed] [Google Scholar]
- 18.Li X, Wang L, Yan S, et al. Clinical characteristics of 25 death cases with COVID-19: a retrospective review of medical records in a single medical center, Wuhan, China. Int J Infect Dis. 2020;94:128–132. doi: 10.1016/j.ijid.2020.03.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iba T, Arakawa M, Di Nisio M, et al. Newly proposed sepsis-induced coagulopathy precedes international society on thrombosis and haemostasis overt-disseminated intravascular coagulation and predicts high mortality. J Intensive Care Med. 2018;885066618773679. [DOI] [PubMed] [Google Scholar]
- 21.Hack CE. Fibrinolysis in disseminated intravascular coagulation. Semin Thromb Hemost. 2001;27(06):633–638. doi: 10.1055/s-2001-18867 [DOI] [PubMed] [Google Scholar]
- 22.Wong RS, Wu A, KF To, et al. Haematological manifestations in patients with severe acute respiratory syndrome: retrospective analysis. BMJ. 2003;326(7403):1358–1362. doi: 10.1136/bmj.326.7403.1358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lillicrap D. Disseminated intravascular coagulation in patients with 2019-nCoV pneumonia. J Thrombosis Haemostasis. 2020;18(4):786–787. doi: 10.1111/jth.14781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chong PY, Chui P, Ling AE, et al. Analysis of deaths during the severe acute respiratory syndrome (SARS) epidemic in Singapore: challenges in determining a SARS diagnosis. Arch Pathol Lab Med. 2004;128:195–204. [DOI] [PubMed] [Google Scholar]
- 25.Assiri A, Al-Tawfiq JA, Al-Rabeeah AA, et al. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: a descriptive study. Lancet Infect Dis. 2013;13(9):752–761. doi: 10.1016/S1473-3099(13)70204-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saad M, Omrani AS, Baig K, et al. Clinical aspects and outcomes of 70 patients with Middle East respiratory syndrome coronavirus infection: a single-center experience in Saudi Arabia. Int J Infect Dis. 2014;29:301–306. doi: 10.1016/j.ijid.2014.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stouthard JM, Levi M, Hack CE, et al. Interleukin-6 stimulates coagulation, not fibrinolysis, in humans. Thromb Haemost. 1996;76:738–742. doi: 10.1055/s-0038-1650653 [DOI] [PubMed] [Google Scholar]
- 28.Undar L, Ertugrul C, Altunbas H, Akca S. Circadian variations in natural coagulation inhibitors protein C, protein S and antithrombin in healthy men: a possible association with interleukin-6. Thromb Haemost. 1999;81:571–575. doi: 10.1055/s-0037-1614526 [DOI] [PubMed] [Google Scholar]
- 29.Song J, Park DW, Moon S, et al. Diagnostic and prognostic value of interleukin-6, pentraxin 3, and procalcitonin levels among sepsis and septic shock patients: a prospective controlled study according to the Sepsis-3 definitions. BMC Infect Dis. 2019;19:968. doi: 10.1186/s12879-019-4618-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou Y, Fu B, Zheng X, et al. Aberrant pathogenic GM-CSF+ T cells and inflammatory CD14+CD16+ monocytes in severe pulmonary syndrome patients of a new coronavirus. bioRxiv. 2020;2020.02.12.945576. [Google Scholar]
- 32.Wong CK, Lam CW, Wu AK, et al. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin Exp Immunol. 2004;136:95–103. doi: 10.1111/j.1365-2249.2004.02415.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Min CK, Cheon S, Ha NY, et al. Comparative and kinetic analysis of viral shedding and immunological responses in MERS patients representing a broad spectrum of disease severity. Sci Rep. 2016;6:25359. doi: 10.1038/srep25359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Inata Y. Should we treat sepsis-induced DIC with anticoagulants? J Intensive Care. 2020;8:18. doi: 10.1186/s40560-020-0435-8 [DOI] [PMC free article] [PubMed] [Google Scholar]