Abstract
Objective
The purpose of this study was to explore the effect of fibroblast growth factor 2 (FGF-2) on collagenous fibre formation and the osteogenic differentiation of human amniotic mesenchymal stem cells (hAMSCs) in vitro, as well as the effect of FGF-2–induced hAMSCs combined with autologous platelet-rich plasma (PRP) on tendon-to-bone healing in vivo.
Methods
In vitro, hAMSCs were induced by various concentrations of FGF-2 (0, 10, 20, and 40 ng/ml) for 14 days, and the outcomes of ligamentous differentiation and osteogenic differentiation were detected by quantitative real-time reverse transcription PCR, Western blot, immunofluorescence, and picrosirius red staining. In addition, a lentivirus carrying the FGF-2 gene was used to transfect hAMSCs, and transfection efficiency was detected by quantitative real time reverse transcription PCR (qRT-PCR) and Western blot. In vivo, the effect of hAMSCs transfected with the FGF-2 gene combined with autologous PRP on tendon-to-bone healing was detected via histological examination, as well as biomechanical analysis and radiographic analysis.
Results
In vitro, different concentrations of FGF-2 (10, 20, and 40 ng/ml) all promoted the ligamentous differentiation and osteogenic differentiation of hAMSCs, and the low concentration of FGF-2 (10 ng/ml) had a good effect on differentiation. In addition, the lentivirus carrying the FGF-2 gene was successfully transfected into hAMSCs with an optimal multiplicity of infection (MOI) (50), and autologous PRP was prepared successfully. In vivo, the hAMSCs transfected with the FGF-2 gene combined with autologous PRP had a better effect on tendon-to-bone healing than the other groups (p < 0.05), as evidenced by histological examination, biomechanical analysis, and radiographic analysis.
Conclusion
hAMSCs transfected with the FGF-2 gene combined with autologous PRP could augment tendon-to-bone healing in a rabbit extra-articular model.
The translational potential of this article
hAMSCs transfected with the FGF-2 gene combined with autologous PRP may be a good clinical treatment for tendon-to-bone healing, especially for acute sports-related tendon–ligament injuries.
Keywords: Human amniotic mesenchymal stem cells, Platelet-rich plasma, Tendon-to-bone junction, Tendon-to-bone healing
Introduction
Tendon/ligament injuries are clinically common diseases, mostly occurring in traffic accidents or because of sport-related injuries [1]. After injury, surgical intervention is necessary to reconstruct the ruptured tissues. Although surgical treatments (reconstruction of injured ligaments/tendons) have been widely performed in clinical settings, the long-term outcomes are far from patients’ expectations owing to poor healing of the tendon-to-bone junction (TBJ). The special structure between the ligament/tendon and the bone is called TBJ (also called “enthesis”), which consists of a transitional series of structures: ligament/tendon, uncalcified fibrocartilage, calcified fibrocartilage, and bone [2]. The TBJ makes it possible to effectively relieve the force transmitted from the ligament/tendon to the bone. However, the TBJ heals slowly, and it is difficult to completely restore its original structure because of the relative avascularity of the fibrocartilage zone [3]. Owing to the special structure and functional significance of the TBJ, accelerating its healing after injury has been a hot research topic. In recent years, many biomimetic strategies to accelerate tendon-to-bone healing have been carried out in basic science orthopaedic research. These strategies include cell-based therapies, using growth factors, and using all kinds of biomimetic materials [4]. However, it is difficult to form effective enthesis that can completely substitute the native interface structurally and functionally. The emergence of tissue engineering techniques that consist of three essential elements, seed cells, growth factors, and scaffolds, provides new ideas and directions for the clinical treatment of diseases [5]. Many kinds of mesenchymal stem cells (MSCs), such as bone marrow mesenchymal stem cells (BMSCs) [6], adipose-derived MSCs [7], and peripheral blood-derived MSCs [8], have been successfully extracted and applied in various basic studies. Among these cell types, BMSCs are the most widely used MSCs. However, there are some potential risks in the process of extracting BMSCs, including haemorrhage, immunological rejection, and donor infection. Recently, human amniotic mesenchymal stem cells (hAMSCs) have been discovered and used in some studies because of their unique advantages, such as their abundant availability and their use in noninvasive operations, and there have been no moral or ethical controversies [9].
Fibroblast growth factor 2 (FGF-2; also known as basic fibroblast growth factor, bFGF), a member of the fibroblast family of factors, is widely distributed in the body with high affinity for heparin [10]. As a mitotic promoter, FGF-2 can promote cell mitosis and accelerate cell proliferation. In addition, it has the potential abilities to promote the formation of collagen and osteogenesis [11,12]. Thus far, FGF-2 has been widely used in promoting the repair of damaged tissues, including in tendon injury [11], articular cartilage damage [13], and bone injury [14]. Recently, some studies have found that FGF-2 plays a significant role in accelerating tendon-to-bone healing [15,16].
Platelet-rich plasma (PRP), which is abundant in various growth factors, including transforming growth factor-β, epidermal growth factor, vascular endothelial growth factor, insulin-like growth factor, and fibroblastic growth factor (FGF) [17], has been widely used in injured cartilage [18] and tendons and for other orthopaedic surgeries [19]. Mechanistically, PRP augments wound healing not only by inhibiting cell apoptosis and reducing the inflammatory response [20] but also by promoting cell proliferation and collagen production [21]. In addition, PRP has also been used as a carrier for some small bioactive molecules, such as simvastatin [22] and kartogenin [23], to accelerate tendon-to-bone healing in a rat model.
Although FGF-2 and PRP have been found to promote tendon-to-bone healing in some studies, the synergistic effect of combining FGF-2 with PRP on tendon-to-bone healing is largely unknown. Therefore, this study aimed to explore whether FGF-2 and PRP could together augment tendon-to-bone healing. Our hypothesis was that FGF-2–induced hAMSCs combined with autologous PRP would have a better effect on tendon-to-bone healing than the use of PRP or PRP combined with hAMSCs.
Materials and methods
Isolation, expansion, and characterisation of hAMSCs
Placentas were harvested from the Obstetrics Department of the Affiliated Hospital of Zunyi Medical University and informed consent was provided. According to a previous protocol, hAMSCs used in this study were isolated from the human placental amniotic membrane of 6 healthy full-term puerperants. Third passage (P3) hAMSCs were applied in subsequent experiments. P3 hAMSCs were subjected to cell marker detection by flow cytometry. The expression of vimentin and cytokeratin19 was detected by immunofluorescence according to the established protocol [24].
hAMSCs were induced by different concentrations of FGF-2
Different concentrations of FGF-2 solution were prepared according to the manufacturer's instructions (Group A: 0 ng/ml, Group B: 10 ng/ml, Group C: 20 ng/ml, and Group D: 40 ng/ml). P3 hAMSCs were seeded in 6-well plates at a density of 1 × 105 cells/ml and cultured in 10% fetal bovine serum (FBS)-containing medium with various concentrations of FGF-2 for 14 days.
Determination of hAMSC proliferation by CCK-8
The cell suspensions of each group were prepared at a density of 1 × 104 cells/ml, and 100 μl of each suspension was seeded into a 96-well plate. Each group consisted of 3 replicate wells and consecutive cultivation for 7 days. Cell proliferation was detected by CCK-8 assays according to the manufacturer's instructions, and growth curves were drawn and recorded.
Quantitative real-time reverse transcription PCR and Western blot analysis
On Day 14, the total RNA was extracted using RNAiso plus (Takara Bio Inc, Japan), and the cDNA was synthesised with a PrimeScript RT Reagent Kit (Takara Bio Inc, Japan). Then, the ligament/tendon-related and bone-related genes were detected by quantitative real-time reverse transcription PCR (qRT-PCR) with TB Green Premix Ex Taq (Takara Bio Inc, Japan). The gene expression levels of collagen I, collagen III (Col III), fibronectin, tenascin-C, alkaline phosphatase, osteocalcin, osteopontin, and runt-related transcription factor 2 (Runx2) were normalised to human glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and calculated by using the 2−ΔΔCt method. The primer sequences are shown in Table 1. On Day 14, total cellular proteins were extracted using the RIPA (RIPA, Solarbio, China, R0010). The protein concentrations were evaluated using a bicinchoninic acid protein assay kit (23325; Thermo) and denatured at 95 °C for 5 min. Then, SDS-PAGE was used to separate proteins. After that, the proteins were transferred from the gels onto polyvinylidene fluoride (PVDF) membranes. The membranes were blocked with 5% evaporated milk for 1 h and incubated overnight at 4 °C with Col III and Runx2. The GAPDH antibody was used as the loading control. The next day, the membranes were incubated with secondary antibody probed with a fluorescent antibody for 2 h at room temperature. The results were normalised to GAPDH.
Table 1.
Primer sequences of ligament-related and bone-related genes.
| Target | Forward: 5'→3' | Reverse: 5'→ 3′ |
|---|---|---|
| Collagen I | CTCCAGGGTTCCAACGAGAT | CATGCCGAATTCCTGGTCTG |
| Collagen III | CCCAACCCAGAGATCCCATT | CCAGTAGGGCAGGATTCACA |
| Fibronectin | AAACTCTGCTCCCCATCCTC | TAGCTTTGTGGTCTCCTGGG |
| Tenascin-C | TACAAGCTGAAGGTGGAGGG | TCTCCCCATCAGGTTGACAC |
| ALP | TGAGCCCAGACCCTACAG | CAGATGCTGCGTTGAGC |
| OCN | ACCGAGACACCATGAGAGC | GCTGCACCTTTGCTGGA |
| OPN | CTCCATTGACTCGAACGAC | GTGAAAACTTCGGTTGCTG |
| Runx2 | CGAGGCAAGAGTTTCACC | GTTCCCGAGGTCCATCTAC |
| GAPDH | GTCGGAGTGAACGGATTTGG | TGACTGTGCCGTGGAATTTG |
ALP = alkaline phosphatase; OCN = osteocalcin; OPN = osteopontin; Runx2 = runt-related transcription factor 2.
Immunofluorescence examination
P3 hAMSCs were seeded onto the 12-well plates at a density of 1 × 104 cells/ml, and DMEM-F12 media with different concentrations of FGF-2 were added for 14 days of successive induction. The cells were fixed with 4% paraformaldehyde for 15 min and then blocked with goat serum for 60 min. Then, the cells were incubated overnight at 4 °C with the primary antibodies against Col III and Runx 2. Thereafter, the cells were incubated with fluorescent secondary antibodies for 1 h at room temperature, and DAPI was used to stain the cell nuclei. The results were observed via inverted fluorescence microscopy (Figure 2).
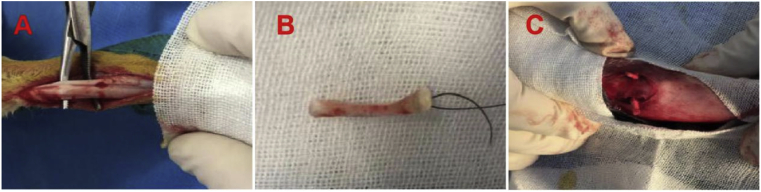
Figure 2.
(A) Fully expose Achilles tendon; (B) Achilles tendon was harvested; (C) Achilles tendon graft was passed through the bone tunnel.
Picrosirius red staining analysis
On Day 14, cells from each group were washed 3 times with phosphate buffer solution (PBS) and fixed with 4% paraformaldehyde for 15 min. Then, picrosirius red staining was performed according to the manufacturer's instructions.
Lentivirus transfected hAMSCs containing the FGF-2 gene
The lentivirus encoded with the FGF-2 gene was purchased from Shanghai Jikai Gene Chemical Technology Co, Ltd. The optimal MOI was detected according to the manufacturer's instructions. Then, lentivirus vectors were transfected into the hAMSCs with the optimal MOI, and the transfection efficiency was measured by the expression of the fluorescent protein. Thereafter, the expression levels of the FGF-2 gene and protein were assessed via qRT-PCR and Western blot.
PRP preparation
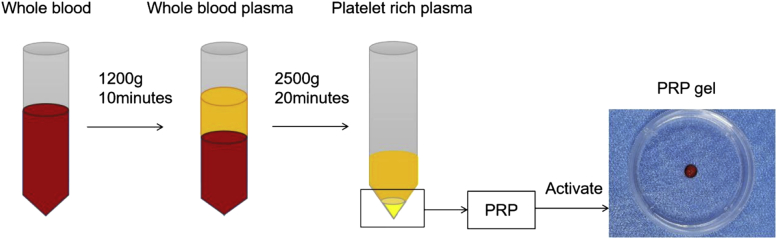
The autologous PRP in rabbits used in the study was made according to a previously published protocol [25]. Briefly, 10 mL of venous blood was drawn from the marginal auricular vein and immediately injected into an anticoagulant tube. The mixture was subjected to centrifugation at 1200×g at 4 °C for 10 min. Then, the supernatant was centrifuged again at 2500×g at 4 °C for 20 min to obtain PRP. The PRP was in an injectable liquid form at this stage, while a soft hydrogel was generated after being activated by thrombin and Cacl2. Similarly, hAMSC-containing PRP gel and FGF-2–transfected hAMSC–containing PRP gel were produced by adding thrombin and Cacl2 into the mixture of PRP and hAMSCs or hAMSCs and FGF-2. A brief flow chart of the PRP preparation is shown in Figure 1.
Figure 1.
Brief preparation process of autologous PRP by double centrifugation. PRP = platelet-rich plasma.
In vivo animal experiments
This study was approved by the Ethics Committee of Affiliated Hospital of Zunyi Medical University. All the experiments were carried out in accordance with the use of Laboratory Animals of the National Institutes of Health and the Animal Welfare Act. Forty healthy New Zealand white rabbits (without bone fracture, osteoporosis, joint deformity and other diseases) (age, 6–7 months; weight, 2.8–3.0 kg; 5 rabbits in each group; twenty rabbits were used for histological examination, and twenty rabbits were used for biomechanical testing and radiographic analysis) were purchased from Chongqing, China. The animal provision licence is Animals for Medical Use (Word) No.2017-0010. The animals underwent surgery to establish an extra articular tendon-to-bone healing model with the autologous Achilles tendon according to a previous protocol [26] (Figure 2). The wounded rabbits were divided into 4 groups with different treatments (Group A: saline; Group B: PRP; Group C: PRP + hAMSCs; Group D: PRP + hAMSCs with FGF-2). Postoperatively, penicillin was administered intramuscularly for three consecutive days. The rabbits were sacrificed 12 weeks after surgery, and the graft–tibia complexes (GTCs) were prepared for the following tests.
Macroscopic observation and histomorphometric analysis
Twelve weeks after surgery, macroscopic pictures of the specimens were taken for evaluation, and histomorphometric analysis was used to evaluate the healing of the bone-tendon interface. Quantitative histomorphometric analysis was performed by two blinded investigators based on the following criteria: fibrocartilage formation, new bone formation, and tendon graft binding to adjacent tissue [27](Table 2).
Table 2.
Histomorphometric analysis to assess healing of the tendon within the bone tunnel (full score = 9 points).
| Characteristic points | Points |
|---|---|
| Fibrocartilage formation | |
| Abundant | 3 |
| Moderate | 2 |
| Slight | 1 |
| None | 0 |
| New bone formation | |
| Abundant | 3 |
| Moderate | 2 |
| Slight | 1 |
| None | 0 |
| Tendon graft bonding to adjacent tissue | |
| 75–100% | 3 |
| 50–75% | 2 |
| 25–50% | 1 |
| 0–25% | 0 |
Histological examination
The GTC samples were fixed in 4% paraformaldehyde, decalcified, and embedded in paraffin. Five-μm-thick sections were sectioned perpendicular to the long axis of the tibial tunnel, and the sections were stained with haematoxylin and eosin (HE), safranin O/fast green (SO/FG), Masson's trichrome, and picrosirius red. The results of the picrosirius red staining were observed by normal inverted phase-contrast microscopy and polarised light microscopy. Reddish yellow represented mature, tight, and well-oriented collagen fibres, whereas green indicated immature and thin collagen fibres [28].
Biomechanical testing
The GTC samples were harvested after sacrifice and immediately prepared for mechanical testing according to a previous procedure [26]. Biomechanical testing was conducted by Nanjing BiaoPu testing technical service Co, Ltd.
Radiographic analysis
Before surgery, all rabbits underwent X-ray examination to rule out relevant diseases that may influence the experimental results. After the rabbits were killed, the GTC samples were scanned perpendicular to the long bone axis covering the entry and exit of the bone tunnel at the Department of Radiology, Affiliated Hospital of Zunyi Medical University.
Statistical analysis
Data were expressed as the mean ± standard deviation. The analysis of variance and Tukey's multiple comparisons were used to evaluate significant differences. p < 0.05 was considered a statistically significant difference. SPSS software (version 18.0; IBM) was used for the data analysis.
Results
Isolation, expansion, and characterisation of hAMSCs
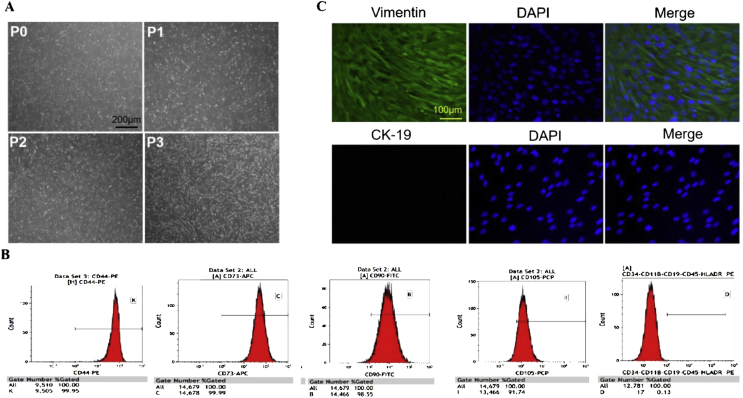
Primary cells (P0) showed a spindle-shaped exterior and were stuck to the culture bottle. After being subcultured for several times, hAMSCs gradually became vortex-like in shape (Figure 3A). P3 hAMSCs highly expressed the mesenchymal markers CD44, CD73, CD90, and CD105 but hardly expressed the haematopoietic markers CD11b, CD19, CD34, CD45, and HLA-DR according to the flow cytometric results (Figure 3B). In addition, P3 hAMSCs highly expressed vimentin (a marker of MSCs) but did not express cytokeratin19 (a specific phenotypic molecule of human amniotic epithelial cells) (Figure 3C).
Figure 3.
(A)Microscopic observation of P0, P1, P2, and P3 hAMSCs (scale bar = 200 μm); (B)Flow cytometry results: P3 hAMSCs highly expressed the mesenchymal markers CD44, CD73, CD90, and CD105 but hardly expressed the haematopoietic markers CD11b, CD19, CD34, CD45, and HLA-DR; (C)P3 hAMSCs highly expressed vimentin but did not express cytokeratin19 (scale bar = 100 μm). P3 = third passage; hAMSCs = human amniotic mesenchymal stem cells.
The viability of hAMSCs detected by CCK8
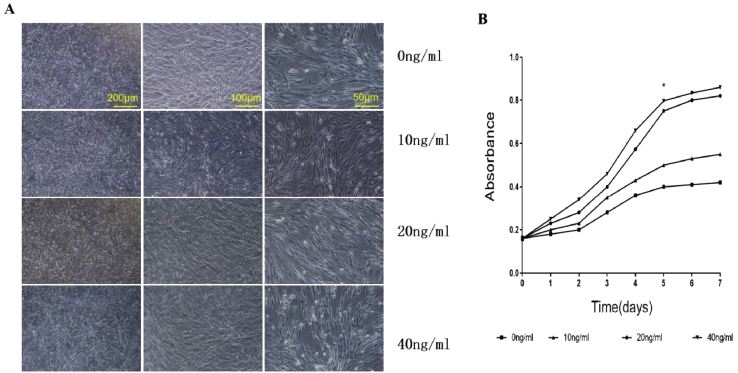
The morphology of the hAMSCs was changed from the original spindle-like appearance to a more deltoid and polygonal in shape after induction with different concentrations of FGF-2, among which the shape of hAMSCs treated with 10 ng/ml of FGF-2 changed greatly (Figure 4A). In addition, the proliferative curve exhibited an “S” pattern. The hAMSCs with high concentrations of FGF-2 (20 and 40 ng/ml) showed faster growth than the other groups (Figure 4B). These results may be explained by the perspective that a low concentration of FGF-2 (10 ng/ml) can facilitate cell differentiation, whereas high concentrations promote cell proliferation, which is consistent with a previous conclusion [29].
Figure 4.
(A)The morphology of the hAMSCs in each group, scale bar = 200 μm, 100 μm, and 50 μm, respectively.(B)The proliferative curve of each group. hAMSCs = human amniotic mesenchymal stem cells.
qRT-PCR and Western blot analysis
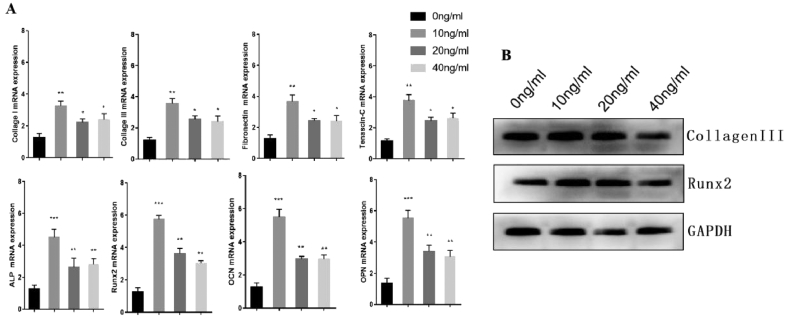
Compared with the hAMSCs treated with 0 ng/ml FGF-2 (control), those treated with other concentrations had significantly increased expression of ligament-related genes. Furthermore, the mRNA expression of ligament-related genes in Group B increased most significantly. The mRNA expression of bone-related genes also drastically increased in Group B, Group C, and Group D compared with that of Group A, and Group B showed the best effect for inducing osteogenic differentiation (Figure 5A). The protein level was detected by Western blot, and the results were consistent with those of qRT-PCR (Figure 5B).
Figure 5.
(A) The mRNA expression of ligament-related genes and bone-related genes were detected by qRT-PCR (B) The protein levels of ligament-related genes and bone-related proteins were detected by Western blot. Note: Group A: 0 ng/ml FGF-2; Group B: 10 ng/ml FGF-2; Group C: 20 ng/ml FGF-2; and Group D: 40 ng/ml FGF-2. qRT-PCR = quantitative real-time reverse transcription PCR; FGF-2 = fibroblast growth factor 2.
Immunofluorescence examination
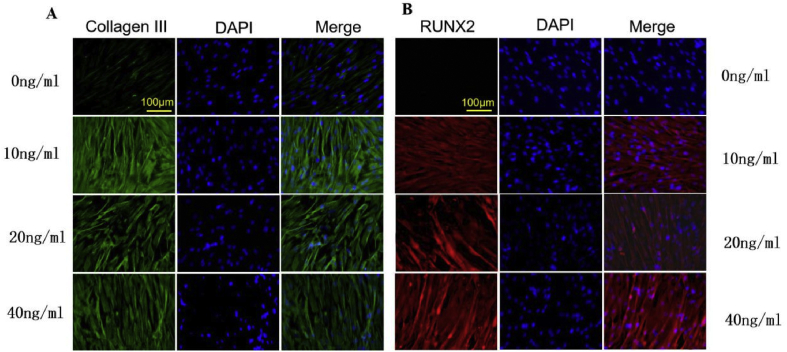
The immunofluorescent results showed that Group A had slight Col III expression (Figure 6A). However, web-like and spreading protein formation was observed in the other groups, which indicated that the Col III expression was distinctly increased in the other groups compared with Group A. In addition, the expression of Col III was higher in Group B than in Group C and Group D. In addition, RUNX2 was also increased in Group B, Group C, and Group D compared with Group A (Figure 6B). Group B expressed the highest level of RUNX2 compared with Group C and Group D. These results demonstrate that 10 ng/ml FGF-2 may be most beneficial in hAMSC differentiation.
Figure 6.
(A) The fluorescent expression of collagen type III; (B) The fluorescent expression of RUNX2 (scale bar = 100 μm). Note: Group A: 0 ng/ml FGF-2; Group B: 10 ng/ml FGF-2; Group C: 20 ng/ml FGF-2; and Group D: 40 ng/ml FGF-2. FGF-2 = fibroblast growth factor 2.
Picrosirius red staining analysis
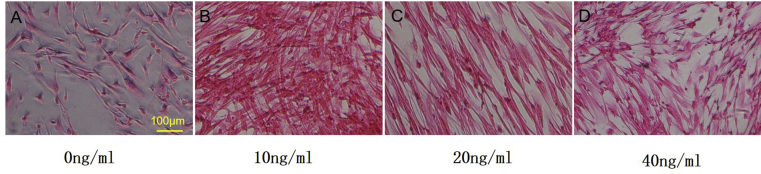
Collagen expression was observed in all groups (Figure 7). The hAMSCs that were not induced with FGF-2 secreted little collagen, while collagen secretion significantly increased in the other groups. The expression of collagen in Group B was markedly increased compared with that in Group C and Group D, which demonstrates that 10 ng/ml FGF-2 can effectively promote collagen formation.
Figure 7.
The expression of collagen fibres (scale bar = 100 μm). Note: Group A: 0 ng/ml FGF-2; Group B: 10 ng/ml FGF-2; Group C: 20 ng/ml FGF-2; and Group D: 40 ng/ml FGF-2. FGF-2 = fibroblast growth factor 2.
Successful transfection of FGF-2
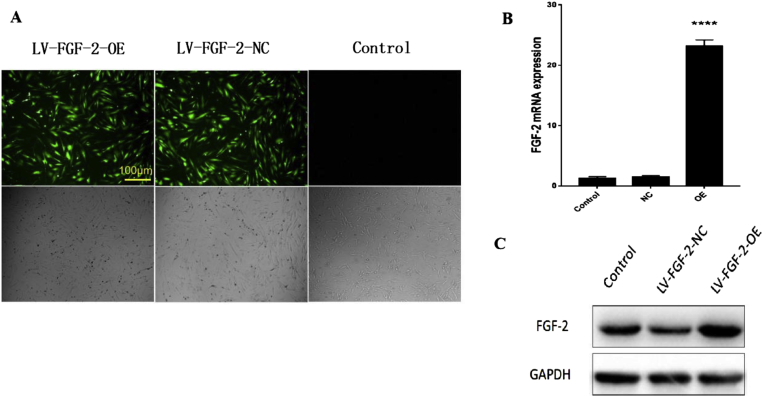
The cellular morphology did not change after transfection with FGF-2 with an optimal MOI value (50). Intense and steady fluorescence was observed at 96 h (Figure 8A). The qRT-PCR results showed that the mRNA expression of FGF-2 in the transfected group was significantly higher than that in the untransfected group and empty virus group (p < 0.05). In addition, no significant difference was found between the untransfected group and the empty virus group (p > 0.05, Figure 8B). The Western blot results were in accordance with the qRT-PCR results (Figure 8C).
Figure 8.
(A) Expression of fluorescent proteins and cellular morphology of each group were observed under fluorescence microscope and ordinary inverted phase contrast microscope, respectively, scale bar = 100 μm; (B) FGF-2 mRNA expression was detected by qRT-PCR; (C) FGF-2 protein expression was detected by Western blot. FGF-2 = fibroblast growth factor 2; qRT-PCR = quantitative real-time reverse transcription PCR.
Macroscopic observation and histomorphometric analysis
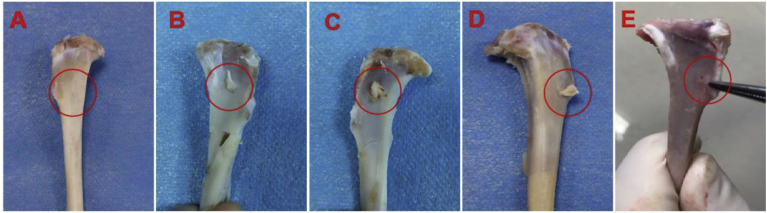
The wounds in Group A were not completely healed and had distinct gaps between the graft and host (Figure 9). In contrast, the wounds in Group B and Group C healed better than those in Group A, as evidenced by gradually narrowed gaps and smooth surfaces. However, the surface of the interface and peripheral colour in the wounds were inferior to those in the normal tissue. Importantly, hardly any gaps were observed, and the colour/lustre of the surface was similar in the wounded tissue to that in the normal tissue in Group D. In addition, the histological score for the TBJ in Group D was significantly higher than that in other groups (Table 3).
Figure 9.
(A) The normal tibial specimen; (B) Group A; (C) Group B; (D) Group C; (E) Group D. Note: Group A: control; Group B: PRP; Group C: PRP+hAMSCs; Group D: PRP + hAMSCs-FGF-2. PRP = platelet-rich plasma; hAMSCs = human amniotic mesenchymal stem cells; FGF-2 = fibroblast growth factor 2.
Table 3.
The reults of histomorphometric analysis.
| Characteristic | Control | PRP | PRP + hAMSCs | PRP + hAMSCs–FGF-2 |
|---|---|---|---|---|
| Fibrocartilage formation | 0 | 0 | 1 | 2 |
| New bone formation | 1 | 2 | 2 | 3 |
| Tendon graft bonding to adjacent tissue | 1 | 2 | 2 | 3 |
PRP = platelet-rich plasma; hAMSCs = human amniotic mesenchymal stem cells; FGF-2 = fibroblast growth factor 2.
Histological examination
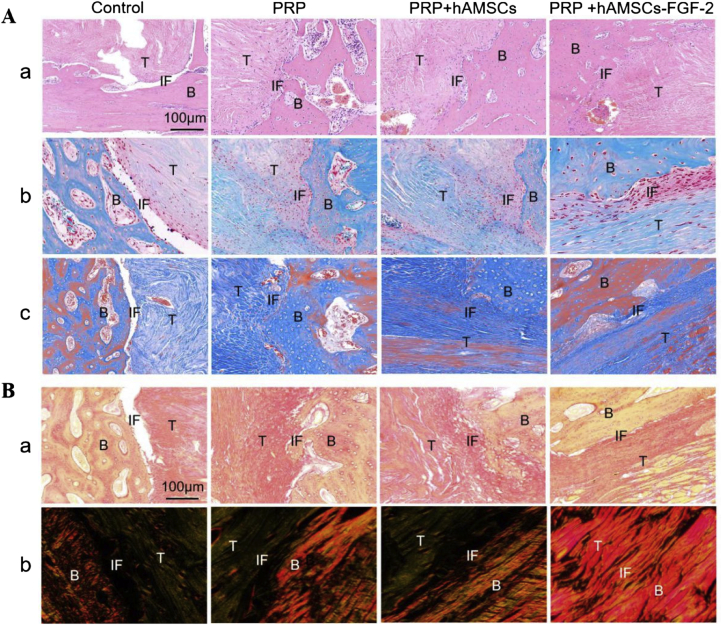
HE staining revealed giant cells existed in the TBJ of Group A, which indicated inflammation in the tissue (Figure 10A-a). In addition, large gaps were observed between the graft and host in Group A, revealing that wound healing was not complete. By contrast, the wounds in Group B and Group C healed better than those in Group A, and a moderate arrangement of cells and fibres was found. Nevertheless, no fibrocartilage-like structures were found in Group A, Group B, or Group C. Above all, fibrocartilage-like structures appeared in Group D, which was evidenced by the presence of chondrocytes in a well-organised parallel arrangement of collagen fibres.
Figure 10.
Histological examination. (A–a)HE staining;(A-b)Safranin O/fast green staining; (A-c) Masson's trichrome staining, scale bar = 100 μm; (B)Picrosirius red staining. The images were observed under normal inverted phase-contrast microscopy (a) and under polarised light microscopy (b), scale bar = 100 μm. Note: B = bone, T = tendon, IF = interface.
Further histochemical staining was performed by SO/FG staining (Figure 10A-b). The results showed that distinct gaps and some inflammatory cells were found in the TBJ of Group A, results that were similar to those acquired from HE staining. Relatively, fewer inflammatory cells and gradually narrowed gaps were observed in Group B and Group C, while there were few chondrocyte-like cells. In contrast, large areas were stained positively with SO/FG in Group D, which indicated the presence of proteoglycan expression in the TBJ.
The Masson's trichrome staining results showed that fibrous cartilage and collagen fibres were widely distributed in the implanted tendons of Group D. In addition, new bone was produced between the tendon and host bone. However, the results of the other groups were inferior to those of Group D (Figure 10A-c).
The picrosirius red staining results showed that the arrangement of collagen was more organised and fairly uniform in Group D than in the other groups (Figure 10B). In addition, the collagen fibres were closely connected to the bone in Group D. In addition, the ratio of the reddish yellow fibre area/total tendon area was distinctly higher in Group D than in the other groups, which suggested that more mature and well-oriented collagen fibres formed in Group D.
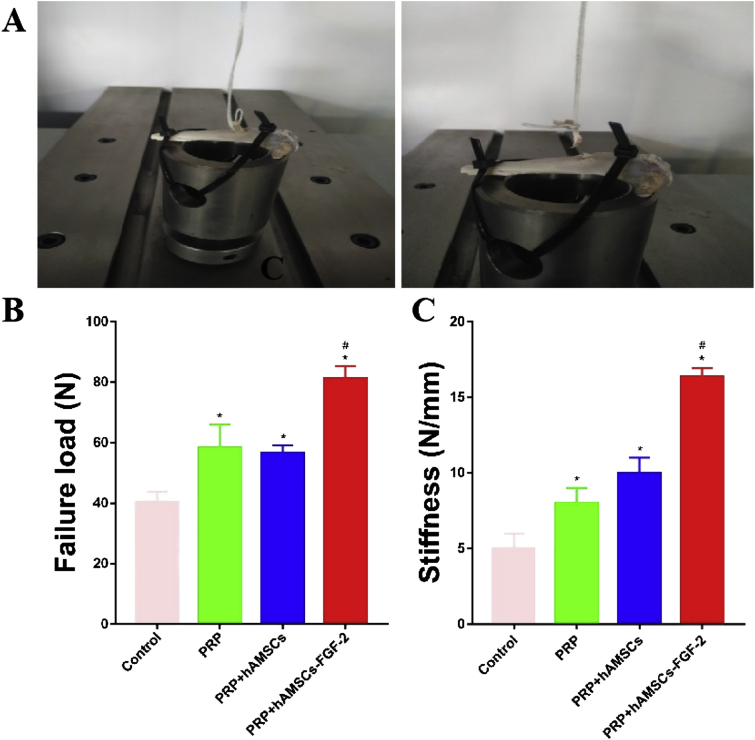
Biomechanical testing
No graft rupture occurred 12 weeks after surgery, and all the grafts were pulled out from the bone tunnel for biomechanical testing (Figure 11A). The ultimate failure load in Group D was significantly higher than that in the other groups. Similarly, the stiffness in Group D was significantly higher than that in the other groups. Although the ultimate failure load (Figure 11B) and the stiffness (Figure 11C) in Group B and Group C were both higher than those in Group A, no statistically significant difference was found between Group B and Group C.
Figure 11.
(A) The brief process of biomechanical testing; (B) Comparison of the ultimate failure load in each group; (C) Comparison of the stiffness in each group. Note: ∗p < 0.05 vs Control; #p < 0.05 vs PRP and RPR þ hAMSCs group. PRP = platelet-rich plasma; hAMSCs = human amniotic mesenchymal stem cells; FGF-2 = fibroblast growth factor 2.
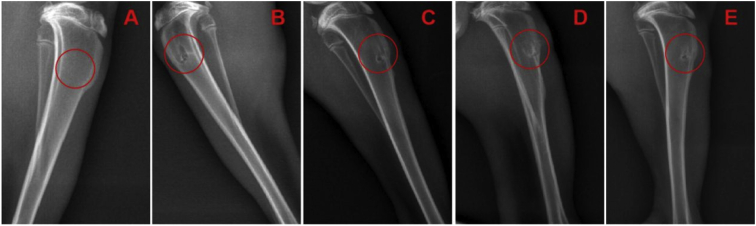
Radiographic analysis
At 12 weeks, new bone formation was observed at the TBJ in all groups. Compared with the other groups, Group D demonstrated a greater amount of new bone formation. In addition, the average bone tunnel area in Group D was significantly smaller than that in the other groups. Although a higher amount of new bone formation and a smaller average bone tunnel area were detected in Group B and Group C than in Group A, there was no significant difference among the groups (Figure 12).
Figure 12.
(A) The normal X-ray scan outcome of the tibia; (B) Group A; (C) Group B; (D) Group C; (E) Group D. Note: Group A: control; Group B: PRP; Group C: PRP þ hAMSCs; roup D: PRP þ hAMSCs-FGF-2. PRP = platelet-rich plasma; hAMSCs = human amniotic mesenchymal stem cells; FGF-2 = fibroblast growth factor 2.
Discussion
The TBJ, which is the attachment point of a ligament/tendon to the bone, is a key anchor that allows articular movements. However, it is sensitive to injury when suffering from chronic inflammatory diseases or overuse, including ageing and excessive mechanical loads [30]. Once the TBJ is injured, the healing process is slow, and its original structure and strength are difficult to restore completely because of its highly organised and specialised structure. Although reconstruction surgery has achieved good results, the long-term outcomes do not satisfy patients’ expectations, especially for athletes. The process of TBJ healing mainly involves the formation and arrangement of collagen fibres and new bone formation. Therefore, any means that can promote collagen fibres and new bone formation may have the potential to accelerate TBJ healing.
FGF-2, as a potent mitogen for various kinds of cells, has been widely used in repairing damaged tissues by facilitating cell proliferation, acting as an initial factor to stimulate the release of additional factors and increasing collagen production [31]. It has been reported that FGF-2 has been used to remodel osteoarthritic cartilage, cure meniscal tears, and facilitate the repair of osteochondral defects. Some studies have also found that FGF-2 has a positive effect on tendon-to-bone healing, including rotator cuff healing and anterior cruciate ligament reconstruction healing [15,16]. In addition, FGF-2 has the ability to induce different MSCs into specific cell lines, including fibroblasts, chondrocytes, and osteocytes.
The healing process of injured tissues involves multiple types of cells, of which the MSCs play a significant role in recruiting other cells and accelerating the healing process. However, the limited number and motivating functions of endogenous MSCs make it necessary to additionally add exogenetic MSCs to synergetically promote the healing of injured tissues. hAMSCs can be a promising seed cell because of their low immunogenicity and extensive sources and the fact that there are no ethical controversies.
According to the previous investigative results and theoretical basis, this study further explored the effect of FGF-2 on the differentiation of hAMSCs in vitro and explored whether hAMSCs induced by FGF-2 can accelerate tendon-to-bone healing in a rabbit extra-articular model. Therefore, this study first used different concentrations (0, 10, 20, and 40 ng/ml) of FGF-2 to induce hAMSCs and detected the ligament-related or bone-related genes and proteins. At 14 days, the mRNA expression levels of ligament-related genes and bone-related genes were obviously increased in all groups except for the control group, indicating that FGF-2 can induce hAMSC differentiation. At the same time, the immunofluorescence and Western blot results were similar to the qRT-PCR results. The picrosirius red staining results showed that FGF-2 could promote collagenous fibre formation. In addition, this study also found that 10 ng/ml FGF-2 had a good effect on the ligamentous differentiation and osteogenic differentiation of hAMSCs but did not have a good effect on cell proliferation, which was consistent with a previous conclusion that a low concentration of FGF-2 (10 ng/ml) could facilitate cell differentiation [29].
Considering that growth factors have shortcomings, such as an exorbitant price, a slow onset time, short half-lives in vivo, and detrimental effects with high-dose or repeated administration [31], a lentivirus carrying the FGF-2 gene was further used to induce hAMSCs and was applied for the in vivo experiments. In this study, although we did not explore the effect of the lentivirus carrying the FGF-2 gene on the ligamentous differentiation and osteogenic differentiation of hAMSCs, the results might be similar to those obtained using the FGF-2 growth factor. This may be because the transfection technique using viruses (adenoviruses, retroviruses, or lentiviruses) is just a means to deliver target genes to specific cells, which have the same effect as growth factors. This experiment did not use a PRP + hAMSCs-empty vector group because the lentivirus-empty vector had no influence on cell proliferation and differentiation, and qRT-PCR results also showed that the mRNA expression of FGF-2 had no significant difference between the untransfected group and the empty virus group.
However, hAMSCs or hAMSCs transfected with the FGF-2 gene could not survive and function at the TBJ because there was no medium that immobilised cells and prevented them from migrating. Some studies have shown that PRP has been used as a carrier for some small bioactive molecules, such as simvastatin and kartogenin, to accelerate tendon-to-bone healing in a rat model [22,23]. In addition, PRP has also been used as a bioscaffold to deliver rat BMSCs to improve tendon-to-bone healing in anterior cruciate ligament reconstruction[25]. Therefore, PRP was used as a carrier for the delivery of hAMSCs and hAMSCs transfected with the FGF-2 gene in this study.
In vivo, a rabbit extra-articular model was used to explore the tendon-to-bone healing. Twelve weeks after surgery, the specimens of the GTCs were harvested, and related detections were performed. The histological examination showed that Group D exhibited the best tendon-to-bone healing. The following factors might be associated with the results. First, hAMSCs transfected with FGF-2 in vivo might promote collagenous fibre formation because FGF-2 has been confirmed to promote collagenous fibre formation in hAMSCs in vitro, and more maturely well-arranged collagenous fibres were observed in vitro, as evidenced by qRT-PCR, Western blot, and picrosirius red staining. Generally, the formation, reorganisation, and maturation of collagen fibres are natural processes in tendon-to-bone healing. Therefore, it was helpful to accelerate tendon-to-bone healing by promoting a greater number and organised collagenous fibre formation. Second, new bone formation is the other pivotal factor involved in tendon-to-bone healing. This experiment not only verified that FGF-2 could induce the osteogenic differentiation of hAMSCs in vitro but also found a smaller average bone tunnel area in Group D than in the other groups in vivo, as evidenced by the radiographic results. Third, FGF-2 might act as a recruiter or stimulant to activate the release of additional significant factors and consequently accelerate tendon-to-bone healing. This speculation was proven by the conclusion that FGF-2 was a vital growth factor in the healing process of injured tissues and that it increased after ligament/tendon injury [32].
In this study, PRP was only used as a carrier for the delivery of hAMSCs and hAMSCs transfected with the FGF-2 gene. However, Group B and Group C showed better healing than Group A, which suggested that PRP played an important role in promoting tendon-to-bone healing. The various growth factors of PRP might play a crucial role in accelerating tendon-to-bone healing although the concrete growth factors and explicit mechanism are still unknown. Further study is necessary to explore how PRP has a positive effect on promoting tendon-to-bone healing. In addition, the hAMSCs group and hAMSCs transfected with the FGF-2 gene without PRP were not used because cells could not survive and function at the TBJ if they were injected directly into the TBJ.
In fact, optimal tendon-to-bone healing involves restoring the original biomechanical properties of mechanical strength and bearing a normal load. In this study, a higher ultimate failure load and stiffness were detected in Group D than in the other groups, which also demonstrate enhanced mechanical strength and better tendon-to-bone healing.
This study also had the following limitations. First, the model of tendon-to-bone healing used in the study was different from that in clinical cases, although this animal model has been well used in some studies [26]. Second, only one time point was set to evaluate the tendon-to-bone healing in this study. The tendon-to-bone healing is a gradually developing process, so different time points should be chosen to explore the healing process in further studies. Third, in this study, microcomputed tomography analysis was not used to evaluate the amount of newly formed mineralised bone tissues, which is necessary in further research.
Conclusion
FGF-2 could effectively promote the ligamentous differentiation and osteogenic differentiation of hAMSCs, and hAMSCs transfected with the FGF-2 gene combined with autologous PRP could augment tendon-to-bone healing in a rabbit extra-articular model, which will be a promising treatment for tendon-to-bone healing.
Data availability
The data used to support the findings of this study are available from the corresponding author upon request.
Funding statement
This work was financially supported by the Science & Technology Program of Guizhou Province China (grant no: LH [2017]7105, Zou Gang) and Qian Wei Ji Ban Han No. 2017-24
Conflict of interest statement
The authors have no conflicts of interest to disclose in relation to this article
Contributor Information
Jun Zhang, Email: 935876478@qq.com.
Ziming Liu, Email: zimingliu88@outlook.com.
Jingfeng Tang, Email: 2394833975@qq.com.
Yuwan Li, Email: 18096517888@163.com.
Qi You, Email: yqkdcg@163.com.
Jibin Yang, Email: 18385113382@163.com.
Ying Jin, Email: jinying0505@qq.com.
Gang Zou, Email: 13885206994@163.com.
Zhen Ge, Email: ezhen7685@163.com.
Xizhong Zhu, Email: 892767381@qq.com.
Qifan Yang, Email: yangqifan@126.com.
Yi Liu, Email: 13308529536@163.com.
References
- 1.Yang G., Rothrauff B.B., Tuan R.S. Tendon and ligament regeneration and repair: clinical relevance and developmental paradigm. Birth Defects Res C Embryo Today. 2013;99:203–222. doi: 10.1002/bdrc.21041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lu H.H., Thomopoulos S. Functional attachment of soft tissues to bone: development, healing, and tissue engineering. Annu Rev Biomed Eng. 2013;15:201–226. doi: 10.1146/annurev-bioeng-071910-124656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Katzel E.B., Wolenski M., Loiselle A.E., Basile P., Flick L.M., Langstein H.N. Impact of Smad3 loss of function on scarring and adhesion formation during tendon healing. J Orthop Res. 2011;29:684–693. doi: 10.1002/jor.21235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Atesok K., Fu F.H., Wolf M.R., Ochi M., Jazrawi L.M., Doral M.N. Augmentation of tendon-to-bone healing. J Bone Joint Surg Am. 2014;96:513–521. doi: 10.2106/JBJS.M.00009. [DOI] [PubMed] [Google Scholar]
- 5.Liguo S., Ling Q., Rui Z., Hongguo L., Yingsen X., Xincheng L. Effects of mechanical stretch on cell proliferation and matrix formation of mesenchymal stem cell and anterior cruciate ligament fibroblast. Stem Cell Int. 2016;2016:1–10. doi: 10.1155/2016/9842075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu J., Chamberlain C.S., Ji M.L., Saether E.E., Leiferman E.M., Li W.J. Tendon-to-Bone healing in a rat extra-articular bone tunnel model: a comparison of fresh autologous bone marrow and bone marrow-derived mesenchymal stem cells. Am J Sports Med. 2019:1–8. doi: 10.1177/0363546519862284. [DOI] [PubMed] [Google Scholar]
- 7.Rbia N., Bulstra L.F., Lewallen E.A., Hovius S.E.R., van Wijnen A.J., Shin A.Y. Seeding decellularized nerve allografts with adipose-derived mesenchymal stromal cells: an in vitro analysis of the gene expression and growth factors produced. J Plast Reconstr Aesthetic Surg. 2019;72:1316–1325. doi: 10.1016/j.bjps.2019.04.014. [DOI] [PubMed] [Google Scholar]
- 8.Yang M., Liu H., Wang Y., Wu G., Qiu S., Liu C. Hypoxia reduces the osteogenic differentiation of peripheral blood mesenchymal stem cells by upregulating Notch-1 expression. Connect Tissue Res. 2019:1–14. doi: 10.1080/03008207.2019.1611792. [DOI] [PubMed] [Google Scholar]
- 9.Qiang Y., Liang G., Yu L. Human amniotic mesenchymal stem cells alleviate lung injury induced by ischemia and reperfusion after cardiopulmonary bypass in dogs. Lab Invest. 2016;96:537–546. doi: 10.1038/labinvest.2016.37. [DOI] [PubMed] [Google Scholar]
- 10.Douglas C.J., Collin H.B., Marie H.M. Fibroblast growth factor 2 and its receptors in bone biology and disease. J Endocr Soc. 2018;2:657–671. doi: 10.1210/js.2018-00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park D.S., Park J.C., Lee J.S., Kim T.W., Kim K.J., Jung B.J. Effect of FGF-2 on collagen tissue regeneration by human vertebral bone marrow stem cells. Stem Cell Dev. 2014;24:228–243. doi: 10.1089/scd.2014.0148. [DOI] [PubMed] [Google Scholar]
- 12.Zhang F., Peng W.X., Wang L., Zhang J., Dong W.T., Wu J.H. Role of FGF-2 transfected bone marrow mesenchymal stem cells in engineered bone tissue for repair of avascular necrosis of femoral head in rabbits. Cell Physiol Biochem. 2018;48:773–784. doi: 10.1159/000491906. [DOI] [PubMed] [Google Scholar]
- 13.Li X., Su G., Wang J., Zhou Z., Li L., Liu L. Exogenous bFGF promotes articular cartilage repair via up-regulation of multiple growth factors. Osteoarthritis Cartilage. 2013;21:1567–1575. doi: 10.1016/j.joca.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 14.Wang H., Zou Q., Boerman O.C., Nijhuis A.W., Jansen J.A., Li Y. Combined delivery of BMP-2 and bFGF from nanostructured colloidal gelatin gels and its effect on bone regeneration in vivo. J Contr Release. 2013;166:172–181. doi: 10.1016/j.jconrel.2012.12.015. [DOI] [PubMed] [Google Scholar]
- 15.Tokunaga T., Shukunami C., Okamoto N., Taniwaki T., Oka K., Sakamoto H. FGF-2 stimulates the growth of tenogenic progenitor cells to facilitate the generation of tenomodulin-positive tenocytes in a rat rotator cuff healing model. Am J Sports Med. 2015;43:2411–2422. doi: 10.1177/0363546515597488. [DOI] [PubMed] [Google Scholar]
- 16.Zhang C., Li Q., Deng S., Fu W., Tang X., Chen G. bFGF- and CaPP-loaded fibrin clots enhance the bioactivity of the tendon-bone interface to augment healing. Am J Sports Med. 2016;44:1972–1982. doi: 10.1177/0363546516637603. [DOI] [PubMed] [Google Scholar]
- 17.Yun Q., Qixin H., Wei C., Song J., Zhao X., Ouyang Y. Platelet-rich plasma derived growth factors contribute to stem cell differentiation in musculoskeletal regeneration. Front Chem. 2017;5:89–96. doi: 10.3389/fchem.2017.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cavallo C., Filardo G., Mariani E., Kon E., Marcacci M., Pereira Ruiz M.T. Comparison of platelet-rich plasma formulations for cartilage healing: an in vitro study. J Bone Joint Surg Am. 2014;96:423–429. doi: 10.2106/JBJS.M.00726. [DOI] [PubMed] [Google Scholar]
- 19.Chen L., Dong S.W., Liu J.P., Tao X., Tang K.L., Xu J.Z. Synergy of tendon stem cells and platelet-rich plasma in tendon healing. J Orthop Res. 2012;30:991–997. doi: 10.1002/jor.22033. [DOI] [PubMed] [Google Scholar]
- 20.Moussa M., Lajeunesse D., Hilal G., El Atat O., Haykal G., Serhal R. Platelet rich plasma (PRP) induces chondroprotection via increasing autophagy, anti-inflammatory markers, and decreasing apoptosis in human osteoarthritic cartilage. Exp Cell Res. 2017;352:146–156. doi: 10.1016/j.yexcr.2017.02.012. [DOI] [PubMed] [Google Scholar]
- 21.Wang X., Qiu Y., Triffitt J., Carr A., Xia Z., Sabokbar A. Proliferation and differentiation of human tenocytes in response to platelet rich plasma: an in vitro and in vivo study. J Orthop Res. 2012;30:982–990. doi: 10.1002/jor.22016. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Y., Yu J., Zhang J., Hua Y. Simvastatin with PRP promotes chondrogenesis of bone marrow stem cells in vitro and wounded rat Achilles tendon-bone interface healing in vivo. Am J Sports Med. 2019;47:729–739. doi: 10.1177/0363546518819108. [DOI] [PubMed] [Google Scholar]
- 23.Zhang J., Yuan T., Zheng N., Zhou Y., Hogan M.V., Wang J.H. The combined use of kartogenin and platelet-rich plasma promotes fibrocartilage formation in the wounded rat Achilles tendon entheses. Bone Joint Res. 2017;6:231–244. doi: 10.1302/2046-3758.64.BJR-2017-0268.R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yuwan L., Ziming L., Ying J., Wang S., Yang J., Ren Y. Differentiation of human amniotic mesenchymal stem cells into human anterior cruciate ligament fibroblast cells by in vitro coculture. BioMed Res Int. 2017;2017:1–15. doi: 10.1155/2017/7360354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Teng C., Zhou C., Xu D., Bi F. Combination of platelet-rich plasma and bone marrow mesenchymal stem cells enhances tendon-to-bone healing in a rabbit model of anterior cruciate ligament reconstruction. J Orthop Surg Res. 2016;11:96. doi: 10.1186/s13018-016-0433-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiangyu C., Juan W., Kaiqiang Y., Li D., Ai C., Sheng D. Dual-layer aligned-random nanofibrous scaffolds for improving gradient microstructure of tendon-to-bone healing in a rabbit extra-articular model. Int J Nanomed. 2018;13:3481–3492. doi: 10.2147/IJN.S165633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee K.W., Lee J.S., Jang J.W., Shim Y.B., Lee K.I. Tendon-bone interface healing using an injectable rhBMP-2-containing collagen gel in a rabbit extra-articular bone tunnel model. J Tissue Eng Regen Med. 2017;11 doi: 10.1002/term.2041. [DOI] [PubMed] [Google Scholar]
- 28.Xie Y., Chen J., Han P., Yang P., Hou J., Kang Y.J. Immunohistochemical detection of differentially localized up-regulation of lysyl oxidase and down-regulation of matrix metalloproteinase-1 in rhesus monkey model of chronic myocardial infarction. Exp Biol Med. 2012;237:853–859. doi: 10.1258/ebm.2012.012070. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Zhe. 2015. Fundamental research on optimal delivery of FGF2 to promote articular cartilage repair. [Google Scholar]
- 30.Yudoh K., Karasawa R. Statin prevents chondrocyte aging and degeneration of articular cartilage in osteoarthritis (OA) Aging. 2010;2:990–998. doi: 10.18632/aging.100213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barrientos S., Stojadinovic O., Golinko M.S., Brem H., Tomic-Canic M. Growth factors and cytokines in wound healing. Wound Repair Regen. 2010;16:585–601. doi: 10.1111/j.1524-475X.2008.00410.x. [DOI] [PubMed] [Google Scholar]
- 32.Chen Zhida, Cai Yiyi, Jin W.U. Study on differentiation of sputum cells from bone marrow mesenchymal stem cells induced by fibroblast growth factor. Chin J Bone and Joint Inj. 2017;32:819–822. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.