Abstracts
Background/Objective
Anterior cervical discectomy and fusion (ACDF), commonly using autogenous iliac bone graft may be limited by donor site availability, donor-site morbidity, lower fusion rate among specific patients and longer surgical time. Surgeons used rhBMP-2 as an alternative in order to fill these clinical needs. However, studies comparing with and without rhBMP-2 in ACDF have reported conflicting results on efficacy and complications. Therefore, the purpose of this article was to evaluate efficacy and complications through dose-related rhBMP-2 and surgical level-dependence in ACDF.
Methods
We comprehensively searched PubMed and the Cochrane Library and performed a systematic review and cumulative meta-analysis of all randomized controlled trials (RCTs), prospective and retrospective comparative studies assessing with and without rhBMP-2 treatments.
Results
1 RCTs, 4 prospective studies and 24 retrospective studies including a total of 1,539,021 cases were identified. Patients in ACDF with rhBMP-2 might benefit from significantly higher fusion rates than that in non-rhBMP-2, not only total value but also in 3 tiers of rhBMP-2 doses. It is worth noting that the low dose of rhBMP-2 (<0.7 mg/level) showed highest fusion rate among all rhBMP-2 doses. Patients in rhBMP-2 also experienced higher complication rate, dysphagia and wound infections than that in non-rhBMP-2. In 2-level ACDF, the fusion rate was significantly better in rhBMP-2 than non-rhBMP-2 but not for complication rate. Surgery operative time, lengths of hospital stay and neurologic symptoms did not differ significantly between two treatments.
Conclusions
rhBMP-2 chosen in ACDF offered higher fusion, but also higher complication rate with more dysphagia and wound infections than non-rhBMP-2. To gain the efficacy and safety, rhBMP-2 dosing recommendations for ACDF would be better < 0.7 mg/level. Moreover, rhBMP-2 may be an option to improve nonunion in high risk of multi-level ACDF.
The translational potential of this article
This article indicated that the product development of facilities used in ACDF, the dose of rhBMP-2 may be lower than 0.7 mg/level was enough to gain the good fusion rates. However, the complications were higher in patients used rhBMP-2, therefore the manufacturers should pay attention to mitigate such side effects.
Keywords: Anterior cervical discectomy and fusion, Efficacy and complications, Infuse® bone graft, Meta-analysis, Recombinant human bone morphogenetic protein-2, Spinal fusion
Introduction
Anterior cervical discectomy and fusion (ACDF), a common surgical procedure, has been used for decades in patients suffering from neck pain and/or neurological deficits without response to conservative managements [[1], [2], [3]]. The procedure of ACDF includes the removal of the herniated or degenerative disc, followed by insertion of an interbody graft to fuse together the bones above and below the disc [[4], [5], [6]]. Iliac crest bone graft (ICBG), the gold-standard graft material, presents its superior osteoconductive, osteoinductive and osteogenic properties [4]. However, autogenous iliac bone graft also possesses several disadvantages, including increased procedure time, limited donor site availability and donor site morbidity [7], including pain, wound infection, haematoma or lateral femoral cutaneous nerve injury [8]. Donor site complication rates caused from autologous bone grafts were reported to be from 9.4 to 50% [9]. These limitations and nontrivial incidence of nonunion have stimulated surgeons and researchers to find potential alternatives to bone matrix, including recombinant human bone morphogenetic proteins (rhBMPs).
rhBMPs, the cytokines with osteoinductive activity, belong to the transforming growth factor β (TGF-β) superfamily, showing better bone healing with the proposal of less morbidity compared to the usual methods of bone graft harvest [10]. Currently, recombinant human bone morphogenetic protein-2 (rhBMP-2) is recognised as the only bone inducer with level I of clinical evidence [10]. Additionally, Chau et al. compared all the bone graft alternatives in ACDF, showing that rhBMPs possessed the best fusion rates, highest osteoinduction and the most effective adjuvant graft without donor site morbidity [11].
However, some independent studies reported complications after rhBMP-2 use in the ACDF, including dysphagia, dysphonia, cervical swelling, readmission, wound complications and ossification [12]. All of the recent research controversies make it difficult for surgeons to understand the proper use of rhBMP-2 in a clinical practice. The intent of exploring this potential alternative to bone matrix was to improve the success of ACDF and the patients’ quality of life. What if patients have difficulties to collect autogenous iliac bone graft or high risks of nonunion rate? Is it possible to use lower dose of rhBMP-2 to avoid adverse events? Does rhBMP-2 show the same efficacy and safety issues in single- and multi-level ACDF? Therefore, we have undertaken a meta-analysis and systematic review that examines the evidence for and against rhBMP-2 at the dose and surgical level, so that it provides insights into new researches in a better effort to provide surgeons with a working framework in which rhBMP-2 could be applied in their clinical practices.
Evidence acquisition
A prospective protocol of objectives, literature-search strategies, inclusion and exclusion criteria, outcome measurements and methods of statistical analysis was prepared a priori according to the Preferred Reporting Items for Systematic Reviews and Meta-analysis of Observational Studies in Epidemiology recommendations for study reporting [13,14].
Literature-search strategy
A literature search was performed in October 2018 without restriction to regions, publication types or languages. The primary sources were the electronic databases of PubMed and the Cochrane Library. The following medical subject headings (MeSH) terms and their combinations were searched in [Title/Abstract]: anterior cervical discectomy and fusion, cervical spine surgery, anterior cervical fusion, anterior cervical spine, cervical revision fusion, spinal fusion, cervical fusion, recombinant human bone morphogenetic protein-2, bone morphogenetic protein, rhBMP-2 and rhBMP, except animal, rat, rabbit and mouse. The Related Articles function was also used to broaden the search, and the computer search was supplemented with manual searches of the reference lists of all retrieved studies, review articles and conference abstracts. When multiple reports describing the same population were published, the most recent or complete report was used.
Inclusion and exclusion criteria
All available randomised controlled trials (RCTs) and retrospective comparative studies (cohort or case-control studies) that evaluated ACDF with rhBMP-2 in all age groups and that had at least one of the quantitative outcomes mentioned in the next section of this paper were included. Editorials, letters to the editor, review articles, case reports and animal experimental studies were excluded.
Data extraction and outcomes of interest
Data from the included studies were extracted and summarised independently by two of the authors (Wen and Shi). Any disagreement was resolved by the adjudicating senior authors (Jiang and Yang). The primary outcomes were fusion rate, complication rate, dysphagia, wound infections and neurologic symptoms. If sufficient data were available, postoperative fusion rate, complications and dysphagia were subdivided by dose of rhBMP-2 and the number of surgical level (ACDF). The secondary outcomes were surgery operative time and lengths of hospital stay.
Quality assessment and statistical analysis
Studies were rated for the level of evidence provided according to the criteria by the Centre for Evidence-Based Medicine in Oxford, UK [15]. The methodological quality of RCTs was assessed by the Cochrane risk of bias tool [16]. The methodological quality of retrospective studies was assessed by the modified Newcastle–Ottawa scale [17], which consists of three factors: patient selection, comparability of the study groups and assessment of outcome. A score of 0–9 (allocated as stars) was allocated to each study except RCTs. RCTs and observational studies achieving six or more stars were considered to be of high quality. All the meta-analyses were performed using Review Manager 5.0 (Cochrane Collaboration, Oxford, UK). The weighted mean difference (WMD) and odds ratio (OR) were used to compare continuous and dichotomous variables, respectively. All results were reported with 95% confidence intervals (CIs). For studies that presented continuous data as means and range values, the standard deviations were calculated using the technique described by Hozo et al. [18]. Statistical heterogeneity between studies was assessed using the chi-squared test with significance set at p < 0.10, and heterogeneity was quantified using the I2 statistic. The random-effects model was used if there was heterogeneity between studies; otherwise, the fixed-effects model was used [16]. Sensitivity analyses were performed for high quality studies. Funnel plots were used for potential publication bias.
Evidence synthesis
In the final analysis, 29 studies including a total of 1,539,021 cases fulfilled the predefined inclusion criteria and were included (Figure 1). These publications were full-text articles [[19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48]]. Examination of the references listed for these studies did not yield any further studies for evaluation. Agreement between the two reviewers was 97% for study selection and 93% for quality assessment of trials.
Figure 1.
Flow diagram of studies identified, included and excluded.
Characteristics of eligible studies
The characteristics of included studies are shown in Table 1. Among the included studies, there was 1 small sampled RCT without clarified confidence interval and <80% follow-up (level of evidence: 2b) [39]; 2 prospective nonrandomised, historically controlled trials (level of evidence: 2b) [24,32]; 2 prospective therapeutic cohort studies (level of evidence: 2b) [35,38]; 1 prospective study that collected data prospectively without controls (level of evidence: 4) [44]; 14 retrospective studies comparing contemporary series of patients (level of evidence: 3b) [22,23,25,26,[28], [29], [30], [31],33,34,37,42,[45], [46], [47]]; 2 retrospective studies using historical series as controls (level of evidence: 4) [36,41] and remaining 7 retrospective studies without controls (level of evidence: 4) [[19], [20], [21],27,40,43,48]. The primary exposure or intervention was rhBMP-2. As for the control groups, there were 4 studies choosing ICBG, 16 studies using non-rhBMP-2, including β-tricalcium phosphate, allograft/demineralised bone matrix, autologous osteophyte, allograft or unstated in control groups, and 9 studies without controls.
Table 1.
Characteristics of included studies.
| Year | Study | Level of evidence | Design | Patients (no.) | Dose of rhBMP-2 | Level of ACDF | Treatments | Follow-up (months) | Quality score |
|---|---|---|---|---|---|---|---|---|---|
| 1999–2000 | Baskin (2003) | 2b | RCT | 33 | 0.6 mg/level | 1-,2- | rhBMP-2 vs. ICBG | 24 | RCT |
| 2002–2003 | Boakye (2005) | 4 | R | 24 | 0.7 mg/level | 1-,2-,3- | rhBMP-2 vs. none | 13.0 | ★★★★ |
| 2011–2013 | Lovasik (2017) | 3b | R | 191 | NA | 1-,2-,3-,4- | rhBMP-2 vs. bTCP | 12 | ★★★★★★ |
| 2007–2009 | Burkus (2017) | 2b | P | 710 | 0.6–1.05 mg/level | 1- | rhBMP-2 vs. non-BMP | 24 | ★★★★★★★★ |
| 2007–2011 | Arnold (2016) | 2b | P | 710 | 0.6–1.05 mg/level | 1- | rhBMP-2 vs. allograft | 24 | ★★★★★★★★ |
| 2007–2011 | Tan (2015) | 3b | R | 146 | 0.9 mg/level | 2- | rhBMP-2 vs. ICBG | 26.8 vs. 27.5 | ★★★★★★★★★ |
| 2009–2011 | Guppy (2014) | 3b | R | 2327 | NA | NA | BMP vs. non-BMP | 7–24 | ★★★★★★★ |
| 1997–2012 | Frenkel (2013) | 3b | R | 45 | 0.26–2.1 mg/level | 2-,3-,4- | BMP vs. non-BMP | 35 vs. 54 | ★★★★★★★★★ |
| NA | Vaidya (2007) | 2b | P | 23 | 1 mg/level | ≥1 | rhBMP-2 vs. demineralised bone matrix | 24 | ★★★★★★ |
| NA | Buttermann (2008) | 2b | P | 66 | 0.9 mg/level | 1-,2-,3- | BMP vs. ICBG | >24 | ★★★★★★ |
| 2007–2012 | Khajavi (2014) | 4 | P | 72 | 0.5–0.7 mg/level | 2-,3-,4- | rhBMP-2 vs. none | 13.8 | ★★★★★★ |
| 2002–2006 | Tumialan (2008) | 4 | R | 200 | 0.7 or 1.05 or 2.2 mg/level | 1-,2-,3-,4- | rhBMP-2 vs. none | 16.7 | ★★★★★ |
| 2008–2011 | Pourtaheri (2015) | 4 | R | 37 | 0.26–0.35 mg/level | 3- | rhBMP-2 vs. none | 48 | ★★★★★★ |
| NA | Klimo (2009) | 4 | R | 22 | 1.1–2.1 mg/level | 1-,2-,3- | rhBMP-2 vs. none | 14.5 | ★★★★ |
| 2011–2012 | Xu (2014) | 3b | R | 40 | 2.1 mg/level | 1-,2- | rhBMP-2 vs. autologous osteophyte | 12 | ★★★★★★★ |
| 2003–2004 | Shields (2006) | 4 | R | 151 | 2.1 mg/level | 1-,2-,3-,4- | rhBMP-2 vs. none | >8 | ★★★ |
| 2006–2008 | Stachniak (2011) | 4 | R | 30 | 0.6 mg/level | 2-,3- | rhBMP-2 vs. none | 9 | ★★★ |
| 2002–2004 | Vaidya (2007) | 3b | R | 46 | 1 mg/level | 1-,2-,3- | rhBMP-2 vs. demineralised bone matrix | 28.03 vs. 23.6 | ★★★★★★ |
| 2007–2012 | Kukreja (2015) | 4 | R | 197 | 0.7 mg/level | 1-,2-,3-,4- | rhBMP-2 vs. none | 24 | ★★★★★ |
| 2002–2009 | Goode (2014) | 3b | R | 57,484 | NA | 2-,3-,4- | BMP vs. non-BMP | 12 | ★★★★★★ |
| 2004–2007 | Williams (2011) | 3b | R | 5184 | NA | NA | BMP vs. non-BMP | NA | ★★★★★ |
| 2002–2009 | Fineberg (2013) | 3b | R | 213,421 | NA | NA | BMP vs. non-BMP | NA | ★★★★★ |
| 2002–2004 | Smucker (2006) | 3b | R | 234 | NA | ≥1 | rhBMP-2 vs. non-BMP | 1.5 | ★★★★★★ |
| 2002–2007 | Lu (2013) | 4 | R | 150 | 0.7–2 mg/level | ≥2 | rhBMP-2 vs. allograft | 35 vs. 25 | ★★★★★★★★★ |
| 2002–2006 | Cahill (2009) | 3b | R | 27,067 | NA | NA | BMP vs. non-BMP | NA | ★★★★★ |
| NA | Shen (2010) | 4 | R | 127 | 4 mg (total) | 3-,4-,5- | rhBMP-2 vs. none | 24 | ★★★★★ |
| 1996–2012 | Riederman (2017) | 4 | R | 400 | 0.7 mg/level | 1-,2-,3-,4- | rhBMP-2 vs. ICBG | NA | ★★★★ |
| 2003–2010 | Jain (2014) | 3b | R | 924,004 | NA | NA | rhBMP vs. non-BMP | NA | ★★★★★ |
| 2005–2011 | Lord (2017) | 3b | R | 215,047 | NA | NA | BMP vs. non-BMP | 3 | ★★★★★ |
| 2006–2010 | Cole (2014) | 3b | R | 91,543 | NA | ≥1 | rhBMP-2 vs. non-rhBMP | >19 | ★★★★★★ |
∗The follow-up months in rh-BMP-2 versus non-rhBMP-2.
Methodological quality of included studies
The quality of included studies is shown in Table 1. True randomisation was used in one RCT. Two prospective nonrandomised trials adopted historical records as controls. None of the retrospective studies adopted an appropriate protocol for treatment assignment, with allocation usually at the discretion of the physician. No studies provided information about allocation concealment or the blinding method. The study-conducting year, dose of rhBMP-2 and level(s) of ACDF were revealed in most studies, benefiting our stratified evaluation. Matching criteria between the groups were variable. Most of studies provided the duration of follow-up. Methods for handling missing data and intention-to-treat analyses were not adequately described in the majority of studies.
Efficacy
The key efficacy outcome, fusion rate, was evaluated in 17 studies, which contained 11 two-arm studies [[22], [23], [24], [25], [26],29,31,35,36,38,39] and 6 one-arm studies [20,21,43,44,46,48]. To investigate the superiority of fusion rates between rhBMP-2 and non-rhBMP-2 in ACDF, 11 studies comparing the two treatments showed that groups choosing rhBMP-2 had significantly higher fusion rate in ACDF than non-rhBMP-2, 98.28% and 95.17%, respectively (OR:7.01, 95% CI: 3.90–12.60, p < 0.00001). To evaluate the influence of doses of rhBMP-2, the index of fusion rate was further divided by low (<0.7 mg/level), middle (0.7–1.1 mg/level) and high (>1.1 mg/level) dose of rhBMP-2, showing higher fusion rate in rhBMP-2 than non-rhBMP-2 (OR: 4.38, 95% CI: 0.21–90.11, p = 0.34; OR: 6.06, 95% CI: 3.19–11.51, p < 0.00001; OR: 3.92, 95% CI: 0.66–23.19, p = 0.13), shown in Figure 2A. The average of fusion rate extracted from 17 studies was 98.34% in patients with rhBMP-2, 95.17% in patients without rhBMP-2, 98.8% in low dose of rhBMP-2, 98.22% in middle dose of rhbmp-2 and 95.29% in high dose of rhBMP-2, as shown in Table 2. A further RCT study using rhBMP-2 in low dose (0.5 mg/level) was being performed by us, showing consistent results (data were not shown).
Figure 2.
Forest plot and meta-analysis of fusion rate. rhBMP-2 = recombinant human bone morphogenetic protein; CI = confidence interval.
Table 2.
Fusion rate and complication rate with or without rhBMP-2.
| rhBMP-2 |
non-rhBMP-2 |
||||
|---|---|---|---|---|---|
| low | middle | high | total | total | |
| Fusion rate | 98.80% | 98.22% | 95.29% | 98.34% | 95.17% |
| Complication rate | 0% | 15.26% | 24.14% | 7.94% | 6.38% |
The score improvements of pain and disability were not extracted to meta-analyse because some papers only revealed the mean values without SD values.
Complications
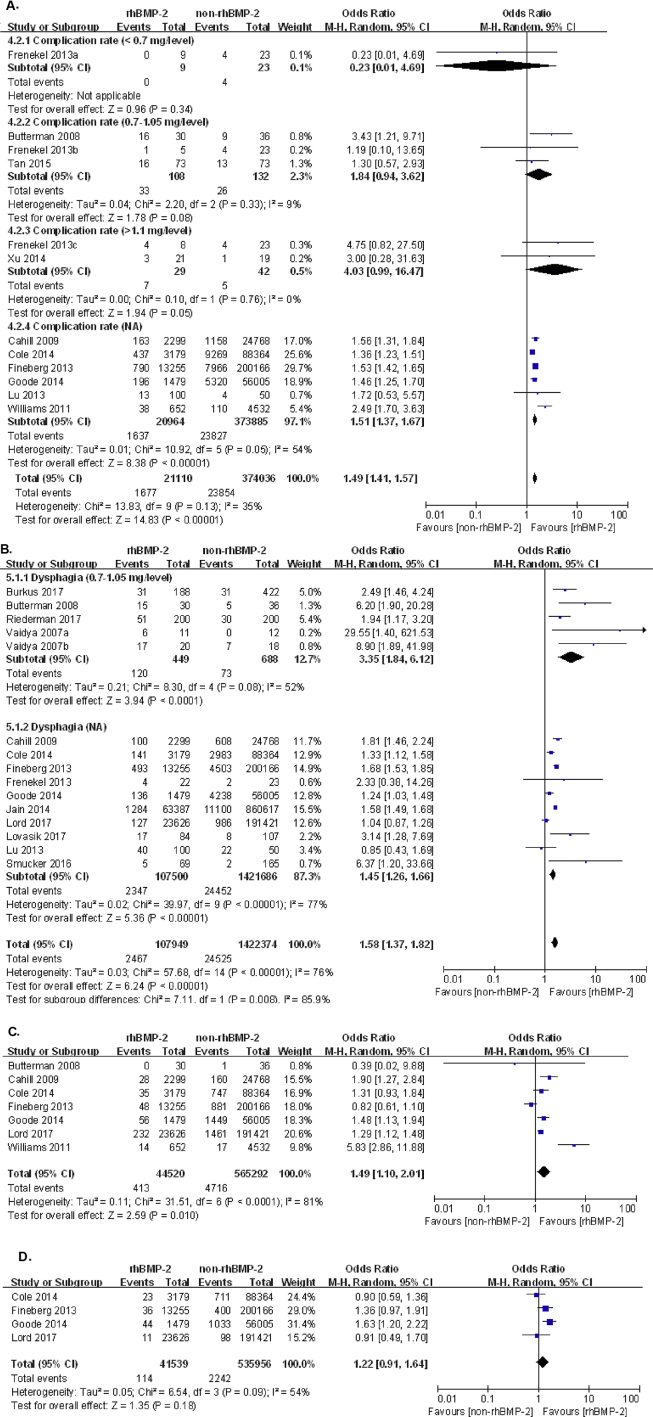
First, pooling the data from nine studies that assessed complication rate in 395,106 patients showed a significant higher complication rate in the rhBMP-2 group than that in the non-rhBMP-2 group, 7.94% and 6.38%, respectively (OR: 1.52, 95% CI: 1.38–1.67, p < 0.00001). Among the nine studies, three studies with 0.7–1.1 mg/level of rhBMP-2 showed higher but nonsignificant change in complication rate between the rhBMP-2 group and non-rhBMP-2 group, 30.56% and 19.70%, respectively (OR: 1.84, 95% CI: 0.94–3.62, p = 0.08). Additionally, two studies that conducted ACDF using high dose (>1.1 mg/level) of rhBMP-2 also showed higher but nonsignificant change in complication rate between two groups, 24.14% and 11.90%, respectively (OR: 4.03, 95% CI: 0.99–16.47, p = 0.06), as shown in Figure 3A. Taking together with one-arm studies, the complication rates of three tiers of rhBMP-2 dose between two groups are shown in Table 2.
Figure 3.
(A) Forest plot and meta-analysis of complication rate; (B) dysphagia; (C) wound infections; (D) neurologic symptoms. rhBMP-2 = recombinant human bone morphogenetic protein; CI = confidence interval.
Second, the most severe and doctor-concerning complication was dysphagia, which was reported in 15 studies in 1,530,323 patients, showing significant more patients with dysphagia in the rhBMP-2 group than in the non-rhBMP-2 group, 2.29% and 1.72%, respectively (OR: 1.58, 95% CI: 1.37–1.82, p < 0.00001), as shown in Figure 3B. Five studies choosing middle dose (0.7–1.1 mg/level) rhBMP-2 reported significantly higher incidence of dysphagia than non-rhBMP-2 patients (OR: 3.35, 95% CI: 1.86–6.12, p < 0.0001). Other studies choosing low or high dose did not reveal the incidence of dysphagia. Therefore, our meta-analysis did not indicate a correlation between rhBMP-2 dose and incidence of dysphagia.
Third, other complications included wound infections and neurologic symptoms. Seven studies in 609,812 patients showed that there were more wound infections in patients treated with rhBMP-2 compared with patients treated without rhBMP-2, 0.93% and 0.83%, respectively (OR: 1.49, 95% CI: 1.10–2.01, p = 0.010). Regarding neurological symptoms, four studies in 577,495 patients reported nonsignificantly adverse symptoms in the group of rhBMP-2 than non-rhBMP-2 (OR: 1.22, 95% CI: 0.91–1.64, p = 0.18), as shown in Figure. 3C and D.
Influences of rhBMP-2 on the levels of ACDF
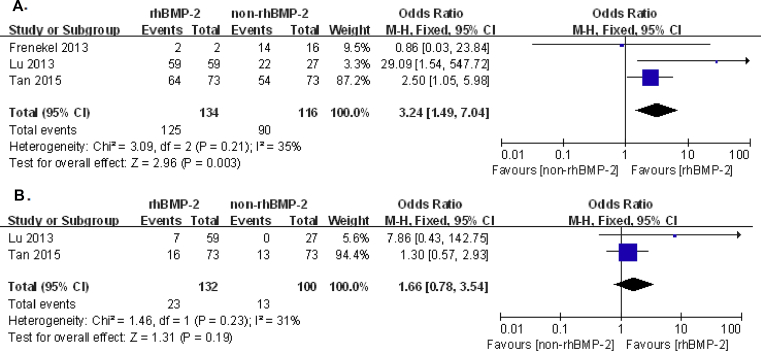
The impacts of rhBMP-2 on the levels of ACDF were divided by the number of ACDF level. But only one study researched influences of rhBMP-2 in 1-level ACDF, which reported fusion rates in postoperative 24 months to be 99.4% versus 87.2%, dysphagia rate 16.4% versus 7.3%, pseudoarthrosis rate 0.4% versus 8.2% and ossification rate 78.6% versus 59.2% (rhBMP-2 group versus non-rhBMP-2) [24]. In 2-level ACDF, fusion rate influenced by rhBMP-2 from three studies was significantly higher in the rhBMP-2 group than in the non-rhBMP-2 group, 93.3% and 77.6% (OR: 3.24, 95% CI: 1.49–7.04, p = 0.003), respectively; complication rate influenced by rhBMP-2 from two studies did not generate significant difference, 17.4% and 13% (OR: 1.66, 95% CI: 0.78–3.54, p = 0.19) in the rhBMP-2 group and non-rhBMP-2 group, respectively. This part of meta-analysis is shown in Fig. 4A and B. Lastly, there is one study conducting one-arm trial to investigate rhBMP-2 effects on 3-level ACDF, which reported one patient who was undertaking an anterior cervical plate removal due to dysphagia among 37 patients; others reached fusion at six months after surgery so that the total fusion rate was 97.3% [46]. Other 27 studies conducting multi-level ACDF did not separate the data according to the level of ACDF. Therefore, our meta-analysis showed that rhBMP-2 increased fusion rate and no significant difference of complication rate on 2-level ACDF. However, current data did not generate the influence of rhBMP-2 on different levels of ACDF.
Figure 4.
Forest plot and meta-analysis of influences of rhBMP-2 on the level of ACDF. (A) fusion rate; (B) complication rate in 2-level ACDF. rhBMP-2 = recombinant human bone morphogenetic protein; CI = confidence interval.
Second outcomes
Three studies with 816 patients reported relative lower but nonsignificant operation time in patients adopting rhBMP-2 than that of non-rhBMP-2 (OR: −10.12, 95% CI: −27.88–7.65, p = 0.26). Other three studies with 214,197 patients revealed similar lengths of hospital stay between patients taken ACDF with rhBMP-2 and without rhBMP-2 (OR: −0.08, 95% CI: −0.34 – 0.18, p = 0.55), as shown in Fig. 5A and B.
Figure 5.
(A) Forest plot and meta-analysis of operation item; (B) length of hospital stay. rhBMP-2 = recombinant human bone morphogenetic protein; SD = standard deviation; IV = inverse variance method; CI = confidence interval.
Sensitivity analysis and publication bias
One RCT and 28 perspective and retrospective studies that scored six or more stars on the modified Newcastle––Ottawa scale were included in sensitivity analysis, shown in Table 3. There was a slight change among these outcomes, but the significance of these outcomes was still in the same range. The degree of between-study heterogeneity decreased dramatically for “length of hospital stay”, “complication rate” and “wound infections” and slightly for “dysphagia”. Between-study heterogeneity remained statistically significant for “operative time” and “dysphagia”.
Table 3.
Sensitivity analysis comparison of rhBMP-2 and non-rhBMP-2.
| Outcomes of interest | Study no. | rhBMP-2 no. | non-rhBMP-2 no. | WMD/OR (95% CI) | p value | Study heterogeneity |
|||
|---|---|---|---|---|---|---|---|---|---|
| χ2 | df | I2, % | p value | ||||||
| Total fusion rate | 11 | 697 | 2980 | 6.96 [3.87, 12.52] | <0.00001 | 11.19 | 9 | 20 | 0.26 |
| Operative time | 3 | 275 | 541 | −10.12 [27.88, 7.65] | 0.26 | 17.38 | 2 | 88 | 0.0002 |
| Hospital stay | 2 | 254 | 522 | 0.02 [-0.08,0.11] | 0.7 | 0.64 | 1 | 0 | 0.42 |
| Complication rate | 7 | 4904 | 144,570 | 1.40 [1.29, 1.52] | <0.00001 | 3.55 | 6 | 0 | 0.68 |
| Dysphagia | 10 | 5182 | 145,202 | 1.96 [1.39, 2.75] | 0.0001 | 30.77 | 9 | 71 | 0.0003 |
| Wound infections | 3 | 3808 | 80,809 | 1.58 [1.26, 1.98] | <0.0001 | 1.72 | 2 | 0 | 0.42 |
| Neurological symptoms | 2 | 4658 | 144,369 | 1.23 [0.68, 2.22] | 0.49 | 5.21 | 1 | 81 | 0.02 |
| Fusion rate in 2-level ACDF | 3 | 134 | 116 | 3.24 [1.49, 7.04] | 0.003 | 3.09 | 2 | 35 | 0.21 |
| Complication rate in 2-level ACDF | 2 | 132 | 100 | 1.66 [0.78, 3.54] | 0.19 | 1.46 | 1 | 31 | 0.23 |
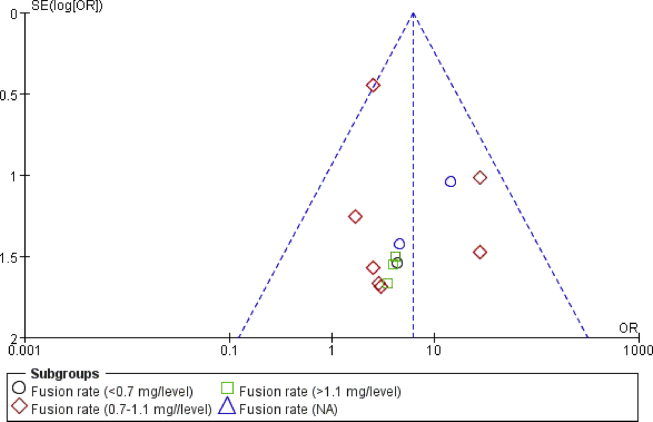
Fig. 6 shows the funnel plot of the studies included in our meta-analysis that reported fusion rates. All recruited studies lied inside the 95% CIs, with an even distribution around the vertical, indicating no obvious publication bias.
Figure 6.
Funnel plot illustrating meta-analysis of fusion rate. SE = standard error; OR = odds ratio.
Discussion
As the multi-functional growth factors, rhBMPs were introduced to several medical scenarios to promote the bone-healing rate with the proposal of less morbidity [10]. Presently, there are two rhBMPs, rhBMP-2 and rhBMP-7 (OP-1, Stryker Biotech, Hopkinton, MA), approved by FDA to treat a variety of bone-related conditions including delayed union and nonunion [49], bringing alternatives to autologous bone graft with significant donor site morbidity. rhBMP-2 was received with the FDA approval in July of 2002 on single-level anterior lumbar interbody fusion from vertebral L2-S1 for the treatments of degenerative disc disease, Grade I spondylolisthesis, and/or retrolisthesis with the lumbar tapered fusion device LT-CAGE (Medtronic Sofamor Danek) [4]. rhBMP-2 has been rapidly used off-label for anterior cervical fusion, but the postoperative complications of soft tissue swelling, dysphagia and respiratory complications raised FDA attentions to release a Public Health Notification at July 2008 [50]. Therefore, 29 studies using rhBMP-2 in ACDF were aggregated to find some clues that dose of rhBMP-2 or levels of ACDF affected the relevant efficacy and safety outcomes.
The rhBMP-2 minimum dose used for single- or multi-level ACDF was nearly 1/10 of maximum dose between studies, which was 0.26 mg/level compared to 2.1 mg/level. To reveal the influence of dose of rhBMP-2 on efficacy and risks, we stratified the data according to the dose of rhBMP-2 to three tiers: high dose (>1.1 mg/level), middle dose (0.7–1.1 mg/level) and low dose (<0.7 mg/level).
The fusion rate was higher in rhBMP-2 groups than in the non-rhBMP-2 group, regardless of the dose of rhBMP-2. Additionally, the fusion rates were associated with the dose of rhBMP-2: higher dose of rhBMP-2, lower fusion rate. These findings indicate surgeons that low dose of rhBMP-2 is enough for the improvement of fusion rate in the ACDF. Another meta-analysis of rhBMP in spinal arthrodesis surgery by Hofstetter et al. reported 100% fusion rate in different doses of BMP in ACDF except 98.88% fusion rate in 0.7–2.1 mg/level, which did not showed the dose-dependent change on postoperative fusion rate [4]. This may be resulted in the only seven publications included, compared to our meta-analysis with 29 studies. Therefore, from better fusion rate, rhBMP-2 was recommended to ACDF, even in the dose of <0.7 mg/level. It is noteworthy that this dose of rhBMP-2 is much lower than the manufacturer's recommendation that was recommended by FDA from 4.2 mg to 12 mg rhBMP-2 per level [4].
High doses of rhBMP-2 were associated with increased complication rates in lumbar interbody fusion, which were consistent with our findings [4]. The included studies using low dose of rhBMP-2 (<0.7 mg/level) did not report the complication rate, while studies using middle and high doses of rhBMP-2 reported higher complication rate than low dose group and non-rhBMP-2 treatment. Frenkel et al. reported that patients had no complication in rhBMP-2 low dosage (<0.5 mg/level), but reported 12.5% (1 patient) in middle dose (0.5–1.1 mg/level) and 50% (4 patients) in high dose (1.4–2.1 mg/level), showing the increasing dose-dependent complication rate. Tumialan et al. also realised that changes of the complication rate may be brought by doses of rhBMP-2 on complication rate, reporting three times dose reduction of rhBMP-2 from 2.1 to 1.05 to 0.7 mg/level to avoid asymptomatic excess interbody bone formation and potential dysphagia [43]. Taking together with peers’ studies, our analysis showed that although rhBMP-2 could improve fusion, rhBMP-2 may induce higher complication rates compared to that in non-rhBMP-2, especially at high and middle doses of rhBMP-2 (>0.7 mg/level).
The most observed complication syndromes, including dysphagia, wound infections and neurologic symptoms, showed higher incidence in the rhBMP-2 group than in the non-rhBMP-2 group. Regarding the dose of rhBMP-2 in these ACDF studies, one RCT that utilised low dose of rhBMP-2 (0.6 mg/level) did not report dysphagia case in its own publication [39], but it was revealed in two Yale Open Data Access (YODA) studies [51,52] that there was 1 dysphagia case from 18 patients in the rhBMP-2 group (5.56%) and 2 from 15 in the ICBG group (13.33%), showing fewer cases of dysphagia in low-dose rhBMP-2. Other two one-arm studies using ultra-low-dose (0.26–0.35 mg/level) [46] and low-dose (0.5–0.7 mg/level) [44] rhBMP-2 reported 2 of 72 (2.78%) and 4 of 37 (11%) incidence of dysphagia, respectively. However, it was hard to meta-analyse dysphagia caused by low-dose rhBMP-2 through the above three studies because of lacking contemporary non-rhBMP-2 series. Additionally, dysphagia incidence could be influenced by female gender, multi-level surgery and surgical site at C3/4 [53]; the wound infections were only considered the wounds in the cervical spine site due to no bone graft collection site in the rhBMP-2 group. We expected that these confounding factors affecting the judges of rhBMP-2 could be eliminated in future well-designed blinded RCTs. Based on our findings of complication rate and symptoms, we recommended that, when spine surgeons consider rhBMP-2 in polyetheretherketone (PEEK), the dose of rhBMP-2 would be better lower than 0.7 mg/level in ACDF for the safety concern.
The quality of life was related to neurological syndromes and pain. Neurological syndromes showed nonsignificantly adverse symptoms in the group of rhBMP-2 than non-rhBMP-2 in our meta-analysis. Regarding pain, the recruited papers applied mean value of pain score without SD, so that the meta-analysis of pain was hard to calculate. However, Basin et al. reported higher improvement of neck pain and arm pain in ACDF with the rhBMP-2 group not in ACDF without rhBMP-2 [39]. Burkus et al. reported no significant difference of neck pain and arm pain between rhBMP-2 and non-rhBMP-2 [24]. These two papers used 20-point numeric rating scale to evaluate pain, and other two recruited papers used VAS to evaluate pain, which also found no difference between the two groups [26,38]. Therefore, although these recruited papers could not generate the meta-analysis of pain, they showed no difference or better improvement of pain in rhBMP-2 not in non-rhBMP-2. From the two aspects—neurological syndromes and pain, ACDF with rhBMP-2 did not influence patients’ quality of life, compared to ACDF without rhBMP-2.
Most studies of rhBMP-2 on ACDF did not separate the efficacy and safety data according to the levels of ACDF. Based on limited data, the trend of effects of rhBMP-2 was not obvious based on the levels of ACDF. In collected papers, one 1-level ACDF study showed fusion rates were 99.4% versus 87.2% in rhBMP-2 and non-rhBMP-2 groups [24]; our meta-analysis of 2-level ACDF showed 93.3% and 77.6%, respectively. Lu et al. analysed their data based on the levels of ACDF, showing stable 100% fusion rate but also increased complication rate and dysphagia on level-dependence in the rhBMP-2 group; these indices did not change on level-dependence in the non-rhBMP-2 group. Meanwhile, Hofstetter et al. reported the decreased fusion rate when fused levels of ACDF increased in the control group, but the fusion rate remained at 98.88–100% even in 4-level ACDF in rhBMP-2 groups, indicating the rhBMP-2 has a high osteoinduction and improved fusion in multilevel ACDF [4]. Based on the data from 29 studies and our meta-analysis, rhBMP-2 improved fusion rate especially in multi-level ACDF, but the influence did not show any level dependence. Therefore, when an ACDF due to the multi-level has a high risk of nonunion, rhBMP-2 may be an option of increasing the fusion.
The current meta-analysis is limited by only one RCT; others are prospective and retrospective nonrandomised studies. Without randomisation and double blind, the data generated may be influenced by confounding factors, like levels of ACDF, other medical conditions and surgeons’ techniques. We well designed and were performing a RCT using low dose (0.5 mg/level) of rhBMP-2 in ACDF under the management of the patient and investigator variables. The data will reveal more associations of efficacy and risks of this osteoinductive cytokine in ACDF soon.
Conclusion
The rhBMP-2 doses used for single- or multi-level ACDF in 29 studies differed greatly from 0.26 to 2.1 mg/level. It is encouraging that lowest dose of rhBMP-2 was enough to achieve the best fusion rate compared to other doses of rhBMP-2. However, it is accompanied by higher complication rate with more dysphagia and wound infections in ACDF with rhBMP-2, compared to that in ACDF without rhBMP-2. Based on the meta-analysis, the lowest dose of rhBMP-2, which gained both the higher bone union and minimum complications, was 0.7 mg/level. Furthermore, considering the higher risk of nonunion in multi-level of ACDF, rhBMP-2 may be an option of increasing the fusion. Even so, healthcare practitioners should weigh the benefits of increasing union and potential risks of dysphagia and other complications drawn by this growth factor before the procedure of ACDF. Therefore, the optimal dose of rhBMP-2 in ACDF is necessary to be redefined by manufacturers in further large-volume, well-designed RCTs with extensive follow-up to achieve association from multiple dimensions.
Conflict of Interest
The authors have no conflicts of interest to disclose in relation to this article.
Acknowledgements
This work was supported by China International Exchanges and Talents Programs of CUS-RF [205458] and China Postdoctoral Science Foundation Grant [2018M643015].
Contributor Information
Ya-Dan Wen, Email: yadan.wen@csu.edu.cn.
Jin-Hui Shi, Email: shijinhui502@126.com.
References
- 1.Wang B., Lü G., Kuang L. Anterior cervical discectomy and fusion with stand-alone anchored cages versus posterior laminectomy and fusion for four-level cervical spondylotic myelopathy: a retrospective study with 2-year follow-up. BMC Muscoskel Disord. 2018;19(1):216. doi: 10.1186/s12891-018-2136-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen Y., Lü G., Wang B., Li L., Kuang L. A comparison of anterior cervical discectomy and fusion (ACDF) using self-locking stand-alone polyetheretherketone (PEEK) cage with ACDF using cage and plate in the treatment of three-level cervical degenerative spondylopathy: a retrospective study with 2-year follow-up. Eur Spine J. 2016;25(7):2255–2262. doi: 10.1007/s00586-016-4391-x. [DOI] [PubMed] [Google Scholar]
- 3.Liu Y., Luo X., Zhou J., Li N., Peng S., Rong P. Prognosis of posterior osteophyte after anterior cervical decompression and fusion in patients with cervical spondylotic myelopathy using three-dimensional computed tomography study. Eur Spine J. 2016;25(6):1861–1868. doi: 10.1007/s00586-016-4390-y. [DOI] [PubMed] [Google Scholar]
- 4.Hofstetter C.P., Hofer A.S., Levi A.D. Exploratory meta-analysis on dose-related efficacy and morbidity of bone morphogenetic protein in spinal arthrodesis surgery. J Neurosurg Spine. 2016;24(3):457–475. doi: 10.3171/2015.4.SPINE141086. [DOI] [PubMed] [Google Scholar]
- 5.Hu Y., Lv G., Ren S., Johansen D. Mid- to long-term Outcomes of cervical disc Arthroplasty versus anterior cervical Discectomy and Fusion for Treatment of symptomatic cervical disc disease: a systematic Review and meta-Analysis of eight prospective randomized controlled trials. PloS One. 2016;11(2) doi: 10.1371/journal.pone.0149312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuang L., Chen Y., Wang B., Li L., Lü G. Cervical disk arthroplasty versus anterior cervical decompression and fusion for the treatment of 2-level cervical spondylopathy: a systematic review and meta-analysis. Clinical Spine Surgery. 2016;(9):29. doi: 10.1097/BSD.0000000000000395. [DOI] [PubMed] [Google Scholar]
- 7.Hustedt J.W., Blizzard D.J. The controversy surrounding bone morphogenetic proteins in the spine: a review of current research. Yale J Biol Med. 2014;87:549–561. [PMC free article] [PubMed] [Google Scholar]
- 8.Kani K.K., Chew F.S. Anterior cervical discectomy and fusion: review and update for radiologists. Skeletal Radiol. 2018;47(1):7–17. doi: 10.1007/s00256-017-2798-z. [DOI] [PubMed] [Google Scholar]
- 9.Silber J.S., Anderson D.G., Daffner S.D., Brislin B.T., Leland J.M., Hilibrand A.S. Donor site morbidity after anterior iliac crest bone harvest for single-level anterior cervical discectomy and fusion. Spine. 2003;28(2):134–139. doi: 10.1097/00007632-200301150-00008. [DOI] [PubMed] [Google Scholar]
- 10.Oliveira O.R., Martins S.P., Lima W.G., Gomes M.M. The use of bone morphogenetic proteins (BMP) and pseudarthrosis, a literature review. Rev Bras Ortop. 2017;52(2):124–140. doi: 10.1016/j.rboe.2016.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chau A.M., Mobbs R.J. Bone graft substitutes in anterior cervical discectomy and fusion. Eur Spine J. 2009;18(4):449–464. doi: 10.1007/s00586-008-0878-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zadegan S.A., Abedi A., Jazayeri S.B., Nasiri Bonaki H., Jazayeri S.B., Vaccaro A.R. Bone morphogenetic proteins in anterior cervical fusion_ A systematic review and meta-analysis. World Neurosurg. 2017;104:752–789. doi: 10.1016/j.wneu.2017.02.098. [DOI] [PubMed] [Google Scholar]
- 13.Stroup D.F., Berlin J.A., Morton S.C., Olkin I., Williamson G.D., Rennie D. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 14.Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gøtzsche P.C., Ioannidis J.P. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.BP, Ball C., Sackett D. Oxford Centre for Evidence-based Medicine Web site; 2012. Levels of evidence and grades of recommendation.http://www.cebm.net/index.aspx?o=1025 [Google Scholar]
- 16.The Cochrane Collaboration . In: Cochrane handbook for systematic reviews of interventions. JPT H., G S., editors. vol. 2011. 2011. http://handbook.cochrane.org Version 5.1.0 [updated March 2011] Available from. [Google Scholar]
- 17.Wells G.A., Shea B., O’Connell D., Peterson J., Welch V., Losos M. Ottawa Hospital Research Institute Web site; 2012. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses.http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp [Google Scholar]
- 18.Hozo S.P., Djulbegovic B., Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5(1):13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shields L.B., Raque G.H., Glassman S.D., Campbell M., Vitaz T., Harpring J. Adverse effects associated with high-dose recombinant human bone morphogenetic protein-2 use in anterior cervical spine fusion. Spine. 2006;31(5):542–547. doi: 10.1097/01.brs.0000201424.27509.72. [DOI] [PubMed] [Google Scholar]
- 20.Stachniak J.B., Diebner J.D., Brunk E.S., Speed S.M. Analysis of prevertebral soft-tissue swelling and dysphagia in multilevel anterior cervical discectomy and fusion with recombinant human bone morphogenetic protein-2 in patients at risk for pseudarthrosis. J Neurosurg Spine. 2011;14(2):244–249. doi: 10.3171/2010.9.SPINE09828. [DOI] [PubMed] [Google Scholar]
- 21.Boakye M., Mummaneni P.V., Garrett M., Rodts G., Haid R. Anterior cervical discectomy and fusion involving a polyetheretherketone spacer and bone morphogenetic protein. J Neurosurg Spine. 2005;2(5):521–525. doi: 10.3171/spi.2005.2.5.0521. [DOI] [PubMed] [Google Scholar]
- 22.Lovasik B.P., Holland C.M., Howard B.M., Baum G.R., Rodts G.E., Refai D. Anterior cervical discectomy and fusion: comparison of fusion, dysphagia, and complication rates between recombinant human bone morphogenetic protein-2 and beta-tricalcium phosphate. World Neurosurg. 2017;97:674–683. doi: 10.1016/j.wneu.2016.10.088. e1. [DOI] [PubMed] [Google Scholar]
- 23.Xu Y., Jin Y., Shi Y., Jiang W.M., Tang T.S. Application of bone repair materials containing recombinant human bone morphogenetic protein-2 in anterior cervical discectomy and fusion. Zhongguo Zuzhi Gongcheng Yanjiu. 2014;18(43):6889–6895. [Google Scholar]
- 24.Burkus J.K., Dryer R.F., Arnold P.M., Foley K.T. Clinical and radiographic outcomes in patients undergoing single-level anterior cervical arthrodesis: a prospective trial comparing allograft to a reduced dose of rhBMP-2. Clin Spine Surg. 2017;30(9):E1321–E1332. doi: 10.1097/BSD.0000000000000409. [DOI] [PubMed] [Google Scholar]
- 25.Tan B., Wang H., Dong J., Yuan Z., Wang D., Wang F. Comparison of rhBMP-2 versus autogenous iliac crest bone Graft for 2-level anterior cervical Discectomy and Fusion for cervical spondylotic myelopathy. Med sci monit. 2015;21:3159–3165. doi: 10.12659/MSM.894656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vaidya R., Carp J., Sethi A., Bartol S., Craig J., Les C.M. Complications of anterior cervical discectomy and fusion using recombinant human bone morphogenetic protein-2. Eur Spine J. 2007;16(8):1257–1265. doi: 10.1007/s00586-007-0351-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kukreja S., Ahmed O.I., Haydel J., Nanda A., Sin A.H. Complications of anterior cervical fusion using a low-dose recombinant human bone morphogenetic protein-2. Korean J Spine. 2015;12(2):68–74. doi: 10.14245/kjs.2015.12.2.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goode A.P., Richardson W.J., Schectman R.M., Carey T.S. Complications, revision fusions, readmissions, and utilization over a 1-year period after bone morphogenetic protein use during primary cervical spine fusions. Spine J. 2014;14(9):2051–2059. doi: 10.1016/j.spinee.2013.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guppy K.H., Paxton E.W., Harris J., Alvarez J., Bernbeck J. Does bone morphogenetic protein change the operative nonunion rates in spine fusions? Spine. 2014;39(22):1831–1839. doi: 10.1097/BRS.0000000000000534. [DOI] [PubMed] [Google Scholar]
- 30.Williams B.J., Smith J.S., Fu K.M., Hamilton D.K., Polly D.W., Jr., Ames C.P. Does bone morphogenetic protein increase the incidence of perioperative complications in spinal fusion? A comparison of 55,862 cases of spinal fusion with and without bone morphogenetic protein. Spine. 2011;36(20):1685–1691. doi: 10.1097/BRS.0b013e318216d825. [DOI] [PubMed] [Google Scholar]
- 31.Frenkel M.B., Cahill K.S., Javahar R.J., Zacur G., Green B.A., Levi A.D. Fusion rates in multilevel, instrumented anterior cervical fusion for degenerative disease with and without the use of bone morphogenetic protein. J Neurosurg Spine. 2013;18(3):269–273. doi: 10.3171/2012.12.SPINE12607. [DOI] [PubMed] [Google Scholar]
- 32.Arnold P.M., Anderson K.K., Selim A., Dryer R.F., Kenneth Burkus J. Heterotopic ossification following single-level anterior cervical discectomy and fusion: results from the prospective, multicenter, historically controlled trial comparing allograft to an optimized dose of rhBMP-2. J Neurosurg Spine. 2016;25(3):292–302. doi: 10.3171/2016.1.SPINE15798. [DOI] [PubMed] [Google Scholar]
- 33.Fineberg S.J., Ahmadinia K., Oglesby M., Patel A.A., Singh K. Hospital outcomes and complications of anterior and posterior cervical fusion with bone morphogenetic protein. Spine. 2013;38(15):1304–1309. doi: 10.1097/BRS.0b013e31828f494c. [DOI] [PubMed] [Google Scholar]
- 34.Smucker J.D., Rhee J.M., Singh K., Yoon S.T., Heller J.G. Increased swelling complications associated with off-label usage of rhBMP-2 in the anterior cervical spine. Spine. 2006;31(24):2813–2819. doi: 10.1097/01.brs.0000245863.52371.c2. [DOI] [PubMed] [Google Scholar]
- 35.Vaidya R., Weir R., Sethi A., Meisterling S., Hakeos W., Wybo C.D. Interbody fusion with allograft and rhBMP-2 leads to consistent fusion but early subsidence. J Bone Joint Surg Br. 2007;89(3):342–345. doi: 10.1302/0301-620X.89B3.18270. [DOI] [PubMed] [Google Scholar]
- 36.Lu D.C., Tumialan L.M., Chou D. Multilevel anterior cervical discectomy and fusion with and without rhBMP-2: a comparison of dysphagia rates and outcomes in 150 patients. J Neurosurg Spine. 2013;18(1):43–49. doi: 10.3171/2012.10.SPINE10231. [DOI] [PubMed] [Google Scholar]
- 37.Cahill K.S., Chi J.H., Day A., Claus E.B. Prevalence, complications, and hospital charges associated with use of bone-morphogenetic proteins in spinal fusion procedures. J Am Med Assoc. 2009;302(1):58–66. doi: 10.1001/jama.2009.956. [DOI] [PubMed] [Google Scholar]
- 38.Buttermann G.R. Prospective nonrandomized comparison of an allograft with bone morphogenic protein versus an iliac-crest autograft in anterior cervical discectomy and fusion. Spine J. 2008;8(3):426–435. doi: 10.1016/j.spinee.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 39.Baskin D.S., Ryan P., Sonntag V., Westmark R., Widmayer M.A. A prospective, randomized, controlled cervical fusion study using recombinant human bone morphogenetic protein-2 with the CORNERSTONE-SR allograft ring and the ATLANTIS anterior cervical plate. Spine. 2003;28(12):1219–1224. doi: 10.1097/01.BRS.0000065486.22141.CA. discussion 1225. [DOI] [PubMed] [Google Scholar]
- 40.Shen H.X., Buchowski J.M., Yeom J.S., Liu G., Lin N., Riew K.D. Pseudarthrosis in multilevel anterior cervical fusion with rhBMP-2 and allograft: analysis of one hundred twenty-seven cases with minimum two-year follow-up. Spine. 2010;35(7):747–753. doi: 10.1097/BRS.0b013e3181bc3420. [DOI] [PubMed] [Google Scholar]
- 41.Riederman B.D., Butler B.A., Lawton C.D., Rosenthal B.D., Balderama E.S., Bernstein A.J. Recombinant human bone morphogenetic protein-2 versus iliac crest bone graft in anterior cervical discectomy and fusion: Dysphagia and dysphonia rates in the early postoperative period with review of the literature. J Clin Neurosci. 2017;44:180–183. doi: 10.1016/j.jocn.2017.06.034. [DOI] [PubMed] [Google Scholar]
- 42.Jain A., Hassanzadeh H., Strike S.A., Skolasky R.L., Riley L.H., 3rd rhBMP use in cervical spine surgery: associated factors and in-hospital complications. J Bone Joint Surg Am. 2014;96(8):617–623. doi: 10.2106/JBJS.M.00666. [DOI] [PubMed] [Google Scholar]
- 43.Tumialan L.M., Pan J., Rodts G.E., Mummaneni P.V. The safety and efficacy of anterior cervical discectomy and fusion with polyetheretherketone spacer and recombinant human bone morphogenetic protein-2: a review of 200 patients. J Neurosurg Spine. 2008;8(6):529–535. doi: 10.3171/SPI/2008/8/6/529. [DOI] [PubMed] [Google Scholar]
- 44.Khajavi K., Shen A. Safety and efficacy of bioabsorbable cervical spacers and low-dose rhBMP-2 in multi-level ACDF. Internet J Spine Surg. 2014:8. doi: 10.14444/1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lord E.L., Cohen J.R., Buser Z., Meisel H.J., Brodke D.S., Yoon S.T. Trends, costs, and complications of anterior cervical discectomy and fusion with and without bone morphogenetic protein in the United States Medicare population. Global Spine J. 2017;7(7):603–608. doi: 10.1177/2192568217699207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pourtaheri S., Hwang K., Faloon M., Issa K., Mease S.J., Mangels D. Ultra-low-dose recombinant human bone morphogenetic protein-2 for 3-level anterior cervical Diskectomy and fusion. Orthopedics. 2015;38(4):241–245. doi: 10.3928/01477447-20150402-04. [DOI] [PubMed] [Google Scholar]
- 47.Cole T., Veeravagu A., Jiang B., Ratliff J.K. Usage of recombinant human bone morphogenetic protein in cervical spine procedures: analysis of the MarketScan longitudinal database. J Bone Joint Surg Am. 2014;96(17):1409–1416. doi: 10.2106/JBJS.M.01016. [DOI] [PubMed] [Google Scholar]
- 48.Klimo P., Jr., Peelle M.W. Use of polyetheretherketone spacer and recombinant human bone morphogenetic protein-2 in the cervical spine: a radiographic analysis. Spine J. 2009;9(12):959–966. doi: 10.1016/j.spinee.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 49.Ong K.L., Villarraga M.L., Lau E., Carreon L.Y., Kurtz S.M., Glassman S.D. Off-label Use of bone morphogenetic Proteins in the United States using administrative data. Spine. 2010;35(19):1794–1800. doi: 10.1097/BRS.0b013e3181ecf6e4. [DOI] [PubMed] [Google Scholar]
- 50.Administration, U.F.a.D . Public Health Notifications; 2008. FDA public Health notification: life-threatening complications associated with recombinant human bone morphogenetic protein in cervical spine fusion. [Google Scholar]
- 51.Rodgers M.A., Brown J.V.E., Heirs M.K., Higgins J.P.T., Mannion R.J., Simmonds M.C. Reporting of industry funded study outcome data: comparison of confidential and published data on the safety and effectiveness of rhBMP-2 for spinal fusion. BMJ. 2013;346:f3981. doi: 10.1136/bmj.f3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Simmonds M.C., Brown J.V.E., Heirs M.K., Higgins J.P.T., Mannion R.J., Rodgers M.A. Safety and Effectiveness of recombinant human bone morphogenetic protein-2 for spinal fusion. Ann Intern Med. 2013;158:877–889. doi: 10.7326/0003-4819-158-12-201306180-00005. [DOI] [PubMed] [Google Scholar]
- 53.Liu F.Y., Yang D.L., Huang W.Z., Huo L.S., Ma L., Wang H. Risk factors for dysphagia after anterior cervical spine surgery: a meta-analysis. Medicine (Baltim) 2017;96(10):e6267. doi: 10.1097/MD.0000000000006267. [DOI] [PMC free article] [PubMed] [Google Scholar]