Abstract
Objective:
To examine the impact of an empty bladder filling protocol on patients receiving radical RT for localised prostate cancer on post RT toxicity and biochemical progression free survival (bPFS).
Methods and materials:
Records of patients receiving radical external beam RT (EBRT) for localised prostate cancer with a full or empty bladder were reviewed. These included the bladder size on planning CT, daily online image guided RT (IGRT) setup data, treatment time and post treatment follow up data.These included bPFS, gastrointestinal(GI) and genitourinary(GU) toxicity scoring post RT using the CTCAE v4.0 scoring system. All patients included in the study were planned and treated under the same departmental clinical protocol with VMAT and daily online IGRT corrections.
Results:
90 patients were treated with 60 Gy in 20 fractions with a median follow up of 48 months. At 4 years bPFS in the empty bladder group was 100 and 98% in the full bladder group (p = 0.27). There were no statistically significant differences in cumulative ≥Grade 2GU (p = 0.10) and GI (p = 0.27) toxicity rates between the two bladder filling protocols. No statistically significant differences in the IGRT setup between the two groups of patients. Although the median treatment times per fraction were not statistically different between the two groups (p = 0.47), patients in the full bladder filling group were required to spend a longer time in the RT department per treatment session for bladder filling.
Conclusion:
An empty bladder filling protocol has non-inferior bPFS, GI and GU toxicities at 4 years in patients with localised prostate cancer using advanced RT techniques in comparison to a full bladder filling protocol. A longer follow up with a larger sample size is required to validate this approach.
Advances in knowledge:
This study suggests that an empty bladder filling protocol can be used in external beam EBRT for localised prostate cancer with non-inferior treatment outcomes.
Background
External beam radiotherapy (EBRT) has been proved to be a well-established treatment modality for localised prostate cancer.1,2 With the recent introductions of advanced radiotherapy techniques including image-guided radiotherapy (IGRT), intensity modulated radiotherapy (IMRT) and volumetric modulated arctherapy (VMAT), these technological improvements facilitate an improvement in tumour control with a decrease in RT-induced toxicity for patients receiving prostate EBRT.1–4
Historically, full bladder filling protocols are used for EBRT to localised prostate cancer. Assuming that the full bladder status can help achieving better dose sparing of the organs at risk (OARs) such as the bladder and small bowel, the post-treatment gastrointestinal (GI) and genitourinary (GU) toxicities should be minimised. Studies exploring this subject focus on theoretical modelling or historical clinical outcome data to support this practice.5,6
Empty bladder filling protocols are increasingly advocated for patients who require RT to the prostate alone.7–9 Non-inferiorities of post-RT GI and GU toxicities at 12 months were found in the adoption of empty bladder filling protocols in EBRT to localised prostate cancer.8,9 Reproducibility of target and OARs positions are important factors to be considered in prostate EBRT as these may influence the treatment outcome in terms of tumour control. So far, no detailed information of tumour control and late post-RT toxicities with the use of an empty bladder filling protocol is available.
Since January 2014, our institution introduced treating localised prostate cancer with EBRT of 60 Gy in 20 fractions using VMAT and an empty bladder filling protocol. This study investigates the impact of an empty bladder filling protocol in patients receiving radical RT to the prostate in terms of tumour control, acute and late post-RT-related toxicities compared to a full bladder filling protocol.
Methods and materials
All patients receiving external beam radiotherapy for localised prostate cancer were eligible for this study. Patients were classified into risk groups according to the D’Amico criteria10 and only low and intermediate favourable risk groups were included in this study. Those with additional nodal volumes or receiving post-prostatectomy salvage radiotherapy were excluded. This work was undertaken as a service evaluation and all patients provided written informed consent for their data to be used in this study.
RT planning and treatment were performed in the supine position with legs supported using a knee rest and ankle stocks. Computer tomography (CT) slices were obtained at 3 mm intervals. All patients received verbal advice on bladder preparation at their CT planning appointments.9 For the full bladder filling protocol, patients were asked to void the bowel and bladder and then drink 300 ml of water within the next 15 min and 30 min later proceed with the RT planning scan. This process was repeated daily prior to each treatment. For the empty bladder filling protocol, the advice was to empty bowel and bladder once at the radiotherapy department immediately before planning CT and each treatment. All patients in the full bladder filling group were required to arrive at the RT department 45 min earlier than their defined appointment time for the bladder preparation. For patients with a rectal distention more than 4 cm, they would be prescribed with micro-enemas to aid emptying the rectum.
Target and OARs including the bladder and rectum were delineated by the attending radiation oncologist on the Varian Eclipse treatment planning system (Varian Medical Systems, Inc., Palo Alto, CA). VMAT was delivered using a 6 MVlinac accelerator treating the prostate and base of seminal vesicles to 60 Gy in 20 fractions with a 10 mm planning target volume (PTV) margin, except posteriorly where 5 mm was used. All patients included in the study were planned according to The International Commission on Radiation Units and Measurements (ICRU) report 8311 and the same departmental clinical protocol with VMAT using dose constraints as shown in Table 1. Daily online IGRT corrections were used for all patients either with 2D kV planar imaging matching to fiducials or 3D volumetric cone beam CT (CBCT) matching to the prostate gland.
Table 1.
PTV and OARs dose objectives used for VMAT treatment planning optimisation
| Volume | DVH objectives | Empty bladder protocol (n = 49) Median (Range) |
Full bladder protocol (n = 41) Median (Range) |
p value |
|---|---|---|---|---|
| PTV | Dose received by 98% of the volume D98% ≥ 57 Gy |
57.9 Gy (57.3–58.5) | 57.8 Gy (57.4–58.4) | 0.35 |
| Dose received by 50% of the volume D50% = 60 Gy |
60 Gy | 60 Gy | N/A | |
| Dose received by 2% of the volume D2% ≤ 63 Gy |
61.5 Gy (61.2–62.2) | 61.6 Gy (61.0–62.0) | 1.00 | |
| Rectum | Volume receiving 42 Gy V42Gy ≤ 60% |
28.3% (17.3–61.0) | 29.0% (10.5–58.9) | 0.75 |
| Volume receiving 50 Gy V50Gy ≤ 50% |
16.8% (9.4–44.5) | 17.1% (2.9–38.6) | 0.72 | |
| Volume receiving 54 Gy V54Gy ≤ 30% |
12.3% (6.6–35.2) | 12.0% (1.4–30.9) | 0.51 | |
| Volume receiving 58 Gy V58Gy ≤ 15% |
6.9% (2.8–21.4) | 6.9% (0.4–18.4) | 0.49 | |
| Bladder | Volume receiving 42 Gy V42Gy ≤ 50% |
51.2% (23.7–84.9) | 24.7% (5.6–72.5) | <0.05 |
| Volume receiving 50 Gy V50Gy ≤ 25% |
39.6% (17.2–66.7) | 18.5% (4.5–61.5) | <0.05 | |
| Volume receiving 62 Gy V62Gy ≤ 5% |
0.1% (0–2.5) | 0% (0–2.0) | 0.09 |
Treatment records and follow-up data of prostate cancer patients who followed the two different bladder filling (empty and full) protocols was collated. This included daily online image-guided radiotherapy setup data, treatment duration, bladder size on planning CT, PTV and OARs dose volume histogram (DVH) data and post-treatment follow-up data.
The daily online IGRT setup data were defined as the absolute vertical (VRT), longitudinal (LNG) and lateral (LAT) couch shifts required for each treatment fraction. The population systematic and random setup errors for both groups of patients were calculated according to the on-target report.12 The treatment time for each fraction was calculated as the time difference between the start of first imaging field and the last treatment field. Mann-Whitney U tests were used to explore differences in DVH data, setup data and treatment times between the two bladder preparation protocols.
Patients were followed at 6 weeks, then 6 months until 5 years with GI/GU toxicity scoring using the Common Terminology Criteria for Adverse Events (CTCAE) v.4.0 protocol, and serial PSA readings. The biochemical progression-free survival (bPFS) was defined as the time to biochemical relapse assigned to patients with PSA rise of ≥2 µg ml−1 above the nadir reading.
Patients free of biochemical recurrence were censored at the date of the last PSA reading. The acute and late GU and GI toxicities were evaluated and recorded at the specified follow-up time intervals. Time zero was defined as the start date of EBRT. Acute toxicity was defined as that occurring within 90 days post-RT; all reported toxicity thereafter was classified as late toxicity.
The bPFS rates for the overall population and for the two bladder filling groups were calculated using the Kaplan-Meier method and the resulting survival curves compared using the Mantel-Cox log rank test. For evaluation of toxicity, patients were analysed according to their bladder filling protocols. The grade distributions of GU and GI toxicities were compared at each follow-up time point. The hazard ratios from the cumulative incidences of toxicities between the two bladder filling groups were compared using the log-rank test. For all tests, a p-value of <0.05 was considered statistically significant. Statistical analysis was performed with SPSS v.22.0 (IBM Corp., Armonk, NY).
Results
Between January 2014 and November 2015, 90 patients in the full (41) and empty (49) bladder groups were included in this study with a median follow-up of 48 months (range 36–60 months). The bPFS at 4 years were 100 and 98% for the empty and full bladder filling groups, respectively, (p = 0.27). There was only one biochemical failure in the whole cohort. On re-staging with whole body bone scan, pelvic CT and magnetic resonance imaging (MRI) there was no local recurrence within the prostate gland.
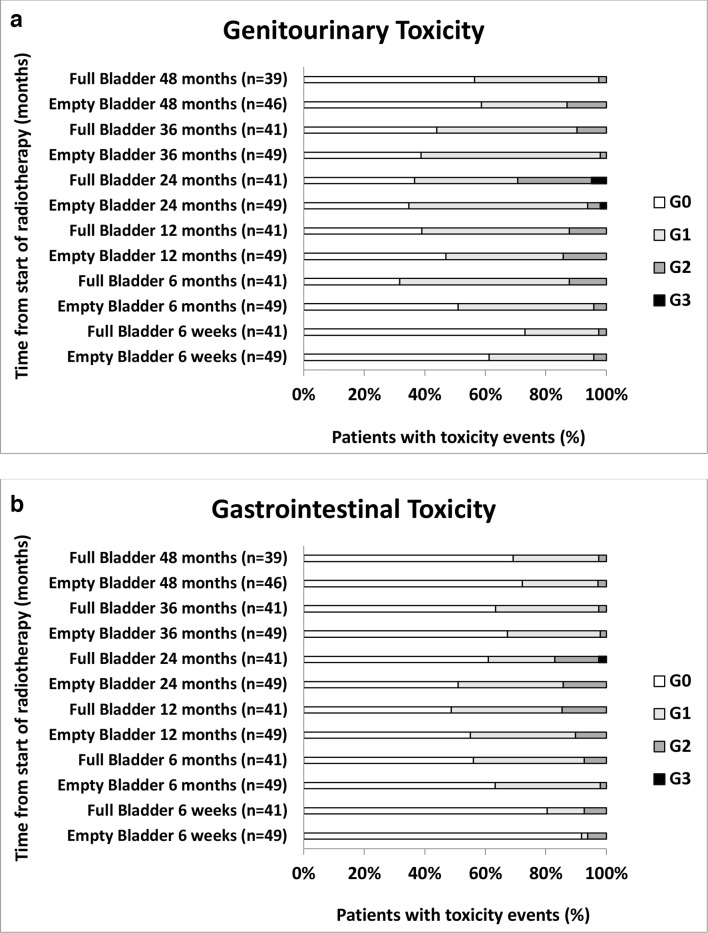
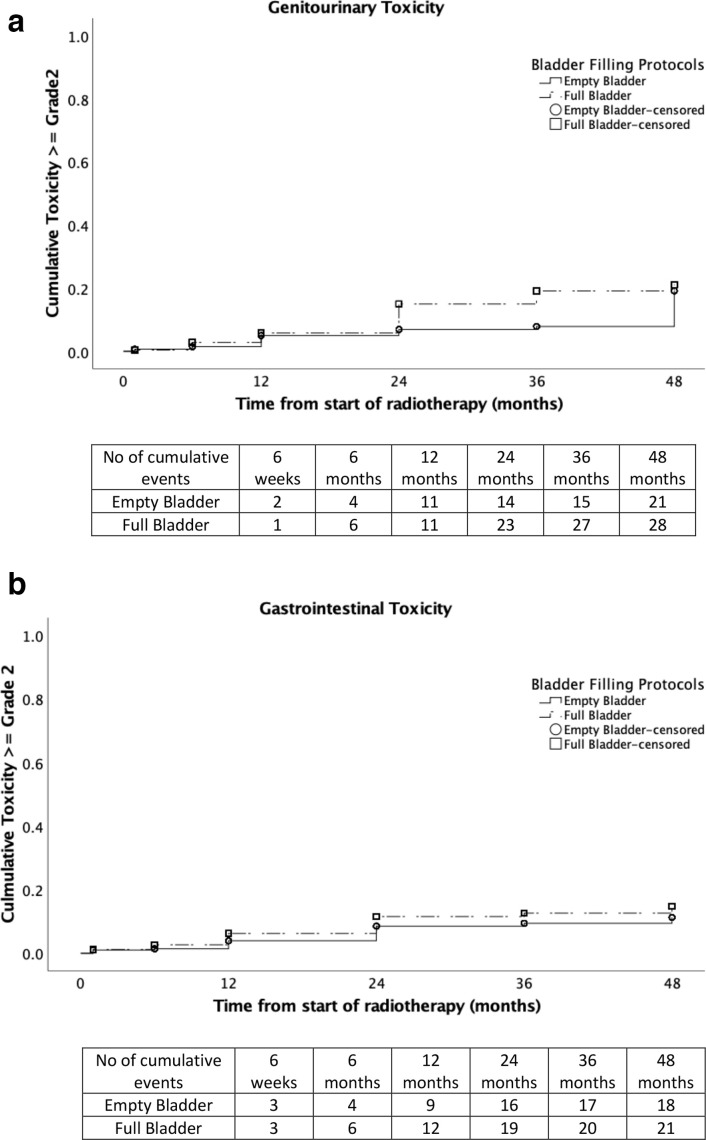
As indicated in Figure 1, post-RT GI and GU toxicities were mild with the prevalence of any ≥Grade 3 toxicity no greater than 4% at any time point. Three cases (one in empty bladder and two in full bladder groups) had Grade 3 GU toxicity with urethral strictures and one case (in full bladder group) had Grade 3 GI toxicity with proctitis. All of these Grade 3 events were reported at 2 years post-RT. There were no statistically significant differences in cumulative ≥Grade 2 GU (p = 0.10) or GI (p = 0.27) toxicity rates between the two bladder filling protocols as shown in Figure 2.
Figure 1.
Grade distributions of genitourinary (figure 1a) and gastrointestinal (figure 1b) toxicity over time measured with CTCAE v.4.0.
Figure 2.
Cumulative rates of ≥ grade 2 genitourinary (figure 2a) and gastrointestinal (figure 2b) toxicity over time.
As shown in Table 1, all plans from both bladder filling protocols achieved all PTV dose objectives. No statistically significant differences were found in PTV and OARs DVH objectives between the two protocols apart from the V42Gy and V50Gy of bladder. The bladder V42Gy dose objective was not met in 24/49 (49.0%) of cases in the empty bladder group and 6 (14.6%) in the full bladder group. For the bladder V50Gy dose objective, this was not met in 36/49 (73.5%) in the empty bladder group and 13/41 (31.7%) in the full bladder group.
Table 2 summarises the treatment record data of the whole patient cohort. Age between the two groups was similar (p = 0.56) as expected, median bladder size was the only statistically significant difference between the two filling protocols (p < 0.05). There were no statistically significant differences in the absolute couch shifts required for the daily IGRT in all directions between the two groups of patients. This finding was in line with the comparable population systematic and random errors of both groups of patients as indicated in Table 3.
Table 2.
The treatment record data including patient’s age, bladder size on planning CT and treatment time per fraction for the two bladder filling protocols
| Empty bladder protocols (n = 49) Median (Range) |
Full bladder protocols (n = 41) Median (Range) |
p value | ||
|---|---|---|---|---|
| Age (years) | 77.6 (60–84) | 76.6 (64–88) | 0.56 | |
| Bladder size on planning CT (cc) | 71.6 (32.8–180.4) | 154.6cc (38.0–581.8) | <0.05 | |
| Treatment time per fraction (minutes) | 3.2 (0.5–69.2) | 3.0 (1.0–73.0) | 0.47 | |
| IGRT corrections | VRT (mm) | 3 (0–17) | 3 (0–15.3) | 0.10 |
| LNG (mm) | 2 (0–20) | 2 (0–12) | 0.69 | |
| LAT (mm) | 2 (0–11) | 2 (0–12) | 0.06 | |
Table 3.
Population systematic and random errors of the two bladder filling protocols
| Empty bladder protocols (n = 49) | Full bladder protocols (n = 41) | ||
|---|---|---|---|
| Population systematic error | VRT | 3.5 mm | 3.5 mm |
| LNG | 2.3 mm | 2.4 mm | |
| LAT | 2.5 mm | 1.8 mm | |
| Population random error | VRT | 0.8 mm | 0.6 mm |
| LNG | 0.6 mm | 0.6 mm | |
| LAT | 1.0 mm | 0.7 mm |
Although the median treatment times per fraction were not statistically different between the two bladder filling groups (p = 0.47), patients in the full bladder filling group would have needed to spend a longer time in the RT department per treatment session they were required to arrive at the RT department 45 min earlier than their defined appointment time for the bladder preparation.
Discussion
Outcomes of radiotherapy are not only measured in tumour control but also in quality of life (QoL) post-treatment. RT-induced GU and GI toxicities are major QoL limiting factors for patients receiving radiotherapy for prostate cancer.13 This study strengthens the findings of two previous publications which showed that there is no difference in bPFS, acute or late post-RT toxicities between treatment using an empty bladder filling protocol or a full bladder filling protocol when treating localised prostate cancer in the low and intermediate favourable risk groups with advanced EBRT techniques.8,9
The bPFS of 98.9% at 4 years is in keeping with the 5 years outcome data of the 60 Gy cohort (90.6% at 5 years) of the Phase 3 conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer (CHHiP) trial.1
In the empty bladder filling group, there was only 1/49 (2%) Grade 3 GU event at 2 years post-RT and no Grade 3 GI toxicity at any time point. Being comparable to the findings of the CHHiP trial, there was a higher frequency of ≥Grade 2 GU and GI toxicity rates reported at 2 years and the 3 years cumulative ≥Grade 2 GU and GI toxicity rates in the empty bladder group was only 10%.1 The 3 years cumulative ≥Grade 2 GU toxicity can be as high as 30% when using 3D conformal prostate EBRT.14,15 The much lower toxicity reported here may be explained by the use of advanced VMAT RT techniques which even with an empty bladder achieves a steep dose fall off to minimise doses to OARs specifically sparing the rectumand bladder while enabling a more conformal delivery of radiation to the prostate.
No statistically significant differences were found in all PTV and OAR DVH dose objectives apart from the V42Gy and V50Gy bladder dose constraints (p < 0.05). Nearly three quarters of the plans in the empty bladder filling group failed to achieve the V50Gy bladder dose constraint. Using an empty bladder filling protocol, the radiation dose to the bladder is expected to be higher when the bladder is not expanded with urine. High dose to the bladder trigone may be more predictive of late RT-induced GU toxicity such as urinary incontinence, frequency and urethral stricture.16 No worse post-RT GU toxicity was observed in the empty bladder filling group. This might be due to the fact that V62Gy bladder dose constraint was kept within the tolerance level in both groups of patients.
Often, prostate cancer patients find it difficult to maintain consistent full bladder volume during RT planning and treatments.17 There are several studies which have investigated the influence of consistent bladder filling between RT planning and treatment on the inter-fraction prostate position.17–19 It is suggested that a more than twofold difference in bladder volume between RT planning and treatment could cause moderate prostate translocations and distortions especially in the anterior/posterior direction and between the prostatic base and the apex.19 This may result in a higher chance of geometrical miss leading to poorer tumour control. The use of daily IGRT in this study will have ensured accurate RT delivery to both bladder filling groups which is supported by the similar population systematic and random errors.
A full or empty bladder is subjective and dependent on an individual’s ability to hold or void. Although the median bladder sizes at planning CT in the full bladder group is significantly larger, there was an overlap in the range of bladder sizes at planning CT between the two bladder filling protocols as indicated in Table 2. Bladder filling is often not dependent solely on how much fluid a patient can take in. This can be mainly related to obstructive urinary symptoms and can also be influenced by renal blood flow which is dependent upon the state of hydration, time of day and concomitant medication.20 Even though patient comfort and duress were not assessed in this study, the benefit for patients of not having to hold a full bladder while enduring acute GU toxicity should not be neglected. It is suggested that there might be a positive correlation between symptoms of overactive bladder or urinary incontinence with anxiety levels.21 Although there was no statistically significant difference in treatment times between the two bladder filling groups, patients using the empty bladder filling protocol do not have the 45 min extra time that those in the full bladder group needed to spend in the department for bladder filling prior to treatment.
It is acknowledged that the obvious limitations of our study are its retrospective nature and the relatively small number of patients included with a relatively short follow-up period. A longer follow-up with a larger sample size is required to validate this empty bladder filling approach to ensure that it does not compromise treatment outcomes in terms of tumour control and post-RT QoL.
Footnotes
Acknowledgment: We thank Miss Catherine Holborn- Senior Lecturer at the Sheffield Hallam University in the UK for her support on this study.
We thank all the patients who participated in this study, and the doctors, nurses, radiographers and physicists at our centre.
We acknowledge the support of Dr Peter Ostler, Dr Robert Hughes, Dr Roberto Alonzi, Dr Nicola Anyamene, Dr Karen Venables, Mr Daniel Megias and Mrs Jagdeep Kudhail at Mount Vernon Cancer Centre.
Funding: Professor Peter Hoskin is supported by the Manchester National Institute of Health Research Biomedical Research Centre.
Contributor Information
Gayan Chetiyawardana, Email: gayan.chetiyawardana@nhs.net.
Peter J. Hoskin, Email: peterhoskin@nhs.net.
Yat Man Tsang, Email: yatmantsang@nhs.net.
REFERENCES
- 1.Dearnaley D, Syndikus I, Mossop H, Khoo V, Birtle A, Bloomfield D, et al. Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: 5-year outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. Lancet Oncol 2016; 17: 1047–60. doi: 10.1016/S1470-2045(16)30102-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wolff RF, Ryder S, Bossi A, Briganti A, Crook J, Henry A, et al. A systematic review of randomised controlled trials of radiotherapy for localised prostate cancer. Eur J Cancer 2015; 51: 2345–67. doi: 10.1016/j.ejca.2015.07.019 [DOI] [PubMed] [Google Scholar]
- 3.Al-Mamgani A, Heemsbergen WD, Peeters STH, Lebesque JV. Role of intensity-modulated radiotherapy in reducing toxicity in dose escalation for localized prostate cancer. Int J Radiat Oncol Biol Phys 2009; 73: 685–91. doi: 10.1016/j.ijrobp.2008.04.063 [DOI] [PubMed] [Google Scholar]
- 4.Matzinger O, Duclos F, van den Bergh A, Carrie C, Villà S, Kitsios P, et al. Acute toxicity of curative radiotherapy for intermediate- and high-risk localised prostate cancer in the EORTC trial 22991. Eur J Cancer 2009; 45: 2825–34. doi: 10.1016/j.ejca.2009.07.009 [DOI] [PubMed] [Google Scholar]
- 5.Nakamura N, Shikama N, Takahashi O, Ito M, Hashimoto M, Uematsu M, et al. Variability in bladder volumes of full bladders in definitive radiotherapy for cases of localized prostate cancer. Strahlenther Onkol 2010; 186: 637–42. doi: 10.1007/s00066-010-2105-6 [DOI] [PubMed] [Google Scholar]
- 6.Olsson CE, Jackson A, Deasy JO, Thor M. A systematic Post-QUANTEC review of tolerance doses for late toxicity after prostate cancer radiation therapy. Int J Radiat Oncol Biol Phys 2018; 102: 1514–32. doi: 10.1016/j.ijrobp.2018.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mullaney LM, O'Shea E, Dunne MT, Finn MA, Thirion PG, Cleary LA, et al. A randomized trial comparing bladder volume consistency during fractionated prostate radiation therapy. Pract Radiat Oncol 2014; 4: e203–12. doi: 10.1016/j.prro.2013.11.006 [DOI] [PubMed] [Google Scholar]
- 8.Morrison S, Ellis T, Fillingham E, Song YP, Birtle A. Less to hold – a comparison of bowel and bladder toxicities in patients undergoing prostate radiotherapy between those treated with an empty bladder and those following a bladder filling protocol. Clin Oncol 2019; 31: e23. doi: 10.1016/j.clon.2018.11.013 [DOI] [Google Scholar]
- 9.Tsang YM, Hoskin P. The impact of bladder preparation protocols on post treatment toxicity in radiotherapy for localised prostate cancer patients. Tech Innov Patient Support Radiat Oncol 2017; 3-4: 37–40. doi: 10.1016/j.tipsro.2017.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.D'Amico AV, Whittington R, Malkowicz SB, Schultz D, Blank K, Broderick GA, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA 1998; 280: 969–74. doi: 10.1001/jama.280.11.969 [DOI] [PubMed] [Google Scholar]
- 11.International Commission on Radiation Units and Measurements Prescribing, recording, and reporting photon beam intensity modulated radiation therapy (IMRT. . . Bethesda (MD: International Commission on Radiation Units and Measurements (ICRU); 2010ICRU Report no. 83. [Google Scholar]
- 12.The Royal College of Radiologists Society and College of Radiographers, Institute of Physics and Engineering in Medicine : On target: ensuring geometric accuracy in radiotherapy. London: The Royal College of Radiologists; 2008. [Google Scholar]
- 13.Jayadevappa R, Chhatre S, Whittington R, Bloom BS, Wein AJ, Malkowicz SB. Health-Related quality of life and satisfaction with care among older men treated for prostate cancer with either radical prostatectomy or external beam radiation therapy. BJU Int 2006; 97: 955–62. doi: 10.1111/j.1464-410X.2006.06128.x [DOI] [PubMed] [Google Scholar]
- 14.Peeters STH, Heemsbergen WD, van Putten WLJ, Slot A, Tabak H, Mens JW, et al. Acute and late complications after radiotherapy for prostate cancer: results of a multicenter randomized trial comparing 68 Gy to 78 Gy. Int J Radiat Oncol Biol Phys 2005; 61: 1019–34. doi: 10.1016/j.ijrobp.2004.07.715 [DOI] [PubMed] [Google Scholar]
- 15.Syndikus I, Morgan RC, Sydes MR, Graham JD, Dearnaley DP, .MRC RT01 collaborators . Late gastrointestinal toxicity after dose-escalated conformal radiotherapy for early prostate cancer: results from the UK medical Research Council RT01 trial (ISRCTN47772397. Int J Radiat Oncol Biol Phys 2010; 77: 773–83. doi: 10.1016/j.ijrobp.2009.05.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schaake W, van der Schaaf A, van Dijk LV, van den Bergh ACM, Langendijk JA. Development of a prediction model for late urinary incontinence, hematuria, pain and voiding frequency among irradiated prostate cancer patients. PLoS One 2018; 13: e0197757. doi: 10.1371/journal.pone.0197757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stam MR, van Lin ENJT, van der Vight LP, Kaanders JHAM, Visser AG. Bladder filling variation during radiation treatment of prostate cancer: can the use of a bladder ultrasound scanner and biofeedback optimize bladder filling? Int J Radiat Oncol Biol Phys 2006; 65: 371–7. doi: 10.1016/j.ijrobp.2005.12.039 [DOI] [PubMed] [Google Scholar]
- 18.Pinkawa M, Asadpour B, Gagel B, Piroth MD, Holy R, Eble MJ. Prostate position variability and dose-volume histograms in radiotherapy for prostate cancer with full and empty bladder. Int J Radiat Oncol Biol Phys 2006; 64: 856–61. doi: 10.1016/j.ijrobp.2005.08.016 [DOI] [PubMed] [Google Scholar]
- 19.Snoj Z, Gill AB, Rundo L, Sushentsev N, Barrett T. Three-Dimensional MRI evaluation of the effect of bladder volume on prostate translocation and distortion. Radiol Oncol 2020; 54: 48–56. doi: 10.2478/raon-2020-0001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hynds S, McGarry CK, Mitchell DM, Early S, Shum L, Stewart DP, et al. Assessing the daily consistency of bladder filling using an ultrasonic Bladderscan device in men receiving radical conformal radiotherapy for prostate cancer. Br J Radiol 2011; 84: 813–8. doi: 10.1259/bjr/50048151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lai HH, Rawal A, Shen B, Vetter J. The relationship between anxiety and overactive bladder or urinary incontinence symptoms in the clinical population. Urology 2016; 98: 50–7. doi: 10.1016/j.urology.2016.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]