Abstract
Objective:
To determine the toxicity reduction required to justify the added costs of MRI-guided radiotherapy (MR-IGRT) over CT-based image guided radiotherapy (CT-IGRT) for the treatment of localized prostate cancer.
Methods:
The costs of delivering prostate cancer radiotherapy with MR-IGRT and CT-IGRT in conventional 39 fractions and stereotactic body radiotherapy (SBRT) 5 fractions schedules were determined using literature values and cost accounting from two institutions. Gastrointestinal and genitourinary toxicity rates associated with CT-IGRT were summarized from 20 studies. Toxicity-related costs and utilities were obtained from literature values and cost databases. Markov modeling was used to determine the savings per patient for every 1% relative reduction in acute and chronic toxicities by MR-IGRT over 15 years. The costs and quality adjusted life years (QALYs) saved with toxicity reduction were juxtaposed with the cost increase of MR-IGRT to determine toxicity reduction thresholds for cost-effectiveness. One way sensitivity analyses were performed. Standard $100,000 and $50,000 per QALY ratios were used.
Results:
The added cost of MR-IGRT was $1,459 per course of SBRT and $10,129 per course of conventionally fractionated radiotherapy. Relative toxicity reductions of 7 and 14% are required for SBRT to be cost-effective using $100,000 and $50,000 per QALY, respectively. Conventional radiotherapy requires relative toxicity reductions of 50 and 94% to be cost-effective.
Conclusion:
From a healthcare perspective, MR-IGRT can reasonably be expected to be cost-effective. Hypofractionated schedules, such a five fraction SBRT, are most likely to be cost-effective as they require only slight reductions in toxicity (7–14%).
Advances in knowledge:
This is the first detailed economic assessment of MR-IGRT, and it suggests that MR-IGRT can be cost-effective for prostate cancer treatment through toxicity reduction alone.
Introduction
MRI-guided radiotherapy (MR-IGRT) systems became available in 2012 in the USA and are available at an increasing number of institutions worldwide.1 MR-IGRT offers enhanced soft tissue contrast over cone beam CT image-guided radiotherapy (CT-IGRT) enabling adaptive radiotherapy and cine imaging during treatment with near real-time target tracking.2,3 These advances promise improved treatment accuracy and smaller treatment margins, which are expected to translate into fewer side-effects and possibly improved cure rates.4,5
Prostate cancer is the most common form of non-cutaneous cancer in males and is a prime target for MR-IGRT.6 The addition of magnetic resonance for daily image-guidance significantly improves visualization of the prostate and the soft tissue bed encasing it. MR-IGRT can identify and correct for differences in prostate position before treatment and prostate motion during treatment caused by gas in the rectum, bladder filling, patient movement, or respirations. Numerous studies have tracked these movements, which can substantially increase the risk of severe and costly toxicities.7 The advances in motion tracking and target delineation of MR-IGRT are anticipated to reduce these toxicities by safely decreasing planning treatment volume (PTV) margins to ≤3 mm, significantly reducing treatment overlap with adjacent normal tissues (e.g. rectum, bladder).8
MR-IGRT is more costly than CT-IGRT, and concerns about overall costs could hinder the technology’s implementation. While some institutions have invested early, some healthcare payers and governments have requested additional cost analyses before investing in MR-IGRT equipment. To date, no randomized clinical trials have demonstrated the technology’s effectiveness, so incremental cost-effective ratios cannot be assessed. However, a threshold analysis can describe the degree of toxicity reduction that would justify the added cost of purchasing and delivering MR-IGRT. This information may help healthcare systems and governments prioritize MR-IGRT investigation and power clinical trials.
Methods
Perspective
A healthcare perspective was used, which shares all costs and benefits between institutions, patients, and payors. This was in accordance with the National Institute for Health and Care Excellence (NICE) guidelines, which recommend that an intervention funded by the public sector with health outcomes should include costs of implementation, downstream costs, and cost/health savings.9 This perspective was intended to maximize the comparability of the results to cost-effectiveness analyses of alternative interventions, such as proton beam therapy. In accordance with NICE guidelines, a separate societal perspective was not performed as the costs outside of a healthcare perspective were minimal.
Radiotherapy definitions
Image guidance was defined by daily imaging with either CT-based or magnetic resonance-based imaging. All patients were assumed to be simulated with a standalone CT simulator, but only patients undergoing MR-IGRT received an MRI simulation as well. Two treatment regimens were modeled, conventional fractionation with 39 daily fractions and stereotactic body radiotherapy (SBRT) with 5 fractions.10 Fiducial markers were assumed to be used with CT-IGRT-based SBRT, but not with conventional fractionation or MR-IGRT.
Toxicity data collection
A literature search for a sample of studies reporting outcomes of daily CT-IGRT published between 2005 and 2019 was conducted using PubMed. The search phrase used was: “toxicity” OR “outcomes” AND “prostate” AND (“IGRT” OR “image guided” OR “image guidance” OR “helical tomography”). Article titles, abstracts, and manuscripts were reviewed for inclusion. Only studies with ≥100 participants were included to allow for adequate observation of toxicity. Cohorts that were reused for different studies were only included once. Only studies that used daily CT-IGRT without fiducial markers were included to avoid overlapping fiducial marker-related toxicity and radiotherapy toxicity. Fiducial marker-related toxicity resulting in hospitalization was included later as part of the added cost analysis.11,12 For the literature review, toxicity rates were assumed to be equal between conventional, moderately hypofractionated, and hypofractionated radiotherapy.13–15 Rectal spacers were not included as they can be used with both CT-IGRT and MR-IGRT. The toxicities collected were acute and chronic gastrointestinal (GI) and genitourinary (GU) Grade 2 and ≥3. Annual toxicity rates were calculated by dividing the average reported toxicity rates by the average number of years of follow-up.
Costs and utilities
Health states represent temporary outcomes of treatment and can be assigned a discrete monetary cost and utility cost measured in quality adjusted life years (QALYs). These values were obtained using previous reports in the literature, cost and utility databases, and 2019 Medicare Physician Fee Schedule. All interventions for toxicities were modified to Common Terminology Criteria for Adverse Events v. 5.0.
Modeling
Markov modeling was performed using MATLAB (v. R2018b, MathWorks, Natick, MA) to simulate annual transitions between health states over 15 years after completing prostate cancer treatment with CT-IGRT. The median age of prostate cancer diagnosis is 66 years old, so 15 yearly cycles were used to simulate life expectancy.16,17 The first cycle used acute side-effect rates and subsequent cycles used chronic side-effect rates. Costs and utilities associated with various health states were discounted 3% and summed over 15 cycles. The base probabilities of acute and chronic toxicities were then reduced by a relative 1% and the Markov model was performed again. The difference in costs and QALYs represented the savings per 1% relative side-effect reduction using MR-IGRT. These outcomes were termed incremental cost savings (ICS) and incremental QALYs gained (IQG).
Transition probabilities
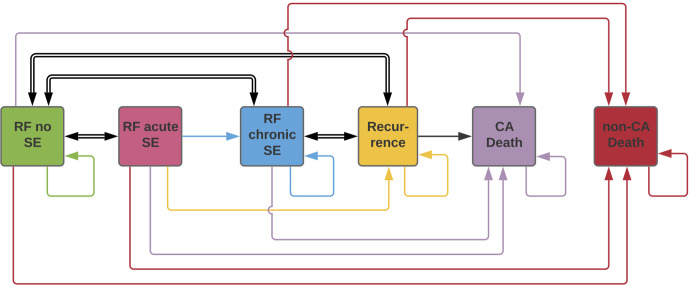
Transition probabilities are the probabilities of moving from one health state to another or remaining in the same health state. A diagram of the transitions used in this analysis is reported in Figure 1. A previously reported hazard ratio of 1.8 was applied to the transition probability between acute and chronic side-effects health states.18 This hazard ratio was assumed to also apply to remaining in the chronic side-effects health state. Cancer outcomes were assumed to be the same for CT-IGRT and MR-IGRT for the purposes of this analysis.
Figure 1.
https://www.lucidchart.com/documents/edit/7d360b60-6bb2-4b23-98be-406dc2fdb9f6/0?callback=close&name=docs&callback_type=back&v=1924&s=612A simplified diagram of the health state transitions used in the Markov model. The specific acute and chronic toxicities (GI Grade 2, GI Grade ≥3, GU Grade 2, and GU Grade ≥3) are not shown, but were included in the model as part of the relapse free acute and chronic side-effects states. The acute side-effects health state was used only for the first cycle and the late side-effects health state was used for cycles 2–15. CA, cancer; GU, genitourinary ; RF, relapse free; SE, side-effects
Added cost of MR-IGRT
The costs of MR-IGRT and CT-IGRT are dependent on the number of unique patients that can be treated with each modality over 15 years. These data were obtained by conducting interviews with staff at the University of Miami and H. Lee Moffitt Cancer Center Radiation Oncology Departments regarding treatment and simulation durations and system operating time. CT-IGRT was assumed to be accompanied by a standalone CT simulator that enabled continuous treatments and concurrent simulations. The MR-IGRT system is used for simulations and treatment, so its daily operating time was divided between both functions.
The costs of purchasing and maintaining CT-IGRT and MR-IGRT units were obtained by reviewing the literature. CT-IGRT costs were assessed for a CT-based image guidance unit with a linear accelerator. MR-IGRT costs were assessed for a MR-based image guidance unit with a linear accelerator. Ranges were obtained from the literature and verified using costs incurred and projected at two institutions. The University of Miami uses the ViewRay (Oakwood Village, OH) MRIdian system (initially Cobalt-60 based system under upgrade to linear accelerator system) and H. Lee Moffitt Cancer Center uses the ViewRay MRIdian with a linear accelerator. The cost of items specific to MR-IGRT not covered under non-disclosure agreements were reported and averaged between the two institutions. The cost of items specific to CT-IGRT were related to fiducial marker implantation and were obtained from the 2019 Medicare Physician Fee Schedule, Pharmaceutical Federal Supply Schedule, and Healthcare Cost and Utilization Project database.
Time-driven activity-based costing was used to determine the cost of all steps of patient care including: consultation, simulation, planning, treatment, on-treatment visits, and follow-up visits (over 15 years). For each step, personnel time and costs were determined using literature values, interviews with staff, and records of staff salaries.
All costs were added together and divided by the estimated total number of unique patients treated over 15 years with each modality. The difference between CT-IGRT and MR-IGRT was the added cost of treatment with MR-IGRT.
Side-effect reduction
The ICS and IQG obtained from Markov modeling were used with the added cost of treatment per patient to determine side-effect reduction thresholds for cost-effectiveness. This was calculated using standard $50,000/QALY and $100,000/QALY metrics in Equation 1.19 This equation was derived using standard equations for cost-effectiveness modeling (Supplementary Material 1)20:
ACWP, added cost willing to pay ($ per QALY, analyzed at $50,000/QALY and $100,000/QALY thresholds); ICS, incremental cost savings ($ per 1% side-effect reduction); IQG, incremental QALYs gained (QALYs per 1% side-effect reduction); %SER, percent relative side-effect reduction (%)
Sensitivity analysis
One-way sensitivity analyses were performed on conventional therapy and SBRT using $50,000/QALY and $100,000/QALY. The ranges for all costs were based on literature values or ±25% of the base estimate, except utilities which were ±0.10 of the base estimate. A Monte-Carlo simulation was not used to generate cost-effectiveness acceptability curves as the model outcomes were percent side-effect reductions (Equation 1) rather than incremental cost-effective ratios.
Results
Unique patients
The number of unique patients treated using conventional fractionation was calculated based on 20 min treatments for CT-IGRT and 30 min treatments and 60 min MRI simulations for MR-IGRT. Both radiotherapy units were assumed to be operational for 8 h/day, 5 days/week, for 15 years with 10 operational days/year subtracted for holidays and maintenance. For conventional fractionation (39 fractions), CT-IGRT could perform 24 treatments per day. MR-IGRT was required to divide operational time between treatments (15.2/day) and simulations (0.4/day). If both machines operated at maximum capacity for 15 years, a total of 2308 unique patients could be treated with CT-IGRT and 1463 with MR-IGRT. For five fraction SBRT, CT-IGRT maintained 24 treatments per day and MR-IGRT decreased to 11.4 treatments per day to accommodate more simulations (2.3/day) for the higher number of unique patients. At maximum capacity over 15 years, 18,000 unique patients could be treated with CT-IGRT and 8571 with MR-IGRT.
Added cost of MR-IGRT
The total cost of each modality was divided by the number of unique patients to determine the cost per patient (Table 1). For conventional fractionation, this was $8707 with CT-IGRT and $18,836 with MR-IGRT. For SBRT, this was $5357 with CT-IGRT and $6816 with MR-IGRT. The net added cost of MR-IGRT was $10,129 per patient for conventional fractionation and $1459 per patient for SBRT.
Table 1.
Added cost of MRI-IGRT
| Conventional radiotherapy (39 fractions) | SBRT (5 Fractions) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CT-IGRT | MR-IGRT | CT-IGRT | MR-IGRT | ||||||||
| Category | Item | Subgroup | Cost (Range) | Quantity | 15 year cost | Quantity | 15 year cost | Quantity | 15 year Cost | Quantity | 15 year Cost |
| Installation costs | Unit | CT-IGRT: $2,965,000 ($2,326,000–3,604,000)21,22,23 MR-IGRT: $9M ($6–12M)23 |
x1 | $2,965,000 | $9,000,000 ($6–12M)x 1 | $9,000,000 | $2,965,000 x 1 | $2,965,000 | $9,000,000 x 1 | $9,000,000 | |
| Renovation | CT-IGRT: N/A MR-IGRT: $2M ($1 M-3M)24 |
N/A | N/A | x 1 | $2,000,000 | N/A | N/A | x1 | $2,000,000 | ||
| Maintenance costs | Annual service contract (first 2 years included) | CT-IGRT: 10% list price21 MR-IGRT: $550K ($300–800K)23,24 |
x13yr | $3,854,500 | x13yr | $7,150,000 | x13 yr | $3,854,500 | x13yr | $7,150,000 | |
| Personnel time/Costs | Consultation | Physician | $233/hr ($320 K-577K/yr)a | 1 hr x Pts (2,308) | $537,764 | 1 hr x Pts (1,463) | $340,879 | 1 hr x Pts (18,000) | $4,194,000 | 1 hr x Pts (8,571) | $1,997,043 |
| Nurse | $42/hr ($57 K-102K/yr)a | 1 hr x Pts (2,308) | $96,936 | 1 hr x Pts (1,463) | $61,446 | 1 hr x Pts (18,000) | $756,000 | 1 hr x Pts (8,571) | $359,982 | ||
| Receptionist | $15/hr ($22 K-37K/yr)a | 1 hr x Pts (2,308) | $34,620 | 1 hr x Pts (1,463) | $21,945 | 1 hr x Pts (18,000) | $270,000 | 1 hr x Pts (8,571) | $128,565 | ||
| Simulation | Therapist | $47/hr ($55 K-126K/yr)a | 0.5 hr x Pts (2,308) | $54,238 | 1.5 hr x Pts (1,463) | $103,142 | 0.5 hr x Pts (18,000) | $423,000 | 1.5 hr x Pts (8,571) | $604,256 | |
| Receptionist | $15/hr ($22 K-37K/yr)a | 0.5 hr x Pts (2,308) | $17,310 | 1.5 hr x Pts (1,463) | $32,918 | 0.5 hr x Pts (18,000) | $135,000 | 1.5 hr x Pts (8,571) | $192,848 | ||
| Planning | Physician | $233/hr ($320 K-577K/yr)a | 1 hr x Pts (2,308) | $537,764 | 1 hr x Pts (1,463) | $340,879 | 1 hr x Pts (18,000) | $4,194,000 | 1 hr x Pts (8,571) | $1,997,043 | |
| Dosimetrist | $61/hr ($106 K-126K/yr)a | 4 hr x Pts (2,308) | $563,152 | 4 hr x Pts (1,463) | $356,972 | 4 hr x Pts (18,000) | $4,392,000 | 4 hr x Pts (8,571) | $2,091,324 | ||
| Physicist | $96/hr ($160–210 K/yr)a | 1 hr x Pts (2,308) | $221,568 | 1 hr x Pts (1,463) | $140,448 | 1 hr x Pts (18,000) | $1,728,000 | 1 hr x Pts (8,571) | $822,816 | ||
| Treatment | Therapist | $47/hr ($55 K-126K/yr)a | 0.33 hr x 39 x Pts (2,308) | $1,395,900 | 0.5 hr x 39 x Pts (1,463) | $1,341,075 | 0.33 hr x 5 x Pts (18,000) | $1,395,900 | 0.5 hr x 5 x Pts (8,571) | $1,007,140 | |
| Receptionist | $15/hr ($22 K-37K/yr)a | 0.33 hr x 39 x Pts (2,308) | $445,500 | 0.5 hr x 39 x Pts (1,463) | $428,003 | 0.33 hr x 5 x Pts (18,000) | $445,500 | 0.5 hr x 5 x Pts (8,571) | $321,428 | ||
| On-treatment visit | Physician | $233/hr ($320 K-577K/yr)a | 0.25 hr x 8 x Pts (2,308) | $1,075,528 | 0.25 hr x 8 x Pts (1,463) | $681,758 | 0.25 hr x 1 x Pts (18,000) | $1,048,500 | 0.25 hr x 1 x Pts (8,571) | $499,261 | |
| Nurse | $42/hr ($57 K-102K/yr)a | 0.25 hr x 8 x Pts (2,308) | $193,872 | 0.25 hr x 8 x Pts (1,463) | $122,892 | 0.25 hr x 1 x Pts (18,000) | $189,000 | 0.25 hr x 1 x Pts (8,571) | $89,996 | ||
| Receptionist | $15/hr ($22 K-37K/yr)a | 0.25 hr x 8 x Pts (2,308) | $69,240 | 0.25 hr x 8 x Pts (1,463) | $43,890 | 0.25 hr x 1 x Pts (18,000) | $67,500 | 0.25 hr x 1 x Pts (8,571) | $32,141 | ||
| Follow-up visit | Physician | $233/hr ($320 K-577K/yr)a | 0.5 hr x 24 x Pts (2,308) | $6,453,168 | 0.5 hr x 24 x Pts (1,463) | $4,090,548 | 0.5 hr x 24 x Pts (18,000) | $50,328,000 | 0.5 hr x 24 x Pts (8,571) | $23,964,516 | |
| Nurse | $42/hr ($57 K-102K/yr)a | 0.5 hr x 24 x Pts (2,308) | $1,163,232 | 0.5 hr x 24 x Pts (1,463) | $737,352 | 0.5 hr x 24 x Pts (18,000) | $9,072,000 | 0.5 hr x 24 x Pts (8,571) | $4,319,784 | ||
| Receptionist | $15/hr ($22 K-37K/yr)a | 0.5 hr x 24 x Pts (2,308) | $415,440 | 0.5 hr x 24 x Pts (1,463) | $263,340 | 0.5 hr x 24 x Pts (18,000) | $3,240,000 | 0.5 hr x 24 x Pts (8,571) | $1,542,780 | ||
| CT-specific costs | Fiducial marker placement (CPT 55876) | $145.96 | _ | _ | _ | _ | 1 x Pts (18,000) | $2,627,280 | _ | _ | |
| Transrectal ultrasound guidance (CPT 76872) | $130.46 | _ | _ | _ | _ | 1 x Pts (18,000) | $2,348,280 | _ | _ | ||
| Prophylactic Ciprofloxacin 500 mg | $0.10 | _ | _ | _ | _ | 1 x Pts (18,000) | $1,800 | _ | _ | ||
| Hospitalization related to fiducial marker | _ | _ | _ | _ | 0.015 x Pts (18,000) | $2,755,080 | _ | _ | |||
| MRI-specific costs | Ancillary MR-equipment (O2 cart, etc) | $12,725 ($4,450–21,000) | _ | _ | x1 | $12,725 | _ | _ | x1 | $12,725 | |
| MR-Immobilization equipment | $26,000 ($0–52,000) | _ | _ | x1 | $26,000 | _ | _ | x1 | $26,000 | ||
| MR-physics equipment | $260,715 ($234,430–287,000) | _ | _ | x1 | $260,715 | _ | _ | x1 | $260,715 | ||
| Total | $20,094,732 | $27,556,925 | $96,430,340 | $58,420,361 | |||||||
| Cost per patient | 2,308 pts | $8,707 | 1,463 pts | $18,836 | 18,000 pts | $5,357 | 8,571 pts | $6,816 | |||
| Added cost of MR-IGRT | $10,129 | $1,459 | |||||||||
CT-IGRT, CT-guided radiotherapy; Hr, hour; K, thousand; M, million; MR, magnetic resonance;MRI-IGRT, MRI-guided radiotherapy; N/A, not applicable; Pts, patients;SBRT, stereotactic body radiotherapy; Txs, treatments; yr, year.
Hourly wages were calculated based on 40 h work weeks and 48 work weeks in a year.
Literature search
The literature search resulted in 554 articles. Of these, 20 articles met the inclusion criteria. Table 2 synthesizes the study designs, oncologic outcomes, and toxicities.
Table 2.
Literature search CT-IGRT outcomes
| Source | Type of study | Treatment | PTV (mm) | Pts | Follow-up (y) | Biochemical relapse | Cancer death | GI A 2 | GI A 3+ | GU A 2 | GU A 3+ | GI C 2 | GI C 3+ | GU C 2 | GU C 3+ |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Raziee25 | Retro | CBCT: IMRT or VMAT | 7–10 | 355 | 4.9 ci | 9% at 5y | 0% at 8y | 7.3 | 0 | NR | NR | 3.1 | NR | 1.4 | |
| Ingrosso26 | Retro | CBCT: CRT (linac or micro MLC) | 5–6 | 294 | 5.24 ci | 13% at 5y | 4% at 5y | 11.5 | 0.6 | 31.9 | 2 | 2.7 | 2.3 | 7.5 | 3.4 |
| Shimizu27 | Pro | MVCT: IMRT (HT) | 5 | 138 | 5 ci | 9.2% at 5y | 1.5% at 5y | NR | NR | NR | NR | 6.3 | 3.1 | 7.9 | 0 |
| Di Muzio28 | Pro | MVCT: IMRT (HT) | 8–10 | 211 | 5-act | 6.3% at 5y | 2.5% at 5y | 12.9 | 0.5 | 29 | 1.9 | 10.7 | 6.3 | 14.3 | 5.9 |
| Ingrosso29 | Pro | CBCT: IMRT | 6–8 | 118 | 4.51 ci | 13% at 4y | NR | 2.5 | 0 | 6.7 | 2.5 | 3.4 | 0 | 0.8 | 3.4 |
| Ishii30 | Pro | CBCT: VMAT | 5–8 | 224 | N/A | NR | NR | 10.7 | 0 | 11.6 | 0 | NR | NR | NR | NR |
| Nishimura31 | NR | MVCT: IMRT | 5 | 117 | 2.67 ci | NR | NR | NR | NR | NR | NR | 4.3 | 3.4 | 6.8 | 0 |
| Guckenberger32 | Pro | CBCT: IMRT | 5–10 | 150 | 4.17 ci | 18% at 5y | NR | 8.7 | 0 | 36.7 | 4 | 2.1 | 1.4 | 25.9 | 8 |
| Marina33 | Retro | CT (helical): IMRT or CRT | NR | 734 | N/A | 8% at 5y | 1% at 5y | NR | NR | NR | NR | NR | NR | NR | NR |
| Guckenberger34 | Pro | CBCT: IMRT | 10 | 100 | 2-i | NR | NR | 8 | 0 | 34 | 1 | 1.5 | 1.5 | 7.7 | 0 |
| Detti35 | Retro | CBCT: IMRT | 6–8 | 394 | 6.7y-ci | NR | NR | 25.6 | 0 | 51.8 | 0.5 | 3.3 | 0 | 3.1 | 0.2 |
| Jereczek-Fossa36 | Retro | CBCT: IMRT | 3–7 | 228 | 4 ci | 6% at 3y | NR | 8.3 | 0.4 | 21.5 | 3.9 | 2.1 | 0.8 | 4.8 | 0 |
| Tomita37 | Retro | MVCT: IMRT (HT) | 4–6 | 208 | 8.5 ci | 18% at 10y | 5% at 10y | NR | NR | NR | NR | 9.6 | 1.9 | 11.5 | 1.4 |
| Yamazaki38 | Retro | MVCT: IMRT (HT) | 3–5 | 270 | 6.17 ci | 10.8% at 5y | 0.2% at 5y | NR | NR | NR | NR | 4.1 | 1.9 | 4.1 | 0.4 |
| Marvaso39 | NR | CBCT: IMRT | 3–5 | 194 | 2.5 ci | 13% at 3y | NR | 2.6 | 0 | 9.8 | 1 | 0.5 | 0.5 | 3.1 | 0.5 |
| Sasaki40 | Retro | MVCT: IMRT (HT) | 3–5 | 274 | 5.17 ci | CNBC | NR | NR | NR | NR | NR | 4 | 1.8 | 4 | 0.4 |
| Arunsingh41 | Retro | CBCT/MVCT: IMRT (HT) | 5–7 | 101 | 2 ci | NR | NR | 20.8 | 0 | 6.9 | 0 | 14.9 | 5.9 | 10.9 | 3 |
| Krupa42 | NR | CBCT: VMAT | 8–10 | 158 | N/A | NR | NR | 1.3 | 0 | 13.9 | 1.9 | NR | NR | NR | NR |
| Girelli43 | Pro | CBCT: IMRT | 5–8 | 104 | 2.17 ci | 3.5% at 3y | 1.5% at 3y | 2.9 | 1 | 2.9 | 1.9 | 7.7 | 1.9 | 4.8 | 0 |
| Cheng44 | Retro | MVCT: IMRT (HT) | 3–6 | 146 | N/A | NR | NR | 32.9 | 0 | 36.9 | 0 | NR | NR | NR | NR |
| Average (%) | 0.0229a | 0.0036a | 11.33 | 0.19 | 22.58 | 1.58 | 5.15 | 2.24 | 7.81 | 1.75 |
A, acute; Act, actuarial; C, chronic; CBCT, cone-beam computed tomography; CNBC, could not be calculated; CRT, conformal radiation therapy;CT-IGRT, CT-guided radiotherapy; GI, gastrointestinal; GU, genitourinary ; HT, helical tomography; I, incidence; IMRT, intensity-modulated radiotherapy; MLC, multileaf collimator; MVCT, megavoltage computed tomography; N/A, not applicable; NR, not reported; PTV, planning treatment volume; Pro, prospective; Pts, patients; Retro, retrospective; SIB, simulVMAT, volumetric-modulated arc therapy; ci, cumulative incidence; y, year.
Incidence per year was calculated by averaging all the values in the column after applying the formula: (1-((1-(%/100))^(1/years).45
Modeling 1% side-effect reduction
The base probabilities, monetary costs, and utilities of each health state used in the Markov model are reported in Table 3. Detailed cost and utility breakdowns of each health state are reported in Supplementary Material 1. Supplementary Material 1 contains all transition probabilities between the comparison groups and the Markov model script used in MatLab. A graph of compounded incidence of each health state over 15 annual cycles is provided in Supplementary Material 1 to demonstrate the model’s replication of reported outcomes of CT-IGRT. For every 1% relative reduction in toxicity, the ICS was $13.76 and IQG was 0.0019.
Table 3.
Health states probability, cost, and utility
| Base probabilitya | Cost ($)b | Utilityb | |
|---|---|---|---|
| Acute GU (first cycle) | |||
| Acute GI Grade 2 toxicity | 0.1133 | 730.0746–48 | 0.786749 |
| Acute GI Grade ≥3 toxicity | 0.0019 | 11,147.7946–48,50 | 0.50351,52 |
| Acute GU Grade 2 toxicity | 0.2258 | 452.7947 | 0.798349 |
| Acute GU Grade ≥3 toxicity | 0.0158 | 8,109.5047,53 | 0.4354,55 |
| Chronic SE (cycles 2+) | |||
| Chronic GI Grade 2 toxicity | 0.0117 | 730.0746–48 | 0.786749 |
| Chronic GI Grade ≥3 toxicity | 0.0051 | 11,147.7946–48,50 | 0.50351,52 |
| Chronic GU Grade 2 toxicity | 0.0178 | 452.7947 | 0.798349 |
| Chronic GU Grade ≥3 toxicity | 0.0040 | 8,109.5047,53 | 0.4354,55 |
| Recurrence (Biochemical) | 0.0229c | 14,011.7556,57 | 0.4958 |
| Non-cancer death | 0.0397d | 0 | 0.00 |
| Cancer death | 0.0036e | 0 | 0.00 |
GI, gastrointestinal;GU, genitourinary.
Base probabilities were obtained from averages in Table 2.
Full cost and utility breakdown is available in Appendix B.
A probability of 0.18 was used for recurrence to remain in the recurrence health state.59
Based on a 66 year old male with a 17.2 year life expectancy.18
A probability of 0.03 was used for recurrence to transition to the cancer death health state.60
Side-effect reduction
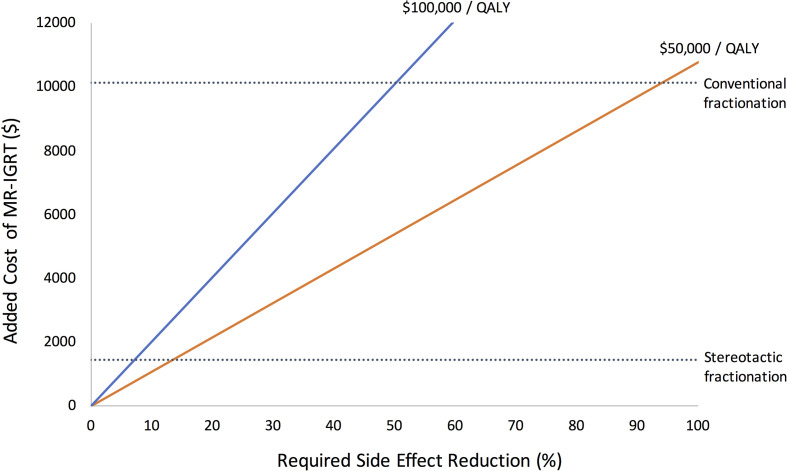
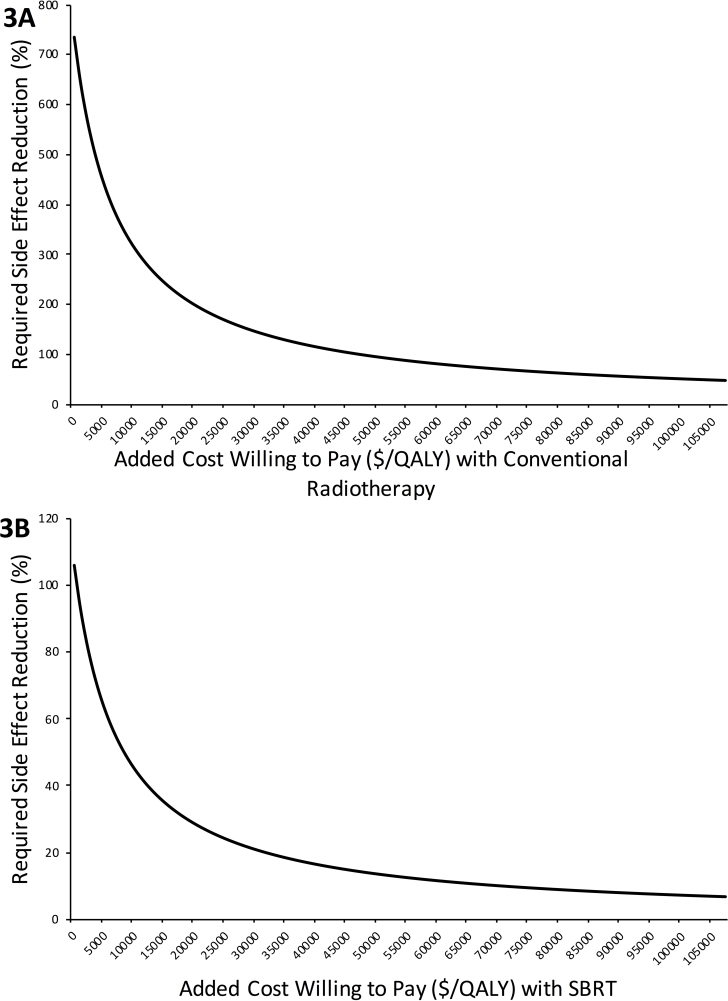
The ICS and IQG per 1% relative toxicity reduction were juxtaposed with the added cost of treatment using Equation 1. The side-effect reduction thresholds for cost-effectiveness of conventional fractionation were 94 and 50% using standard ratios of $50,000/QALY and $100,000/QALY, respectively. For SBRT, side-effect reduction thresholds were 14 and 7%, respectively. These thresholds are presented in Figure 2. The side-effect reductions required to justify MR-IGRT’s added costs based on a range of added costs willing to pay per QALY are presented in Figure 3A (conventional fractionation) and 3B (SBRT).
Figure 2.
Side-effect reduction thresholds for a range of added costs of MR-IGRT The two solid cost/QALY lines demonstrate the direct relationships between added cost of MR-IGRT and side-effect reduction needed to be cost-effective. As added costs of MR-IGRT increases, more side-effect reduction is needed to justify its use. The dotted lines represent the added costs of the two fractionation schedules determined by the cost assessment. SBRT’s added cost is $1,459 per patient and conventionally fractionated radiotherapy added cost is $10,129 per patient. The points where the dotted lines intersect the solid lines represent the minimum side-effect reductions MR-IGRT need to achieve to be cost-effective. For SBRT, the thresholds are 14% using $50,000/QALY and 7% using $100,000/QALY. For conventional fractionation, the thresholds are 94% using $50,000/QALY and 50% using $100,000/QALY. MR-IGRT, MRI-guided radiotherapy; QALY, quality adjusted life year; SBRT, stereotactic body radiotherapy.
Figure 3.
Figures 3A and 3B demonstrate the side-effect reductions that are required for a range of added costs that healthcare systems may be willing to pay per QALY for MR-IGRT. These were generated with Equation 1 using the values for “incremental cost savings per 1% toxicity reduction” and “incremental QALYs gained per 1% toxicity reduction” obtained from Markov modeling and cost values obtained from the added cost assessment. Figure 3A is for conventionally fractionated MR-IGRT and Figure 3B is for SBRT. MR-IGRT, MRI-guided radiotherapy; QALY, quality adjusted life year; SBRT, stereotactic body radiotherapy.
Sensitivity
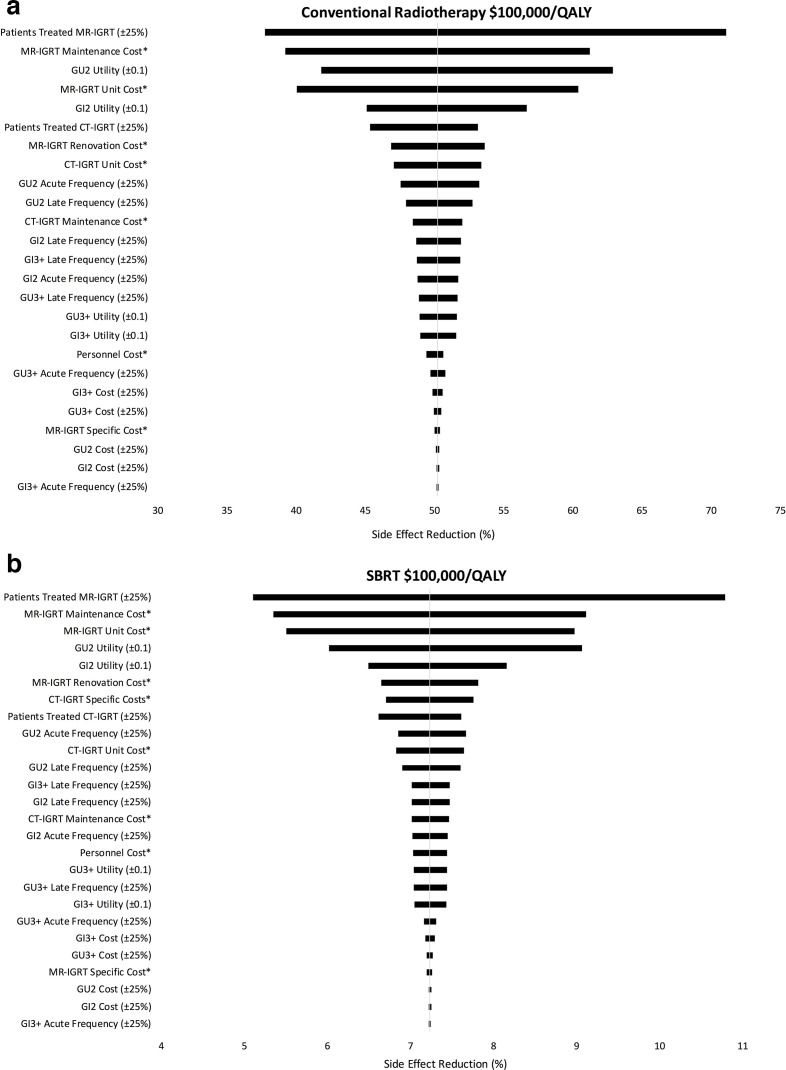
One-way sensitivity analyses were performed for each fractionation regimen and cost/QALY threshold. Figure 4 reports these findings in a tornado diagram for conventional radiotherapy (4A) and SBRT (4B) using $100,000/QALY. The factors with the highest impact on side-effect reduction were: number of unique patients treated with MR-IGRT and CT-IGRT, costs of MR-IGRT units and their maintenance, and GU and GI Grade 2 utility. Supplementary Material 1 contains additional sensitivity diagrams.
Figure 4.
A: One way sensitivity of conventional radiotherapy using $100,000/QALY. 4B: One way sensitivity of SBRT using $100,000/QALY. Table 1The tornado diagrams demonstrate the impact each variable’s range has on the overall outcome of side-effect reduction threshold for cost-effectiveness. Variables at the top have the largest impact on the results while variables at the bottom have the smallest impact. * Range is reported in Table 1. CT-IGRT, CT-guided radiotherapy; GI, gastrointestinal; GU, genitourinary; MR-IGRT, MRI-guided radiotherapy; QALY, quality adjusted life year
Discussion
This study describes the relative side-effect reductions that MR-IGRT needs to achieve to be considered a cost-effective alternative to CT-IGRT for treatment of localized prostate cancer. Our findings demonstrate that even a slight reduction in overall toxicity of 7% with stereotactic body MR-IGRT can render the technology cost-effective using a standard cost/QALY metric of $100,000/QALY. A conventionally fractionated schedule requires greater toxicity reduction of 50% using $100,000/QALY, but cost-effectiveness remains possible.
The results of this study are put into perspective when compared to proton beam therapy (PBT). A study comparing the costs of PBT to conventional therapy with intensity modulated radiotherapy (IMRT) found the added cost of PBT to be $36,445 per patient.61 By using Equation 1 to juxtapose the added cost of PBT with ICS and IQG for every 1% side-effect reduction, PBT is calculated to require 179% relative side-effect reduction to be cost-effective using $100,000/QALY. Because relative side-effect reduction cannot exceed 100%, this indicates PBT cannot be cost-effective through toxicity reduction alone. In contrast, MR-IGRT with conventional radiotherapy can be cost-effective through toxicity reduction alone using both $100,000/QALY and $50,000/QALY (50 and 94% side-effect reduction, respectively). Further, prostate SBRT is not commonly proposed for PBT, but SBRT is commonly proposed for MR-IGRT.4,8 The low number of fractions with SBRT and relatively low cost of MR-IGRT compared to PBT reduces the necessary clinical improvement for cost-effectiveness to less than 1/25th of that needed for PBT (7% vs 179%). These findings put MR-IGRT’s likelihood of achieving cost-effectiveness into perspective. Even small degrees of clinical benefit with MR-IGRT can make it cost-effective for prostate radiotherapy.
One key finding is that SBRT has considerably lower side-effect reduction thresholds for cost-effectiveness than conventional schedules. This can be explained by the higher volume of patients treated over 15 years when only five fractions are used per patient. It allows the static costs of purchasing, installing, and maintaining the machines to be distributed over nearly eight times as many patients, assuming both CT-IGRT and MR-IGRT operate at maximum capacity over 15 years.
Maximum treatment capacity has the largest impact on the overall outcome of the model, as seen in the sensitivity analyses. An institution that cannot maintain CT-IGRT at maximum treatment capacity of 24 treatments per day would likely have a lower threshold for cost-effectiveness (i.e. easier for MR-IGRT to achieve cost-effectiveness) as the cost per patient of CT-IGRT increases. In contrast, an institution with MR-IGRT treatment and simulation times longer than 30 and 60 min would likely have increased added costs of MR-IGRT per patient making cost-effectiveness more difficult to achieve (Figure 2).
Other large influences on the model stem from high cost items, such as MR-IGRT units and their maintenance. The mean of large publicly available ranges, verified with costs from two institutions, were used in this model. The estimates are supported by a previous economic analysis by Parikh on CT-IGRT and MR-IGRT for stereotactic liver radiotherapy.62 Although the published abstract did not report individual costs of services and items, the overall added cost assessment ($1,107) is similar to our two institution study ($1,459). This suggests that any potentially large cost differences in individual services or items between studies were balanced by the overall estimates of the remaining costs of radiotherapy.
The toxicity rates of CT-IGRT without fiducial markers reported in this study are similar to rates reported in a meta-analysis of 38 SBRT studies with fiducial markers.63 All late toxicities were within 2% except GU Grade 2 toxicity, which was 7.8% in our study and 12.1% in the meta-analysis. Our study assumed radiotherapy toxicity rates were equal between the regimens. However, if the incidence of late GU Grade 2 toxicity is higher with SBRT with fiducial markers, then the side-effect reduction threshold for cost-effectiveness of MR-IGRT would likely be lower than 7% using $100,000/QALY. This is because every 1% relative toxicity reduction results in a larger decrease in absolute toxicity (i.e. more costs and QALYs saved) when a higher incidence of toxicity exists. As a result, less relative toxicity reduction is needed for cost-effectiveness.
The healthcare perspective of the model views the benefits of reduced toxicities, borne primarily by the payor and patient, as compensation for the added costs of MR-IGRT, borne primarily by the institution delivering radiotherapy. A narrower perspective, such as a patient or institution perspective, is likely to see an uneven distribution of costs and benefits within the healthcare system that could result in vastly different thresholds for cost-effectiveness. A broader societal perspective is unlikely to change the results significantly as most prostate radiotherapy patients are retired (median age 66), and therefore are not contributing to the workforce.17 The few societal factors that could be included, such as transportation related to side-effect treatment, were unlikely to substantially change overall outcomes and warrant a separate analysis. This is supported by the sensitivity analysis demonstrating that even a 25% increase in side-effect costs had minimal influence on thresholds for cost-effectiveness (<1% for each toxicity, Figure 3). Results can therefore be considered similar to a societal perspective.
The findings in this study on prostate cancer may apply to treatment of cancer at other sites. MR-IGRT’s enhanced soft tissue contrast and capability to pause, or even adjust, the beam when targets move beyond treatment margins has been speculated to enable PTVs to be safely reduced to ≤3 mm.8,64,65 In head/neck cancer, a dosimetric study predicted these advances will reduce incidences of dysphagia ≥Grade 2 by 11%, feeding tube dependence by 4%, xerostomia by 1%, and hypothyroidism by 5%.66 Similar benefits may also be realized in advanced pancreatic cancer where high doses of IMRT have been shown to improve overall survival, but can cause high toxicity to radiosensitive organs surrounding the pancreas.5,67 The smaller treatment margins with MR-IGRT may increase the total tolerable radiation dose delivered to improve survival. Given the findings in our prostate cancer study demonstrating that slight reductions in toxicity can be cost-effective, the potential for toxicity reductions and possibly even improved oncologic outcomes at other sites would expand the cost-effective feasibility of MR-IGRT.
One primary limitation of this study is the lack of a universal “standard therapy” definition. Cone beam CT with or without fiducials, X-ray methods with fiducials, no image guidance with lower radiotherapy doses, and ranges of fractionation are used in practice. Any of these techniques could be considered standard therapy so the CT-IGRT standard used in this study may not apply to all institutions. A randomized clinical trial comparing MR-IGRT to CT-IGRT would better control for variations in treatment and a host of other assumptions required to produce this model. The assumptions described in this study will need to be updated as more data regarding prostate cancer treatment become available.
Conclusion
MRI-IGRT can easily be cost-effective for stereotactic prostate cancer treatment as only a slight reduction in overall side-effects is required (7% using $100,000/QALY). Conventional fractionation would require a greater side-effect reduction (50% using $100,000/QALY), but cost-effectiveness remains possible. Thresholds for both of these regimens are low compared to a previous report on PBT’s added cost, which would require >100% toxicity reduction to be cost-effective. The costs and equations reported in this study can be used to tailor threshold estimates to individual institutions and to update cost-effective estimates as future clinical trials report outcomes of prostate cancer radiotherapy with MR-IGRT.
Footnotes
Acknowledgements: The authors would like to thank Richard Fitterer, Executive Director, Radiation Oncology Services at the University of Miami and Stuart Wasserman, Division Administrator Radiation Oncology and Director Clinical Physics at the H. Lee Moffitt Cancer Center for their assistance with financial and workflow data used within these models.
Conflict of Interest : Dr. Mellon received travel funding from ViewRay in 2018. Authors are otherwise without conflicts of interest, including relevant financial interests, activities, relationships, and affiliations.
Funding: The project described was partially supported by the National Cancer Institute of the National Institutes of Health under Award Number K12CA226330. The authors would also like to thank the Sylvester Comprehensive Cancer Center for their support of this work.
Contributor Information
Alan Dal Pra, Email: alan.dalpra@med.miami.edu.
Sarah E Hoffe, Email: sarah.hoffe@moffitt.org.
Eric A Mellon, Email: eric.mellon@med.miami.edu.
REFERENCES
- 1.Mutic S, Dempsey JF. The ViewRay system: magnetic resonance-guided and controlled radiotherapy. Semin Radiat Oncol 2014; 24: 196–9. doi: 10.1016/j.semradonc.2014.02.008 [DOI] [PubMed] [Google Scholar]
- 2.El-Bared N, Portelance L, Spieler BO, Kwon D, Padgett KR, Brown KM, et al. Dosimetric benefits and practical pitfalls of daily online adaptive MRI-guided stereotactic radiation therapy for pancreatic cancer. Pract Radiat Oncol 2019; 9: e46–54. doi: 10.1016/j.prro.2018.08.010 [DOI] [PubMed] [Google Scholar]
- 3.Spieler BO, Mellon EA, Jones PD, Giap H, Feun L, Venkat S, et al. Stereotactic ablative radiotherapy for hepatocellular carcinoma. Hepatoma Res 2019; 54. [Google Scholar]
- 4.Kupelian P, Sonke J-J. Magnetic resonance-guided adaptive radiotherapy: a solution to the future. Semin Radiat Oncol 2014; 24: 227–32. doi: 10.1016/j.semradonc.2014.02.013 [DOI] [PubMed] [Google Scholar]
- 5.Rudra S, Jiang N, Rosenberg SA, Olsen JR, Roach MC, Wan L, et al. Using adaptive magnetic resonance image-guided radiation therapy for treatment of inoperable pancreatic cancer. Cancer Med 2019; 8: 2123–32. doi: 10.1002/cam4.2100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bashir MN. Epidemiology of prostate cancer. Asian Pac J Cancer Prev 2015; 16: 5137–41. doi: 10.7314/APJCP.2015.16.13.5137 [DOI] [PubMed] [Google Scholar]
- 7.Byrne TE. A review of prostate motion with considerations for the treatment of prostate cancer. Med Dosim 2005; 30: 155–61. doi: 10.1016/j.meddos.2005.03.005 [DOI] [PubMed] [Google Scholar]
- 8.McPartlin AJ, Li XA, Kershaw LE, Heide U, Kerkmeijer L, Lawton C, et al. MRI-guided prostate adaptive radiotherapy - A systematic review. Radiother Oncol 2016; 119: 371–80. doi: 10.1016/j.radonc.2016.04.014 [DOI] [PubMed] [Google Scholar]
- 9.nice.org.uk National Institute for health and care excellence, c2020. 2020. Available from: https://www.nice.org.uk/process/pmg20/chapter/incorporating-economic-evaluation[2020 Apr 1].
- 10.Pollack A, Zagars GK, Smith LG, Lee JJ, von Eschenbach AC, Antolak JA, et al. Preliminary results of a randomized radiotherapy dose-escalation study comparing 70 Gy with 78 Gy for prostate cancer. J Clin Oncol 2000; 18: 3904–11. doi: 10.1200/JCO.2000.18.23.3904 [DOI] [PubMed] [Google Scholar]
- 11.Moman MR, van der Heide UA, Kotte ANTJ, van Moorselaar RJA, Bol GH, Franken SPG, et al. Long-Term experience with transrectal and transperineal implantations of fiducial gold markers in the prostate for position verification in external beam radiotherapy; feasibility, toxicity and quality of life. Radiotherapy and Oncology 2010; 96: 38–42. doi: 10.1016/j.radonc.2010.02.027 [DOI] [PubMed] [Google Scholar]
- 12.Schaeffer EM. Re: infections after fiducial marker implantation for prostate radiotherapy: are we underestimating the risks? Journal of Urology 2016; 196443. doi: 10.1016/j.juro.2016.05.025 [DOI] [PubMed] [Google Scholar]
- 13.Yu JB, Cramer LD, Herrin J, Soulos PR, Potosky AL, Gross CP. Stereotactic body radiation therapy versus intensity-modulated radiation therapy for prostate cancer: comparison of toxicity. J Clin Oncol 2014; 32: 1195–201. doi: 10.1200/JCO.2013.53.8652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Evans JR, Zhao S, Daignault S, Sanda MG, Michalski J, Sandler HM, et al. Patient-Reported quality of life after stereotactic body radiotherapy (SBRT), intensity modulated radiotherapy (IMRT), and brachytherapy. Radiother Oncol 2015; 116: 179–84. doi: 10.1016/j.radonc.2015.07.016 [DOI] [PubMed] [Google Scholar]
- 15.Van As NJ, Brand D, Tree A, Ostler PJ, Chu W, Loblaw A, et al. Pace: analysis of acute toxicity in PACE-B, an international phase III randomized controlled trial comparing stereotactic body radiotherapy (SBRT) to conventionally fractionated or moderately hypofractionated external beam radiotherapy (CFMHRT) for localized prostate cancer (LPCa. JCO 2019; 37(7_suppl): 1. doi: 10.1200/JCO.2019.37.7_suppl.1 [DOI] [Google Scholar]
- 16.Cancer.gov SEER Cancer Statistics Review 1975–2013. National Cancer Institute. 2016. Available from: https://seer.cancer.gov/archive/csr/1975_2013/ [2019 Aug 30].
- 17.SSA.gov Period Life Table, 2016. USA Social Security Administration. 2016. Available from: https://www.ssa.gov/oact/STATS/table4c6.html [cited 2019 Aug 30].
- 18.Heemsbergen WD, Peeters STH, Koper PCM, Hoogeman MS, Lebesque JV. Acute and late gastrointestinal toxicity after radiotherapy in prostate cancer patients: consequential late damage. Int J Radiat Oncol Biol Phys 2006; 66: 3–10. doi: 10.1016/j.ijrobp.2006.03.055 [DOI] [PubMed] [Google Scholar]
- 19.Cameron D, Ubels J, Norström F. On what basis are medical cost-effectiveness thresholds set? Clashing opinions and an absence of data: a systematic review. Glob Health Action 2018; 11: 1447828. doi: 10.1080/16549716.2018.1447828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hounton S, Newlands D. Applying the net-benefit framework for assessing cost-effectiveness of interventions towards universal health coverage. Cost Eff Resour Alloc 2012; 10: 8: 8. doi: 10.1186/1478-7547-10-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Dyk J, Zubizarreta E, Lievens Y. Cost evaluation to optimise radiation therapy implementation in different income settings: a time-driven activity-based analysis. Radiother Oncol 2017; 125: 178–85. doi: 10.1016/j.radonc.2017.08.021 [DOI] [PubMed] [Google Scholar]
- 22.Perrier L, Morelle M, Pommier P, Lagrange J-L, Laplanche A, Dudouet P, et al. Cost of prostate image-guided radiation therapy: results of a randomized trial. Radiother Oncol 2013; 106: 50–8. doi: 10.1016/j.radonc.2012.11.011 [DOI] [PubMed] [Google Scholar]
- 23.Dushyanth A, Marckx B. VRAY: 2017 Financial Highlights. What To Expect In 2018? Zacks. 2018. Available from: http://s1.q4cdn.com/460208960/files/News/2018/January-16-2018_VRAY_Dushyanth.pdf [2019 Apr 1].
- 24.Das S, Liu T, Jani AB, Rossi P, Shelton J, Shi Z, et al. Comparison of image-guided radiotherapy technologies for prostate cancer. Am J Clin Oncol 2014; 37: 616–23. doi: 10.1097/COC.0b013e31827e4eb9 [DOI] [PubMed] [Google Scholar]
- 25.Raziee H, Moraes FY, Murgic J, Chua MLK, Pintilie M, Chung P, et al. Improved outcomes with dose escalation in localized prostate cancer treated with precision image-guided radiotherapy. Radiother Oncol 2017; 123: 459–65. doi: 10.1016/j.radonc.2017.04.003 [DOI] [PubMed] [Google Scholar]
- 26.Ingrosso G, Carosi A, Cristino Ddi, Ponti E, Lancia A, Bottero M, et al. Volumetric image-guided conformal radiotherapy for localized prostate cancer: analysis of dosimetric and clinical factors affecting acute and late toxicity. Rep Pract Oncol Radiother 2018; 23: 315–21. doi: 10.1016/j.rpor.2018.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shimizu D, Yamazaki H, Nishimura T, Aibe N, Okabe H. Long-Term tumor control and late toxicity in patients with prostate cancer receiving hypofractionated (2.2 Gy) soft-tissue-matched image-guided intensity-modulated radiotherapy. Anticancer Res 2017; 37: 5829–35. doi: 10.21873/anticanres.12026 [DOI] [PubMed] [Google Scholar]
- 28.Di Muzio NG, Fodor A, Noris Chiorda B, Broggi S, Mangili P, Valdagni R, et al. Moderate hypofractionation with simultaneous integrated boost in prostate cancer: long-term results of a phase I-II study. Clin Oncol 2016; 28: 490–500. doi: 10.1016/j.clon.2016.02.005 [DOI] [PubMed] [Google Scholar]
- 29.Ingrosso G, Carosi A, di Cristino D, Ponti E, Lancia A, Murgia A, et al. Volumetric image-guided highly conformal radiotherapy of the prostate bed: toxicity analysis. Rep Pract Oncol Radiother 2017; 22: 64–70. doi: 10.1016/j.rpor.2016.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ishii K, Ogino R, Hosokawa Y, Fujioka C, Okada W, Nakahara R, et al. Comparison of dosimetric parameters and acute toxicity after whole-pelvic vs prostate-only volumetric-modulated Arc therapy with daily image guidance for prostate cancer. Br J Radiol 2016; 89: 20150930. doi: 10.1259/bjr.20150930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nishimura T, Yamazaki H, Aibe N, Nakamura S, Yoshida K, Okabe H. Exceptionally high incidence of grade 2-3 late rectal toxicity in patients with prostate cancer receiving hypofractionated (2.2 Gy) soft tissue-matched image-guided intensity-modulated radiotherapy. Anticancer Res 2013; 33: 5507–10. [PubMed] [Google Scholar]
- 32.Guckenberger M, Lawrenz I, Flentje M. Moderately hypofractionated radiotherapy for localized prostate cancer: long-term outcome using IMRT and volumetric IGRT. Strahlenther Onkol 2014; 190: 48–53. doi: 10.1007/s00066-013-0443-x [DOI] [PubMed] [Google Scholar]
- 33.Marina O, Gustafson GS, Kestin LL, Brabbins DS, Chen PY, Ye H, et al. Comparison of dose-escalated, image-guided radiotherapy vs. dose-escalated, high-dose-rate brachytherapy boost in a modern cohort of intermediate-risk prostate cancer patients. Brachytherapy 2014; 13: 59–67. doi: 10.1016/j.brachy.2013.05.004 [DOI] [PubMed] [Google Scholar]
- 34.Guckenberger M, Ok S, Polat B, Sweeney RA, Flentje M. Toxicity after intensity-modulated, image-guided radiotherapy for prostate cancer. Strahlenther Onkol 2010; 186: 535–43. doi: 10.1007/s00066-010-2144-z [DOI] [PubMed] [Google Scholar]
- 35.Detti B, Baki M, Becherini C, Saieva C, Scartoni D, Giacomelli I, et al. High-Dose intensity-modulated radiation therapy as primary treatment of prostate cancer: genitourinary/gastrointestinal toxicity and outcomes, a single-institution experience. Radiol Med 2019; 124: 422–31. doi: 10.1007/s11547-018-0977-1 [DOI] [PubMed] [Google Scholar]
- 36.Jereczek-Fossa BA, Maucieri A, Marvaso G, Gandini S, Fodor C, Zerini D, et al. Impact of image guidance on toxicity and tumour outcome in moderately hypofractionated external-beam radiotherapy for prostate cancer. Med Oncol 2018; 36: 9: 9. doi: 10.1007/s12032-018-1233-1 [DOI] [PubMed] [Google Scholar]
- 37.Tomita N, Soga N, Ogura Y, Furusawa J, Tanaka H, Koide Y, et al. Favorable 10-year outcomes of image-guided intensity-modulated radiotherapy combined with long-term androgen deprivation for Japanese patients with nonmetastatic prostate cancer. Asia Pac J Clin Oncol 2019; 15: 18–25. doi: 10.1111/ajco.13097 [DOI] [PubMed] [Google Scholar]
- 38.Yamazaki H, Masui K, Suzuki G, Nakamura S, Shimizu D, Nishikawa T, et al. High-dose-rate brachytherapy monotherapy versus image-guided intensity-modulated radiotherapy with helical tomotherapy for patients with localized prostate cancer. Cancers 2018; 10: e322. doi: 10.3390/cancers10090322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marvaso G, Riva G, Ciardo D, Gandini S, Fodor C, Zerini D, et al. "Give me five" ultra-hypofractionated radiotherapy for localized prostate cancer: non-invasive ablative approach. Med Oncol 2018; 35: 96: 96. doi: 10.1007/s12032-018-1155-y [DOI] [PubMed] [Google Scholar]
- 40.Sasaki N, Yamazaki H, Shimizu D, Suzuki G, Masui K, Nakamura S, et al. Long-Term outcomes of a dose-reduction trial to decrease late gastrointestinal toxicity in patients with prostate cancer receiving soft tissue-matched image-guided intensity-modulated radiotherapy. Anticancer Res 2018; 38: 385–91. doi: 10.21873/anticanres.12234 [DOI] [PubMed] [Google Scholar]
- 41.Arunsingh M, Mallick I, Prasath S, Arun B, Sarkar S, Shrimali RK, et al. Acute toxicity and its dosimetric correlates for high-risk prostate cancer treated with moderately hypofractionated radiotherapy. Medical Dosimetry 2017; 42: 18–23. doi: 10.1016/j.meddos.2016.10.002 [DOI] [PubMed] [Google Scholar]
- 42.Krupa P, Ticha H, Kazda T, Dymackova R, Zitterbartova J, Odlozilikova A, et al. Early toxicity of hypofractionated radiotherapy for prostate cancer. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2016; 160: 435–41. doi: 10.5507/bp.2016.008 [DOI] [PubMed] [Google Scholar]
- 43.Girelli G, Franco P, Sciacero P, Cante D, Borca VC, Pasquino M, et al. Image-Guided intensity-modulated radiotherapy for prostate cancer employing hypofractionation and simultaneous integrated boost: results of a consecutive case series with focus on erectile function. Anticancer Res 2015; 35: 4177–82. [PubMed] [Google Scholar]
- 44.Cheng JC, Schultheiss TE, Nguyen KH, Wong JYC. Acute toxicity in definitive versus postprostatectomy image-guided radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys 2008; 71: 351–7. doi: 10.1016/j.ijrobp.2007.09.043 [DOI] [PubMed] [Google Scholar]
- 45.Cooper NJ, Abrams KR, Sutton AJ, Turner D, Lambert PC. A Bayesian approach to Markov modelling in cost-effectiveness analyses: application to taxane use in advanced breast cancer. J R Stat Soc Ser A Stat Soc 2003; 166: 389–405. doi: 10.1111/1467-985X.00283 [DOI] [Google Scholar]
- 46.Hutchinson RC, Sundaram V, Folkert M, Lotan Y. Decision analysis model evaluating the cost of a temporary hydrogel rectal spacer before prostate radiation therapy to reduce the incidence of rectal complications. Urol Oncol 2016; 34: 291.e19–291.e26. doi: 10.1016/j.urolonc.2016.02.024 [DOI] [PubMed] [Google Scholar]
- 47.Zemplényi AT, Kaló Z, Kovács G, Farkas R, Beöthe T, Bányai D, et al. Cost-Effectiveness analysis of intensity-modulated radiation therapy with normal and hypofractionated schemes for the treatment of localised prostate cancer. Eur J Cancer Care 2018; 27: e12430 10.1111/ecc.12430 [DOI] [PubMed] [Google Scholar]
- 48.Andreyev HJN, Muls AC, Norton C, Ralph C, Watson L, Shaw C, et al. Guidance: the practical management of the gastrointestinal symptoms of pelvic radiation disease. Frontline Gastroenterol 2015; 6: 53–72. doi: 10.1136/flgastro-2014-100468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shimizu F, Fujino K, Ito YM, Fukuda T, Kawachi Y, Minowada S, et al. Factors associated with variation in utility scores among patients with prostate cancer. Value Health 2008; 11: 1190–3. doi: 10.1111/j.1524-4733.2008.00336.x [DOI] [PubMed] [Google Scholar]
- 50.Raphaeli T, Menon R. Current treatment of lower gastrointestinal hemorrhage. Clin Colon Rectal Surg 2012; 25: 219–27. doi: 10.1055/s-0032-1329393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hall F, de Freitas HM, Kerr C, Ito T, Nafees B, Lloyd AJ, et al. Estimating utilities/disutilities for high-risk metastatic hormone-sensitive prostate cancer (mHSPC) and treatment-related adverse events. Qual Life Res 2019; 28: 1191–9. doi: 10.1007/s11136-019-02117-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nafees B, Stafford M, Gavriel S, Bhalla S, Watkins J. Health state utilities for non small cell lung cancer. Health Qual Life Outcomes 2008; 6: 84. doi: 10.1186/1477-7525-6-84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Constable L, Cotterill N, Cooper D, Glazener C, Drake MJ, Forrest M, et al. Male synthetic sling versus artificial urinary sphincter trial for men with urodynamic stress incontinence after prostate surgery (master): study protocol for a randomised controlled trial. Trials 2018; 19: 131. doi: 10.1186/s13063-018-2501-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cher DJ, Miyamoto J, Lenert LA. Incorporating risk attitude into Markov-process decision models: importance for individual decision making. Med Decis Making 1997; 17: 340–50. doi: 10.1177/0272989X9701700311 [DOI] [PubMed] [Google Scholar]
- 55.Krahn MD, Mahoney JE, Eckman MH, Trachtenberg J, Pauker SG, Detsky AS. Screening for prostate cancer. A decision analytic view. JAMA 1994; 272: 773–80. [PubMed] [Google Scholar]
- 56.Penson DF, Moul JW, Evans CP, Doyle JJ, Gandhi S, Lamerato L. The economic burden of metastatic and prostate specific antigen progression in patients with prostate cancer: findings from a retrospective analysis of health plan data. J Urol 2004; 171(6 Pt 1): 2250–4. doi: 10.1097/01.ju.0000127732.63726.4c [DOI] [PubMed] [Google Scholar]
- 57.Showalter TN, Foley KA, Jutkowitz E, Lallas CD, Trabulsi EJ, Gomella LG, et al. Costs of early adjuvant radiation therapy after radical prostatectomy: a decision analysis. Ann Oncol 2012; 23: 701–6. doi: 10.1093/annonc/mdr281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stewart ST, Lenert L, Bhatnagar V, Kaplan RM. Utilities for prostate cancer health states in men aged 60 and older. Med Care 2005; 43: 347–55. doi: 10.1097/01.mlr.0000156862.33341.45 [DOI] [PubMed] [Google Scholar]
- 59.Stephenson AJ, Scardino PT, Kattan MW, Pisansky TM, Slawin KM, Klein EA, et al. Predicting the outcome of salvage radiation therapy for recurrent prostate cancer after radical prostatectomy. J Clin Oncol 2007; 25: 2035–41. doi: 10.1200/JCO.2006.08.9607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Freedland SJ, Humphreys EB, Mangold LA, Eisenberger M, Dorey FJ, Walsh PC, et al. Risk of prostate cancer-specific mortality following biochemical recurrence after radical prostatectomy. JAMA 2005; 294: 433–9. doi: 10.1001/jama.294.4.433 [DOI] [PubMed] [Google Scholar]
- 61.Parthan A, Pruttivarasin N, Davies D, Taylor DCA, Pawar V, Bijlani A, et al. Comparative cost-effectiveness of stereotactic body radiation therapy versus intensity-modulated and proton radiation therapy for localized prostate cancer. Front Oncol 2012; 2: 81. doi: 10.3389/fonc.2012.00081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Parikh NR, Lee P, Raman S, Cao M, Tyran M, Lamb JM, et al. Time-Driven activity based costing of CT-guided vs. MR-guided SBRT in patients with unresectable hepatocellular carcinoma. Int J Radiat Oncol Biol Phys 2018; 102: e402–3. doi: 10.1016/j.ijrobp.2018.07.1187 [DOI] [Google Scholar]
- 63.Jackson WC, Silva J, Hartman HE, Dess RT, Kishan AU, Beeler WH, et al. Stereotactic body radiation therapy for localized prostate cancer: a systematic review and meta-analysis of over 6,000 patients treated on prospective studies. Int J Radiat Oncol Biol Phys 2019; 104: 778–89. doi: 10.1016/j.ijrobp.2019.03.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen AM, Cao M, Hsu S, Lamb J, Mikaeilian A, Yang Y, et al. Magnetic resonance imaging guided reirradiation of recurrent and second primary head and neck cancer. Advances in Radiation Oncology 2017; 2: 167–75. doi: 10.1016/j.adro.2017.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rwigema JC, Thomas DH, Cao M, Yoshizaki T, Chen AM. Intrafraction organ motion tracking with real-time MRI-guided radiation therapy for head and neck cancer. Int J Radiat Oncol Biol Phys 2016; 94: 878. doi: 10.1016/j.ijrobp.2015.12.052 [DOI] [Google Scholar]
- 66.Mohamed ASR, Aristophanous M, Blanchard P, Kamal M, Ding Y, Garden AS, et al. Prospective in silico study of the feasibility and dosimetric advantages of MRI-guided dose adaptation for human papillomavirus positive oropharyngeal cancer patients compared with standard IMRT. Int J Radiat Oncol Biol Phys 2017; 99: E699–700. doi: 10.1016/j.ijrobp.2017.06.2287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Henke L, Kashani R, Robinson C, Curcuru A, DeWees T, Bradley J, et al. Phase I trial of stereotactic MR-guided online adaptive radiation therapy (smart) for the treatment of oligometastatic or unresectable primary malignancies of the abdomen. Radiother Oncol 2018; 126: 519–26. doi: 10.1016/j.radonc.2017.11.032 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.