Abstract
Objective:
Better cosmetic outcome after vacuum assisted excision (VAE) compared to surgical excision of benign breast lesions is suggested in previous studies but has never been evaluated with validated outcome measures. In this study, patient reported cosmetic outcome after VAE was evaluated.
Methods:
Patients who underwent VAE between July 2017 and December 2018 were invited to complete the cosmetic subscale of the Dutch Breast Cancer Treatment Outcome Scale, comparing the treated with the untreated breast. Response mode ranged from 1 (no difference) to 4 (large difference) and cosmetic outcome was calculated as the unweighted mean. Clinical outcomes included: tumor size, number of cores, complications, residual lesions and recurrences.
Results:
Response rate was 73.4% (47 of 64 patients). Median tumor size was 15 mm (range 5–51 mm) and median number of cores 6.5 (range 1–85), complete excision was confirmed in all but two patients. Mean cosmetic outcome was good (mean score ≤1.75) in 74% of patients and no patients reported a poor cosmetic outcome (mean score >3.25). A hematoma occurred in five patients (one needed aspiration) and a skin rash in one patient, no patients developed an infection or seroma.
Conclusion:
In this study VAE is safe and effective for tumors up to 5 cm and patient reported cosmetic outcome was good. Patients with benign lesions could benefit from VAE as an alternative for surgical excision.
Advances in knowledge:
A formal quantitative measurement of cosmetic outcome after vacuum assisted excision for benign breast lesions was still lacking. This study shows that this cosmetic outcome is overall good in benign lesions up to 5 cm.
Introduction
In the Netherlands, more than 17.000 females are diagnosed with breast cancer each year. Even more females are diagnosed with benign breast lesions,1–4 although exact prevalence data are lacking. Surgical excision of all high-risk lesions is performed in 2.5% of all recalled females after screening,5 and over 70% of these excised lesions are benign lesions.5 This does not even include females referred from the general physician with palpable lesions. Benign or high-risk lesion are excised if: (1) the lesion is symptomatic or (2) when there is the need for additional tissue for diagnostic purposes.5–8 Surgical excision is still the most performed procedure for benign or high-risk breast lesions in the Netherlands. However, vacuum-assisted excision (VAE) offers an alternative therapy for benign and high-risk lesions and surgery can then be avoided.9,10
VAE is a method in which the tumor is removed under ultrasound guidance, using large tissue samples.8 With VAE high complete excision rates (>94%) and low complication rates (5%) are reported.10,11 Although, previous studies suggest a better cosmetic outcome after VAE, it has never been evaluated with validated PROMs or clinical assessment tools.10,12–21 The role of PROMs for the evaluation of cosmesis after breast cancer treatment is emphasized in recent literature.22–25 Therefore, and to minimize the burden for patients, we evaluated cosmetic outcome with PROMs only.
No validated questionnaires are available for patient reported cosmetic outcome after benign breast lesion excision but similar questionnaires are available for the evaluation of breast cancer surgery. Amongst others, the Breast Cancer Treatment Outcome Scale (BCTOS) is a widely used questionnaire in which the patient compares the treated with the untreated breast on several important aspects of cosmetic outcome.26–33 The Dutch BCTOS-13 was recently validated in patients with early-stage breast cancer.28,33
The aim of this study is to report on a cohort of patients with lesions up to 5 cm treated with VAE and to evaluate cosmetic outcome of this treatment using a validated PROM.
Methods
Design and patients
In this single-center cross-sectional study, all patients who underwent a VAE between July 2017 and December 2018 in a large secondary teaching hospital (Franciscus Gasthuis & Vlietland, Rotterdam, the Netherlands) were retrieved from the electronic patient record system, using the code of the procedure. These patients were contacted from January to April 2019, to complete the cosmetic subscale of the Breast Cancer Treatment Outcome Scale (BCTOS-cs). No questionnaires were sent to patients unable to understand Dutch, patients that underwent an operation on the same breast after VAE or had bilateral tumors. A benign or uncertain benign lesion was excised when it was symptomatic or when there was a need for additional tissue for diagnostic purposes. These lesions could be treated by VAE when they were smaller than 5 cm. Lesions with a strong suspicion on microcalcifications, phyllodes lesions or atypical ductal hyperplasia were not eligible for VAE. The final treatment decision (VAE or surgical excision) was made in a multidisciplinary team meeting and discussed with the patient.34
VAE procedure
All VAE procedures were preceded by core needle biopsy (CNB) and executed by experienced breast radiologists with a minimum of 7 years of experience in breast biopsies. The ENCOR ULTRA® Breast Biopsy System in combination with a 7 Gauge needle was used under real-time ultrasound guidance. When no residual lesion could be identified by the performing radiologist in axial and longitudinal planes on ultrasound, the procedure was completed. After every VAE, a marker was placed and mammography was performed. Lesions in proximity of the skin, nipple and pectoralis muscle were not eligible for VAE at the discretion of the radiologist. The goal of the VAE procedure was to achieve complete excision of the lesion.
Outcome measures and data collection
The primary outcome measure was the cosmetic result after VAE, according to the 9-item cosmetic subscale of the Dutch BCTOS-13.33 The response mode per item varies from no difference (1) to a large difference (4) when the treated breast is compared to the untreated breast. The final cosmetic outcome score was the unweighted mean cosmetic outcome of all nine items, and was categorized into good (1.00–1.75), intermediate (1.76–2.50), fair (2.51–3.25) and poor (3.26–4.00).28 These categories were later dichotomized into good vs suboptimal (intermediate, fair or poor).
Data on patient characteristics, tumor characteristics (including pathology results), procedure-related characteristics and short-term complications was retrospectively collected from electronic patient records. Complications were classified using the Clavien-Dindo Classification System35 and complete excision was defined as no residual lesion on ultrasound and mammography. Routine follow-up with ultrasound and/or mammography and a clinical consultation was only performed for the uncertain benign lesions, according to the Dutch breast cancer guideline.34 All patients with clear benign lesions were instructed to return in case of complaints or concerns. Non-response selection bias was checked by comparing the characteristics of patients who completed BCTOS-cs with those who did not complete the BCTOS-cs.
Statistical analysis
Descriptive analyses were used to evaluate patient characteristics, tumor characteristics and cosmetic outcome. Cronbach’s α was used to assess the internal consistency of the BCTOS-cs, which should exceed 0.70 to be acceptable.36 Floor and ceiling effects were measured by calculating the percentage of the minimum (1) and maximum (4) score per item and were considered low when <20%.37 The impact of clinically relevant variables was tested for the cosmetic outcome score as well as the dichotomized cosmetic outcome using Pearson or Spearman’s correlation coefficients, one-way ANOVA or the Kruskal–Wallis H test, and the unpaired Student’s t-test or Mann–Whitney U test or χ2 as appropriate, depending on measurement scale and skewness of data. Variables considered as clinically relevant were: tumor size, tumor weight, number resected cores, follow-up complications, BIRADS classification, histology class before and after VAE, age, side of the tumor, year of procedure, number of tumors resected on one day, time between procedure and questionnaire, indication for excision, and executing radiologist.
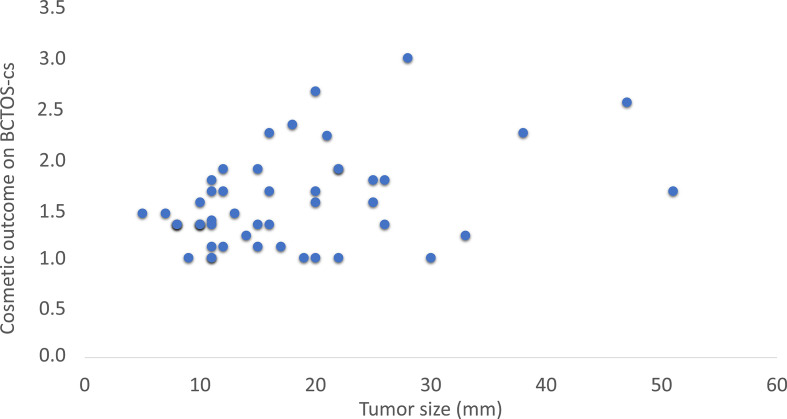
All possibly associated variables (p < 0.2) were included in a weighted least squares (WLS) multiple linear regression analysis (for the cosmetic outcome score as dependent variable) and a binary multivariable logistic regression analysis (for dichotomized cosmetic outcome as dependent variable). A multivariable WLS regression instead of multivariable OLS regression was chosen to account for heteroscedasticity (Figure 1). All analyses were performed using IBM SPSS 25 (IBM Corp. Armonk, NY) and p < 0.05 (two-sided) was considered to be of statistical significance.
Figure 1.
Association between tumor size and cosmetic outcome
Results
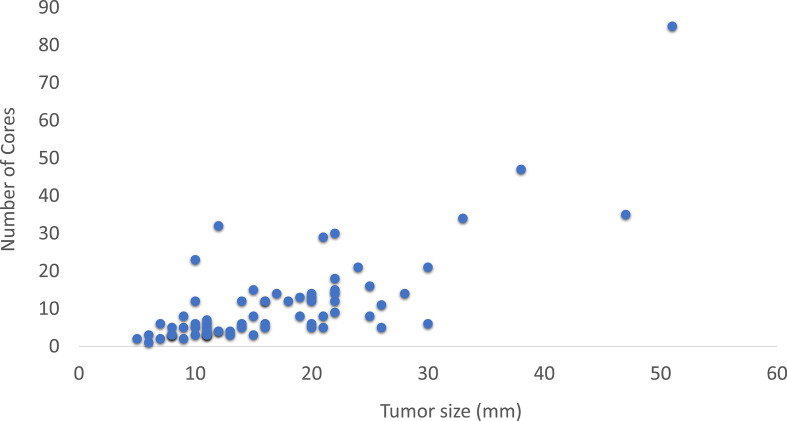
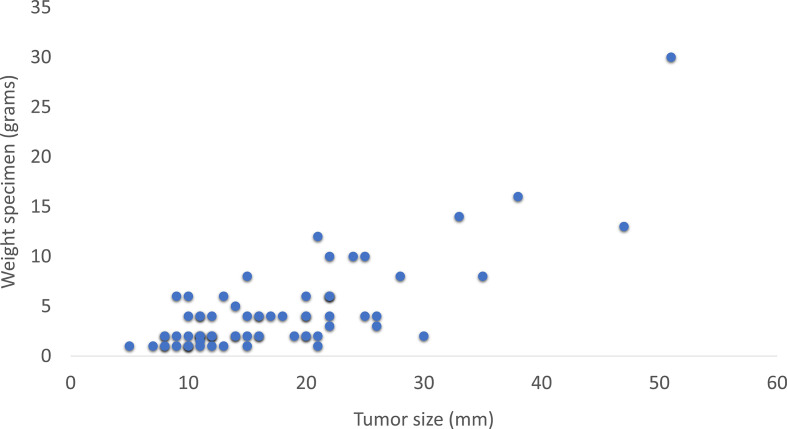
An overview of the baseline characteristics is shown in Table 1. VAE was performed for 77 tumors in 69 patients (71 procedures), with 7 tumors being >3 cm. Three patients were excluded from cosmetic evaluation because VAE was performed in both breasts, two because they did not understand Dutch, and one because surgery was performed before the questionnaire could be sent. Analysis of excluded patients or patients that did not respond to the BCTOS-cs showed no difference in these baseline characteristics, thus selective non-response was not found (data not shown). Tumor size correlated with the weight of the excised specimen (r = 0.65, p < 0.01) and the number of resected cores (r = 0.71, p < 0.01) (Figures 2 and 3). The number of resected cores was comparable in patients with (median 8.0) and without (median 6.5) complications (p = 0.79).
Table 1.
Baseline characteristics of all patients
| Patientcharacteristics (N = 69) | |
|---|---|
| Age Median (range) | 35.0 (16–61) |
| Tumor characteristics (N = 78) | |
| Tumor size Median (range) | 15.0 (5–51) |
| Weight specimen Median (range) in grams | 2.5 (1–30) |
| Number of cores median (range) | 7.0 (1–85) |
| Side (left) | 38 (49%) |
| Indication | |
| Request patient | 50 (64%) |
| Excision MDM advice | 28 (36%) |
| Birads classification | |
| 0 | 1 (1.3%) |
| I | - |
| II | 13 (16.9%) |
| III | 43 (55.8%) |
| IV | 18 (23.4%) |
| V | 2 (2.6%) |
| VI | - |
| Histologyresultafter VAE | |
| Benign | 61 (79%) |
| Uncertain benign | 13 (16.9%) |
| DCIS | 1 (1.3%) |
| Other | 2 (2.6%) |
| Procedure characteristics (N = 71) | |
| Year of procedure (2018) | 64 (90%) |
| FU complications (yes) | 6 (8.5%) |
| Number of resected tumors per procedure | |
| 1 | 65 (92%) |
| 2 | 5 (7%) |
| 3 | 1 (1%) |
| Radiologist | |
| A | 57 (80%) |
| B | 11 (16%) |
| C | 1 (1%) |
| D | 3 (3%) |
DCIS, ductal carcinoma in situ.
Figure 2.
Association between resected number of cores and tumor size.
Figure 3.
Association between weight of the resected specimen and tumor size.
Cosmetic outcome
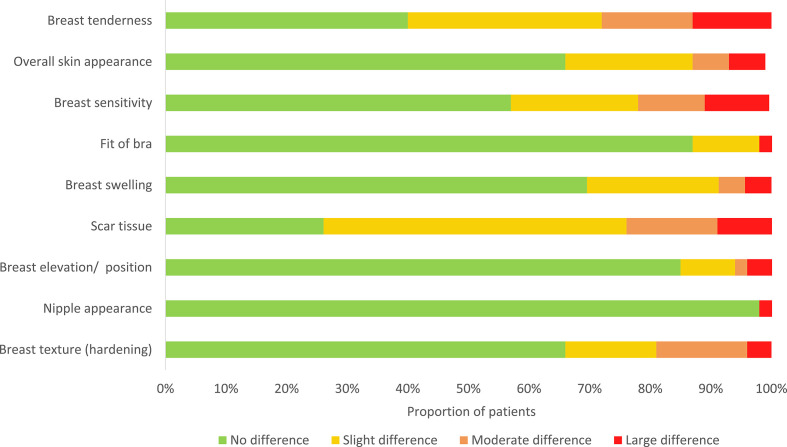
Within 10–79 weeks (mean 38 ± 17 weeks) after VAE, 47 of 64 contacted patients (73.4%) completed the BCTOS-cs. Cronbach’s α of the BCTOS-cs was 0.75. The difference between the treated and the untreated breast was most noticeable in terms of “scar tissue” and “breast tenderness” according to patients (Figure 4). The overall median cosmetic outcome score was 1.4 (range 1–3, SD). Cosmetic outcome was good in 34 patients (73.9%), intermediate in 9 patients (19.6%), and fair in 3 patients (6.5%). None of the patients reported a poor cosmetic outcome. A floor effect >20% was reached in all items, a ceiling effect >20% was reached in none of the items.
Figure 4.
Distribution of scores per questions on the BCTOS-cs, BCTOS, Breast Cancer Treatment Outcome Scale.
Cosmetic outcome was not significantly different between tumors ≥3 cm and <3 cm (mean ± SD: 1.74 ± 0.66 vs 1.53 ± 0.45, p = 0.36, respectively).
Factors associated with cosmetic outcome
A smaller tumor size (r = 0.311, p = 0.03) and older age (r = −0.301, p = 0.04) were significantly associated with a better cosmetic outcome. Patients without complications reported a better cosmetic outcome than patients with complications during follow-up (respectively mean rank 21.7 vs mean rank 39.8, p = 0.002). The weight of the specimen (r = 0.232, p = 0.11), the number of cores (r = 0.264, p = 0.07), and the time between procedure and questionnaire (r = −0.090, p = 0.55) did not significantly correlate to cosmetic outcome after a univariate analysis, nor did all other tested variables which were considered to be clinically relevant.
Variables included in the WLS multivariable linear regression were: number of cores, weight of the specimen, age, tumor size, number of tumors resected on one day, executing radiologist, and complications during follow-up (FU-complications). In this analysis, only the presence of FU-complications (β = 0.332, SE = 0.150) was significantly associated with cosmetic outcome (p = 0.03, adjusted R2 = 0.155). Additionally, after dichotomizing cosmetic outcome into good vs suboptimal, none of the variables had a significant impact on cosmetic outcome in the multiple binary logistic regression (data not shown).
Histology results
In 1 out of 77 lesions (1.3 %), ductal carcinoma in situ (DCIS) was found after VAE, while the lesion was classified as a fibroadenoma on core needle biopsy (CNB) (Table 2). Surgical excision was performed. No questionnaire regarding cosmetic outcome was sent to this patients, because she underwent surgery before questionnaires were administered.
Table 2.
Histology results before VAE, N = 77
| Histology result | Before VAE N (%) |
|---|---|
| Fibroadenoma | 40 (51.9%) |
| Other fibrocystic lesions | 3 (3.9%) |
| Fibroadenoma dd phyllodes | 11 (14.3%) |
| Tubular adenoma | 2 (2.6%) |
| Adenosis | 1 (1.3%) |
| PASH | 2 (2.6%) |
| Intraductal papilloma | 5 (6.5%) |
| CCL/FEA | 1 (1.3%) |
| Radial scar | 3 (3.9%) |
| Other lesions | 2 (2.6%) |
| Not performeda | 7 (9.1%) |
VAE, vacuum assisted excision.
Histology was previously performed, performed in another hospital, or no specimen was available because not all lesions in one breast were biopsied.
Complete excision, recurrence and complications
Complete excision could not be ascertained in 2 of 77 lesions (2.6 %) immediately after VAE. In one patient, complete excision could not reliably be assessed due to the presence of hematoma. In another patient, a doubtful residual lesion was seen on the mammography after the procedure but no palpable lesion was found at the 1 year follow-up visit. Two patients returned to the hospital with a recurrent lesion that both proved to be fibroadenomas.These lesions were found after 20.7 and 6.1 months of follow-up; a new VAE of this fibroadenoma was performed because of complaints in only one of these patients. Additionally, one recurrent radial scar was seen 5.5 months after VAE for which surgical excision was performed. All recurrent lesions or possibly incomplete excisions occurred in tumors < 3 cm.
6 complications occurred after 71 procedures (8.5%). A hematoma occurred in five patients (one needed aspiration), and a skin rash in one patient, no patient developed an infection or seroma.
Discussion
In this study, 69 patients with tumors up to 5 cm were successfully treated with VAE, and complication rate was low. Overall, a good cosmetic outcome was reported. Interestingly, treatment of larger tumors (>3 cm) with VAE did not result in more complications or impaired cosmetic outcome in this study. Additional surgical excision was needed in only two cases, because of histological outcome after VAE (DCIS and radial scar).
The current study showed that the internal consistency and content validity of the BCTOS-cs was good in patients with benign lesions excised by VAE, which supports that cosmetic outcome was measured reliably. A ceiling effect >20% was not reached in any of the items of the BCTOS-cs, which was expected because of an overall good cosmetic outcome. This result is in line with previous studies using the BCTOS.28,33 Because the response was incomplete but not selective, we consider our results to be representative for the entire patient group.
A few papers have described the cosmetic outcome after VAE. In one study,17 97% of patients were “a little bit”-“very much“ satisfied, and in another study,15 85–90% of the patients were satisfied with the cosmetic result. These results are in line with the cosmetic outcome in our study (93.5% intermediate–good cosmetic outcome). However, as the previously published studies did not use a validated measure to evaluate the cosmetic results after VAE, a valid comparison of our results to existing literature cannot be made.
Several studies have suggested that VAE has better cosmetic results as compared to surgical excision. A previous study described the cosmetic outcome after surgical excision of benign lesions: 48.5% excellent, 26.7% good, 12.9% fair, and 11.9% poor.38 However, these results were not patient reported, but evaluated by the physician. Another study reported the outcome on the Dutch BCTOS-cs in patients after completing breast conserving therapy being 1.95; however, this outcome might be influenced by radiation therapy.33 The cosmetic results after VAE in our study are better than after surgical excision reported in literature. However, a formal conclusion on this cosmetic benefit of VAE can only be made after performing a comparative study.
Most authors advice to excise tumors at a maximum of 3 cm because of decreased effectiveness and increased complication rates in larger tumors.11,39 In our study, no severe complications or residual lesions during or after VAE were seen in tumors >3 cm. Complication rate in our study was slightly higher than in a previous study10 but only one of these complications occurred in a tumor >3 cm. As this was a novel technique, especially for larger lesions we chose to report complication rates very conservatively. Patients were instructed to return to the clinic even if a low suspicion of complications. This might have resulted in overreporting of clinically irrelevant Grade 1 hematomas, as reporting such hematomas is somewhat arbitrary. No significant difference in cosmetic outcome for larger tumors (>3 cm vs <.3 cm) was found. Moreover, the cosmetic outcome in tumors >3 cm was good (1.74). Since residual lesions are mostly asymptomatic and cosmetic results remain good, VAE could be indicated for benign tumors > 3 cm when visibility on ultrasound remains good, which is in line with previous findings.10,13
Limitations of our study are the small number of patients included in the cosmetic outcome evaluation and the use of patient reported outcome measures only. Therefore, we could not conclude on clinician reported cosmetic outcome; nonetheless, the importance of PROMs is increasingly emphasized in current literature22–25 and is highly applicable for this benign condition in which patient’s preference has an important role in treatment decision.
In our hospital (Franciscus Gasthuis & Vlietland, Rotterdam, the Netherlands), the total number of surgeries was comparable before and after the use of VAE, but the attribution of benign excisions decreased from 25 to 15%. Therefore, it is expected that VAE reduces waiting lists for malignant diseases and decreases costs. However, this hypothesis should be verified in a comparative study of VAE vs surgical excision.An additional variable of interest, in future cost-effectiveness analysis could be duration of the procedure, since specialist time is an important aspect of the costs of the procedure.
In this study, VAE proved safe and effective for tumors up to 5 cm and patient reported cosmetic outcome is good, and was independent from tumor size and specimen weight. Patients with benign lesions could benefit from VAE as an alternative for surgical excision, and the findings of this study can provide helpful data to clinicians who are routinely consenting these patients. The true cosmetic and economic advantages of VAE over surgical excision of benign breast lesions should be corroborated in a comparative study.
Footnotes
Author statement and Conflicts of interest: All co-authors have contributed to the development of the manuscript, approved its content and its submission to the journal, and have authorized the corresponding author to represent all co-authors in pre-publication discussion with the journal. The authors guarantee that the manuscript, or one with substantially the same content, was not published previously and is not being considered for publication elsewhere. The manuscript contains original unpublished work. There are no conflicts of interest to disclose.
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study protocol was reviewed by the institutional reviewing board of our hospital (ACW Franciscus, the Netherlands) and ethical clearance was granted (internal protocol number 2018–099).
Informed consent: Informed consent was obtained from all individual participants included in the study.
Contributor Information
Elles M.F. van de Voort, Email: e.voort@franciscus.nl.
Taco M.A.L. Klem, Email: t.klem@franciscus.nl.
Gerson M. Struik, Email: g.struik@rdgg.nl.
Erwin Birnie, Email: e.birnie@franciscus.nl.
Renata H.J.A. Sinke, Email: r.sinke@pathan.nl.
Ali Ghandi, Email: aghandi78@gmail.com.
REFERENCES
- 1.Integraal Kankercentrum Nederland kn Overlevingscijfers borstkanker, Stadiumverdeling bij diagnose in 2015 van borstkanker. 2015. Available from: https://www.kanker.nl/kankersoorten/borstkanker/wat-is/overlevingscijfers-borstkanker [cited 2019 25-01-2019].
- 2.ACV MJJCP, Gommer AM. Borstkanker, Sterfte en Overleving. Volksgezondheid en Zorg. 2018. Available from: https://www.volksgezondheidenzorg.info/onderwerp/borstkanker/cijfers-context/sterfte-en-overleving#node-sterfte-borstkanker [cited 2019 15-02-2019].
- 3.Nederland IK. Cijfers over Kanker. 2017. Available from: https://www.cijfersoverkanker.nl/selecties/dataset_1/img5c29e0f938f6d [cited 2019 25-01-2019].
- 4.(RIVM) RvVeM monitor bevolkingsonderzoek borstkanker 2016. 2018. Available from: https://www.rivm.nl/monitoring-en-evaluatie-bevolkingsonderzoek-borstkanker [cited 2019 29-10-2019].
- 5.Luiten JD, Korte B, Voogd AC, Vreuls W, Luiten EJT, Strobbe LJ, et al. Trends in frequency and outcome of high-risk breast lesions at core needle biopsy in women recalled at biennial screening mammography, a multiinstitutional study. Int J Cancer 2019; 145: 2720–7. doi: 10.1002/ijc.32353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lakoma A, Kim ES. Minimally invasive surgical management of benign breast lesions. Gland Surg 2014; 3: 142–8. doi: 10.3978/j.issn.2227-684X.2014.04.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gur D, Wallace LP, Klym AH, Hardesty LA, Abrams GS, Shah R, et al. Trends in recall, biopsy, and positive biopsy rates for screening mammography in an academic practice. Radiology 2005; 235: 396–401. doi: 10.1148/radiol.2352040422 [DOI] [PubMed] [Google Scholar]
- 8.Park H-L, Hong J. Vacuum-assisted breast biopsy for breast cancer. Gland Surg 2014; 3: 120–7. doi: 10.3978/j.issn.2227-684X.2014.02.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Strachan C, Horgan K, Millican-Slater RA, Shaaban AM, Sharma N. Outcome of a new patient pathway for managing B3 breast lesions by vacuum-assisted biopsy: time to change current UK practice? J Clin Pathol 2016; 69: 248–54. doi: 10.1136/jclinpath-2015-203018 [DOI] [PubMed] [Google Scholar]
- 10.Luo H-jun, Chen X, Tu G, Wang J, Wu C-yi, Yang G-lun. Therapeutic application of ultrasound-guided 8-gauge Mammotome system in presumed benign breast lesions. Breast J 2011; 17: 490–7. doi: 10.1111/j.1524-4741.2011.01125.x [DOI] [PubMed] [Google Scholar]
- 11.Park H-L, Kim KY, Park JS, Shin J-E, Kim H-R, Yang B, et al. Clinicopathological analysis of ultrasound-guided Vacuum-assisted breast biopsy for the diagnosis and treatment of breast disease. Anticancer Res 2018; 38: 2455–62. doi: 10.21873/anticanres.12499 [DOI] [PubMed] [Google Scholar]
- 12.Johnson AT, Henry-Tillman RS, Smith LF, Harshfield D, Korourian S, Brown H, et al. Percutaneous excisional breast biopsy. American Journal of surgery. 2002; 184: 550–4discussion 54. [DOI] [PubMed] [Google Scholar]
- 13.Yao F, Li J, Wan Y, Zhong Y, Wei W, Tu Y, et al. Sonographically guided vacuum-assisted breast biopsy for complete excision of presumed benign breast lesions. J Ultrasound Med 2012; 31: 1951–7. doi: 10.7863/jum.2012.31.12.1951 [DOI] [PubMed] [Google Scholar]
- 14.Wang WJ, Wang Q, Cai QP, Zhang JQ. Ultrasonographically guided vacuum-assisted excision for multiple breast masses: non-randomized comparison with conventional open excision. J Surg Oncol 2009; 100: 675–80. doi: 10.1002/jso.21394 [DOI] [PubMed] [Google Scholar]
- 15.Thurley P, Evans A, Hamilton L, James J, Wilson R. Patient satisfaction and efficacy of vacuum-assisted excision biopsy of fibroadenomas. Clin Radiol 2009; 64: 381–5. doi: 10.1016/j.crad.2008.09.013 [DOI] [PubMed] [Google Scholar]
- 16.March DE, Coughlin BF, Barham RB, Goulart RA, Klein SV, Bur ME, et al. Breast masses: removal of all US evidence during biopsy by using a handheld vacuum-assisted device--initial experience. Radiology 2003; 227: 549–55. doi: 10.1148/radiol.2272020476 [DOI] [PubMed] [Google Scholar]
- 17.Fine RE, Boyd BA, Whitworth PW, Kim JA, Harness JK, Burak WE. Percutaneous removal of benign breast masses using a vacuum-assisted hand-held device with ultrasound guidance. Am J Surg 2002; 184: 332–6. doi: 10.1016/S0002-9610(02)00951-0 [DOI] [PubMed] [Google Scholar]
- 18.Bowling A. Just one question: if one question works, why ask several? J Epidemiol Community Health 2005; 59: 342–5. doi: 10.1136/jech.2004.021204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manning WG NJ, Ware JE. The status of health in demand estimation: beyond excellent, good, fair and poor : Fuchs V. R, Economic aspects of health. Chicago, IL: University of Chicago Press; 1982. [Google Scholar]
- 20.McHorney CA, Ware, Jr JE, Rogers W, Raczek AE, Lu JF. The validity and relative precision of mos short- and long-form health status scales and Dartmouth coop charts. results from the medical outcomes study. Med Care 1992; 30(5 Suppl): Ms253–65. doi: 10.1097/00005650-199205001-00025 [DOI] [PubMed] [Google Scholar]
- 21.Ware JE, Phillips J, Yody BB, Adamczyk J. Assessment tools: functional health status and patient satisfaction. Am J Med Qual 1996; 11: S50–3. [PubMed] [Google Scholar]
- 22.Hollen PJ, Msaouel P, Gralla RJ. Determining issues of importance for the evaluation of quality of life and patient-reported outcomes in breast cancer: results of a survey of 1072 patients. Breast Cancer Res Treat 2015; 151: 679–86. doi: 10.1007/s10549-015-3420-5 [DOI] [PubMed] [Google Scholar]
- 23.Lagendijk M, van Egdom LSE, Richel C, van Leeuwen N, Verhoef C, Lingsma HF, et al. Patient reported outcome measures in breast cancer patients. Eur J Surg Oncol 2018; 44: 963–8. doi: 10.1016/j.ejso.2018.03.009 [DOI] [PubMed] [Google Scholar]
- 24.Kool M, van der Sijp JRM, Kroep JR, Liefers G-J, Jannink I, Guicherit OR, et al. Importance of patient reported outcome measures versus clinical outcomes for breast cancer patients evaluation on quality of care. Breast 2016; 27: 62–8. doi: 10.1016/j.breast.2016.02.015 [DOI] [PubMed] [Google Scholar]
- 25.Mori S, Lee EH. Beyond the physician's perspective: a review of patient-reported outcomes in dermatologic surgery and cosmetic dermatology. Int J Womens Dermatol 2019; 5: 21–6. doi: 10.1016/j.ijwd.2018.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heil J, Czink E, Golatta M, Schott S, Hof H, Jenetzky E, et al. Change of aesthetic and functional outcome over time and their relationship to quality of life after breast conserving therapy. Eur J Surg Oncol 2011; 37: 116–21. doi: 10.1016/j.ejso.2010.11.007 [DOI] [PubMed] [Google Scholar]
- 27.Heil J, Holl S, Golatta M, Rauch G, Rom J, Marmé F, et al. Aesthetic and functional results after breast conserving surgery as correlates of quality of life measured by a German version of the breast cancer treatment outcome scale (BCTOS. Breast 2010; 19: 470–4. doi: 10.1016/j.breast.2010.05.004 [DOI] [PubMed] [Google Scholar]
- 28.Hennigs A, Heil J, Wagner A, Rath M, Moosbrugger H, Kelava A, et al. Development and psychometric validation of a shorter version of the breast cancer treatment outcome scale (BCTOS-12. Breast 2018; 38: 58–65. doi: 10.1016/j.breast.2017.12.002 [DOI] [PubMed] [Google Scholar]
- 29.Pignol J-P, Truong P, Rakovitch E, Sattler MG, Whelan TJ, Olivotto IA. Ten years results of the Canadian breast intensity modulated radiation therapy (IMRT) randomized controlled trial. Radiother Oncol 2016; 121: 414–9. doi: 10.1016/j.radonc.2016.08.021 [DOI] [PubMed] [Google Scholar]
- 30.Costa Vieira R, Brandini Silva F, da Silva J, Ferreira L, Santos Paulista J, Alves de Lima M, et al. Aesthetic and functional results of quality of life after breast conserving surgery evaluated by Portuguese/Brazil version of breast cancer treatment outcome scale (BCTOS. The Breast 2017; 32: S131. doi: 10.1016/S0960-9776(17)30408-3 [DOI] [Google Scholar]
- 31.Chen CM, Klassen AF, Cano SJ, Pusic AL. BCTOS in measuring HR-QoL after breast-conserving therapy. Breast J 2011; 17: 443–43. doi: 10.1111/j.1524-4741.2011.01107.x [DOI] [Google Scholar]
- 32.Kanatas A, Velikova G, Roe B, Horgan K, Ghazali N, Shaw RJ, et al. Patient-Reported outcomes in breast oncology: a review of validated outcome instruments. Tumori 2012; 98: 678–88. doi: 10.1177/030089161209800602 [DOI] [PubMed] [Google Scholar]
- 33.Struik GM, de Jongh FW, Birnie E, Pignol J-P, Klem TM. Development and psychometric evaluation of a Dutch-translated shorter breast cancer treatment outcome scale (Dutch BCTOS-13. J Patient Rep Outcomes 2018; 2: 60. doi: 10.1186/s41687-018-0085-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nortier JWR, Rutgers EJT, van der Sangen MJC, van Tienhoven G, van Vegchel T, Wesseling J, et al. Richtlijn: Mammacarcinoom (2.0. 2013;.
- 35.Clavien PA, Sanabria JR, Strasberg SM. Proposed classification of complications of surgery with examples of utility in cholecystectomy. Surgery 1992; 111: 518–26. [PubMed] [Google Scholar]
- 36.Bland JM, Altman DG. Cronbach's alpha. BMJ 1997; 314: 572. doi: 10.1136/bmj.314.7080.572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Winters ZE, Afzal M, Rutherford C, Holzner B, Rumpold G, da Costa Vieira RA, et al. International validation of the European organisation for research and treatment of cancer QLQ-BRECON23 quality-of-life questionnaire for women undergoing breast reconstruction. Br J Surg 2018; 105: 209–22. doi: 10.1002/bjs.10656 [DOI] [PubMed] [Google Scholar]
- 38.Saarela AO, Kiviniemi HO, Rissanen TJ, Haukipuro K, Kaarela O. Cosmetic results after wire-guided biopsy of benign breast lesions. J Am Coll Surg 1998; 187: 610–5. doi: 10.1016/S1072-7515(98)00252-X [DOI] [PubMed] [Google Scholar]
- 39.Salazar JP, Miranda I, de Torres J, Rus MN, Espinosa-Bravo M, Esgueva A, et al. Percutaneous ultrasound-guided vacuum-assisted excision of benign breast lesions: a learning curve to assess outcomes. Br J Radiol 2019; 92: 20180626. doi: 10.1259/bjr.20180626 [DOI] [PMC free article] [PubMed] [Google Scholar]