Abstract
The first hospital-based treatment facilities for particle therapy started operation about thirty years ago. Since then, the clinical experience with protons and carbon ions has grown continuously and more than 200,000 patients have been treated to date. The promising clinical results led to a rapidly increasing number of treatment facilities and many new facilities are planned or under construction all over the world. An inverted depth–dose profile combined with potential radiobiological advantages make charged particles a precious tool for the treatment of tumours that are particularly radioresistant or located nearby sensitive structures. A rising number of trials have already confirmed the benefits of particle therapy in selected clinical situations and further improvements in beam delivery, image guidance and treatment planning are expected. This review summarises some physical and biological characteristics of accelerated charged particles and gives some examples of their clinical application. Furthermore, challenges and future perspectives of particle therapy will be discussed.
Background
Only a few decades after the first patient treatment with X-rays, another type of radiation has slowly found its way into cancer treatment: particle beams. Back in 1929, Ernest Orlando Lawrence and M.S. Livingston picked up the idea of linear particle accelerators already described by Rolf Widerøe and extended it to a more compact and powerful circular accelerator: the cyclotron.1 In 1934, E.O. Lawrence patented the first cyclotron at the University of California in Berkeley.2 Together with his brother John Lawrence, who was both a physicist and a physician, he conducted first experiments on rats with accelerated particles in 1936. They used fast neutrons, produced by shooting a beryllium target with accelerated deuterons.3 Two years later, in 1938, fast neutrons were used for the first time to treat patients with malignant disease.4 However, treatment with fast neutrons will only gain limited acceptance in the following decades, since the biological advantages are accompanied by heavier side-effects, due to unfavourable depth–dose distributions. This ultimately led to a significant decrease of clinical application since the 1990’s.5–7
In 1946, R.R. Wilson suggested for the first time to use accelerated protons in radiotherapy. He investigated the depth–dose profile of protons produced at the cyclotron in Berkeley and observed a steep increase of energy deposition at the end of the particle range, known as Bragg peak.8 In the same year, simultaneous descriptions of phase stability by Veksler and McMillan enabled further improvements in accelerator technology with the introduction of synchrocyclotrons, isochronous cyclotron and finally synchrotrons. The first proton synchrotron was designed in 1952 by Sir M. Oliphant.9
Proton and deuteron beams were used for the first time on animal tissue at the Lawrence Radiation Laboratory in Berkeley.10 In 1958, the same group reported on the first clinical use of accelerated protons: 26 patients with advanced breast cancer received 340 MeV proton beam therapy to the pituitary gland for hormone suppression in a palliative intention.11 In the following decades, several proton therapy programs expanded and clinical application was further investigated, notably at the Harvard Cyclotron Laboratory in Boston and at the Gustaf Werner Institute in Uppsala, Sweden. The accelerator facilities used had initially been developed for nuclear physics programs, therefore they only had little beam time dedicated to medical purposes and the beam lines were not ideal for patient treatment. With the construction of the proton cyclotron at the Clatterbridge Oncology Center in 1989 and the proton synchrotron at Loma Linda University in 1990, the first dedicated treatment facilities for proton therapy started operation.12 In the meantime, radiotherapy with heavier charged particles, including Helium-, Carbon-, Argon- and Neon-ions, had also been implemented into pre-clinical and clinical trials from the 1970’s on, notably at the BEVALAC in Berkeley.13 Nevertheless, it took some other 20 years, until 1994, for the first dedicated treatment facility for carbon ion radiotherapy, the HIMAC in Chiba (Japan), to start with patient treatment.14
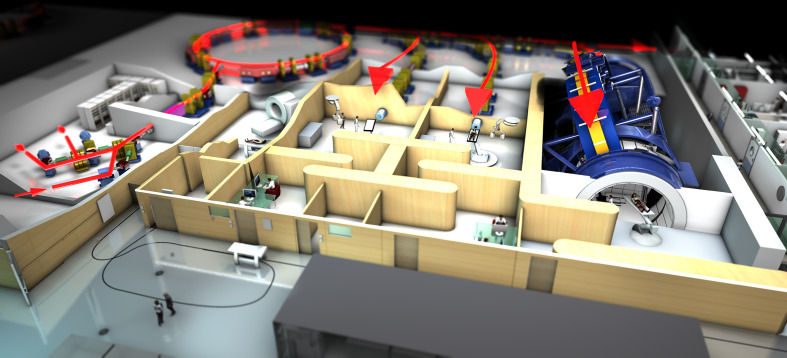
As of December 2018, over 200,000 patients have been treated with particle therapy, mainly protons and carbon ions. The number of treatment facilities has increased rapidly in the last years, especially for proton therapy, and spreads throughout the USA, Europe and Asia.15 To date, only 13 institutions are treating patients with carbon ion beams and there is an ongoing discussion whether the potential benefits can justify higher costs for construction and maintenance of heavy ion facilities (Figure 1).
Figure 1.
Plan of the HIT Center in Germany. This hospital-based facility can treat patients with protons and heavy ions. Particles are accelerated in a synchrotron (at the back left) and three rooms are available for patient treatment (red arrows). The third treatment room on the right features a heavy ion gantry, enabling flexible positioning of the beam delivery system. HIT, Heidelberg Ion Beam Therapy.
In this review, physical and biological properties of particle beams in clinical use today, i.e. carbon ions and protons, will be summarised. Some of the clinical results will be presented and future perspectives of particle therapy will be discussed. In this context, the authors would also like to refer to a recently published special issue dedicated to proton therapy, which summarises clinical findings and current developments and points out future challenges and opportunities.16
The principle of particle therapy
Physical aspects
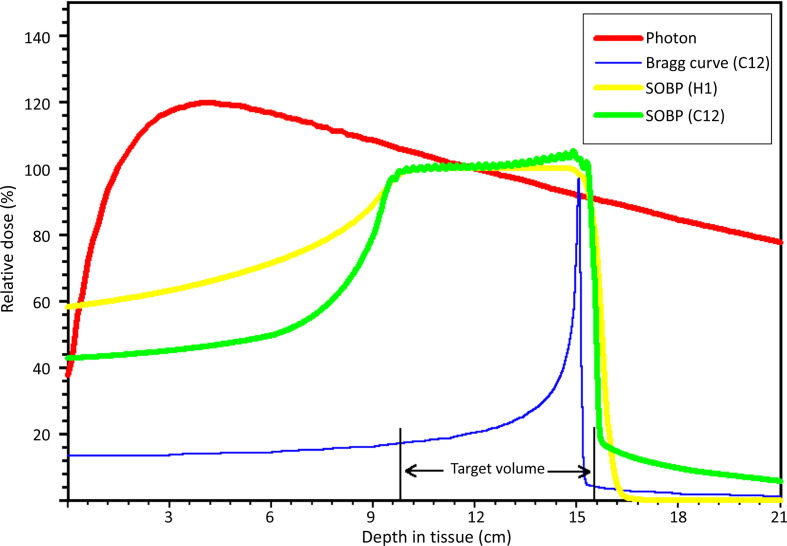
The observations R.R. Wilson made in the 1940’s already revealed essential characteristics of particle beams. The energy deposition in irradiated tissue and therefore the dose distribution differs much from conventional radiotherapy with photons. The main difference is an inverted depth–dose profile leading to sharply increasing energy deposition in narrowly defined areas at the end of the particle’s range. This phenomenon, also called Bragg peak, was first described by Sir William H. Bragg in 1904 on α particles originating from radium salt.17 Charged particles are slowed down in matter and lose energy due to interactions with electrons and nuclei. The probability for these interactions increases as particles get slower, leading to a sudden increment in energy and dose deposition just before the particle stops in a depth defined by its energy. This results in a characteristic Bragg curve, as illustrated for carbon ions in Figure 2. This figure also sketches the main difference when compared to the depth–dose profile of photons (red curve). The low energy deposition in the entrance path and beyond the target volume seem to make charged particles particularly interesting for the means of radiotherapy.
Figure 2.
Schematic diagram of depth–dose distributions of different radiation modalities.
A three-dimensional target volume can generally not be covered by a single monoenergetic particle beam, even when considering a broadened Bragg peak resulting from energy and thus range straggling of different particles.18 Thus, during treatment planning, a set of beams with decreasing energies needs to be superimposed to produce a so-called spread-out Bragg peak (SOBP) (Figure 2). In this way, complex shaped target volumes can receive the prescribed dose with high conformality and homogeneous dose distribution.
Furthermore, charged particles undergo coulomb interactions with nuclei and electrons that cause lateral deviation and consequently beam-broadening. This lateral scattering is more important for lighter ions such as protons, especially for lower energies and deeper penetration depths. For heavier particles such as carbon ions, the lateral scattering is almost negligible.19 In clinical practice and during treatment planning, this difference has meaningful consequences when striving sharp dose gradients in order to spare tissue at risk. A more detailed discussion and comparison of physical properties of protons and carbon ions are provided for instance by Weber et Kraft20 and Durante et al.21,22
Biological aspects
Radiotherapy generally attempts to achieve high dose depositions in a target volume while sparing surrounding healthy tissue. The physical dose distribution of charged particles is particularly well suited for this purpose, as described previously. The density of this dose deposition and the resulting biological effects can be quantified by the linear energy transfer (LET), defined as the amount of energy loss per unit of length of the particle’s track. Higher LET radiation can produce more severe damage on a biomolecular scale. Direct interactions of accelerated heavy charged particles (e.g. carbon ions) with a cell’s DNA can cause cluster lesions with single-strand breaks, cross-links or double-strand breaks that occur close to each other in time and space.23 This disables the tumour cell’s capacity to repair the lesions, resulting in a greater potential for tumour cell inactivation.
To account for the different biological effects of higher-LET radiation, the relative biological effectiveness (RBE) has been introduced. It is the dose ratio of a reference beam (250 keV X-rays) to a particle beam producing the same biological effect. The physical dose of a particle beam needs to be multiplied with a specific RBE in order to obtain the biologically effective dose in Gy (RBE). The RBE depends on several factors, most importantly the LET of the particle, the position within the Bragg peak, dose levels, fractionation, oxygenation, cell lines, cell position within the cell replication cycle and chosen clinical endpoints.24
For protons, the LET stays relatively low and rises only a little towards the end of the SOBP.21 In clinical practice, it is common to define a constant RBE of 1.1 for treatment planning with protons. However, this represents an average value deduced by in vivo measurements, eventually underestimating a local “hot spot” in the distal millimetres of the SOBP, where the RBE can increase steeply.25
For carbon ions, the RBE increases in function of LET within the SOBP while staying low in the entrance path of the beam. Depending on several factors, as mentioned previously, the RBE of carbon ion beams can reach values up to 3–5 in the distal parts of the SOBP.19,26 This selective increase of RBE within the SOBP represents a significant advantage of heavier charged particles in radiotherapy, as the therapeutic ratio of high dose to a target volume and low dose to surrounding tissues can be enhanced by higher biological effects. Also, the oxygen enhancement ratio (OER), i.e. the ratio of doses required to produce the same clinical effect in hypoxic and normoxic conditions, is lower for high LET radiation,27 potentially increasing the cell killing effect in hypoxic and generally radioresistant tumour areas. Furthermore, carbon ion beams might be able to generate stronger immunological responses, although this is still subject to preclinical research at this point in time.28
Beam delivery
Two main beam delivery systems can be distinguished in particle therapy: passive scattering and active scanning.
The passive scattering technique, at first, modulates a monoenergetic beam to different energy levels, e.g. with a rotating modulator wheel, producing the depth–dose distribution of a SOBP. The thin pencil beam is then spread laterally after passing through scattering material. Finally, the beam is shaped by collimators and compensators in order to adapt to the three-dimensional target volume.29
In active scanning techniques, the penetration depth of the beam can be modulated actively by modifying the output energy of the accelerator. The target volume is then split up into isoenergetic slices that are scanned voxel wise by a pencil beam with predefined energy. During the scanning process, the thin pencil beam is deflected by fast moving magnetic dipoles.30 The result is a very flexible, intensity modulated particle therapy with highly conformal three-dimensional dose distributions, also for complexly shaped target volumes. Active scanning techniques offer some other advantages, including sparing of normal tissue in the entrance path, absence collimators and compensators and reduced secondary radiation resulting from particle interaction with scattering material.31
More compact accelerators and beam delivery systems have been developed in the past decades and single-room solutions are available now for proton beam therapy, integrating a synchrocyclotron, a gantry and image guidance systems.32,33 But also for heavy ion treatment facilities, a more compact gantry solution has been designed and started operation in 2017 at the HIMAC in Chiba, Japan.34
Clinical results
The true impact of particle therapy on local tumour control (LC) or overall survival (OAS) can only be determined by clinical trials. The following section will briefly review some of the results of particle beam therapy in use today, i.e. carbon ion beam RT (CIRT) and proton beam RT (PRT), in selected clinical situations.
Chordoma and chondrosarcoma of the skull base
Tumours growing at the skull base are challenging, both for surgery and radiotherapy. Because of vicinity to sensitive organs at risk such as the optical system, cranial nerves, the brainstem, the carotid arteries or the inner ear, surgery is often incomplete or not feasible, requiring definitive or additional radiotherapy.
Chordomas are rare bone tumours, most likely arising from notochordal remnants and therefore generally located at the midline along the spinal axis. About 30% are located at the skull base.35 Tumour growth is generally slow but locally invasive with high recurrence rates. Additional radiotherapy is therefore recommended, even after macroscopic complete resection.36 As suggested by Schulz-Ertner et al. in 2007 and later by McDonald et al,37,38 there is a strong dose–response relationship for chordomas. However, dose escalation with conventional radiotherapy has physical boundaries and is often not sufficient. A selection of studies analysing particle beam therapy after partial resection or biopsy of skull base and cervical spine chordoma are summarised in Table 1. Results with both, CIRT and PRT are promising, yielding LC in 70% and more after 5 years, with only few or no Grade 3 or higher toxicities. For 106 patients treated in a combined protocol with photons and protons, Fung et al have found LC rates of 75% after 5 years with Grade 3 or higher toxicities in seven patients (6.6%).43 A metanalysis conducted by Zhou et al. in 2018 compared overall survival after conventional radiotherapy, stereotactic radiotherapy or particle therapy for skull base chordomas after surgery, suggesting long term survival benefits after particle therapy.44
Table 1.
Selected studies of outcome after particle therapy for skull base/cervical spine chordoma
| Reference | Study design | Modality | n | Median age (range) | Median dose in Gy (RBE) (range) | Fx | Gy (RBE)/Fx | Follow-up in months (range) | LC | OAS |
|---|---|---|---|---|---|---|---|---|---|---|
| Schulz–Ertner et al., 200737 | R | CIRT | 96 | 47 (11–80) | 60 (60–70) | 20 | 3 | 31 (3–91) | 81% (3y) 70% (5y) |
92% (3y) 89% (5y) |
| Mizoe et al., 200939 | P | CIRT | 34 | 47 (16–76) | 60.8 (48.0–60.8) | 16 | 3–3.8 | 53 (8–129) | 85% (5y) 64% (10y) |
88% (5y) 67% (10y) |
| Uhl et al., 201440 | R | CIRT | 155 | 48 (15–85) | 60 (60–70) | 20 | 3 | 72 (12–165) | 82% (3y) 72% (5y) 54% (10y) |
95% (3y) 85% (5y) 75% (10y) |
| Noel et al., 200541 | R | PhRT +PRT | 100 | 53 (8–85) | 45 (29–55) PhRT +2212–38 PRT | 30–39 | 1.8–2.0 | 31 (0–87) | 86% (2y) 54% (4y) |
94% (2y) 81% (5y) |
| Weber et al., 201642 | R | PRT | 151 | 43.3a (18,1 SD) | 72.5 ± 2.2a/b | 35–40 | 1.8–2.0 | 50 (4–176)b | 76% (5y) 71% (7y) |
73% (7y) |
| McDonald et al., 201638 | R | PRT | 39 | 52 (17–78) | 77.4 (70.2–79.2) | 35–40 | 2 | 51 (2–106) | 70% (5y) | 81% (5y) |
| Fung et al., 201843 | R | PhRT +PRT | 106 | 72 (68.4–73.8) | 38–41 | 1.8 | 61 (11–119) | 89% (2y) 78% (4y) 75% (5y) |
99% (2y) 90% (4y) 88% (5y) |
CIRT: carbon ion beam radiotherapy.Fx, Fractions; LC, local control; OAS, overall survival; P, Prospective; PRT, proton beam radiotherapy; PhRT, conventional radiotherapy with photons; R, Retrospective.
Mean values.
Weber et al analysed 222 patients with chordoma (151) or chondrosarcoma (71)of the skull base. Characteristics marked with b refer to the whole patient group.
Low-grade chondrosarcomas are similarly known to be relatively radioresistant, causing the same dilemma of required high doses in a highly sensitive region when located at the skull base. Nevertheless, evidence of safety and high efficacy for particle therapy has been continuously growing, making them indispensable in the multidisciplinary treatment approach for these tumours. Mattke et al. for instance analysed 101 patients with residual tumour at the skull base who had received either CIRT (median 60 Gy(RBE) at 3 Gy (RBE)/fraction) or PRT (median 70 Gy (RBE) at 2 Gy (RBE)/fraction). Control rates were high, with 100% after 1, 2 and 4 years for PRT and 100%, 98.5% and 92.9% after 1, 2 and 4 years for CIRT. No Grade 3 or higher toxicity was observed.45
There is growing evidence demonstrating that particle therapy is beneficial for the treatment of skull base chordomas and chondrosarcomas, although prospective analyses remain sparse. Two ongoing prospective randomised controlled trials are comparing CIRT and PRT for the treatment of skull base chordomas and chondrosarcomas.46,47 The results will also be important to clarify whether the higher RBE of CIRT can further improve the effectiveness of the treatment.
Meningioma
Meningioma are generally benign and slow growing tumours, over 90% classified WHO Grade I.48 Surgery is the treatment of choice, but especially when located at the skull base, e.g. in the petroclival region, resection is often incomplete or not feasible. Postoperative or primary conventional or stereotactic radiotherapy is well established for meningioma of the skull base, achieving promising local control rates of more than 90% after 10 years, a frequent relief of cranial nerve affections and acceptable complication rates.49–51 However, the vicinity to vital structures such as the brain stem or cranial nerves requires steep dose gradients in many situations. Furthermore, reduction of possible late side-effects is indispensable, considering the very good prognosis of these patients. PRT offers high conformality and steep dose gradients, reducing the integral dose exposure of surrounding tissues. Dosimetric treatment plan comparisons confirmed possible advantages over conventional radiotherapy.52,53 The clinical outcome after particle therapy of skull base meningioma has been reported in several studies, some of them are summarised in Table 2. Results were promising, with high local control rates and low toxicities. Besides conventional fractionation, stereotactic radiosurgery with protons has also been used in analogy to modern hypofractionated RT or SRS with photons, suggesting feasibility and good tolerance.55
Table 2.
Selected clinical studies of particle beam therapy for meningioma of the skull base
| Reference | Study design | Histology | % located at skull base | Median/Mean Follow up (range) | Modality | n | Median/Mean age (range) | Median dose in Gy (RBE) (range) | Number of Fx | Gy(RBE)/Fx | LC in % (years) | OAS in % (years) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Noel et al, 200554 | R | WHO°I | 100 | 21 (1–90) | PhRT +PRT | 51 | 56 (11–75) | 60.6 (54–64) 30.6 Gy +30 Gy(RBE) |
30–32 | 1.8–2 | 98% (4y) | 100% (4y) |
| Halasz et al., 201155 | R | WHO°I | 69 | 32 (6–133) | PRT | 50 | 60 (33–85) | 13 (10.0–15.5) | 1 | 13 (10.0–15.5) | 94% (3y) | n.s. |
| El Shafie et al., 201856 | R | WHO°I-III | 100 | 47 | PRT (WHO°I) PhRT +CIRT (WHO°II/III) |
104/6 | 52 (45–59) | 54 Gy(RBE) / (50 Gy)+18 Gy (RBE) | 28–30/25–28+6 | 1.8/3 | 100% (3y) 97% (5y) |
96% (5y) |
| Vlachogiannis et al., 201757 | R | WHO°I | 91 | 84 | PRT | 170 | 54 (22–85) | 21.9 (14–46) | 4–23 | 2–6 | 93% (5y) 85% (10y) |
n.s. |
| Murray et al., 201758 | R | WHO°I-III | 67 | 57 (12–207) | PRT | 96 (64 skull base) | 53 (3–77) | WHO°I: 54 (50.4–64) WHO°II/III: 62.0 (54–68) |
28–34 | 1.8–2 | 86% (5y) | 88% (5y) |
CIRT, carbon ion beam RT; LC, local control; OAS, overall survival; PRT, proton beam RT;PhRT, conventional radiotherapy with photons.
Atypical and anaplastic meningioma (WHO Grade II + III) are less frequent, but treatment is complicated by high local recurrence rates and necessity of high radiation dose to achieve local control. In fact, McDonald et al showed that dose escalation to >60 Gy (RBE) for high-risk meningioma significantly increases local control rates.59 This dose escalation in highly sensitive regions such as the skull-base is difficult, even with modern IMRT techniques or photon SRS. CIRT has the potential to increase dose without threatening organs at risk (OAR) due to favourable dose distributions and enhanced biological effects. Safety and feasibility of CIRT combined with photons has been shown in a Phase I/II trial by Combs et al.60 and the efficacy of this bimodal approach after incomplete resection of atypical meningiomas is currently being investigated in the prospective MARCIE-Trial at HIT in Heidelberg.61
Head and neck cancer
Postoperative or primary radiotherapy is one of the central pillars in the treatment of advanced head and neck cancer (HNC). Nevertheless, morbidity associated with chemoradiotherapy or radiotherapy alone is high, even though the past decades have shown significant improvements with the introduction of intensity modulated radiotherapy (IMRT).62 But even with IMRT, integral dose to normal tissue remains high, due to physical limitations of photon beams in complex shaped target volumes. In 2014, Patel et al. have reviewed 43 cohorts treated with either protons or photons (incl. IMRT) for malignant disease of the nasal cavity and paranasal sinuses. Disease free survival after 5 years and locoregional control at longest follow-up differed significantly in favour of PRT, HR 1.44 (CI 1.01–2.05) and 1.26 (1.05–1.51).63 Other, although retrospective, analyses report lower toxicities after PRT. Some of them are summarised in Table 3. Dosimetric analyses in those trials showed reduced dose maxima to the brainstem and spinal cord as well as reduced mean dose to the contralateral salivary glands and oral cavity.66 Considering the growing interest in individualised target volume delineation and identification of low-risk patients, e.g. HPV-positive oropharyngeal cancer, PRT might be an important asset to further reduce acute and late toxicities. Numerous prospective trials are planned or already recruiting in order to generate reliable data on patient outcome after PRT.67
Table 3.
Selected studies analysing side-effects of PRT vs photon IMRT in head and neck cancer
| Reference | Location | n/modality | Endpoint | Outcome for protons (%) | Outcome for photons (%) | p value |
|---|---|---|---|---|---|---|
| Sio et al., 201664 | Oropharynx | 35 PRT +CHT 46 IMRT +CHT |
Subacute mucus symptoms Subacute appetite symptoms Chronic appetite symptoms Subacute Dysgeusia |
62% 4.68a 2.12a 5.76a |
84% 6.37a 4.14a 7.70a |
0.038 0.048 0.036 0.01 |
| Blanchard et al., 201665 | Oropharynx | 50 PRT 100 IMRT |
Xerostomia°II-III (patient reported, 3 months after RT) Gastrostomy tube or weight loss (>20%) (1 year after RT) |
42% 8% |
61% 24.7% |
0.009 0.01 |
| Romesser et al., 201666 | Major salivary gland or cutaneous SCC (unilateral) | 18 PRT 23 IMRT |
Acute Mucositis >= °II Acute Dysgeusia >= °II Acute Nausea >= °II |
16.7% 5.6% 11.1% |
52.2% 65.2% 56.5% |
0.019 <0.001 0.003 |
CHT, Chemotherapy; IMRT, intensity modulated radiotherapy; PRT, proton beam RT.
Score of the MD Anderson Symptom Inventory-Head and Neck Module (MDASI-HN).
Heavier charged particles can be beneficial when dose escalation is required but not feasible with low LET radiation due to adjacent OAR. This is the case for instance for sinonasal malignancies, where dose escalation with IMRT alone is limited by adjacent eyes, optical nerves or the brain stem.68,69 First results on CIRT alone (median dose 64 Gy (RBE)) for advanced HNC were published at NIRS in Chiba, Japan.70 Specific results for advanced sinonasal malignancies have been published by Koto et al. in 2014 and 2018, yielding promising local control rates of 84% after 2 years, although visual impairment remained the most common Grade 3 or higher toxicity. 71,72 A prospective trial analysing safety and effectiveness of a combination of CIRT+IMRT has finished recruiting in Heidelberg and first results are to be expected soon.73
A particular challenge in the head and neck region are salivary gland tumours with low radiosensitivity. A typical example is adenoid cystic carcinoma (ACC), an uncommon salivary gland tumour with high recurrence rates and perineural spread towards the skull base. As shown in the late last century with fast neutrons, higher LET radiation can achieve higher local control rates.74–76 Based on this rationale, a combination treatment of IMRT and carbon ion boost was tested, yielding promising results and comparing favourably to historical cohorts of IMRT only.77 Subsequently, dose escalation up to 24 Gy (RBE) CIRT followed by 50 Gy IMRT was tested in the COSMIC-Trial, resulting in local control rates of 82% after 3 y with moderate toxicity.78 CIRT has also been used alone to treat salivary gland tumours in Japan, with a median dose of 64 Gy (RBE) in 16 fractions.79 Other retrospective analyses confirmed the good results of CIRT for ACC of different localisations, with or without previous surgery. Some of them are summarised in Table 4. Based on the rationale of enhanced LC with high LET radiation and given that local recurrence of ACC is often in-field, a prospective randomised controlled trial has been launched at the HIT in Heidelberg, comparing CIRT alone (cumulative dose of 66 Gy(RBE)) with the established bimodal concept (50 Gy +24 Gy (RBE)) (NCT04214366, www.clinicaltrials.gov).
Table 4.
Selected clinical studies of CIRT for ACC in the head and neck region
| Reference | Type | Location | n | modality | Dose prescription | LC in % (years) | PFS in % (years) | OAS in % (years) |
|---|---|---|---|---|---|---|---|---|
| Jensen et al., 201577 | R | Paranasal sinus Parotid gland Nasopharynx |
58 37 |
CIRT + IMRT IMRT/FSRT |
18 Gy (RBE)+54 Gy 66 Gy |
59.6% (5y) 39.9% (5y) |
48.4% (5y) 27% (5y) |
76.5% (5y) 58.7% (5y) |
| Jensen et al., 201578 | P | Paranasal sinus, submandibular gland, palate | 53 | CIRT + IMRT | 24 Gy (RBE)+50 Gy | 81.9% (3y) | 57.9% (3y) | 78.4% (3y) |
| Lang et al., 201880 | R | Oral cavity | 67 (postoperative RT) | CIRT + IMRT | 24 Gy (RBE)+50 Gy | 85.5% (5y) | 57.4% (5y) | 74.9% (5y) |
| Akbaba et al., 201981 | R | Paranasal sinuses | 137 (primary RT) 90 (postoperative RT) |
CIRT + IMRT | 18–24 Gy (RBE)+48–56 Gy | 79% (3y) 82% (3y) |
67% (3y) 74% (3y) |
64% (3y) 79% (3y) |
ACC, adenoid cystic carcinoma; CIRT, carbon ion beam RT; FSRT, Fractionated stereotactic radiotherapy; IMRT, intensity-modulated radiotherapy; LC, local control; OAS, overall survival; RBE, relative biological effectiveness.
Re-irradiation
Locoregional recurrence of HNC after primary or postoperative (chemo-)radiotherapy is a challenging situation. Local treatment options with potential curative intent are sparse, surgery is often not feasible and re-irradiation is limited by cumulative dose constraints for surrounding OAR. However, local control of the recurrent disease is of major importance in terms of overall survival but also to preclude uncontrolled tumour growth in the head and neck region, causing significant morbidity and quality of life impairment. Retrospective analyses of re-irradiations in the head and neck region with PRT showed acceptable toxicity and reasonable local control rates.82–84 For instance, Romesser et al. analysed 92 patients with recurrent HNC, treated with a median dose of 60.6 Gy (RBE). Locoregional failure, OAS and distant PFS at 12 months were 25.1%, 65.2% and 84.0% respectively. Major acute toxicities Grade 3 included mucositis, dysphagia, esophagitis and dermatitis (all <10%). Two bleeding-related deaths occurred. Dysphagia and skin toxicity were the only other Grade 3 or higher toxicities, observed in 7.1 and 8.6% respectively.82
CIRT might be of particular use in the treatment of recurrent disease. The rationale are steep dose gradients but also a higher biological effectiveness, potentially valuable in overcoming lower radiosensitivity of recurrent tumour disease. In 2011, Combs et al. analysed first results of re-irradiation with active raster scanning CIRT at GSI in Darmstadt, Germany. In 28 patients with mostly chordoma or chondrosarcoma of the skull base or sacral bone and few cases of other head and neck malignancies, re-irradiation was generally well tolerated with no severe acute or late toxicity, yielding an OAS rate of 86% after 2y.85 Gao et al. have recently analysed 141 patients with recurrent HNC after primary treatment involving radiotherapy, in most cases IMRT. Patients underwent re-irradiation with CIRT in Shanghai, China with a median dose of 60 Gy (RBE). After a median follow-up of 14.7 months, LC and OAS rates 1 year after re-irradiation were 84.9% and 95.9%, respectively. Grade 3 or higher late toxicities were observed in 10.6% of the patients, most common toxicity being mucosal necrosis. The death of four patients was related to haemorrhage after mucosal necrosis.86 In 32 patients treated with CIRT for recurrent HNC with a cumulative dose of 128.6 Gy (RBE; EQD2), Held et al. found local control rates of 66% after 1 y, no Grade 3 or higher toxicities were observed.87 El Shafie et al. have analysed re-irradiation of recurrent intracranial meningioma with CIRT (n = 34) and PRT (n = 8). Local control was promising with 71 and 56.5% after 1 and 2 y, especially considering that 74% were classified high-risk, i.e. WHO Grade II or III. No Grade IV or higher toxicities were observed.88
These results indicate that particle therapy might be a safe and effective option, widening the therapeutic window for this heavily pre-treated patient group that is often not suitable for other local treatments. Nevertheless, those findings need to be validated in prospective trials with meticulous documentation of early and late side-effects.
Younger patients and integral dose reduction
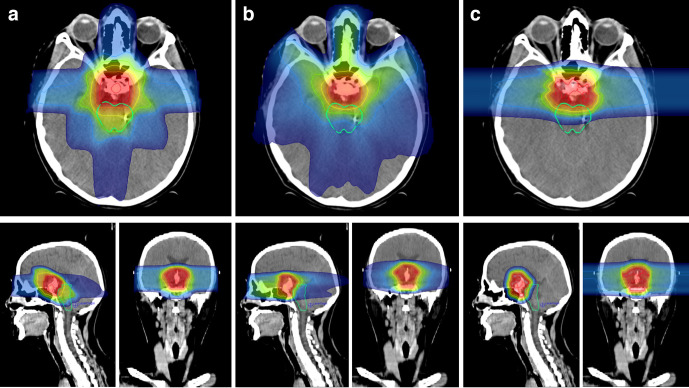
In younger patients and children, exposure of healthy tissue to ionising radiation must be reduced to a minimum, not only because of acute toxicities, but also because they are more likely to experience long-term sequelae and secondary malignancies. Furthermore, damage to organs with central functions in child development, e.g. the pituitary gland, can cause developmental delay and long-term deficiencies. The results of a Phase II trial published by Yock et al. in 2016, analysing ototoxicity, neurocognitive and neuroendocrinological function in young patients with Medulloblastoma, suggest that PRT is a safe modality for craniospinal irradiation, with no difference in PFS (80%) and OAS (83%) after 5 years compared to photon IMRT. After a median follow-up of 7 years, no late cardiac, gastrointestinal or pulmonary toxicities were observed, comparing favourably to historical photon RT studies.89,90 The same group prospectively compared health-related quality of life in paediatric patients with brain tumours after PRT (n = 57) and RT with photons (n = 63), finding significantly higher scores in the psychosocial and physical domains for PRT.91 Tabrizi et al. have recently updated the results of their prospective cohort of 20 patients with low-grade glioma treated with PBRT (54 Gy (RBE)). No overall decline in neurocognitive functioning was observed after a median follow-up of 6.8 years.92 The contralateral hippocampus could be successfully spared (median Dmean: 0 Gy (RBE)), comparing favourably to doses with photon IMRT after hippocampal sparing (median Dmean 23.5 Gy).93 These results are in line with the analysis of 74 young patients and adults with low-grade glioma who underwent PRT at the HIT in Heidelberg. 3D-CRT plans were generated and dosimetric comparison showed significantly lower maximum and mean doses to critical neurological structures and areas of neurogenesis with PRT.94 Figure 3 illustrates the potential benefits of particle therapy, comparing treatment plans of different modalities in an 8-year-old boy with craniopharyngioma. Integral dose can be reduced with PRT and surrounding structures, e.g. the brainstem, are more likely to be spared.
Figure 3.
Exemplary treatment plan comparison in a young patient with craniopharyngioma. A: 3D-CRT with photons, B: IMRT (VMAT) with photons, C: PRT with two opposing beams. Highlighted OAR: Brain stem. IMRT, intensity modulated radiation therapy; OAR, organ at risk; PRT, proton beam radiotherapy; VMAT, volumetric modulated arc therapy
Discussion and perspectives
Particle therapy has the strength of producing highly conformal dose distributions while reducing integral doses to surrounding tissue. But this potential can only be fully exploited when limiting factors concerning beam delivery and target motion are reduced. Especially, active scanning techniques are particularly sensitive to intra- or interfractional anatomical variations within the beam’s path. The scanning process has a time and space dimension and can therefore interfere with time-dependent target motion, also known as the interplay effect.95 As a result, small subvolumes might not receive the prescribed and intended dose. Although this can be partly prevented by optimising treatment plans, some target motions and anatomical changes are unpredictable, requesting better motion control or compensation. A common approach is to predict motion patterns during treatment planning with 4D-computed tomography in order to define an internal target volume (ITV) that accounts for target motions.96 However, this often leads to larger target volumes and correspondingly higher doses to surrounding tissue. Gating techniques compensate organ motion by enabling beam delivery only within predefined thresholds (e.g. end of expiration). Nevertheless, some downsides remain, as for instance uncertainties of external surrogate parameters (respiratory sensors or skin surface monitoring) or required implantation of fiducial markers when using internal surrogates.97 Another method to mitigate interplay effects is rescanning, i.e. repeated irradiation of isoenergetic layers or whole target volumes with smaller doses in order to statistically reduce dose inhomogeneities.98 Newer approaches, e.g. respiratory-correlated rescanning, can adapt to cyclic organ motion and further increase precision of particle beam delivery.95 However, a drawback of these methods is the extended treatment time. Tumour tracking could therefore be an effective approach to account for target motion and mitigate interplay effects.99 A major challenge that will need to be addressed in the future is the tracking speed of beam delivery systems. Although fast lateral displacements are already feasible with fast scanning dipole magnets, adaptation of the beam’s range is still too slow in most particle accelerators. Some solutions combining superconducting cyclotrons with linear accelerators have already been proposed.100 Also, future improvements in computational power to extrapolate and predict organ motion as well as image guidance that is sensitive to soft tissue, e.g. 4D-MRI, will help moving towards a real 4D-conformal dose distribution.
Concerning biological effects of particle therapy, a better understanding of the variability of RBE is required to further improve homogeneity of the biological effective dose distribution. The use of a fixed RBE of 1.1 for protons seems to be too simplistic. Numerous in vitro trials have detected greater RBE values, especially at the end of the SOBP.101–103 The consideration of this RBE variability during treatment planning could further increase effectiveness of PRT and reduce potential side-effects. For CIRT, biological dose computation and treatment planning is more complex and time-consuming. Different calculation models accounting for different parameters that influence the RBE have been developed and are continuously improved and adapted: e.g. the local effect model (LEM) and the Microdosimetric Kinetic Model (MKM) for active scanning techniques.104,105 However, the model used is still choice of the treating institution and a consensus is required to achieve better comparability throughout all CIRT-facilities.106
Since the pioneer work in the 1970–1980s on different particles including Helium (4He) and Neon (20Ne) at the LBL in California,107 mainly protons and carbon ions have been used for particle therapy to date. Nevertheless, there is a growing interest in widening the spectrum of particles used in radiotherapy and preclinical research has led to a better understanding of other particles.108 Helium ions exhibit radiobiological characteristics similar to protons but have some physical benefits such as reduced lateral scattering. First treatment plan comparison studies have shown the potential clinical benefit of 4He ions for selected tumour geometries and localisations.109,110
The use of heavier ions such as oxygen (16O) might seem tempting, considering that increased LET can enhance tumour cell inactivation, especially for hypoxic areas with low radiosensitivity.111 But this gain in RBE within the target volume comes along with higher RBE values in the entrance path of the beam, potentially increasing toxicity. Nevertheless, heavier charged particles might still be beneficial, especially when their attributes of higher LET can be exploited while minimising drawbacks of higher LET in surrounding tissue. A strategy that is currently subject of investigations is dose painting, or LET painting with multiple ions.112 A combination of heavy ions and lighter ones, e.g. 16O and 4He, can result in conformal and uniform high LET distributions in the target volume, as shown in silico by Sokol et al.113 The combination of multiple ions for particle therapy has a great potential to further improve biological dose distribution while sparing surrounding tissue.
Conclusion
There is growing evidence for safety and effectiveness of particle therapy for a variety of clinical situations. Especially for protons, the development of more compact treatment solutions integrating superconducting synchrocyclotrons, gantries and image guidance systems led to a rapidly increasing number of hospital-based treatment facilities.114 About 200,000 patients have been treated with PRT to date. However, there is still a lack of high-level evidence directly comparing PRT with modern conventional radiotherapy techniques. These trials are underway, e.g. for low-grade glioma, oropharyngeal cancer, lung cancer, prostate cancer and liver cancer.115
CIRT has been used in the past three decades in Asia and Europe based on the rationale of favourable dose distribution and higher biological effectiveness. Expansion of heavy charged particle therapy has been much slower, as it demands large investments, sufficient space for bigger accelerators and a team of specialists who are familiar with the physical and biological peculiarities. About 30,000 patients have been treated with CIRT so far, most of them at the NIRS in Japan.116 For some entities such as chordomas, chondrosarcomas and salivary gland tumours, CIRT has proven to be highly effective. For many other tumour entities such as early stage lung cancer, oesophageal cancer, hepatocellular carcinoma, pancreatic cancer or prostate cancer, treatment with CIRT is being investigated.117 Comparing CIRT with conventional radiotherapy remains difficult due to unconventional fractionation regimens and heterogeneous biological dose calculation models. This needs to be addressed in future clinical trials and improved understanding of RBE variability is required. Early data suggest high effectiveness of CIRT for selected tumour entities, but the full potential of heavy charged particles remains unclear and must be determined by further preclinical and clinical research. This should be considered when outweighing costs and benefits of the construction of a heavy ion facility. To our knowledge, new heavy ion facilities are planned or under construction in the USA, France, Taiwan, South Korea, Japan and China.118
Contributor Information
Lukas Schaub, Email: lukas.schaub@med.uni-heidelberg.de.
Semi Ben Harrabi, Email: semi.harrabi@med.uni-heidelberg.de.
Juergen Debus, Email: juergen.debus@med.uni-heidelberg.de.
REFERENCES
- 1.Degiovanni A, Amaldi U. History of hadron therapy accelerators. Phys Med 2015; 31: 322–32. doi: 10.1016/j.ejmp.2015.03.002 [DOI] [PubMed] [Google Scholar]
- 2.Lawrence EO, Patent US. 1948384. „Method and apparatus for the acceleration of ions“ 1934;. [Google Scholar]
- 3.Lawrence JH, Aebersold PC, Lawrence EO. Comparative effects of x-rays and neutrons on normal and tumor tissue. Proc Natl Acad Sci U S A 1936; 22: 543–57. doi: 10.1073/pnas.22.9.543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stone RS, Lawrence JH, Aebersold PC. A preliminary report on the use of fast neutrons in the treatment of malignant disease. Radiology 1940; 35: 322–7. doi: 10.1148/35.3.322 [DOI] [Google Scholar]
- 5.Koh W-J, Griffin TW, Laramore GE, Stelzer KJ, Russell KJ. Fast neutron radiation therapy: results of phase III randomized trials in head and neck, lung, and prostate cancers. Acta Oncol 1994; 33: 293–8. doi: 10.3109/02841869409098420 [DOI] [PubMed] [Google Scholar]
- 6.Maor MH, Errington RD, Caplan RJ, Griffin TW, Laramore GE, Parker RG, et al. . Fast-neutron therapy in advanced head and neck cancer: a Col laborative international randomized trial. Int J Radiat Oncol Biol Phys 1995; 32: 599–604. doi: 10.1016/0360-3016(94)00595-C [DOI] [PubMed] [Google Scholar]
- 7.MacDougall RH, Orr JA, Kerr GR, Duncan W. Fast neutron treatment for squamous cell carcinoma of the head and neck: final report of Edinburgh randomised trial. BMJ 1990; 301: 1241–2. doi: 10.1136/bmj.301.6763.1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilson RR. Radiological use of fast protons. Radiology 1946; 47: 487–91. doi: 10.1148/47.5.487 [DOI] [PubMed] [Google Scholar]
- 9.Rotblat J. Mark Oliphant (1901–2000. Nature 2000; 407: 468. doi: 10.1038/35035202 [DOI] [PubMed] [Google Scholar]
- 10.Tobias CA, Anger HO, Lawrence JH. Radiological use of high energy deuterons and alpha particles. Am J Roentgenol Radium Ther Nucl Med 1952; 67: 1–27. [PubMed] [Google Scholar]
- 11.Lawrence JH, Tobias CA, Born JL, CR M, Roberts JE, Anger HO, et al. . Pituitary irradiation with high-energy proton beams: a preliminary report. Cancer Res 1958; 18: 121–34. [PubMed] [Google Scholar]
- 12.Slater JM, Archambeau JO, Miller DW, Notarus MI, Preston W, Slater JD. The proton treatment center at Loma Linda University medical center: rationale for and description of its development. Int J Radiat Oncol Biol Phys 1992; 22: 383–9. doi: 10.1016/0360-3016(92)90058-P [DOI] [PubMed] [Google Scholar]
- 13.Tobias CA, Blakely EA, Alpen EL, Castro JR, Ainsworth EJ, Curtis SB, et al. . Molecular and cellular radiobiology of heavy ions. Int J Radiat Oncol Biol Phys 1982; 8: 2109–20. doi: 10.1016/0360-3016(82)90554-5 [DOI] [PubMed] [Google Scholar]
- 14.Mohamad O, Makishima H, Kamada T. Evolution of carbon ion radiotherapy at the National Institute of radiological sciences in Japan. Cancers 2018; 10: 66. doi: 10.3390/cancers10030066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jermann M. Particle therapy patient statistics (per end of 2018) (data collected by the particle therapy co-operative group. 2018;.
- 16.Held KD, Lomax AJ, Troost EGC. Proton therapy special feature: introductory editorial. Br J Radiol 2020; 93: 20209004. doi: 10.1259/bjr.20209004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bragg WH, Kleeman R. XXXIX. On the α particles of radium, and their loss of range in passing through various atoms and molecules. The London, Edinburgh, and Dublin Philosophical Magazine and Journal of Science 1905; 10: 318–40. doi: 10.1080/14786440509463378 [DOI] [Google Scholar]
- 18.Bortfeld T. An analytical approximation of the Bragg curve for therapeutic proton beams. Med Phys 1997; 24: 2024–33. doi: 10.1118/1.598116 [DOI] [PubMed] [Google Scholar]
- 19.Suit H, DeLaney T, Goldberg S, Paganetti H, Clasie B, Gerweck L, et al. . Proton vs carbon ion beams in the definitive radiation treatment of cancer patients. Radiotherapy and Oncology 2010; 95: 3–22. doi: 10.1016/j.radonc.2010.01.015 [DOI] [PubMed] [Google Scholar]
- 20.Weber U, Kraft G. Comparison of carbon ions versus protons. The Cancer Journal 2009; 15: 325–32. doi: 10.1097/PPO.0b013e3181b01935 [DOI] [PubMed] [Google Scholar]
- 21.Durante M, Debus J. Heavy charged particles: does improved precision and higher biological effectiveness translate to better outcome in patients? Semin Radiat Oncol 2018; 28: 160–7. doi: 10.1016/j.semradonc.2017.11.00429735192 [DOI] [Google Scholar]
- 22.Durante M, Paganetti H. Nuclear physics in particle therapy: a review. Rep. Prog. Phys. 2016; 79: 096702. doi: 10.1088/0034-4885/79/9/096702 [DOI] [PubMed] [Google Scholar]
- 23.Ward JF. Dna damage produced by ionizing radiation in mammalian cells: identities, mechanisms of formation, and reparability. Prog Nucleic Acid Res Mol Biol 1988; 35: 95–125. [DOI] [PubMed] [Google Scholar]
- 24.Kraft G. Tumor therapy with heavy charged particles. Progress in Particle and Nuclear Physics 2000; 45: S473–544. doi: 10.1016/S0146-6410(00)00112-5 [DOI] [Google Scholar]
- 25.Paganetti H. Relative biological effectiveness (RBE) values for proton beam therapy. variations as a function of biological endpoint, dose, and linear energy transfer. Phys Med Biol 2014; 59: R419–72. doi: 10.1088/0031-9155/59/22/R419 [DOI] [PubMed] [Google Scholar]
- 26.Weyrather WK, Kraft G. RBE of carbon ions: experimental data and the strategy of RBE calculation for treatment planning. Radiotherapy and Oncology 2004; 73(Suppl 2): S161–9. doi: 10.1016/S0167-8140(04)80041-0 [DOI] [PubMed] [Google Scholar]
- 27.Furusawa Y, Fukutsu K, Aoki M, Itsukaichi H, Eguchi-Kasai K, Ohara H, et al. . Inactivation of aerobic and hypoxic cells from three different cell lines by accelerated (3)He-, (12)C- and (20)Ne-ion beams. Radiat Res 2000; 154: 485–96. [DOI] [PubMed] [Google Scholar]
- 28.Durante M, Reppingen N, Held KD. Immunologically augmented cancer treatment using modern radiotherapy. Trends Mol Med 2013; 19: 565–82. doi: 10.1016/j.molmed.2013.05.007 [DOI] [PubMed] [Google Scholar]
- 29.Mohan R, Grosshans D. Proton therapy – present and future. Adv Drug Deliv Rev 2017; 109: 26–44. doi: 10.1016/j.addr.2016.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haberer T, Becher W, Schardt D, Kraft G. Magnetic scanning system for heavy ion therapy. Nuclear Instruments and Methods in Physics Research Section A: Accelerators, Spectrometers, Detectors and Associated Equipment 1993; 330: 296–305. doi: 10.1016/0168-9002(93)91335-K [DOI] [Google Scholar]
- 31.Paganetti H, Bortfeld T, Therapy P. In: Schlegel W, Bortfeld T, Grosu A-L, editors. New technologies in radiation oncology. Berlin, Heidelberg: Springer Berlin Heidelberg 2006;: 345 -–63p.. [Google Scholar]
- 32.Farr JB, Flanz JB, Gerbershagen A, Moyers MF. New horizons in particle therapy systems. Med Phys 2018; 45: e953–83. doi: 10.1002/mp.13193 [DOI] [PubMed] [Google Scholar]
- 33.Contreras J, Zhao T, Perkins S, Sun B, Goddu S, Mutic S, et al. . The world’s first single-room proton therapy facility: Two-year experience. Pract Radiat Oncol 2017; 7: e71–6. doi: 10.1016/j.prro.2016.07.003 [DOI] [PubMed] [Google Scholar]
- 34.Iwata Y, Noda K, Shirai T, Murakami T, Furukawa T, Mori S, et al. . Design of a superconducting rotating gantry for heavy-ion therapy. Physical Review Special Topics - Accelerators and Beams 2012; 15: 044701. doi: 10.1103/PhysRevSTAB.15.044701 [DOI] [Google Scholar]
- 35.McMaster ML, Goldstein AM, Bromley CM, Ishibe N, Parry DM. Chordoma: incidence and survival patterns in the United States, 1973-1995. Cancer Causes Control 2001; 12: 1–11. doi: 10.1023/A:1008947301735 [DOI] [PubMed] [Google Scholar]
- 36.Stacchiotti S, Sommer J. Chordoma global consensus G. building a global consensus approach to chordoma. a position paper from the medical and patient community. Lancet Oncol 2015; 16: e71–83. [DOI] [PubMed] [Google Scholar]
- 37.Schulz-Ertner D, Karger CP, Feuerhake A, Nikoghosyan A, Combs SE, Jäkel O, et al. . Effectiveness of carbon ion radiotherapy in the treatment of skull-base chordomas. Int J Radiat Oncol Biol Phys 2007; 68: 449–57. doi: 10.1016/j.ijrobp.2006.12.059 [DOI] [PubMed] [Google Scholar]
- 38.McDonald MW, Linton OR, Moore MG, Ting JY, Cohen-Gadol AA, Shah MV. Influence of residual tumor volume and radiation dose coverage in outcomes for clival chordoma. Int J Radiat Oncol Biol Phys 2016; 95: 304–11. doi: 10.1016/j.ijrobp.2015.08.011 [DOI] [PubMed] [Google Scholar]
- 39.Mizoe J–etsu, Hasegawa A, Takagi R, Bessho H, Onda T, Tsujii H. Carbon ion radiotherapy for skull base chordoma. Skull Base 2009; 19: 219–24. doi: 10.1055/s-0028-1114295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Uhl M, Mattke M, Welzel T, Roeder F, Oelmann J, Habl G, et al. . Highly effective treatment of skull base chordoma with carbon ion irradiation using a raster scan technique in 155 patients: first long-term results. Cancer 2014; 120: 3410–7. doi: 10.1002/cncr.28877 [DOI] [PubMed] [Google Scholar]
- 41.Noël G, Feuvret L, Calugaru V, Dhermain F, Mammar H, Haie-Méder C, et al. . Chordomas of the base of the skull and upper cervical spine. one hundred patients irradiated by a 3D conformal technique combining photon and proton beams. Acta Oncol 2005; 44: 700–8. doi: 10.1080/02841860500326257 [DOI] [PubMed] [Google Scholar]
- 42.Weber DC, Malyapa R, Albertini F, Bolsi A, Kliebsch U, Walser M, et al. . Long term outcomes of patients with skull-base low-grade chondrosarcoma and chordoma patients treated with pencil beam scanning proton therapy. Radiotherapy and Oncology 2016; 120: 169–74. doi: 10.1016/j.radonc.2016.05.011 [DOI] [PubMed] [Google Scholar]
- 43.Fung V, Calugaru V, Bolle S, Mammar H, Alapetite C, Maingon P, et al. . Proton beam therapy for skull base chordomas in 106 patients: a dose adaptive radiation protocol. Radiotherapy and Oncology 2018; 128: 198–202. doi: 10.1016/j.radonc.2017.12.017 [DOI] [PubMed] [Google Scholar]
- 44.Zhou J, Yang B, Wang X, Jing Z. Comparison of the effectiveness of radiotherapy with photons and particles for chordoma after surgery: a meta-analysis. World Neurosurg 2018; 117: 46–53. doi: 10.1016/j.wneu.2018.05.209 [DOI] [PubMed] [Google Scholar]
- 45.Mattke M, Vogt K, Bougatf N, Welzel T, Oelmann-Avendano J, Hauswald H, et al. . High control rates of proton- and carbon-ion-beam treatment with intensity-modulated active raster scanning in 101 patients with skull base chondrosarcoma at the Heidelberg ion beam therapy center. Cancer 2018; 124: 2036–44. doi: 10.1002/cncr.31298 [DOI] [PubMed] [Google Scholar]
- 46.Nikoghosyan AV, Karapanagiotou-Schenkel I, Münter MW, Jensen AD, Combs SE, Debus J. Randomised trial of proton vs. carbon ion radiation therapy in patients with chordoma of the skull base, clinical phase III study HIT-1-Study. BMC Cancer 2010; 10: 607. doi: 10.1186/1471-2407-10-607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nikoghosyan AV, Rauch G, Münter MW, Jensen AD, Combs SE, Kieser M, et al. . Randomised trial of proton vs. carbon ion radiation therapy in patients with low and intermediate grade chondrosarcoma of the skull base, clinical phase III study. BMC Cancer 2010; 10: 606. doi: 10.1186/1471-2407-10-606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wilson CB. Meningiomas: genetics, malignancy, and the role of radiation in induction and treatment. J Neurosurg 1994; 81: 666–75. doi: 10.3171/jns.1994.81.5.0666 [DOI] [PubMed] [Google Scholar]
- 49.Debus J, Wuendrich M, Pirzkall A, Hoess A, Schlegel W, Zuna I, et al. . High efficacy of fractionated stereotactic radiotherapy of large base-of-skull meningiomas: long-term results. JCO 2001; 19: 3547–53. doi: 10.1200/JCO.2001.19.15.3547 [DOI] [PubMed] [Google Scholar]
- 50.Mendenhall WM, Morris CG, Amdur RJ, Foote KD, Friedman WA. Radiotherapy alone or after subtotal resection for benign skull base meningiomas. Cancer 2003; 98: 1473–82. doi: 10.1002/cncr.11645 [DOI] [PubMed] [Google Scholar]
- 51.Combs SE, Adeberg S, Dittmar J-O, Welzel T, Rieken S, Habermehl D, et al. . Skull base meningiomas: long-term results and patient self-reported outcome in 507 patients treated with fractionated stereotactic radiotherapy (FSRT) or intensity modulated radiotherapy (IMRT. Radiotherapy and Oncology 2013; 106: 186–91. doi: 10.1016/j.radonc.2012.07.008 [DOI] [PubMed] [Google Scholar]
- 52.Kosaki K, Ecker S, Habermehl D, Rieken S, Jäkel O, Herfarth K, et al. . Comparison of intensity modulated radiotherapy (IMRT) with intensity modulated particle therapy (IMPT) using fixed beams or an ion gantry for the treatment of patients with skull base meningiomas. Radiat Oncol 2012; 7: 44. doi: 10.1186/1748-717X-7-44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bolsi A, Fogliata A, Cozzi L. Radiotherapy of small intracranial tumours with different advanced techniques using photon and proton beams: a treatment planning study. Radiotherapy and Oncology 2003; 68: 1–14. doi: 10.1016/S0167-8140(03)00117-8 [DOI] [PubMed] [Google Scholar]
- 54.Noël G, Bollet MA, Calugaru V, Feuvret L, Haie-Meder C, Dhermain F, et al. . Functional outcome of patients with benign meningioma treated by 3D conformal irradiation with a combination of photons and protons. Int J Radiat Oncol Biol Phys 2005; 62: 1412–22. doi: 10.1016/j.ijrobp.2004.12.048 [DOI] [PubMed] [Google Scholar]
- 55.Halasz LM, Bussière MR, Dennis ER, Niemierko A, Chapman PH, Loeffler JS, et al. . Proton stereotactic radiosurgery for the treatment of benign meningiomas. Int J Radiat Oncol Biol Phys 2011; 81: 1428–35. doi: 10.1016/j.ijrobp.2010.07.1991 [DOI] [PubMed] [Google Scholar]
- 56.El Shafie RA, Czech M, Kessel KA, Habermehl D, Weber D, Rieken S, et al. . Clinical outcome after particle therapy for meningiomas of the skull base: toxicity and local control in patients treated with active rasterscanning. Radiat Oncol 2018; 13: 54. doi: 10.1186/s13014-018-1002-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vlachogiannis P, Gudjonsson O, Montelius A, Grusell E, Isacsson U, Nilsson K, et al. . Hypofractionated high-energy proton-beam irradiation is an alternative treatment for who grade I meningiomas. Acta Neurochir 2017; 159: 2391–400. doi: 10.1007/s00701-017-3352-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Murray FR, Snider JW, Bolsi A, Lomax AJ, Walser M, Kliebsch U, et al. . Long-Term clinical outcomes of pencil beam scanning proton therapy for benign and non-benign intracranial meningiomas. Int J Radiat Oncol Biol Phys 2017; 99: 1190–8. doi: 10.1016/j.ijrobp.2017.08.005 [DOI] [PubMed] [Google Scholar]
- 59.McDonald MW, Plankenhorn DA, McMullen KP, Henderson MA, Dropcho EJ, Shah MV, et al. . Proton therapy for atypical meningiomas. J Neurooncol 2015; 123: 123–8. doi: 10.1007/s11060-015-1770-9 [DOI] [PubMed] [Google Scholar]
- 60.Combs SE, Hartmann C, Nikoghosyan A, Jäkel O, Karger CP, Haberer T, et al. . Carbon ion radiation therapy for high-risk meningiomas. Radiotherapy and Oncology 2010; 95: 54–9. doi: 10.1016/j.radonc.2009.12.029 [DOI] [PubMed] [Google Scholar]
- 61.Combs SE, Edler L, Burkholder I, Rieken S, Habermehl D, Jäkel O, et al. . Treatment of patients with atypical meningiomas Simpson grade 4 and 5 with a carbon ion boost in combination with postoperative photon radiotherapy: the MARCIE trial. BMC Cancer 2010; 10: 615. doi: 10.1186/1471-2407-10-615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.van der Veen J, Nuyts S. Can Intensity-Modulated-Radiotherapy reduce toxicity in head and neck squamous cell carcinoma? Cancers 2017; 9: 135. doi: 10.3390/cancers9100135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Patel SH, Wang Z, Wong WW, Murad MH, Buckey CR, Mohammed K, et al. . Charged particle therapy versus photon therapy for paranasal sinus and nasal cavity malignant diseases: a systematic review and meta-analysis. Lancet Oncol 2014; 15: 1027–38. doi: 10.1016/S1470-2045(14)70268-2 [DOI] [PubMed] [Google Scholar]
- 64.Sio TT, Lin H-K, Shi Q, Gunn GB, Cleeland CS, Lee JJ, et al. . Intensity modulated proton therapy versus intensity modulated photon radiation therapy for oropharyngeal cancer: first comparative results of patient-reported outcomes. Int J Radiat Oncol Biol Phys 2016; 95: 1107–14. doi: 10.1016/j.ijrobp.2016.02.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Blanchard P, Garden AS, Gunn GB, Rosenthal DI, Morrison WH, Hernandez M, et al. . Intensity-Modulated proton beam therapy (IMPT) versus intensity-modulated photon therapy (IMRT) for patients with oropharynx cancer – a case matched analysis. Radiotherapy and Oncology 2016; 120: 48–55. doi: 10.1016/j.radonc.2016.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Romesser PB, Cahlon O, Scher E, Zhou Y, Berry SL, Rybkin A, et al. . Proton beam radiation therapy results in significantly reduced toxicity compared with intensity-modulated radiation therapy for head and neck tumors that require ipsilateral radiation. Radiotherapy and Oncology 2016; 118: 286–92. doi: 10.1016/j.radonc.2015.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hu M, Jiang L, Cui X, Zhang J, Yu J. Proton beam therapy for cancer in the era of precision medicine. J Hematol Oncol 2018; 11: 136. doi: 10.1186/s13045-018-0683-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hoppe BS, Nelson CJ, Gomez DR, Stegman LD, Wu AJ, Wolden SL, et al. . Unresectable carcinoma of the paranasal sinuses: outcomes and toxicities. Int J Radiat Oncol Biol Phys 2008; 72: 763–9. doi: 10.1016/j.ijrobp.2008.01.038 [DOI] [PubMed] [Google Scholar]
- 69.Madani I, Bonte K, Vakaet L, Boterberg T, De Neve W. Intensity-Modulated radiotherapy for sinonasal tumors: Ghent university hospital update. Int J Radiat Oncol Biol Phys 2009; 73: 424–32. doi: 10.1016/j.ijrobp.2008.04.037 [DOI] [PubMed] [Google Scholar]
- 70.Mizoe J-etsu, Hasegawa A, Jingu K, Takagi R, Bessyo H, Morikawa T, et al. . Results of carbon ion radiotherapy for head and neck cancer. Radiotherapy and Oncology 2012; 103: 32–7. doi: 10.1016/j.radonc.2011.12.013 [DOI] [PubMed] [Google Scholar]
- 71.Koto M, Hasegawa A, Takagi R, Sasahara G, Ikawa H, Mizoe J-etsu, et al. . Feasibility of carbon ion radiotherapy for locally advanced sinonasal adenocarcinoma. Radiotherapy and Oncology 2014; 113: 60–5. doi: 10.1016/j.radonc.2014.09.009 [DOI] [PubMed] [Google Scholar]
- 72.Koto M, Demizu Y, Saitoh J-ichi, Suefuji H, Tsuji H, Okimoto T, et al. . Definitive Carbon-Ion radiation therapy for locally advanced sinonasal malignant tumors: subgroup analysis of a multicenter study by the Japan Carbon-Ion radiation oncology Study Group (J-CROS. Int J Radiat Oncol Biol Phys 2018; 102: 353–61. doi: 10.1016/j.ijrobp.2018.05.074 [DOI] [PubMed] [Google Scholar]
- 73.Jensen AD, Nikoghosyan AV, Windemuth-Kieselbach C, Debus J, Münter MW. Treatment of malignant sinonasal tumours with intensity-modulated radiotherapy (IMRT) and carbon ion boost (C12. BMC Cancer 2011; 11: 190. doi: 10.1186/1471-2407-11-190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Douglas JG, Koh W-jin, Austin-Seymour M, Laramore GE. Treatment of salivary gland neoplasms with fast neutron radiotherapy. Arch Otolaryngol Head Neck Surg 2003; 129: 944–8. doi: 10.1001/archotol.129.9.944 [DOI] [PubMed] [Google Scholar]
- 75.Douglas JG, Laramore GE, Austin-Seymour M, Koh W-J, Lindsley KL, Cho P, et al. . Neutron radiotherapy for adenoid cystic carcinoma of minor salivary glands. Int J Radiat Oncol Biol Phys 1996; 36: 87–93. doi: 10.1016/S0360-3016(96)00213-1 [DOI] [PubMed] [Google Scholar]
- 76.Huber PE, Debus J, Latz D, Zierhut D, Bischof M, Wannenmacher M, et al. . Radiotherapy for advanced adenoid cystic carcinoma: neutrons. photons or mixed beam? Radiother Oncol 2001; 59: 161–7. [DOI] [PubMed] [Google Scholar]
- 77.Jensen AD, Nikoghosyan AV, Poulakis M, Höss A, Haberer T, Jäkel O, et al. . Combined intensity-modulated radiotherapy plus raster-scanned carbon ion boost for advanced adenoid cystic carcinoma of the head and neck results in superior locoregional control and overall survival. Cancer 2015; 121: 3001–9. doi: 10.1002/cncr.29443 [DOI] [PubMed] [Google Scholar]
- 78.Jensen AD, Nikoghosyan AV, Lossner K, Haberer T, Jäkel O, Münter MW, et al. . Cosmic: a regimen of intensity modulated radiation therapy plus dose-escalated, Raster-Scanned carbon ion boost for malignant salivary gland tumors: results of the prospective phase 2 trial. Int J Radiat Oncol Biol Phys 2015; 93: 37–46. doi: 10.1016/j.ijrobp.2015.05.013 [DOI] [PubMed] [Google Scholar]
- 79.Hayashi K, Koto M, Demizu Y, Saitoh J-ichi, Suefuji H, Okimoto T, et al. . A retrospective multicenter study of carbon-ion radiotherapy for major salivary gland carcinomas: subanalysis of J-CROS 1402 HN. Cancer Sci 2018; 109: 1576–82. doi: 10.1111/cas.13558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lang K, Baur M, Akbaba S, Held T, Kargus S, Bougatf N, et al. . Intensity modulated radiotherapy (IMRT) + carbon ion boost for adenoid cystic carcinoma of the minor salivary glands in the oral cavity. Cancers 2018; 10: 488. doi: 10.3390/cancers10120488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Akbaba S, Ahmed D, Mock A, Held T, Bahadir S, Lang K, et al. . Treatment outcome of 227 patients with sinonasal adenoid cystic carcinoma (ACC) after intensity modulated radiotherapy and active Raster-Scanning carbon ion boost: a 10-year single-center experience. Cancers 2019; 11: 1705. doi: 10.3390/cancers11111705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Romesser PB, Cahlon O, Scher ED, Hug EB, Sine K, DeSelm C, et al. . Proton beam reirradiation for recurrent head and neck cancer: multi-institutional report on feasibility and early outcomes. Int J Radiat Oncol Biol Phys 2016; 95: 386–95. doi: 10.1016/j.ijrobp.2016.02.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.McDonald MW, Zolali-Meybodi O, Lehnert SJ, Estabrook NC, Liu Y, Cohen-Gadol AA, et al. . Reirradiation of recurrent and second primary head and neck cancer with proton therapy. Int J Radiat Oncol Biol Phys 2016; 96: 808–19. doi: 10.1016/j.ijrobp.2016.07.037 [DOI] [PubMed] [Google Scholar]
- 84.Phan J, Sio TT, Nguyen TP, Takiar V, Gunn GB, Garden AS, et al. . Reirradiation of head and neck cancers with proton therapy: outcomes and analyses. Int J Radiat Oncol Biol Phys 2016; 96: 30–41. doi: 10.1016/j.ijrobp.2016.03.053 [DOI] [PubMed] [Google Scholar]
- 85.Combs SE, Kalbe A, Nikoghosyan A, Ackermann B, Jäkel O, Haberer T, et al. . Carbon ion radiotherapy performed as re-irradiation using active beam delivery in patients with tumors of the brain, skull base and sacral region. Radiotherapy and Oncology 2011; 98: 63–7. doi: 10.1016/j.radonc.2010.10.010 [DOI] [PubMed] [Google Scholar]
- 86.Gao J, Hu J, Guan X, Yang J, Hu W, Kong L, et al. . Salvage Carbon-Ion radiation therapy for locoregionally recurrent head and neck malignancies. Sci Rep 2019; 9: 4259. doi: 10.1038/s41598-019-39241-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Held T, Windisch P, Akbaba S, Lang K, Farnia B, Liermann J, et al. . Rare entities in head-and-neck cancer: salvage re-irradiation with carbon ions. Radiat Oncol 2019; 14: 202. doi: 10.1186/s13014-019-1406-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.El Shafie RA, Czech M, Kessel KA, Habermehl D, Weber D, Rieken S, et al. . Evaluation of particle radiotherapy for the re-irradiation of recurrent intracranial meningioma. Radiat Oncol 2018; 13: 86. doi: 10.1186/s13014-018-1026-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yock TI, Yeap BY, Ebb DH, Weyman E, Eaton BR, Sherry NA, et al. . Long-Term toxic effects of proton radiotherapy for paediatric medulloblastoma: a phase 2 single-arm study. Lancet Oncol 2016; 17: 287–98. doi: 10.1016/S1470-2045(15)00167-9 [DOI] [PubMed] [Google Scholar]
- 90.Huang TT, Chen Y, Dietz AC, Yasui Y, Donaldson SS, Stokes DC, et al. . Pulmonary outcomes in survivors of childhood central nervous system malignancies: a report from the childhood cancer Survivor study. Pediatr Blood Cancer 2014; 61: 319–25. doi: 10.1002/pbc.24819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yock TI, Bhat S, Szymonifka J, Yeap BY, Delahaye J, Donaldson SS, et al. . Quality of life outcomes in proton and photon treated pediatric brain tumor survivors. Radiotherapy and Oncology 2014; 113: 89–94. doi: 10.1016/j.radonc.2014.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tabrizi S, Yeap BY, Sherman JC, Nachtigall LB, Colvin MK, Dworkin M, et al. . Long-Term outcomes and late adverse effects of a prospective study on proton radiotherapy for patients with low-grade glioma. Radiotherapy and Oncology 2019; 137: 95–101. doi: 10.1016/j.radonc.2019.04.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Marsh JC, Godbole R, Diaz AZ, Gielda BT, Turian JV. Sparing of the hippocampus, limbic circuit and neural stem cell compartment during partial brain radiotherapy for glioma: a dosimetric feasibility study. J Med Imaging Radiat Oncol 2011; 55: 442–9. doi: 10.1111/j.1754-9485.2011.02282.x [DOI] [PubMed] [Google Scholar]
- 94.Harrabi SB, Bougatf N, Mohr A, Haberer T, Herfarth K, Combs SE, et al. . Dosimetric advantages of proton therapy over conventional radiotherapy with photons in young patients and adults with low-grade glioma. Strahlenther Onkol 2016; 192: 759–69. doi: 10.1007/s00066-016-1005-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mori S, Knopf Antje‐Christin, Umegaki K. Motion management in particle therapy. Med Phys 2018; 45: e994–1010. doi: 10.1002/mp.12679 [DOI] [PubMed] [Google Scholar]
- 96.Tashiro M, Ishii T, Koya J-ichi, Okada R, Kurosawa Y, Arai K, et al. . Technical approach to individualized respiratory-gated carbon-ion therapy for mobile organs. Radiol Phys Technol 2013; 6: 356–66. doi: 10.1007/s12194-013-0208-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shimizu S, Miyamoto N, Matsuura T, Fujii Y, Umezawa M, Umegaki K, et al. . A proton beam therapy system dedicated to spot-scanning increases accuracy with moving tumors by real-time imaging and gating and reduces equipment size. PLoS One 2014; 9: e94971. doi: 10.1371/journal.pone.0094971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Phillips MH, Pedroni E, Blattmann H, Boehringer T, Coray A, Scheib S. Effects of respiratory motion on dose uniformity with a charged particle scanning method. Phys Med Biol 1992; 37: 223–33. doi: 10.1088/0031-9155/37/1/016 [DOI] [PubMed] [Google Scholar]
- 99.Bert C, Saito N, Schmidt A, Chaudhri N, Schardt D, Rietzel E. Target motion tracking with a scanned particle beam. Med Phys 2007; 34: 4768–71. doi: 10.1118/1.2815934 [DOI] [PubMed] [Google Scholar]
- 100.Garonna A, Amaldi U, Bonomi R, Campo D, Degiovanni A, Garlasché M, et al. . Cyclinac medical accelerators using pulsed C6+/H2+ion sources. Journal of Instrumentation 2010; 5C09004-C. [Google Scholar]
- 101.Maeda K, Yasui H, Matsuura T, Yamamori T, Suzuki M, Nagane M, et al. . Evaluation of the relative biological effectiveness of spot-scanning proton irradiation in vitro. J Radiat Res 2016; 57: 307–11. doi: 10.1093/jrr/rrv101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jones B, Underwood TSA, Dale RG. The potential impact of relative biological effectiveness uncertainty on charged particle treatment prescriptions. Br J Radiol 2011; 84 Spec No 1;:: S61–984 Spec No. doi: 10.1259/bjr/36792876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jones B. Clinical radiobiology of proton therapy: modeling of RBE. Acta Oncol 2017; 56: 1374–8. doi: 10.1080/0284186X.2017.1343496 [DOI] [PubMed] [Google Scholar]
- 104.Scholz M. Kraft G. A parameter-free track structure model for heavy ion action cross sections. United Kingdom: Adam Hilger; 1992. [Google Scholar]
- 105.Inaniwa T, Kanematsu N, Matsufuji N, Kanai T, Shirai T, Noda K, et al. . Reformulation of a clinical-dose system for carbon-ion radiotherapy treatment planning at the National Institute of radiological sciences, Japan. Phys Med Biol 2015; 60: 3271–86. doi: 10.1088/0031-9155/60/8/3271 [DOI] [PubMed] [Google Scholar]
- 106.Fossati P, Matsufuji N, Kamada T, Karger CP. Radiobiological issues in prospective carbon ion therapy trials. Med Phys 2018; 45: e1096–110. doi: 10.1002/mp.12506 [DOI] [PubMed] [Google Scholar]
- 107.Castro JR. Results of heavy ion radiotherapy. Radiat Environ Biophys 1995; 34: 45–8. doi: 10.1007/BF01210545 [DOI] [PubMed] [Google Scholar]
- 108.Tommasino F, Scifoni E, Durante M. New ions for therapy. Int J Part Ther 2016; 2: 428–38. doi: 10.14338/IJPT-15-00027.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Knäusl B, Fuchs H, Dieckmann K, Georg D. Can particle beam therapy be improved using helium ions? - a planning study focusing on pediatric patients. Acta Oncol 2016; 55: 751–9. doi: 10.3109/0284186X.2015.1125016 [DOI] [PubMed] [Google Scholar]
- 110.Tessonnier T, Mairani A, Chen W, Sala P, Cerutti F, Ferrari A, et al. . Proton and helium ion radiotherapy for meningioma tumors: a Monte Carlo-based treatment planning comparison. Radiat Oncol 2018; 13: 2. doi: 10.1186/s13014-017-0944-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sokol O, Scifoni E, Tinganelli W, Kraft-Weyrather W, Wiedemann J, Maier A, et al. . Oxygen beams for therapy: advanced biological treatment planning and experimental verification. Phys Med Biol 2017; 62: 7798–813. doi: 10.1088/1361-6560/aa88a0 [DOI] [PubMed] [Google Scholar]
- 112.Bassler N, Jäkel O, Søndergaard CS, Petersen JB. Dose- and LET-painting with particle therapy. Acta Oncol 2010; 49: 1170–6. doi: 10.3109/0284186X.2010.510640 [DOI] [PubMed] [Google Scholar]
- 113.Sokol O, Krämer M, Hild S, Durante M, Scifoni E. Kill painting of hypoxic tumors with multiple ion beams. Phys Med Biol 2019; 64: 045008. doi: 10.1088/1361-6560/aafe40 [DOI] [PubMed] [Google Scholar]
- 114.Zhao T, Sun B, Grantham K, Rankine L, Cai B, Goddu SM, et al. . Commissioning and initial experience with the first clinical gantry-mounted proton therapy system. J Appl Clin Med Phys 2016; 17: 24–40. doi: 10.1120/jacmp.v17i2.5868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mishra MV, Aggarwal S, Bentzen SM, Knight N, Mehta MP, Regine WF. Establishing evidence-based indications for proton therapy: an overview of current clinical trials. Int J Radiat Oncol Biol Phys 2017; 97: 228–35. doi: 10.1016/j.ijrobp.2016.10.045 [DOI] [PubMed] [Google Scholar]
- 116.Kamada T, Tsujii H, Blakely EA, Debus J, De Neve W, Durante M, et al. . Carbon ion radiotherapy in Japan: an assessment of 20 years of clinical experience. Lancet Oncol 2015; 16: e93–100. doi: 10.1016/S1470-2045(14)70412-7 [DOI] [PubMed] [Google Scholar]
- 117.Lazar AA, Schulte R, Faddegon B, Blakely EA, Roach M. Clinical trials involving carbon-ion radiation therapy and the path forward. Cancer 2018; 124: 4467–76. doi: 10.1002/cncr.31662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Malouff TD, Mahajan A, Krishnan S, Beltran C, Seneviratne DS, Trifiletti DM. Carbon ion therapy: a modern review of an emerging technology. Front Oncol 2020; 10: 82. doi: 10.3389/fonc.2020.00082 [DOI] [PMC free article] [PubMed] [Google Scholar]