Abstract
Background
Respiratory syncytial virus (RSV) is responsible for most respiratory tract infections and hospitalizations in infants and represents a significant economic burden for public health. The development of a safe, effective, and affordable vaccine is a priority for the WHO.
Methods
We conducted a double-blinded, escalating-dose phase 1 clinical trial in healthy males aged 18-50 years to evaluate safety, tolerability, and immunogenicity of a recombinant Mycobacterium bovis BCG vaccine expressing the nucleoprotein of RSV (rBCG-N-hRSV). Once inclusion criteria were met, volunteers were enrolled in three cohorts in an open and successive design. Each cohort included six volunteers vaccinated with 5 × 103, 5 × 104, or 1 × 105 CFU, as well as two volunteers vaccinated with the full dose of the standard BCG vaccine. This clinical trial (clinicaltrials.gov NCT03213405) was conducted in Santiago, Chile.
Findings
The rBCG-N-RSV vaccine was safe, well-tolerated, and no serious adverse events related to the vaccine were recorded. Serum IgG-antibodies directed against Mycobacterium and the N-protein of RSV increased after vaccination, which were capable of neutralizing RSV in vitro. Additionally, all volunteers displayed increased cellular response consisting of IFN-γ and IL-2 production against PPD and the N-protein, starting at day 14 and 30 post-vaccination respectively.
Interpretation
The rBCG-N-hRSV vaccine had a good safety profile and induced specific cellular and humoral responses.
Funding
This work was supported by Millennium Institute on Immunology and Immunotherapy from Chile (P09/016), FONDECYT 1190830, and FONDEF D11E1098.
Keywords: Human respiratory syncytial virus, BCG vaccine, Phase I clinical trial, Safety, Transmissibility, Immunogenicity
Research in Context.
Evidence before this study
In 2008, two recombinant Mycobacterium bovis BCGs were able to induce a protective response against the infection caused by human respiratory syncytial virus (RSV), mediated by a T cell protective response, in a murine model. Reports from 2010 indicate that this protective response was best with the recombinant BCG expressing the N protein (rBCG-N-hRSV) and that it was mediated by both CD4+ and CD8+ T cells, in mice. In 2017, a single, low dose of the cGMP rBCG-N-hRSV vaccine in mice was able to protect against the disease caused by RSV, as shown in previous reports. Also, immunization was safe with no adverse events in mice; and a PPD response could be detected up to 6 months after immunization. Finally, in 2018 immunization of mice with rBCG-N-hRSV was able to induce the secretion of antibodies against several viral proteins, with increased neutralizing capacities. Moreover, sera transfer assays were able to protect naïve mice from infection with RSV.
Added value of this study
This is the first time that the cGMP rBCG-N-hRSV is administered to humans (healthy male adults), and safety and immunogenicity responses are evaluated. We show that the vaccine is safe and well-tolerated, and the immune response elicited by the highest dose tested can last for at least six months.
Implications of all the available evidence
This study suggests that this vaccine is an adequate candidate to proceed with new clinical trials that may allow this vaccine candidate to be eventually administered to newborns to prevent the disease caused by RSV.
Alt-text: Unlabelled box
1. Introduction
Respiratory syncytial virus (RSV) is one of the leading causes of acute respiratory tract infections (ARTIs) in children worldwide and is associated with significant health and economic burden, frequently leading to severe diseases such as bronchiolitis and pneumonia [1], [2], [3]. Extrapulmonary symptoms have also been associated with RSV infection [4,5]. Currently, the only prophylactic approach against RSV consists of a humanized IgG monoclonal antibody (palivizumab) that prevents epithelial cell infection; although it is mainly aimed for high-risk infants and its protection is short-lived [6]. Therefore, vaccines against RSV are highly needed [7]. Ideally, these vaccines should avoid T helper (Th)-2 cell responses, that lead to enhanced pulmonary disease as previously seen [8], [9], [10], [11], [12]. According to PATH (www.path.com), currently, there are approximately fifteen vaccine prototypes against RSV being evaluated in clinical trials [13]. rBCG-N-hRSV is a recombinant Mycobacterium bovis bacillus Calmette-Guérin (BCG) that expresses the nucleoprotein (N) of RSV [14]. BCG, the vector of this vaccine, is currently routinely administered to newborns in most countries -such as Japan, Mexico, China, Brazil, and Chile, among others [15]- and has been used for almost a century to prevent tuberculosis meningitis and miliary disease in children [16]. A final volume of 0.05 mL of a reconstituted vial (which usually contains about 2-8 × 106 CFU of the bacteria per 0.1 mL) of this vaccine is administered intradermally [17], resulting in the administration of about 1–4 × 105 CFU of BCG to a newborn. BCG induces a strong Th-1 immune response and therefore is likely a suitable vector for a vaccine against RSV, since a Th-1 response may be better for the clearance of viral pathogens [14,18]. In murine models, rBCG-N-hRSV vaccination prevented RSV associated-lung damage, decreased pro-inflammatory infiltration into this tissue, and induced early recruitment of CD4+ and CD8+ T cells into the lungs [14,19,20]. rBCG-N-hRSV vaccination also induced protective T cells and the secretion of antibodies against several RSV proteins other than the N-protein upon infection -a response associated with a mechanism previously described as Linked Recognition [21] – with neutralizing capacity in vitro and an antibody isotype suitable for viral clearance [19,21].
Here, we report the results of a double-blind dose-escalation phase 1 clinical trial to evaluate the safety, tolerability, and immunogenicity of three different doses of cGMP rBCG-N-hRSV in healthy males aged 18–50 years.
The vaccine was well tolerated in healthy adults with no severe adverse effects related to the immunization, while inducing both T cell and antibody responses against RSV -either viral antigens or a clinical isolate- with increasing neutralizing capacities. Our results suggest that rBCG-N-hRSV is a safe vaccine that could induce protection against RSV-infection.
2. Material and methods
2.1. Vaccine
The study vaccine is a live attenuated recombinant Mycobacterium bovis BCG based on the Danish strain 1331 that expresses the nucleoprotein (N) of RSV (rBCG-N-hRSV) [14,19,21]. The vaccine was designed at the Pontificia Universidad Católica de Chile and manufactured under cGMP conditions at IDT Biologika (USA). The control vaccine is a live attenuated Mycobacterium bovis BCG Serum Institute of India (SII) strain. Both vaccines were administered intradermally over the deltoid area.
2.2. Ethical statements and clinical trial information
This clinical trial (clinicaltrials.gov NCT03213405) was conducted in Santiago, Chile, at a single site in the Clinical Research Center of the Pontificia Universidad Católica de Chile (CICUC). The final posting date of the study in clinicaltrails.gov was defined in compliance of regulatory requirements from local authorities.
The study protocol was conducted according to the current guidelines of the Tripartite Guidelines for Good Clinical Practices, the Declaration of Helsinki [22], and local regulations. The clinical trial was approved by both the Institutional Ethical Committee (number 15-216) and the Chilean Public Health Institute (ISP Chile, number EC819077/16). Written informed consent was obtained from each participant before study entry. Volunteers did not receive any payment for their participation.
2.3. Study design
This phase 1 study was double-blinded and with dose-escalation to evaluate the safety, tolerability, and immunogenicity of rBCG-N-hRSV in healthy males, aged 18-50 years.
2.3.1. Inclusion/exclusion criteria and cohorts
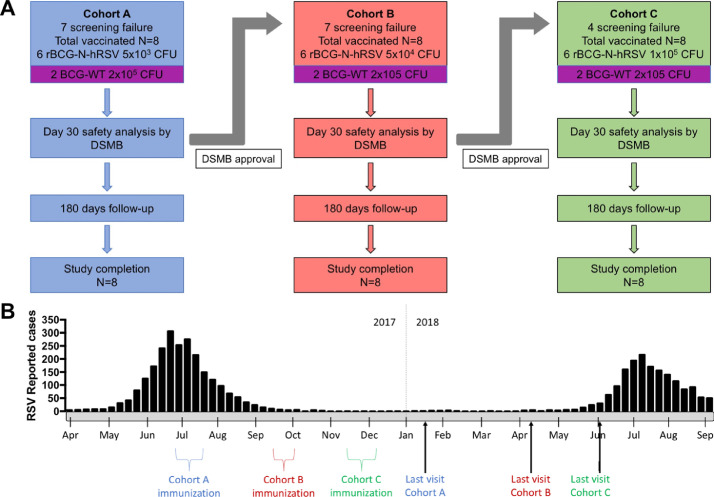
A sample of 24 subjects was recruited, considering a usual phase I studies sample size (20-100 subjects). Once inclusion criteria were met, volunteers were enrolled in one of three cohorts, each containing eight subjects (6 receiving the study vaccine and two the standard BCG as control vaccine) in an open and ascending dose cohort design (Table S1 and Fig. A). In each cohort, they were vaccinated with 0.1 mL of the vaccine, as follows: 6 volunteers in Cohort A were vaccinated with the lowest dose (5 × 103 CFU); 6 volunteers in Cohort B received the middle dose (5 × 104 CFU); 6 volunteers in Cohort C received the highest dose (1 × 105 CFU) of the study vaccine. Each cohort included two volunteers vaccinated with 0.1 mL of the standard BCG vaccine (BCG-WT; a full dose of 1-33 × 105 CFU) (Fig. 1A). As the adult dose of BCG vaccine is in the order of 105 CFU, we decided to use this dose as the highest, with 103 and 104 as the lower doses.
Fig. 1.
Clinical study flow diagram and timeline. This phase 1 clinical study was double-blinded and dose-escalated. (A) Each cohort included 6 volunteers vaccinated with escalating doses of rBCG-N-hRSV (Cohort A: 5 × 103 CFU (blue); Cohort B: 5 × 104 CFU (red); Cohort C: 1 × 105 CFU (green)) and 2 volunteers vaccinated with the standard BCG at full dose (BCG-WT 2 × 105 CFU (purple)). A DSMB evaluated the safety data of each cohort after the first 30 days of follow-up and decided if escalation could continue. (B) A timeline indicating the periods of immunization and end of visits is shown. RSV peaks reported during 2017 and 2018 are also indicated.
2.3.2. Randomization
Volunteers in each cohort were randomized to study vaccine (n=6) or to BCG control vaccine (n=2), using a statistical application (random.org). The study had one unblinded nurse who only vaccinated the participants. The rest of the study team was kept blinded. A Data and Safety Monitoring Board (DSMB) evaluated the safety data of each cohort after the first 30 days of follow up and decided if the next cohort could be vaccinated. Further information of the study can be found in the Study protocol included as Appendix 1.
2.4. Study schedule and follow-up
Inclusion criteria included healthy -as determined by medical history, physical examination, and laboratory tests- male adults ranging between 18-50 years old, previously vaccinated with 1 or 2 doses of BCG. A detailed list of the inclusion and exclusion criteria is presented in the supplementary material (Table S1).
Participants received a single dose of the vaccine, administered intradermally in the deltoid area following the standard national protocol for BCG vaccination used in Chile, and were observed during the following 3 hours. Participants were then evaluated at 1–3, 7, 14, 30, 60, 120, and 180 days post-vaccination (dpv) (Table 1). Follow up phone calls were performed at 4, 21, 45, 90, and 150 dpv. The study was performed between July 2017 and June 2018 (Fig. 1B).
Table 1.
Follow up schedule.
| Screening | Va | Follow up | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day | -10 | -3 | 0 | 1 | 2 | 3 | 4 | 7 | 14 | 21 | 30 | 45 | 60 | 90 | 120 | 150 | 180 |
| Clinical evaluation | x | x | x | x | x | x | x | x | x | x | x | x | |||||
| Informed Consent | x | ||||||||||||||||
| Transmissibility samples | x | x | x | ||||||||||||||
| Immunogenicity samples | x | x | x | x | x | x | |||||||||||
| Adverse Events report | x | x | x | x | x | x | x | x | x | x | x | x | x | x | x | ||
*Va=Vaccination.
2.5. Primary outcome: safety evaluation
2.5.1. Clinical evaluation
Adverse events (AEs): Local solicited AEs included pain, sensitivity, erythema, induration, pustule, scab, and axillary lymphadenopathy; these AEs were registered until the end of the study. General (systemic) solicited AEs included fever, tachycardia, hypo/hypertension, headache, fatigue, myalgia, nausea/vomiting, and diarrhea. These AEs were recorded for 30 dpv.
General AEs included general solicited AEs occurring beyond 30 dpv, and any other general AEs up to 180 dpv, as well as concomitant medications administered to volunteers. AEs were collected by self-report on diary cards, in study visits, and study phone calls.
Serious adverse event (SAEs) were defined as any untoward medical occurrence that: resulted in death; was life-threatening; required hospitalization or prolongation of existing hospitalization; resulted in disability/incapacity or resulted in a congenital anomaly/congenital disability in the offspring of a study subject. For this study, grade 4 laboratory abnormalities were also considered as SAEs.
AEs intensity: pain and sensitivity were graded as I: mild pain or tactile discomfort, II: pain or discomfort upon movement, the requirement for analgesics, III: significant pain or discomfort at rest, use of narcotic analgesics, or prevention of regular activities and IV: AEs requiring emergency unit consultation. Erythema and induration were graded as I: 25–50 mm, II: 51–100 mm, III: >100 mm. Pustule and scab were graded as I: 1–9 mm, II: 10-30 mm, III: >30 mm, IV: necrosis. Lymphadenopathy was graded as I: regional, up to 1 cm, II: regional 1,1 to 5 cm, III: multiple, or unique > 5 cm or suppurated, IV: disseminated. Other AEs were graded as I: does not interfere with normal activities, II: some interference, III: prevents normal activities, and IV: AEs requires emergency unit consultation [23].
2.5.2. Laboratory tests
Hematological and biochemical parameters included blood counts (hemoglobin, leukocytes, neutrophils, lymphocytes, and platelet count), transaminases, bilirubin, cholesterol, creatinine, ureic nitrogen, plasmatic electrolytes, coagulation test (aPTT and prothrombin time), creatine phosphokinase (CPK), and urine analyses. Laboratory tests were performed at 7, 14, 30, 60, 120, and 180 dpv. Grading of laboratory adverse events was assessed by the Toxicity Grading Scale for Healthy Adult and Adolescent Volunteers Enrolled in Preventive Vaccine Clinical Trials of the U.S. Department of Health and Human Services [23].
2.5.3. Transmissibility assays
rBCG-N-hRSV in blood, urine, and saliva of vaccinated volunteers was evaluated by PCR-evaluating the presence of both Mycobacterium genes and the n gene of RSV- and by conventional mycobacterial culture. DNA was extracted from body fluids by QIAamp kits (Qiagen). Further information on molecular analysis is included in the Supplementary Material. For conventional cultures, samples of blood, urine, and saliva were seeded on selective culture media for Mycobacteria and observed 21 days after to evaluate the presence or absence of colony-forming units. Transmissibility of the vaccine was evaluated in all cohorts at three different time points (7, 30, and 180 dpv).
2.5.4. Criteria for vaccine safety and tolerability
We determined that immunization was safe and well-tolerated if no subjects presented: (1) grade 4 local or general solicited AEs; (2) grade 4 general or laboratory AEs related to the vaccine; (3) SAEs considered related to the vaccine, or (4) transmissibility of any of the vaccine components demonstrated in body fluids.
Assessment of causality included the following categories: non-related (occurrence of the AEs is not reasonably related temporarily with the vaccine, or there is a cause that explains the event), possibly-related (administration of the study vaccine and the AEs are reasonably related temporarily, but an alternative cause is more likely to be responsible for AEs), and probably-related (administration of the study vaccine and the AEs are reasonably related temporarily, and the vaccine is more likely to be responsible for AEs than other causes).
2.6. Secondary outcomes: immunogenicity assays
2.6.1. Humoral immunogenicity assays
All humoral immunogenicity assays were performed with serum obtained from peripheral blood of volunteers at 0, 14, 30, 60, 120, and 180 dpv. Samples were used for indirect ELISA assays and neutralization assays, as indicated in the Supplementary Material section. Quantification of specific anti-N and anti-PPD antibody concentrations were performed by ELISA, and RSV neutralization capacities of these antibodies were determined by plaque reduction assays without complement, as described in [24]. Further information can be found in the Supplementary Material.
2.6.2. Cellular immunogenicity assays
All cellular immunogenicity assays were performed using PBMCs obtained from volunteers. Briefly, 20 mL of venous blood were collected in heparinized tubes (BD Vacutainer), and PBMCs were purified by a gradient of centrifugation with Leukocyte Separation Medium (LSM, Corning, Virginia, USA). Cells were collected, washed, counted in a Neubauer chamber, and finally frozen. All samples were stored in a −150 °C Ultrafreezer until ELISPOT and flow cytometry assays were performed. Flow cytometry assays and ELISPOT assays were performed as indicated in the Supplementary Material section. Once used, all samples were evaluated in a blinded manner. Flow cytometry assays were performed to characterize cellular populations and the production of cytokines by these populations in PBMC samples. ELISPOT assays were performed to detect cells secreting either IL-2 or IFN-γ.
2.7. Statistical analyses
A sample of 24 subjects was recruited, considering a usual phase I studies sample size (20–100 subjects). A power calculation to estimate frequency of AE was not performed, as we used a conventional sample size for a phase 1 study (20–100 subjects). Descriptive analyses were performed for local solicited, general, and laboratory adverse events (AEs). All immunogenicity statistical analyses were performed using GraphPad Prism version 7.0 Software. Statistical significances were assessed using one-way or two-way ANOVA test with a posteriori Tukey test when corresponded. Additional information is indicated in each figure legend.
2.8. Role of the funding sources
The funding sources had no role during the study design; in the collection, analysis, or interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
3. Results
3.1. Subjects
42 males were screened, and 24 healthy males with ages ranging from 19 to 44 were enrolled and vaccinated, 8 in each cohort (Fig. 1A). All participants were Hispanic and Chilean. Screening failures were due to detection of Gilbert Syndrome [n=5], hepatitis B and latent Mycobacterium tuberculosis infection [n=1], HIV infection [n=1], BCG scars [n=1], and screening tests out of the normal range [n=10]. Weight ranged from 57.3 to 91 kg, height from 167 to 183 cm, and body mass index (BMI) from 19.2 to 29.9 kg/m2 (Table S2). Follow up of vaccinated participants was performed as described in Table 1. The successive cohort vaccinations were conducted without interruption, as approved by the DSMB.
3.2. Safety and reactogenicity
All participants were followed for six months after vaccination, with no withdrawals or lost-to-follow ups. All vaccines were well tolerated; there were two SAEs, none considered related to the vaccine; no participants were discontinued due to an AEs.
3.2.1. Clinical adverse events (AEs)
Local solicited AEs: most frequent were sensitivity, pustule, and scab, occurring in all participants (N=24). Pain was present in 23 individuals, erythema in 19, induration in 13, and lymphadenopathy in 2 (in both cases, lymphadenopathy was less than 2 cm, non-suppurative, and resolved without treatment). Erythema was detected more frequently with increasing doses of the study vaccine (Table 2, sub-table 1). Days (median) post-vaccination for the onset of pustule and scab decreased with increasing doses of the study vaccine (Table 2, sub-table 2). The median duration of pustule and pain increased with increasing doses of the study vaccine (Table 2, sub-table 3). The intensity of local solicited AEs was mostly mild (grade 1: 70.9%) and moderate (grade 2: 27.6%). Only one participant, who received the highest dose of the study vaccine, reported severe intensity (grade 3) for sensitivity and pain; however, his symptoms lasted for just one day as grade 3. When analyzing the subset of local solicited AEs with moderate (grade 2) intensity, subjects with pustule and scab increased in number with increasing doses of the study vaccine (Table 2, sub-table 4).
General solicited AEs: eighty general solicited AEs were reported, 72 (90.0%) were classified as mild intensity, and 8 (10.0%) as moderate intensity. The most frequent were headache (37.5%), fatigue (17.5%), diarrhea (15.0%), and myalgia (12.5%). Considering all general solicited AEs, we found no association with increasing doses of the study vaccine (Table 2, sub-table 5).
General AEs: there was a total of 77 general AEs (Table S3). There was no tendency in the frequency of general AEs with the dose of the vaccine received. Sixty-eight were considered not related to the vaccine, and nine were considered possibly related. General AEs considered possibly related to the vaccine were diarrhea: (2), nausea (1), bilateral otalgia (1), retro-orbital pain (1), headache (1), leg pain (1), scalp pain (1), malaise (1). Urticaria occurred in one subject of the highest dose vaccine group 12 days after vaccination, which was considered not related to the vaccine.
3.2.2. Laboratory examinations
Overall, 123 laboratory AEs were reported, as shown in detail in Table S4. None was considered probably related to the study vaccine. 92 (74.8%) were classified as mild or grade 1; 22 (17.9%) as moderate or grade 2; 8 (6.5%) as severe or grade 3; and 1 (0.8%) as grade 4, which was classified as SAEs (see below). Grade 3 and 4 AEs are presented more in detail in Table S5.
3.2.3. Severe adverse events (SAEs)
Two SAEs were reported, neither considered related to study vaccine: (i) a grade 4 increase in CPK 15 days after vaccination with the control BCG, related to intense physical exercise two days before the exam. CPK values returned to normal levels in an analysis performed 16 days later; (ii) a one-day hospitalization due to thoracic pain secondary to supraventricular tachycardia (previous condition of the subject) detected 51 days after vaccination with the control BCG (not included).
3.2.4. Transmissibility
None of the subjects evaluated were positive at any of the time points for the n gene of RSV in the samples evaluated. Non-pathogenic Mycobacteria were identified by culture in saliva samples in 3 subjects; in one of them, PCR and sequencing were also performed. In addition, urine samples at 30 and 180 dpv of one subject of the intermediate cohort were positive for the 16S RNA of M. bovis. In this subject, two extra control genes were tested to evaluate a possible relationship to the immunization: the is6110 and the esat6 genes, which have been previously reported to be used for the characterization of Mycobacterium, specifically indicating whether the identified bacterium belongs to the Tuberculosis complex -such as M. bovis- or not. In this sample, these genes were undetected, suggesting that the Mycobacteria was not M. bovis, and therefore should not be associated with the study vaccine [25,26]. Table S6 shows the non-pathogenic Mycobacteria species identified, and additional information can be found in the Supplementary Material.
3.3. Immunogenicity
3.3.1. Humoral immune response
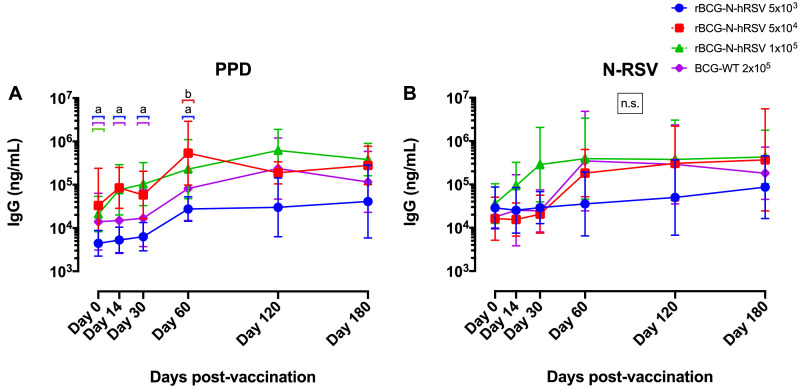
We evaluated antibody production against BCG (anti-PPD) and RSV (anti-N-RSV) in response to rBCG-N-hRSV immunization (Fig. 2). For PPD, we detected antibodies in subjects vaccinated with all the doses tested for rBCG-N-hRSV, as well as in the subjects vaccinated with BCG-WT (Fig. 2A). The highest anti-PPD antibody production was observed at 60 and 120 dpv, with the intermediate dose of the rBCG-N-hRSV (Cohort B: 5 × 104 CFU) exhibiting the highest concentration at 60 dpv, as compared to the other doses used and the BCG control (BCG-WT: 2 × 105 CFU) (Fig. 2A). This point -60 dpv for Cohort B- was statistically different as compared to all the other groups at 0 dpv and also to the lowest dose of the study vaccine (Cohort A: 5 × 103 CFU) and the BCG-WT control group, at 14 and 30 dpv. For anti-N-RSV antibodies, we observed an increase in the concentrations of all the groups at 60-, 120- and 180- dpv, although this was not statistically significant. We also observed an early increase in the levels of anti-N antibodies at 30 dpv in the subjects who received the highest dose of rBCG-N-hRSV (Cohort C: 1 × 105), as compared to the subjects in Cohort A or BCG-WT (Fig. 2B). Accordingly, from 14 to 60 dpv, Cohort C exhibited the highest concentration of anti-N antibodies, supporting our dose-escalated study. At 120 dpv, all cohorts exhibited increased levels of anti-N-RSV antibodies, suggesting possible natural exposure to RSV -due to seasonal outbreak-, which would promote antibody production in all the cohorts for both vaccines (Fig. 2B). However, in BCG-WT immunized volunteers antibodies concentrations starts to wane at 120 dpv, while rBCG-N-hRSV immunized volunteers keep their antibody levels high, up until 180 dpv. To better understand the effect of natural exposure to RSV in the anti-N antibody response, we determined the anti-N antibody fold-increase -normalized to day 0- for each volunteer (Fig. S1). In cohort A, which was immunized in the middle of the RSV seasonal outbreak (Fig. 2B), we observed a more rapid and pronounced fold-increase of anti-N antibodies at 14 dpv in 4 out of 6 volunteers immunized with the rBCG-N-hRSV vaccine, as compared to volunteers of the same cohort immunized with BCG WT (Fig. S1A and D). In Cohort B, which was immunized at the end of the RSV outbreak, fold-increase of anti-N antibodies started to rise at 60 dpv, suggesting that -probably due to recent RSV exposure- levels of anti-N antibodies were already high (Fig. S1B). In cohort C, which was immunized when the RSV outbreak was over for at least 2 months, fold-increase of anti-N antibodies started immediately at 14 dpv, and it was even more pronounced than the one seen for cohort A (Fig. S1C). Therefore, we can suggest that rBCG-N-hRSV induce a sustained production of anti-N antibodies, at least for Cohorts A and C.
Fig. 2.
PPD- and N-RSV-specific humoral immune response in rBCG-N-hRSV-immunized volunteers. Anti-PPD and anti-N-RSV IgG levels were measured in the sera of all the volunteers at 0, 14, 30, 60, 120, and 180 dpv with rBCG-N-hRSV doses 5 × 103 (blue), 5 × 104 (red), and 1 × 105 (green) CFU and the full dose of BCG-WT (Purple). IgG levels are expressed as the geometric mean (and geometric standard deviation) of the concentrations of IgG (ng/mL) on a logarithmic scale. The responses against (A) PPD and (B) N-RSV antigens are shown. Statistical differences were evaluated by a two-way ANOVA with a posterior Tukey test. If different letters are shown above a specific time point (with their respective color-bar representing the corresponding group), then statistical differences (p<0.05) were found among those groups. If no letters are indicated above a group, then no statistical differences were found among them. n.s. = no statistical differences were found among any of the groups.
3.3.2. Antibody neutralization assays
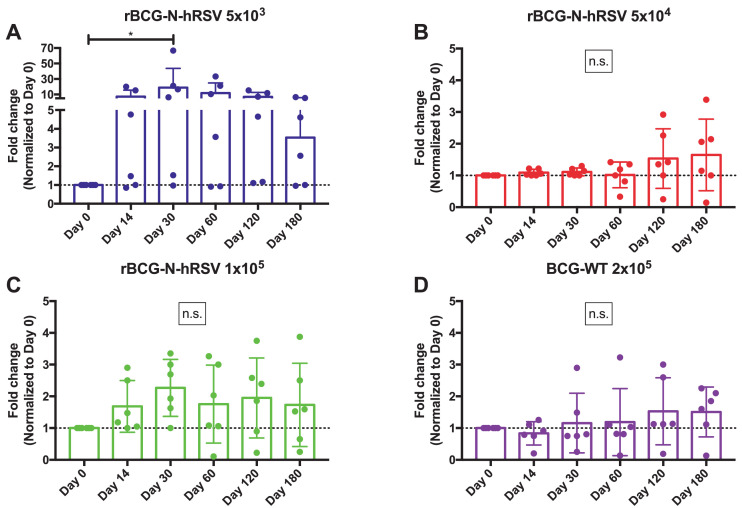
Since the production of total anti-N antibodies in the immunized subjects could be due to seasonal exposure to RSV (Fig. 1B), neutralization assays were performed as described previously [21], in order to determine whether immunization with rBCG-N-hRSV resulted in improved neutralizing capacities of the produced antibodies. Fig. 3 shows the neutralizing capacities -depicted as Fold Change normalized to day 0- of the antibodies obtained from the escalating doses of the rBCG-N-hRSV (Fig. 3A-C) and the BCG-WT (Fig. 3D) immunized subjects. These assays were performed evaluating the neutralizing capacity detected in a fixed amount of total IgG for all the volunteers in the three cohorts (0.75 µg, equivalent to a dilution 1/2,560), using a neutralization assay protocol described previously, with modifications [24]. We observed increased neutralization capacities of viral particles, starting from 14 dpv, in the sera obtained from subjects immunized with rBCG-N-hRSV, as compared to the neutralization capacity detected at day 0. In agreement with the results shown in Fig. S1, higher increases of neutralization capacities were observed for volunteers immunized with rBCG-N-hRSV in cohort A, as compared to cohort B and C. These could be related to recent seasonal RSV exposure of some volunteers included in cohorts B and C, which showed >50% neutralizing capacities at day 0 (data not shown). Nevertheless, in each cohort immunized with rBCG-N-hRSV the neutralization capacity of the sera increased over time (Fig. 2A–C). No significant differences in the neutralization ability of the sera were detected for BCG-WT volunteers, although a subtle increase in the percentage of neutralization was observed at 120 dpv. Fig. S2 shows the fold change of the neutralization capacities for each volunteer, while figure S3A shows pictures of the changes in the neutralization capacities of one volunteer for each cohort, for all the time points evaluated. Two positive controls -a polyclonal pool of anti-RSV antibodies from BEI resources (NR-21973) and palivizumab- were included in the assays to confirm reproducibility of the results, and their neutralizing effects are shown in Fig. S3B.
Fig. 3.
Evaluation of the neutralizing capacity of antibodies obtained from vaccinated subjects. The neutralizing capacities of the sera obtained from the immunized subjects were tested and are shown as fold change (Normalized to Day 0 for each subject). The sera samples were tested at 0, 14, 30, 60, 120, and 180 dpv with rBCG-N-hRSV doses 5 × 103 (A - Blue), 5 × 104 (B - Red), and 1 × 105 (C - Green) CFU and the full dose of BCG-WT (D - Purple). Neutralization was tested with amounts of IgG ranging from 10 µg to 0.1 µg, which are equivalent to dilutions 1/160 and 1/20.480, respectively. Highest amounts resulted in 100% neutralization, while lowest amounts resulted in 0% neutralization. Therefore, the level of neutralization for 0.75 µg of total antibodies -dilution 1/2.560- is shown. Statistical differences were evaluated by a one-way ANOVA with a posterior Tukey test. n.s.= No statistical differences; *=P<0.05.
The results obtained from these neutralization assays suggest that immunization with rBCG-N-hRSV most-likely promotes the production of antibodies with increasing neutralizing capacities -as compared to the BCG-WT group- as early as 14 dpv.
3.3.3. Cellular immune response
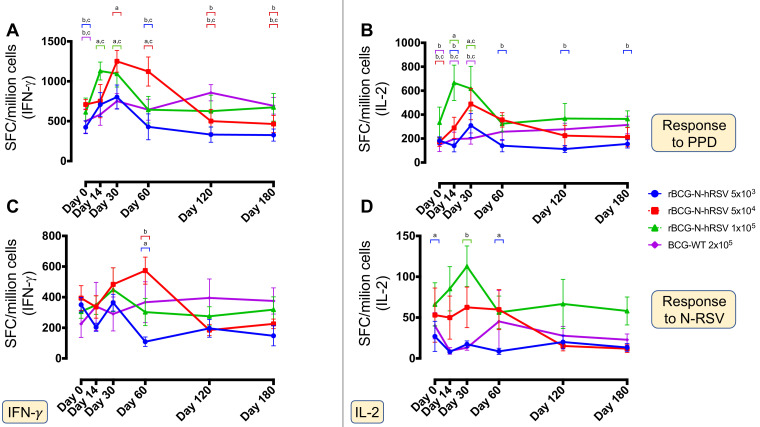
PPD and N-RSV-specific immune responses in vaccinated subjects were evaluated by ELISPOT (Fig. 4) and flow cytometry assays (Fig. S4). The number of IFN-γ+- and IL-2+-producing cells were evaluated by ELISPOT and are shown as the number of spot-forming-cells (SFC) per million cells (Fig. 4). The data obtained show a dose-dependent increase in the number of IFN-γ+ and IL-2+ secreting cells in response to PPD at 14 dpv, which was at least 4-fold times higher -and statistically different- in Cohort C, as compared to the BCG-WT group (Fig. 4A and B). A similar response was observed at 30 dpv, in which the response against PPD was dose-dependent. At later time points -180 dpv-, the response detected in the volunteers from Cohort A and B and BCG-WT was not higher than the one observed in Cohort C volunteers. An increased response of IFN-γ+-secreting cells upon stimulation with N-RSV was detected in Cohorts B and C, while an increased response in IL-2+-secreting cells was detected in volunteers included in Cohort C, particularly at 14 dpv (Fig. 4C and D). This increased response was only detected in subjects vaccinated with rBCG-N-hRSV, since vaccination with BCG-WT mostly exhibited an invariable response. Also, levels of IFN-γ+− and IL-2+-secreting cells remained stable during the 180 days of monitoring (Fig. 4C and D).
Fig. 4.
PPD- and N-RSV-specific cellular immune response in the rBCG-N-hRSV-immunized subjects evaluated by ELISPOT. Cellular responses to PPD (A and B) and N-RSV (C and D) antigens in all PBMCs samples were measured at 0, 14, 30, 60, 120, and 180 dpv with rBCG-N-hRSV doses 5 × 103 (blue), 5 × 104 (red), and 1 × 105 (green) CFU and the full dose of BCG-WT (Purple). The IFN-γ- (A and C) and IL-2- (B and D) secreting cells were detected by ELISPOT and plotted as spot-forming cells (SFC) per million cells. Statistical differences were evaluated by a two-way ANOVA with a posterior Tukey test. If different letters are shown above a specific time point (with their respective color-bar representing the corresponding group), then statistical differences (p<0.05) were found among those groups. If no letters are indicated above a group, then no statistical differences were found among them.
Additionally, specific IFN-γ+-IL-2+ (γ−2) and TNF-α+-IL-2+ (α−2) secreting CD4+ and CD8+ T cells were evaluated by flow cytometry (Fig. S4). The highest numbers of γ−2 and α−2 cells were detected starting at 30 dpv against PPD, mainly in Cohort C (Fig. S3A–D). This response was similarly found in both CD4+ and CD8+ T cells. A lower but similar response was observed against N-RSV in Cohort C, exhibiting higher numbers of γ−2 and α−2 -both CD4+ and CD8+- secreting T cells (Fig. S3E-H). The response to both PPD and N-RSV antigens showed a constant increase in time when γ−2 T cells were evaluated, as compared to α−2 T cells, which was not constant.
4. Discussion
The rBCG-N-hRSV vaccine candidate was safe, well-tolerated, and immunogenic in healthy male adult volunteers. No SAEs related to the vaccine were reported up to 180 dpv. Local response to the BCG component of the vaccine (development of pustule and then a scab) occurred as expected. Although none of the local reactions were severe, higher reactogenicity was observed in some parameters as the study vaccine dose increased. All local reactions were well tolerated and resolved spontaneously. Additionally, no general AEs nor laboratory abnormalities probably-related to the study vaccine were found. Mycobacterium bovis and the RSV component in the vaccine were not observed in the different fluids evaluated, suggesting that there is no vaccine excretion. These data suggest that this BCG based vaccine has an adequate safety profile, similar to the routinely used BCG [27], and therefore it could be used in the future to replace the regular vaccine administered to newborns.
We measured immunogenicity against PPD and the N-RSV protein by evaluating the production of specific IgGs and the T cell response against these antigens. Specific IgGs increased upon immunization in all the subjects evaluated for antibodies against both PPD and N-RSV. However, this response was dependent on the dose used and may be influenced by the time of the year when cohorts were immunized, as some of the subjects were probably exposed to natural RSV infection during autumn and winter seasons. In this line, recruitment and immunization of volunteers per cohort are shown in Fig. 1B. Cohort A was mostly recruited during July, the month when the outbreak of RSV was reported. This overlap could result in low levels of both cells and antibodies specific for RSV at day 0 in Cohort A, as compared to the levels found in Cohort B and C at the same time (Fig S3), and this could have a masking effect over the interpretation of the results obtained by these particular cohorts. Despite this, subjects immunized with the highest dose of rBCG-N-hRSV showed the earliest increase of anti-N antibodies -14 dpv-, which lasted longer -up to 180 dpv. Importantly, this effect in cohort C was unlikely affected by seasonal exposure to RSV during the study (Figure 1B). Subjects immunized with BCG-WT were distributed among the three cohorts, which resulted in subjects exposed to the same conditions as each cohort. Nevertheless, we observed increasing neutralization abilities in the subjects immunized with rBCG-N-hRSV, as compared to the subjects immunized with BCG-WT, which was more evident for cohort A. This is depicted by the fold-increases at 60 and 120 dpv in the rBCG-N-hRSV immunized subjects, as compared to the BCG-WT immunized subjects. These results support the notion that immunization with rBCG-N-hRSV could promote the production of anti-RSV antibodies more effectively. However, future clinical trials need to be performed when the circulation of RSV is low, including more BCG-WT immunized as well as non-immunized volunteers, in order to evaluate immunogenicity specifically induced by the study vaccine, ruling out the effect of seasonal RSV exposure.
We found that cells from all volunteers responded to an in vitro stimulation with the RSV N protein, as determined by the secretion of IFN-γ, IL-2, and TNF-α (Figs. 3 and S6). Consistent with these data, all participants exhibited increased numbers of SFC and higher frequencies of IFN-γ-secreting CD4+ and CD8+ T cells after in vitro stimulation with the RSV N protein, as determined by ELISPOT. However, this general response may be due to a recall immune response after seasonal RSV infection, which is known to occur throughout life [3,28,29]. This recall immunity could also have an impact on further studies that we could perform, and it will be particularly significant if we evaluate this vaccine in newborns, where no pre-existing RSV immune response should be found. In that particular population, this could have further implications and lead to different outcomes like the ones seen for the adult volunteers. As seen for PPD measurements, N-RSV immune responses increased with higher doses of the vaccine, indicating that it is possible to detect a specific immune response against the N-RSV protein. Importantly, polyfunctional T cell immune responses, as well as IFN-γ- and IL-2-positive cells were more pronounced at 30 dpv, as seen in the cellular immunogenicity assays (Fig. S6). Prior to vaccination, all participants displayed a modest frequency of IFN-γ and IL-2 secreting cells in response to PPD stimulation, likely due to a prior vaccination in their childhood with BCG (Table S1). Therefore, we expected to find a specific recall immune response after in vitro cell stimulation with PPD [30]. Upon immunization, all the volunteers exhibited a sustained increase in IFN-γ and IL-2 secretion, starting from 14 dpv (Figs. 3 and S6).
Both immunogenicity and reactogenicity responses were lower in BCG-WT immunized volunteers as compared to volunteers immunized with the study vaccine. Theses apparent lower responses of the BCG control vaccine were unexpected. A possible explanation is that the manufacturers of both vaccines are different, as previously indicated. The immunogenic response of our study vaccine -for both BCG and RSV antigens- appears to be higher than that of the control BCG, although we considered that tolerability and safety are not compromised. The findings reported above with our vaccine are consistent with previous studies that showed that it induces a Th-1 RSV-specific immune response in mice, which was protective against RSV challenge [20]. Furthermore, we have previously observed that rBCG-N-hRSV is immunogenic and does not cause exacerbated immune disease in mice [14,20], which is consistent with the observation that vaccinated individuals followed for up to 180 dpv did not show enhancement of RSV disease, although they were likely naturally exposed to this virus at the time being. As stated above, this was the first phase 1 clinical trial held in Chile. In following clinical trials, we expect to assess more variables that our current study could not address, i.e., immunization previous to RSV season, inclusion of non-immunized groups, addition of women into the study cohort, and the evaluation of safety and immunogenicity in populations of different ethnicity. This will allow us to further advance into Phase 2 clinical trials.
Taken together, the present phase I clinical study indicates that the rBCG-N-hRSV vaccine is safe and immunogenic in humans and a promising vaccine to further advance into following clinical trials where we could evaluate its protective capacities in young infants, the most susceptible population to RSV-related disease.
Declaration of Competing Interest
Authors KA, ERJ, NMD, YV, JS, NG, JV, CI, MU, AB, JC, VM, PG, JG, SB, and AMK report grants from Millennium Institute on Immunology and Immunotherapy from Chile, grants from Fondo Nacional de Desarrollo Científico y Tecnológico, grants from Fondo de Fomento al Desarrollo Tecnológico, personal fees from Pontificia Universidad Católica de Chile, during the conduct of the study. LV reports personal fees from Pontificia Universidad Católica de Chile, during the conduct of the study .
Acknowledgments
Funding
This work was supported by the Millennium Institute on Immunology and Immunotherapy from Chile (P09/016-F). The "Fondo Nacional de Desarrollo Científico y Tecnológico” (FONDECYT) # 1190830 and the “Fondo de Fomento al Desarrollo Tecnológico” (FONDEF) #D11E1098. The funding sources had no role during the study design; in the collection, analysis, or interpretation of data; in the writing of the report; or in the decision to submit the paper for publication. AMK is a Helen C. Levitt Visiting Professor at the Department of Microbiology and Immunology of the University of Iowa.
Acknowledgments
A patent concerning the rBCG-N-hRSV vaccine has been filed by the Pontificia Universidad Católica de Chile (PCT/US2008/076682).
Data Sharing
All the data reported in this manuscript is available upon request to the corresponding author.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2020.100517.
Contributor Information
Katia Abarca, Email: katia@med.puc.cl.
Susan M. Bueno, Email: sbueno@bio.puc.cl.
Alexis M. Kalergis, Email: akalergis@bio.puc.cl.
Appendix. Supplementary materials
References
- 1.Nair H, Nokes DJ, Gessner BD. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet. 2010;375:1545–1555. doi: 10.1016/S0140-6736(10)60206-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Collins PL, Melero JA. Progress in understanding and controlling respiratory syncytial virus: Still crazy after all these years. Virus Res. 2011;162:80–99. doi: 10.1016/j.virusres.2011.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Breese Hall C. The burgeoning burden of respiratory syncytial virus among children. Infect Disord – Drug Targets. 2012;12:92–97. doi: 10.2174/187152612800100099. [DOI] [PubMed] [Google Scholar]
- 4.Bohmwald K, Gálvez NMS, Ríos M, Kalergis AM. Neurologic alterations due to respiratory virus infections. Front Cell Neurosci. 2018;12:386. doi: 10.3389/fncel.2018.00386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andrade CA, Pacheco GA, Gálvez NMS, Soto JA, Bueno SM, Kalergis AM. Innate immune components that regulate the pathogenesis and resolution of hRSV and hMPV Infections. Viruses. 2020;12:637. doi: 10.3390/v12060637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang K, Incognito L, Cheng X, Ulbrandt ND, Wu H. Respiratory syncytial virus-neutralizing monoclonal antibodies motavizumab and palivizumab inhibit fusion. J Virol. 2010;84:8132–8140. doi: 10.1128/JVI.02699-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vekemans J, Moorthy V, Giersing B. Respiratory syncytial virus vaccine research and development: World Health Organization technological roadmap and preferred product characteristics. Vaccine. 2019;37:7394–7395. doi: 10.1016/j.vaccine.2017.09.092. [DOI] [PubMed] [Google Scholar]
- 8.Kruijsen D, Schijf MA, Lukens M V. et al. Local innate and adaptive immune responses regulate inflammatory cell influx into the lungs after vaccination with formalin inactivated RSV. Vaccine. 2011;29:2730–2741. doi: 10.1016/j.vaccine.2011.01.087. [DOI] [PubMed] [Google Scholar]
- 9.Knudson CJ, Hartwig SM, Meyerholz DK, Varga SM. RSV Vaccine-enhanced disease is orchestrated by the combined actions of distinct CD4 T cell subsets. PLoS Pathog. 2015;11:1–23. doi: 10.1371/journal.ppat.1004757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim HW, Canchola JG, Brandt CD. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol. 1969;89:422–434. doi: 10.1093/oxfordjournals.aje.a120955. [DOI] [PubMed] [Google Scholar]
- 11.Bendelja K, Gagro A, Baće A. Predominant type-2 response in infants with respiratory syncytial virus (RSV) infection demonstrated by cytokine flow cytometry. Clin Exp Immunol. 2000;121:332–338. doi: 10.1046/j.1365-2249.2000.01297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Acosta PL, Caballero MT, Polack FP. Brief history and characterization of enhanced respiratory syncytial virus disease. Clin Vaccine Immunol. 2016;23:189–195. doi: 10.1128/CVI.00609-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.PATH . Program for Appropriate Technology in Health; 2020. RSV vaccine and mAb Snapshot; p. 1.https://path.azureedge.net/media/documents/RSV-snapshot-2020_03_26_High_Resolution_PDF.pdf [Google Scholar]
- 14.Bueno SM, González PA, Cautivo KM. Protective T cell immunity against respiratory syncytial virus is efficiently induced by recombinant BCG. Proc Natl Acad Sci. 2008;105:20822–20827. doi: 10.1073/pnas.0806244105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zwerling A, Behr MA, Verma A, Brewer TF, Menzies D, Pai M. The BCG World atlas: a database of global BCG vaccination policies and practices. PLoS Med. 2011;8 doi: 10.1371/journal.pmed.1001012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abubakar I, Pimpin L, Ariti C. Systematic review and meta-analysis of the current evidence on the duration of protection by bacillus Calmette-Guérin vaccination against tuberculosis. Health Technol Assess. 2013;17:1–4. doi: 10.3310/hta17370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aggarwal A, Dutta AK. Timing and dose of BCG vaccination in infants as assessed by postvaccination tuberculin sensitivity. Indian Pediatr. 1995;32:635–639. [PubMed] [Google Scholar]
- 18.Marchant A, Goetghebuer T, Ota MO. Newborns develop a Th1-type immune response to Mycobacterium bovis bacillus Calmette-Guerin vaccination. J Immunol. 1999;163:2249–2255. [PubMed] [Google Scholar]
- 19.Cautivo KM, Bueno SM, Cortes CM, Wozniak A, Riedel CA, Kalergis AM. Efficient lung recruitment of respiratory syncytial virus-specific Th1 cells induced by recombinant bacillus calmette-guerin promotes virus clearance and protects from infection. J Immunol. 2010;185:7633–7645. doi: 10.4049/jimmunol.0903452. [DOI] [PubMed] [Google Scholar]
- 20.Céspedes PF, Rey-Jurado E, Espinoza JA. A single, low dose of a cGMP recombinant BCG vaccine elicits protective T cell immunity against the human respiratory syncytial virus infection and prevents lung pathology in mice. Vaccine. 2017;35:757–766. doi: 10.1016/j.vaccine.2016.12.048. [DOI] [PubMed] [Google Scholar]
- 21.Soto JA, Gálvez NMS, Rivera CA. Recombinant BCG vaccines reduce pneumovirus-caused airway pathology by inducing protective humoral immunity. Front Immunol. 2018;9:2875. doi: 10.3389/fimmu.2018.02875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Valdespino Gómez JL, García García MDL. Declaración de Helsinki. Gac Med Mex. 2001;137:391. [PubMed] [Google Scholar]
- 23.U. S. Department of Health and Human Services Toxicity Grading Scale for Healthy Adult and Adolescent Volunteers Enrolled in Preventive Vaccine Clinical Trials. Guid Ind. 2007;1:1–10. doi: 10.1016/j.vaccine.2023.07.072. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/toxicity-grading-scale-healthy-adult-and-adolescent-volunteers-enrolled-preventive-vaccine-clinical [DOI] [PubMed] [Google Scholar]
- 24.van Remmerden Y, Xu F, van Eldik M, Heldens JGM, Huisman W, Widjojoatmodjo MN. An improved respiratory syncytial virus neutralization assay based on the detection of green fluorescent protein expression and automated plaque counting. Virol J. 2012;9:253. doi: 10.1186/1743-422X-9-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raj A, Singh N, Gupta KB. Comparative evaluation of several gene targets for designing a multiplex-PCR for an early diagnosis of extrapulmonary tuberculosis. Yonsei Med J. 2016;57:88. doi: 10.3349/ymj.2016.57.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Pinxteren LAH, Ravn P, Agger EM, Pollock J, Andersen P. Diagnosis of tuberculosis based on the two specific antigens ESAT-6 and CFP10. Clin Vaccine Immunol. 2000;7:155–160. doi: 10.1128/cdli.7.2.155-160.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nissen TN, Birk NM, Kjærgaard J. Adverse reactions to the Bacillus Calmette-Guérin (BCG) vaccine in new-born infants-an evaluation of the Danish strain 1331 SSI in a randomized clinical trial. Vaccine. 2016;34:2477–2482. doi: 10.1016/j.vaccine.2016.03.100. [DOI] [PubMed] [Google Scholar]
- 28.Bont L, Versteegh J, Swelsen WTN. Natural reinfection with respiratory syncytial virus does not boost virus-specific T-cell immunity. Pediatr Res. 2002;52:363–367. doi: 10.1203/00006450-200209000-00009. [DOI] [PubMed] [Google Scholar]
- 29.Hall CB, Walsh EE, Long CE, Schnabel KC. Immunity to and frequency of reinfection with respiratory syncytial virus. J Infect Dis. 1991;163:693–698. doi: 10.1093/infdis/163.4.693. [DOI] [PubMed] [Google Scholar]
- 30.Whelan KT, Pathan AA, Sander CR. Safety and immunogenicity of boosting BCG vaccinated subjects with BCG: comparison with boosting with a new TB vaccine, MVA85A. PLoS One. 2009;4:e5934. doi: 10.1371/journal.pone.0005934. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.