Abstract
Background:
Toxoplasma gondii (T. gondii) causes an important parasitic infection known as toxoplasmosis, which is a globally distributed important zoonosis. One of the major serious characteristics of T. gondii is its ability to manipulate the behavior of intermediate hosts. We performed a cross-sectional study to determine toxoplasmosis in schizophrenic patients, as one of the major neuropsychiatric disorders, using loop-mediated isothermal amplification (LAMP) technic by targeting parasite B1 gene.
Methods:
Blood samples were taken from 118 schizophrenic patients hospitalized in tow hospitals including Baharan, Clinic of Psychiatric Ali-ibn-Abi-Talib Hospital (in Zahedan City), and Amir-al Momenin Psychiatric Hospital (in Zabol City), Sistan and Baluchestan Province, southeast Iran in 2016. They were analyzed using LAMP, and compared with the previous data of nested-PCR and serology.
Results:
Out of the 118 schizophrenic individuals, 56 patients (47.4%) were found to be infected with T. gondii. The diagnosis of toxoplasmosis was confirmed in 41 patients (34.7%) via the nested-PCR. The seroprevalence of toxoplasmosis in schizophrenic patients was 55.9% (66/118).
Conclusion:
We found a high efficiency of LAMP method in identifying toxoplasmosis and its high prevalence among schizophrenic patients. Our findings could provide viable offer implications for the prevention of schizophrenia.
Keywords: Loop-mediated isothermal amplification (LAMP), Toxoplasma gondii, Toxoplasmosis, Schizophrenia
Introduction
With uncertain causes and a very high global prevalence, schizophrenia as a neurological disorder has harmful impacts upon affected patients and their relatives (1, 2). The disease inflicts both direct and indirect costs on human society in terms of the real resources concerning medical service activities, lost work productivity, and associated socioeconomic difficulties (2). According to the literature, the main cause of its development is commonly unknown; however, various scientists assume that causes may include both genetic and environmental factors. Among those factors, one of the main etiological agents is being infected with infectious pathogens such as intracellular parasite Toxoplasma gondii (T. gondii) which is related to the parasite neurotropism and its effects on brain dysfunction (3, 4).
T. gondii, the causative agent of toxoplasmosis, is a coccidian protozoan parasite of the apicomplexan family, and one of the major zoonotic disease whose life cycle can be fulfilled solely in the felids (cats) as the final definitive hosts (5–7). Warm-blooded animals such as humans act as intermediate hosts. Toxoplasma parasite infects almost 30% of the world’s human population in all countries (1, 8). Major risk factors for being infected with this protozoan parasite in humans’ society are the use of inadequately cooked and/or raw meat containing tissue cysts of Toxoplasma, ingest of sporulated oocysts from different sources such as contaminated water or soil, and the vertical transmission of parasite via the placenta (8).
Different studies have been carried out to describe the linkage between T. gondii infection and schizophrenia and other mental disorders (4, 9). Although several studies have elucidated that schizophrenic patients have an increased seroprevalence of anti-Toxoplasma IgG and IgM antibodies (3, 4), other studies have failed to show a significant connection between developing schizophrenia and toxoplasmosis (10–12). Various recent experiments with contradictory findings in distinct parts of Iran have been performed to evaluate the association of schizophrenia with toxoplasmosis (1, 13–15). Furthermore, studies in animal models have demonstrated that toxoplasmosis can manipulate the behavior in mice, increase the chances when a mouse is hunted and eaten by a cat, and consequently enable the Toxoplasma parasite to fulfil its complicated life cycle (16).
LAMP method is a novel and user-friendly DNA amplification technic with a very high efficiency, sensitivity, simplicity, and specificity under isothermal conditions (17, 18).
This technic has already been successfully implemented for rapid identification and/or detection of several parasitic pathogens including Schistosoma spp. (19), Fasciola spp. (20) Babesia spp. (21), Trypanosoma spp. (22), Cryptosporidium spp. (23), Entamoeba histolytica (24), Plasmodium spp. (25) and Toxoplasma (18, 26).
Due to lack of any documented report on the possible link between T. gondii infection and schizophrenia in Sistan and Baluchestan province (southeast Iran) and more importantly its molecular identification in this region, the present study aimed to investigate the molecular diagnosis of toxoplasmosis in schizophrenic individuals using LAMP method and targeting the repetitive conserved region of B1 gene in T. gondii via comparing it with the previously published data
Materials and Methods
Study population
In the present cross-sectional study, a total of 118 confirmed schizophrenic individuals who referred to distinct hospitals (Baharan, Clinic of Psychiatric Ali-ibn-Abi-Talib hospital in Zahedan city, and Amir-al Momenin Psychiatric hospital in Zabol City) in two main cites of Sistan and Baluchestan Province, southeast Iran including Zahedan and Zabol were enrolled (Fig. 1). The study was done within a six-month period from Jun to Nov in 2016. The main inclusion criteria in the present study were: 1) schizophrenic patients who were chosen under the supervision of neurology consultants of Zahedan University of Medical Sciences, Zahedan, Iran (based on the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV-TR)) (27); 2) the individuals who aged above 18 yr; 3) individuals who volunteered to take part and signed written informed consent questionnaire before participating in the study.
Fig. 1:
Location of sampling sites (Zabol and Zahedan cities) in Sistan and Baluchestan Province, Southeast of Iran (Created by Arc GIS version 10.2)
Ethical approval was obtained from the Ethical Committee of Zahedan University of Medical Sciences, Zahedan, Iran (ethical code: IR.ZAUMS.REC.1395.14).
Sample collection
From each schizophrenic patient who fulfilled the above-mentioned inclusion criteria, 4 ml whole blood in total was taken. All the samples under the cold-chain storage conditions were immediately transferred to the Department of Medical Parasitology, Zahedan University of Medical Sciences, Zahedan, Iran. These samples were centrifuged for 5 min at 4000 rpm. Then, sera were isolated to examine the specific anti-Toxoplasma IgM and IgG antibodies ELISA, as already described (28). Moreover, buffy coat extractions for molecular evaluations were performed for all samples. Both separated sera and buffy coats were separately poured in sterile 2 ml tubes and then were kept at −20 °C until they were used.
DNA extraction and Nested-PCR
The purification of genomic DNA from buffy coat samples was done using the commercial DNA extraction kit [DynaBio Tm Blood/Tissue DNA Extraction MiniKit (Takapoozist, Iran)] according to the manufacturer’s guidelines, as described previously (28). Nested-PCR was done for molecular identifications via B1 gene, according to the procedures were described in our previous publication (28, 29).
LAMP assay
As shown in Table 1, four main T. gondiispecific primers targeting six highly conserved and repeated regions of the B1 gene (AF179871.1) were utilized for the LAMP identification assay, as previously described elsewhere (30). Briefly, the LAMP amplification was conducted in 25 μl of reaction mixtures including 40 pmol of each inner primers (FIP and BIP), 5 pmol of each outer primer (F3 and B3), 8 U of Bst DNA polymerase (New England Biolabs, USA), 1.4 mmol/μl of dNTP, 1X reaction buffer (Sigma-Aldrich), 6.5 μl of distilled water (DW), and 1 μl of purified template DNA. After adding 1 μl of fluorescent detection dye, the reaction mixture was incubated at 66 °C for 60 min and heated at 95 °C for 2 min to inactivate the DNA Polymerase. Both positive (purified DNA of T. gondii RH-strain) and negative controls (DDW) were added in each run of the assay.
Table 1:
Nucleotide sequences of LAMP primers used for Toxoplasma identification in patients with schizophrenia
| Molecular Technique | Target gene | Sequence (5′-3′) |
|---|---|---|
| LAMP | B1 | BIP-TCGCAACGGAGTTCTTCCCAGTTTTGGCCTGATATTACGACGGAC FIP-TGACGCCTTTAGCACATCTGGT TTTTGATGCTCAAAGTCGACCGC F3-GGGAGCAAGAGTTGGGACTA B3-CAGACAGCGAACAGAACAGA |
Statistical analysis
In the present study, the data was analyzed using Graph-Pad Prism 6.0 for Windows (Graph-Pad Prism, San Diego, California, USA). The P-values less than 0.05 (P<0.05) were considered statistically significant.
Results
Overall 118 individuals with confirmed schizophrenia and inclusion criteria, who lived in Zahedan and Zabol (Fig. 1), were recruited for the present study. Out of the 118 confirmed schizophrenic patients, 19 were female (16.1%) and 99 were male (83.9%), with a median age of 38 yr (range: 19 to 55 yr). The diagnosis of toxoplasmosis was confirmed in 41 patients (34.7%) using the nested-PCR. The results of our study using LAMP assay showed that, out of the 118 schizophrenic individuals, 56 patients (47.4%) were found to be infected with T. gondii (Figs. 2 and 3). The comparison between LAMP and nested-PCR results, which was described our previous publication, showed that the efficiency of the former was higher than that of the latter one (P < 0.05) (Table 2). The serology data in our previous work showed that the seroprevalence of toxoplasmosis in schizophrenic patients was 55.9% (66/118). Distinct titers of the anti-Toxoplasma IgG were found in 48 cases (40.67%), while the anti-Toxoplasma IgM and IgG/IgM were positive in 4 cases (3.37%) and 14 cases (11.86%), respectively (Table 2).
Fig. 2:
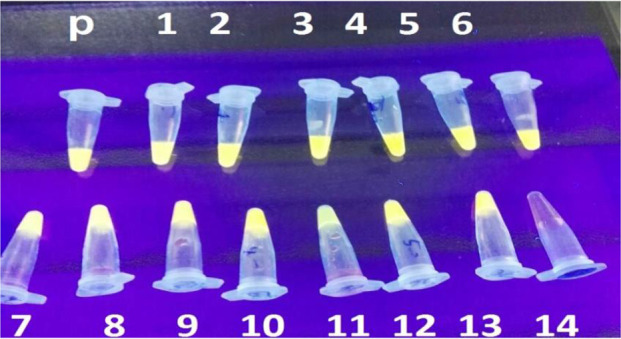
LAMP monitor of T. gondii DNA in schizophrenic patients. P: positive control, N: negative control; Tubes 1–14 represent patients samples
Fig. 3:

Agarose gel analysis of LAMP assay for the specific detection of T. gondii DNA based on B1 gene amplification. Lane 1: 100 bp molecular weight marker; Lane 2: positive control; Lane 3: negative control; lanes 4–9: negative test group from patients; lanes 10–12: positive test group from patients
Table 2:
Comparative results of Nested-PCR and LAMP techniques in Toxoplasma-seropositive schizophrenic patients based on B1 gene
| Diagnostic methods | ELISA | Total of 66/118 seropositive | |||
|---|---|---|---|---|---|
| IgG+/IgM+ | IgG-/IgM- | IgG-/IgM+ | IgG+/IgM- | ||
| Nested-PCR | 12 (86%) | 2 (3%) | 4 (100%) | 25 (52%) | 43 (62%) |
| LAMP | 14(100%) | 3 (4.5%) | 4 (100%) | 35 (73%) | 56 (85%) |
Discussion
Since the 1950s, hidden association between toxoplasmosis, as a globally important parasitic disease, and schizophrenia, as one of the major neuropsychiatric disorders, has been investigated (2, 9). In the mid-1990s, the infectious hypothesis of schizophrenia development via close contact with cats shed light on the plausible roles of T. gondii in this mental disorder (31). The prevalence of toxoplasmosis in schizophrenic patients is very high, and the parasitic pathogen increases the risk of getting schizophrenia (3, 4, 32). Furthermore, a study in Lorestan Province (Western Iran) have shown that psychiatric individuals such as bipolar and schizophrenia cases had a remarkably higher frequency of T. gondii infection than healthy patients (33).
In the present study, for the first time, the prevalence rate of toxoplasmosis in confirmed schizophrenic patients was investigated using LAMP method and was compared with the serology and molecular data of our previous publication in Southeastern Iran (28). We found that 47.4%, 34.7%, and 55.9% of schizophrenic patients were positive for T. gondii via LAMP, nested-PCR, and serology, respectively. Furthermore, distinct titers of the anti-Toxoplasma IgG were found in 48 cases (40.67%) which represented the chronic phase of disease in the schizophrenic patients. In addition, the anti-Toxoplasma IgM was positive in 4 cases (3.37%) revealing the existence of a relationship with the acute phase of the infection. Due to the mainly hot and dry climate in the Southeast of Iran, the prevalence of T. gondii was lower than it was in other regions of the country. In this regard, different studies in the Southeast of Iran showed that the prevalence rates of T. gondii in different human groups such as general population, pregnant women, and healthy blood donors were 22.8%, 25%, and 22.7%, respectively (15, 34–36). Various studies have elucidated that environmental conditions such as different climates are very important for Toxoplasma oocysts survival; therefore, the possible explanation for this phenomenon can be the reduction of parasite oocysts due to hot and dry climates (37, 38).
Distinct molecular assessments based on several targets and methods have been investigated and reported for the diagnosis of Toxoplasma parasite (26). A rapid screening of the individuals infected by T. gondii dispensed extremely remarkable information for the surveillance of parasite control and the precise prevalence estimation of the disease in the endemic regions of the country (18, 26). In this respect, we used an exceedingly sensitive LAMP technic targeting the B1 gene to diagnose rapid identification of T. gondii DNA in the schizophrenic patients (18, 26). Molecular markers that target the genes such as B1 and conserve the repeated region of the Toxoplasma genome show excellent sensitivity and specificity for isolated clinical samples; therefore, genomic target of B1 was chosen in the present study (18). Our data elucidated that the LAMP method was highly specific for the identification of the T. gondii, providing more evidence for the other studies (18, 26, 39).
The LAMP assay has higher diagnostic sensitivity due to the 4–6 primers utilized in the current reaction which target 6–8 different internal regions on the B1 gene as the target DNA (18). Owing to the isothermal conditions in this simple method and less specialized tools, the amplification of DNA can be gained via ordinary incubators including block heater and/or water bath; therefore, this assay can be utilized to detect toxoplasmosis in the field study. Furthermore, one of the main advantages of the LAMP is that the amplification of DNA can be quickly investigated through visual inspection. The LAMP assay, as a fast and feasible field diagnostic equipment, was validated in the schizophrenic patients infected by T. gondii. LAMP assay is very efficient and sensitive in the infected patients of distinct parasitic diseases such as leishmaniasis and toxoplasmosis (26, 40).
The results of the present study should be interpreted considering several limitations. First, there was no control groups (healthy individuals) to evaluate plausible associations between the diseases. Second, no specific questionnaire was designed to compare the related risk factors such as contact with cats and sources of drinking water between toxoplasmosis and schizophrenia.
Conclusion
Our findings in the present and previous studies demonstrated a high prevalence of toxoplasmosis in schizophrenic patients; hence, due to simplicity and sensitivity of LAMP assay, it is recommended as a suitable technic for the regular molecular detection of T. gondii DNA.
Ethical considerations
Ethical issues (Including plagiarism, informed consent, misconduct, data fabrication and/or falsification, double publication and/or submission, redundancy, etc.) have been completely observed by the authors.
Acknowledgements
We are very grateful to the Baharan, Clinic of Psychiatric Ali-ibn-Abi-Talib hospital staffs in Zahedan city, and Amir-al Momenin Psychiatric hospital staffs in Zabol city, Sistan and Baluchestan province, for their assistance in this study. Also, we would like to thank Dr. Zahra Asadgol (Department of Environmental Health Engineering, School of Public Health, Iran University of Medical Sciences, Tehran, Iran) for her great technical assistance in designing the GIS map.
This article has been extracted from the MSc thesis written by Raheleh Hasanzadeh from the Department of Parasitology and Mycology, Faculty of Medicine, Zahedan University of Medical Sciences, Zahedan, Iran. The present study was financially supported by the Zahedan University of Medical Sciences, Zahedan, Iran under Grant number 7691 to Raheleh Hasanzadeh.
Footnotes
Conflict of interest
The authors declare that there is no conflict of interest.
References
- 1.Ansari-Lari M, Farashbandi H, Mohammadi F. Association of Toxoplasma gondii infection with schizophrenia and its relationship with suicide attempts in these patients. Trop Med Int Health. 2017; 22(10): 1322–1327. [DOI] [PubMed] [Google Scholar]
- 2.Yolken R, Dickerson F, Fuller Torrey E. Toxoplasma and schizophrenia. Parasite Immunol. 2009; 31(11):706–15. [DOI] [PubMed] [Google Scholar]
- 3.Torrey EF, Bartko JJ, Lun Z-R, et al. Antibodies to Toxoplasma gondii in patients with schizophrenia: a meta-analysis. Schizophr Bull. 2007; 33(3):729–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Torrey EF, Bartko JJ, Yolken RH. Toxoplasma gondii and other risk factors for schizophrenia: an update. Schizophr Bull. 2012; 38(3):642–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ullmann LS, Gravinatti ML, Yamatogi RS, et al. Serosurvey of anti- Leptospira sp. and anti- Toxoplasma gondii antibodies in capybaras and collared and white-lipped peccaries. Revista da Sociedade Brasileira de Medicina Tropical. 2017; 50(2):248–50. [DOI] [PubMed] [Google Scholar]
- 6.Mahmoudvand H, Sheibani V, Shojaee S, et al. Toxoplasma gondii infection potentiates cognitive impairments of Alzheimer’s disease in the BALB/c mice. J Parasitol. 2016; 102(6): 629–635. [DOI] [PubMed] [Google Scholar]
- 7.Alipour A, Shojaee S, Mohebali M, et al. Toxoplasma infection in schizophrenia patients: a comparative study with control group. Iran J Parasitol. 2011; 6(2): 31–37. [PMC free article] [PubMed] [Google Scholar]
- 8.Rostami A, Seyyedtabaei SJ, Aghamolaie S, et al. Seroprevalence and risk factors associated with Toxoplasma gondii infection among rural communities in northern Iran. Rev Inst Med Trop Sao Paulo. 2016; 58:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flegr J. Schizophrenia and Toxoplasma gondii: an undervalued association? Expert Rev Anti Infect Ther. 2015; 13(7):817–820. [DOI] [PubMed] [Google Scholar]
- 10.Sugden K, Moffitt TE, Pinto L, et al. Is Toxoplasma gondii infection related to brain and behavior impairments in humans? Evidence from a population-representative birth cohort. PLoS One. 2016; 11(2):e0148435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karabulut N, Bilgiç S, Gürok MG, et al. Is there any role of latent toxoplasmosis in schizophrenia disease? Journal of the Chinese Medical Association. 2015; 78(9):533–7. [DOI] [PubMed] [Google Scholar]
- 12.De Witte LD, Van Mierlo HC, Litjens M, et al. The association between antibodies to neurotropic pathogens and schizophrenia: a case-control study. npj Schizophrenia. 2015; 1:15041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abdollahian E, Shafiei R, Mokhber N, et al. Seroepidemiological study of Toxoplasma gondii infection among psychiatric patients in Mashhad, northeast of Iran. Iran J Parasitol. 2017; 12(1):117–122. [PMC free article] [PubMed] [Google Scholar]
- 14.Khademvatan S, Khajeddin N, Izadi S, et al. Investigation of anti-Toxocara and anti-Toxoplasma antibodies in patients with schizophrenia disorder. Schizophr Res Treatment. 2014; 2014:230349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Daryani A, Sarvi S, Aarabi M, et al. Seroprevalence of Toxoplasma gondii in the Iranian general population: a systematic review and meta-analysis. Acta Trop. 2014; 137:185–94. [DOI] [PubMed] [Google Scholar]
- 16.Webster JP. The effect of Toxoplasma gondii on animal behavior: playing cat and mouse. Schizophr Bull. 2007; 33(3):752–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mahmoudvand H, Ziaali N, Aghaei I, et al. The possible association between Toxoplasma gondii infection and risk of anxiety and cognitive disorders in BALB/c mice. Pathog Glob Health. 2015; 109(8): 369–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fallahi S, Seyyed Tabaei SJ, Pournia Y, et al. Comparison of loop-mediated isothermal amplification (LAMP) and nested-PCR assay targeting the RE and B1 gene for detection of Toxoplasma gondii in blood samples of children with leukaemia. Diagn Microbiol Infect Dis. 2014; 79(3):347–54. [DOI] [PubMed] [Google Scholar]
- 19.Kumagai T, Furushima-Shimogawara R, Ohmae H, et al. Detection of early and single infections of Schistosoma japonicum in the intermediate host snail, Oncomelania hupensis, by PCR and loop-mediated isothermal amplification (LAMP) assay. Am J Trop Med Hyg. 2010; 83(3):542–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ai L, Li C, Elsheikha H, et al. Rapid identification and differentiation of Fasciola hepatica and Fasciola gigantica by a loop-mediated isothermal amplification (LAMP) assay. Vet Parasitol. 2010; 174(3–4):228–33. [DOI] [PubMed] [Google Scholar]
- 21.Ikadai H, Tanaka H, Shibahara N, et al. Molecular evidence of infections with Babesia gibsoni parasites in Japan and evaluation of the diagnostic potential of a loop-mediated isothermal amplification method. J Clin Microbiol. 2004; 42(6):2465–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thekisoe OM, Inoue N, Kuboki N, et al. Evaluation of loop-mediated isothermal amplification (LAMP), PCR and parasitological tests for detection of Trypanosoma evansi in experimentally infected pigs. Vet Parasitol. 2005; 130(3–4):327–30. [DOI] [PubMed] [Google Scholar]
- 23.Bakheit MA, Torra D, Palomino LA, et al. Sensitive and specific detection of Cryptosporidium species in PCR-negative samples by loop-mediated isothermal DNA amplification and confirmation of generated LAMP products by sequencing. Vet Parasitol. 2008; 158(1–2):11–22. [DOI] [PubMed] [Google Scholar]
- 24.Rivera WL, Ong VA. Development of loop-mediated isothermal amplification for rapid detection of Entamoeba histolytica. Asian Pac J Trop Med. 2013; 6(6):457–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paris DH, Imwong M, Faiz AM, et al. Loop-mediated isothermal PCR (LAMP) for the diagnosis of falciparum malaria. Am J Trop Med Hyg. 2007; 77(5):972–6. [PubMed] [Google Scholar]
- 26.Fallahi S, Mazar ZA, Ghasemian M, et al. Challenging loop—mediated isothermal amplification (LAMP) technique for molecular detection of Toxoplasma gondii. Asian Pac J Trop Med. 2015; 8(5):366–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.First MB, Spitzer RL, Gibbon M, et al. The structured clinical interview for DSM-III-R personality disorders (SCID-II). Part II: Multi-site test-retest reliability study. J Pers Disord. 1995; 9(2):92–104. [Google Scholar]
- 28.Jafari Modrek M, Hasanzadeh R, Foroutan M, et al. Seroprevalence and molecular evaluation of Toxoplasma gondii in Schizophrenic patients hospitalized in Sistan and Baluchestan province, Southeast of Iran. Trop Biomed. 2018; 36(2): 422–429. [PubMed] [Google Scholar]
- 29.Keshavarz Valian H, Mirhendi H, Mohebali M, et al. Comparison of the RE-529 sequence and B1 gene for Toxoplasma gondii detection in blood samples of the at-risk seropositive cases using uracil DNA glycosylase supplemented loop-mediated isothermal amplification (UDG-LAMP) assay. Microb Pathog. 2020; 140):103938. [DOI] [PubMed] [Google Scholar]
- 30.Sotiriadou I, Karanis P. Evaluation of loop-mediated isothermal amplification for detection of Toxoplasma gondii in water samples and comparative findings by polymerase chain reaction and immunofluorescence test (IFT). Diagn Microbiol Infect Dis. 2008; 62(4):357–65. [DOI] [PubMed] [Google Scholar]
- 31.Torrey EF, Yolken RH. Could schizophrenia be a viral zoonosis transmitted from house cats. Schizophr Bull. 1995; 21(2):167–71. [DOI] [PubMed] [Google Scholar]
- 32.Zhou P, Chen Z, Li H-L, et al. Toxoplasma gondii infection in humans in China. Parasites & Vectors. 2011; 4(1):165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kheirandish F, Nazari H, Mahmoudvand H, et al. Possible Link Between Toxoplasma gondii Infection and Mood Disorders in Lorestan Province, Western Iran. Clin Infect Dis. 2016; 11(4):e36602. [Google Scholar]
- 34.Teimouri A, Mohtasebi S, Kazemirad E, et al. Role of Toxoplasma gondii IgG avidity testing in discriminating between acute and chronic toxoplasmosis in pregnancy. J Clin Microbiol. 2020; 58(9):e00505–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mousavi P, Mirhendi H, Mohebali M, et al. Detection of Toxoplasma gondii in acute and chronic phases of infection in immunocompromised patients and pregnant women with real-time PCR assay using TaqMan fluorescent probe. Iran J Parasitol. 2018; 13(3): 373–381. [PMC free article] [PubMed] [Google Scholar]
- 36.Modrek MJ, Mousavi M, Saravani R. Toxoplasma gondii seroprevalence among blood donors in Zahedan, southeastern Iran. International Journal of Infection. 2014; 1(2). [Google Scholar]
- 37.Meerburg BG, Kijlstra A. Changing climate— changing pathogens: Toxoplasma gondii in North-Western Europe. Parasitol Res. 2009; 105(1):17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tenter AM, Heckeroth AR, Weiss LM. Toxoplasma gondii: from animals to humans. Int J Parasitol. 2000; 30(12–13):1217–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kong Q-M, Lu S-H, Tong Q-B, et al. Loop-mediated isothermal amplification (LAMP): early detection of Toxoplasma gondii infection in mice. Parasit Vectors. 2012; 5:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ghodrati M, Spotin A, Hazratian T, et al. Diagnostic Accuracy of Loop-mediated Isothermal Amplification Assay as a Field Molecular Tool for Rapid Mass Screening of Old World Leishmania Infections in Sand Flies and In Vitro Culture. Iran J Parasitol. 2017; 12(4):506–515. [PMC free article] [PubMed] [Google Scholar]