Abstract
Aim
Cerebrovascular disease is the leading cause of death and disability in China, causing a huge burden among patients and their families. Hence, stroke prevention is critical, especially in the high-risk population. Here, we present the evidence-based guideline suitable for the Chinese population.
Methods
Literature search of PubMed and Cochrane library (from January 1964 to June 2019) was done. After thorough discussion among the writing group members, recommendations were listed and summarised. This guideline was reviewed and discussed by the fellow writing committees of the Chinese Stroke Association’s Stroke.
Results
This evidence-based guideline was written in three parts: controlling the risk factors of stroke, utilisation of antiplatelet agents and assessing the risks of first-ever stroke. All recommendations were listed along with the recommending classes and levels of evidence.
Conclusions
This guideline provides recommendations for primary prevention of cerebrovascular disease among high-risk population in China. Controlling related risk factors, appropriately using antiplatelet agents, assessing the risk of developing first-ever stroke should help reduce the rate of cerebrovascular disease in China.
Keywords: stroke, atherosclerosis, blood pressure, platelets
Introduction
In the past two decades, stroke has been a big healthcare burden in China. From the results of 2013 national survey, the age-adjusted stroke incidence and mortality rates were 246.8/100 000 and 114.8/100 000 person-years, respectively. Stroke prevalence was 1114.8/100 000. Based on this calculation, there are >11 million stroke survivors, >2.4 million new strokes and >1.1 million people dying of stroke every year in China. The disability-adjusted loss of life-years was 2010.0/100 000 person-years. In addition, there are approximately 270 million patients with hypertension, 110 million with diabetes mellitus and 160 million with dyslipidaemia and the number of people with these risk factors of stroke continues to rise in China.1
The best treatment of stroke is prevention. Many studies showed that primary prevention is the key to reduce stroke incidence and mortality. The aim of this guideline is to recommend the appropriate prevention strategies for population at high risk for stroke. This executive summary offers a simplified version of the comprehensive recommendations that have been published, but with an update since the publication of the original version. The recommendations and levels of evidence are in line with the American Heart Association (AHA) guidelines for the primary prevention of stroke.2 3
Controlling the risk factors of stroke
Genetic factors
Recommendations
Inquiring a family history can help identify individuals at increased risk of stroke (class IIa; level of evidence A).
For patients with rare genetic causes of stroke (such as cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy, Fabry disease, COL4A1-related intracerebral haemorrhage, autosomal-dominant polycystic kidney disease, Ehlers-Danlos type IV), referral for genetic counselling may be considered (Class IIb; level of evidence C). In general population routine genetic screening for the prevention of a first stroke is not recommended (class III; level of evidence C).
For patients with ≥2 first-degree relatives with SAH or intracranial aneurysms, non-invasive screening such as CT Angiography (CTA) or MR Angiography (MRA) of brain for detecting any unruptured intracranial aneurysm (UIA) may be reasonable (class IIb; level of evidence C).
For patients with autosomal-dominant polycystic kidney disease, ≥1 relatives with autosomal-dominant polycystic kidney disease and subarachnoid hemorrhage (SAH) or ≥1 relatives with autosomal-dominant polycystic kidney disease and intracranial aneurysms, non-invasive screening such as CTA or MRA of brain for detecting any UIAs may be considered (class IIb; level of evidence C).
For patients with (cervical) fibromuscular dysplasia, non-invasive screening such as CTA or MRA brain for detecting any UIAs may be considered (class IIb; level of evidence C).
Modifiable risk factors
Well-documented modifiable risk factors
Hypertension
Recommendations
Patients with hypertension are suggested to check their blood pressure (BP) regularly. Treatments including lifestyle changes and pharmacological therapies are recommended (class I; level of evidence A).
For prehypertensive patients (SBP 120–139 mm Hg or DBP 80–89 mm Hg), annual BP screening and hypertension-related health check are recommended (class I; level of evidence A).
Patients with hypertension should be treated with antihypertensive drugs to a target BP of 140/90 mm Hg or lower (class I; level of evidence A); patients with diabetes or renal dysfunction can set lower targets depending on their BP tolerance (class I; level of evidence B).
Patients over 65 years old can set a target BP of <150/90 mm Hg at first, and <140/90 mm Hg if tolerable (class I; level of evidence A).
Choosing appropriate drugs to successfully reduce BP is important. Doctors should individualise treatment and use drugs according to patients’ characteristics (class I; level of evidence A).
Self-monitoring of BP is recommended in order to improve compliance and BP control. Ambulatory BP monitoring is effective to differentiate white-coat hypertension and masked hypertension (class I; level of evidence B).
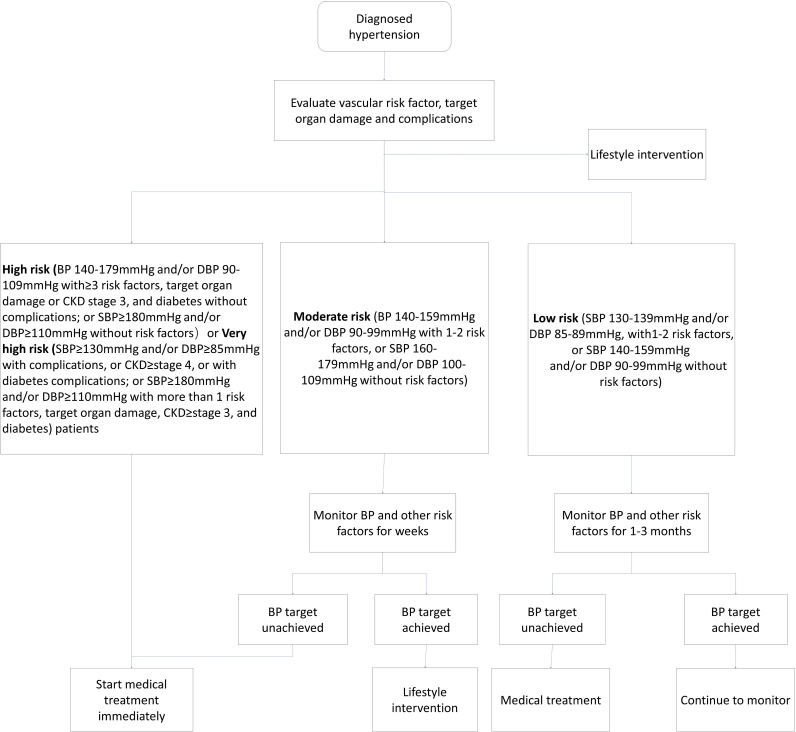
The management of patients with hypertension (figure 1).
Figure 1.
Flow chart for the management of patients with hypertension hypertension is defined as an average SBP ≥135 mm Hg or DBP ≥85 mm Hg during the day, SBP ≥120 mm Hg or DBP ≥70 mm Hg during the night or SBP ≥130 mm Hg or DBP ≥80 mm Hg in 24 hours by ambulatory blood pressure (BP) monitoring; or SBP ≥135 mm Hg or DBP ≥85 mm Hg by self-monitoring. details about risk factors and target organ damage is described on the publication: Prevention and Treatment of Cardio_Cerebral_Vascular Disease Jan 2018. Vol 19. No 1, DOI: 10.3969/j.issn.1007–5410.2019.01.002. CKD, chronic kidney disease. SBP, systolic blood pressure, DBP, diastolic blood pressure.
Diabetes and pre-diabetes
Recommendations
Diabetes and pre-diabetes are risk factors of stroke. Patients with stroke risk factors should monitor blood glucose regularly, and HbA1c or oral glucose tolerance test if need, in order to discover diabetes and pre-diabetes as early as possible (class I; level of evidence A).
Health-promoting lifestyle modification is important. Patients should first obtain a healthy diet and increase physical activity, then add pharmacological treatment if needed. Targeting Hemoglobin A1c (HbA1c) to lower than 7.0% is recommended (class I; level of evidence A).
Patients with both diabetes and hypertension should control their BP to a goal of lower than 130/80 mm Hg (class I; level of evidence A).
With glucose and BP under control, patients are recommended to be treated with a statin to lower the risk of stroke (class I; level of evidence A).
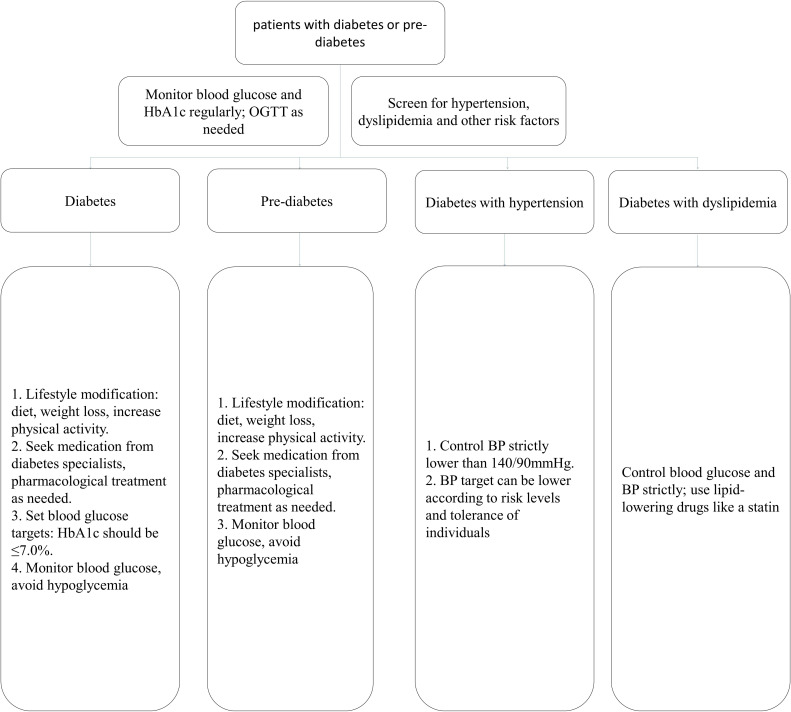
The management of patients with diabetes or pre-diabetes (figure 2).
Figure 2.
Flow chart for the management of patients with diabetes or pre-diabetes. BP, blood pressure; OGTT, oral glucose tolerance test.
Dyslipidaemia
Recommendations
For patients at high or very high risk of atherosclerotic cerebrovascular disease, in addition to therapeutic lifestyle changes, statin treatment is recommended for the primary prevention of stroke (class I; level of evidence A).4–10
Patients need to set goals for lipid-lowering treatments. Low-density lipoprotein cholesterol (LDL-C) is recommended to be the primary treating target (class I; level of evidence A).
Target LDL-C is rated according to the rating scales of cerebrovascular disease. LDL-C <1.4 mmol/L (55 mg/dL) for very-high-risk patients (class IIb; level of evidence B), and those with high bleeding risk should be treated with extra care: LDL-C <1.8 mmol/L (70 mg/dL) for high-risk patients (class IIb; level of evidence B). LDL-C <2.6 mmol/L (100 mg/dL) for middle-risk patients, LDL-C <3.0 mmol/L (116 mg/dL) for low-risk patients (class IIb; level of evidence B).
Treatment for high-risk or very-high-risk patients can be started with moderate-intensity statin, and modify treatment intensity according to their therapeutic effect and tolerance. Other lipid-lowering drugs can be added if target cholesterol levels cannot be reached (class I; level of evidence B).
Niacin can be taken into consideration for patients with low HDL cholesterol or high lipoprotein (a) (Lp(a)), but the efficacy in primary prevention of stroke is still lack of evidence (class IIb; level of evidence B).
For patients who cannot tolerate statins or reach their cholesterol targets, non-statin lipid-lowering drugs like fibric acid derivatives and bile acid sequestrants, or ezetimibe and PCSK9 inhibitors can be added, but their efficacy in preventing stroke is not evidenced (class IIb; level of evidence C).
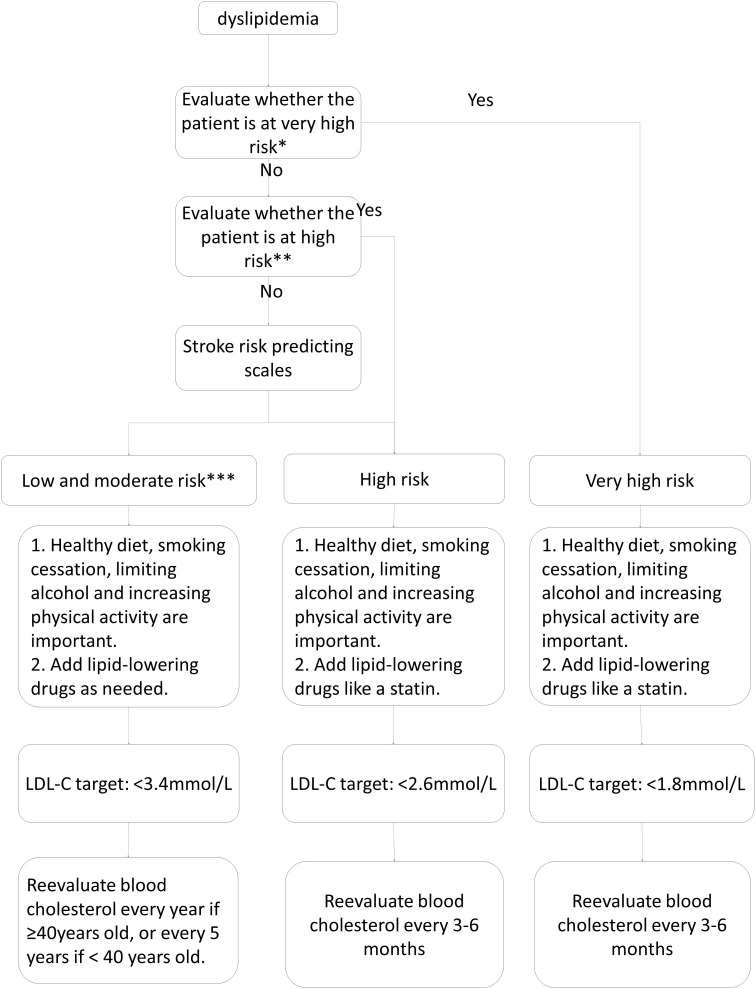
The management of patients with dyslipidaemia (figure 3).
Figure 3.
Flow chart for the management of patients with dyslipidaemia. *Very-high-risk patients diagnosed with acute cardiovascular syndrome, stable coronary artery disease, postpercutaneous coronary intervention, ischaemic cardiomyopathy and peripheral artery diseases. **High risk: (1) LDL-C ≥4.9 mmol/L or TC ≥7.2 mmol/L; (2) patients with diabetes aged over 40 with LDL-C 1.8–4.9 mmol/L, or TC 3.1–7.2 mmol/L. ***Low risk and moderate risk: TC 3.1–7.2 mmol/L or LDL-C 1.8–4.9 mmol/L.
Atrial fibrillation
Recommendations
For patients ≥65 years of age, active screening for atrial fibrillation (AF) in community health centres or primary carehospitals by checking pulses combined with electrocardiogram (ECG) is recommended (class I; level of evidence B). For high-risk patients, long-term ECG monitoring can increase the detection of undiagnosed AF, which is cost-effective (class IIa; level of evidence A).
For patients with non-valvular AF, a CHA2DS2-VASc score of ≥2 (table 1), and low risk of haemorrhage, anticoagulant therapy is recommended. Options include warfarin (INR2.0–INR3.0), or New Oral Anticoagulants (NOACs) such as dabigatran, rivaroxaban, apixaban (class I; level of evidence A), and edoxaban (class I; level of evidence B). The evidence on effective adjustment of warfarin dosage by genotypes is not sufficient (class IIb; level of evidence C).
For patients with non-valvular AF and severe renal impairment (Ccr <15 mL/min), certain NOACs (dabigatran and rivaroxaban) should not be used (class III; level of evidence C).
For patients with non-valvular AF and a CHA2DS2-VASc score of 0, antithrombotic therapy is not recommended (class III; level of evidence B).
For patients with non-valvular AF, a CHA2DS2-VASc score of 1, low risk of haemorrhage, and no previous antithrombotic therapy, anticoagulant therapy or aspirin therapy may be considered. The selection of antithrombotics should be determined on an individual basis (class IIb; level of evidence C).
For high-risk patients with AF who are unsuitable for anticoagulation, closure of the left atrial appendage (LAA) may be considered when performed at a centre with low rates of periprocedural complications and the patient can tolerate the risk of at least 45 days of postprocedural anticoagulant therapy(class IIb; level of evidence B).
Table 1.
CHA2DS2-VASc Score
| CHA2DS2-VASc | Score |
| Heart failure | 1 |
| Hypertension | 1 |
| Age ≥75 years | 2 |
| Diabetes mellitus | 1 |
| Prior stroke/TIA | 2 |
| Peripheral vascular disease | 1 |
| Age 65−74 years | 1 |
| Female sex | 1 |
TIA, transient ischaemic attack.
Other cardiac conditions
Recommendations
For patients with ST-elevation myocardial infarction (STEMI) and asymptomatic left ventricular mural thrombi, vitamin K antagonist therapy can be used (class IIa; level of evidence C).
For patients with STEMI and anterior apical akinesis or dyskinesis, anticoagulant therapy may be considered (class IIb; level of evidence C).
For patients with heart failure in sinus rhythm and no other indications for anticoagulation, vitamin K antagonist therapy is not recommended (class III; level of evidence B).
For patients with mitral stenosis who have a previous thromboembolic event or left atrial thrombi, vitamin K antagonist therapy is recommended (class I; level of evidence B).
Patients after valve replacement should visit the cardiovascular disease clinic and receive specific stroke prevention programmes based on the valve condition and other risk factors (class I).
For patients with infective endocarditis, anticoagulant therapy is not recommended unless there is a specific indication. Antibiotic therapy is the most important way to reduce the incidence of thromboembolic event in patients with endocarditis. For patients with non-bacterial thrombotic endocarditis, the key principle of treatment is to control the underlying diseases (class I; level of evidence C).
For patients with Patent foramen ovale (PFO), antithrombotic therapy or percutaneous closure is not recommended for the primary prevention of stroke (class III; level of evidence C).
Asymptomatic extracranial or intracranial stenosis
Recommendations
Patients with asymptomatic carotid stenosis are recommended to take aspirin and statin on a daily basis (class IIa; level of evidence C).
Patients with carotid stenosis >50% should be screened for other treatable risk factors of stroke. Appropriate medical therapies and a healthy lifestyle should be implimented, such as smoke-quitting, healthy diet and proper physical exercise (class I; level of evidence C).
In patients with carotid stenosis of 60%–99% and expected lifetime over 5 years, it is reasonable to consider performing carotid endarterectomy (CEA) and be done in qualified hospitals if the risk of perioperative stroke and death is low (<3%) (class IIa; level of evidence B). Unless contraindicated, patients are recommended to take aspirin perioperatively and postoperatively (class I; level of evidence C).
Prophylactic carotid artery stenting (CAS) might be considered in patients with carotid stenosis of 69%–99% but unsuitable for CEA. CAS can be conducted in qualified hospitals if the risk of perioperative stroke and death is low (<3%), but the effectiveness compared with medical therapy has not been well established (class IIb; level of evidence B).
Screening and following-up with ultrasound in qualified hospitals are recommended in patients with more than two risk factors, in order to better evaluate the progress of stenosis as well as the risk of stroke (class IIa; level of evidence C).
For patients with asymptomatic intracerebral stenosis, it is reasonable to consider antiplatelet and statin treatment (class IIa; level of evidence C).
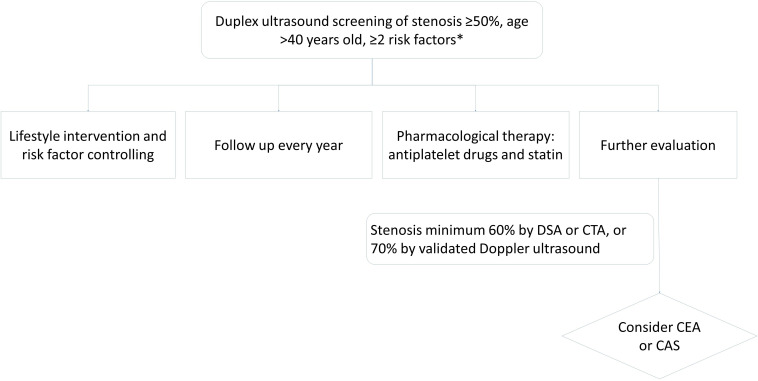
The management of patients with asymptomatic carotid stenosis (figure 4).
Figure 4.
Flow chart for the management of patients with asymptomatic carotid stenosis. *Risk factors include hypertension, dyslipidaemia, diabetes, atrial fibrillation, smoking, obesity, lack of physical activity and stroke family history. DSA, Digital Subtraction Angiography. CTA; CT Angiography; CEA, carotid endarterectomy;, CAS, carotid artery stenting.
Unruptured intracranial aneurysms
Recommendations
Smoking can increase the risk of UIA formation. Patients should be counselled the importance of smoking cessation (class I; level of evidence B).
Hypertension may be involved in the growth and rupture of intracranial aneurysms. Patients should monitor BP and receive BP lowering treatment (class I; level of evidence B).
Aneurysmal SAH (aSAH) can be regarded as a risk factor for the rupture of other coexistent UIAs. Patients with a history of aneurysmal SAH are probably recommended to proper treatment (class IIa; level of evidence B).
Patients with single anterior circulation aneurysms (size <5 mm) can be managed conservatively. For posterior circulation aneurysms, or size of aneurysm of >5 mm, with a history of aneurysmal rupture, symptomatic aneurysms, image-based risk factors (including the enlargement, increased number of daughter sacs and blebs, blood blister-like aneurysms), individualised treatment should be considered. Age, neurological function, overall condition, medical comorbidities and the risk of rupture should be considered in the decision making process (class IIa; level of evidence B).
Radiographic follow-up is indicated regularly for patients with UIAs managed non-invasively. For those with detected enlargement of the aneurysms during the follow-up, unless contraindicated, proper treatment is recommended (class I; level of evidence B).
Radiographic screening for follow-up of UIA can be conducted with CTA or MRA (classI; level of evidence B). For patients without contraindications to MRI, it is reasonable to consider MRA for long-term follow-up. DSA can be conducted if needed. It is reasonable to consider a first follow-up at 6–12 months after initial discovery of UIA, and follow-up every 1–2 years (class IIa; level of evidence C).
Treatment includes surgical clipping and endovascular treatment. Treatment selection is related to multiple factors including aneurysm location, size and morphology; documented growth of aneurysms during the follow-up; the age of the patient; history of aSAH; family history of cerebral aneurysm; the presence of multiple aneurysms or the presence of concurrent pathology such as an arteriovenous malformation or other cerebrovascular or inherited pathology (class I; level of evidence C).
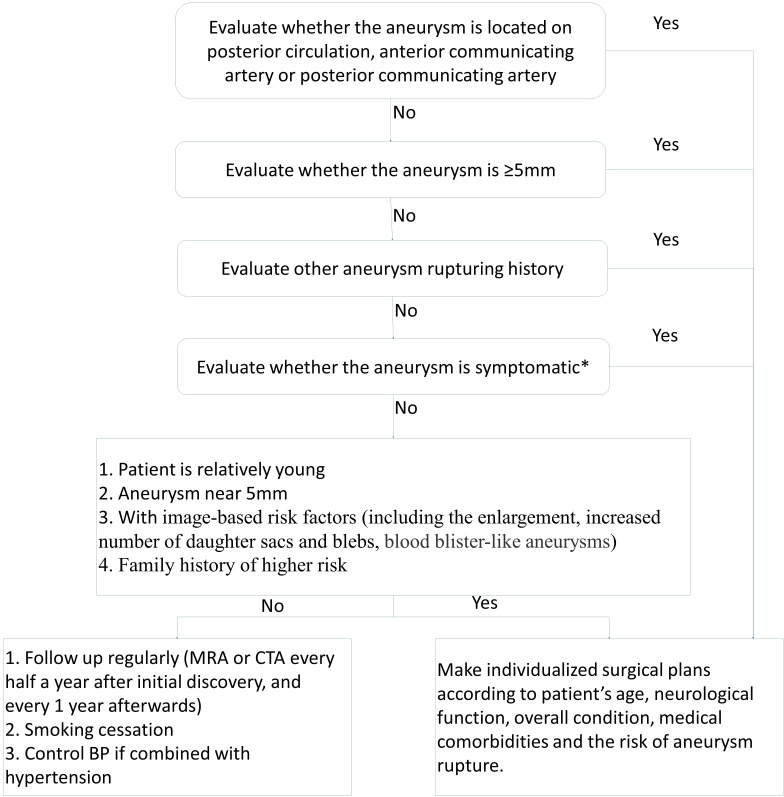
The management of patients with UIAs (figure 5).
Figure 5.
Flow chart for the management of patients with unruptured intracranial aneurysms. *Symptomatic aneurysm: patients show symptoms related to the aneurysm, such as ptosis, severe headache, vomiting, neck stiffness, drowsiness, enlarged pupils, disorder of consciousness and so on. MRA, MR Angiography; CTA, CT Angiography; BP, blood pressure.
Cigarette smoking
Recommendations
Smoking cessation ought to involve both smokers and the whole society, by adopting comprehensive tobacco control measures including psychological counselling, nicotine replacement, smoking cessation medications, and intervention in the community (class I; level of evidence A).
Non-smokers should avoid passive smoking (class I; level of evidence B).
Publicity and education should continue to be strengthened to raise public awareness of the harm of active and passive smoking. Local government should legislate against smoking in public places as soon as possible, such as smoking bans in offices, meeting rooms, airplanes or trains, to reduce the harm of smoking (class IIa; level of evidence B).
Alcohol consumption
Recommendations
Drinkers should reduce or eliminate alcohol consumption (class I; level of evidence A).
Drinking only in moderation should be recommended among individuals who choose to consume alcohol. Moderate alcohol consumption of ≤25 g/day for men and half for women is reasonable (class IIb; level of evidence B).
Obesity and body fat distribution
Recommendations
Overweight and obese individuals can reduce weight by a healthy lifestyle, a healthy diet, regular exercises, (class I; level of evidence C).
For overweight and obese individuals, weight reduction is recommended to help control BP (class I; level of evidence A), and reduce the risk of stroke (class I; level of evidence B).
Diet and nutrition
Recommendations
Daily diet should be diverse to make the intake of energy and nutrition more reasonable; a balanced diet including whole grains, legumes, tubers, fruits, vegetables, dairy product and a low intake of total fat and saturated fat is recommended (class I; level of evidence A).
Reducing the intake of salt and increasing the intake of potassium are recommended to reduce the risk of stroke by lowing BP. A salt intake of ≤6 g/day is recommended (class I; level of evidence A).
Encouraging more intake of fruits, vegetables and certain dairy product, and reducing the intake of saturated fat and trans fatty acids (class I; level of evidence A). The total fat intake per day should be less than 30% of total calories and trans fatty acids of less than 2 g; fresh vegetables intake of 400–500 g; fruits intake of 200–400 g; moderate consumption of fish, poultry, eggs and lean meat, with an average total intake of 120–200 g; various dairy product, with an intake equivalent to 300 g of liquid milk; vegetable oil intake of <25 g; added sugar (or free sugar, ie, the monosaccharide added in food, such as rock candy, refined cane sugar, etc) intake of <50 g/day, preferably <25 g/day.
Physical inactivity
Recommendations
Appropriate physical activities are recommended to reduce the risk of stroke (class I; level of evidence B). In elderly and high-risk people, individualised exercise programme should be planned, after conducting a safer maximal exercise tolerating test.
Healthy adults should perform at least moderate-to-vigorous-intensity aerobic physical activity (such as brisk walking, jogging, cycling or other aerobics) for at least 40 min per day for 3–4 days per week (class I; level of evidence B).
Standing up and moving limbs around for a few minutes after an hour of sitting still is recommended for people who are sedentary in their daily work, including those who have already had enough regular exercise weekly is recommended (class I; level of evidence C).
Evidence insufficient or potential modifiable risk factors
Metabolic syndrome
Recommendations
Metabolic syndrome is a clinical condition in which multiple metabolic abnormalities occur in the human body, including central obesity (abdominal obesity), dyslipidaemia, hypertension and elevated fasting blood glucose. Metabolic syndrome is increasingly common. Interventions of every single risk factor of the metabolic syndrome are recommended, including lifestyle measures and pharmacotherapy (ie, medications for BP lowering, lipid-lowering, glycaemic control, etc). Detailed interventions refer to relevant sections on ‘hypertension’, ‘abnormal glucose metabolism’, ‘dyslipidaemia’ of this guideline.
Sleep-disordered breathing
Recommendations
Screening for patients with sleep apnoea requires a detailed history. The screening methods include structured questionnaires such as the Epworth Sleepiness Scale, Berlin Questionnaire, physical examination. And polysomnography may be considered depending on the patients’ condition (class IIb; level of evidence C).
The effectiveness of treatment for sleep apnoea in stroke prevention is still not well established (class IIb; level of evidence C).
Migraine
Recommendations
For women and geriatric patients with migraine with aura, smoking cessation is recommended (class I; level of evidence B).
For women with active migraine with aura, withdrawal of oral contraceptives (OCs), especially those containing oestrogen, should be considered (class IIa; level of evidence B).
Treatments to reduce migraine frequency (especially for migraines with specific aura symptoms) might be able to reduce the risk of stroke (class IIa; level of evidence B).
For patients with migraine, the clinical benefit of closing PFO for the prevention of stroke is unproven (class III; level of evidence B).
Hyperhomocysteinaemia
Recommendations
Hyperhomocysteinaemia is a risk factor of stroke (level of evidence A). For patients with hyperhomocysteinaemia, the use of folic acid or folic acid in combination with cobalamin (B12) and pyridoxine (B6) might be effective in the prevention of stroke (class IIa; level of evidence B).
Elevated Lp(a)
Recommendations
The clinical benefit of using lipoprotein(a) [Lp(a)] in stroke risk prediction is not well established (class IIb; level of evidence B).
The effectiveness of niacin in lowing Lp(a) for the prevention of ischaemic stroke is not well established (class IIb; level of evidence B).
Hypercoagulability
Recommendations
For patients with inherited hypercoagulable states, the effectiveness of genetic screening for the prevention of stroke is not well established (class IIb; level of evidence C).
The effectiveness of treatments for the primary prevention of stroke in asymptomatic patients with a hereditary or acquired hypercoagulable state is not well established (class IIb; level of evidence C).
For patients who are antiphospholipid antibody (aPL) positive, low-dose aspirin is not recommended for the primary prevention of stroke (class III; level of evidence B).
For high-risk pregnant individuals, low-molecular-weight heparin is not recommended for the prevention of cerebrovascular disease (class III; level of evidence A).
Inflammation and infection
Recommendations
Patients with rheumatoid arthritis (RA) or systemic lupus erythematosus (SLE) are at an increased risk of stroke (level of evidence B).
Annual influenza vaccination might help to reduce stroke risk in high-risk patients with stroke, which still need more clinical research to confirm (class IIb; level of evidence B).
For high-risk patients with stroke, measurement of inflammatory markers such as high-sensitivity C reactive protein (hs-CRP) or Lp(a)-associated phospholipase A2 might be useful in assessing stroke risk (class IIb; level of evidence B).
For patients with myocardial infarction followed by hs-CRP >2.0 mg/L, anti-inflammatory therapy with a canakinumab may reduce stroke risk (class IIa; level of evidence B). However, its role in the risk of recurrent stroke prevention needs to be confirmed by large clinical trials mainly in patients with cerebral infarction. Whether statins can reduce the risk of cerebrovascular disease by their anti-inflammatory effects still needs to be confirmed by large clinical trials (class IIb; level of evidence B).
Treatment with antibiotics for chronic infections for the prevention of stroke is not recommended (class III; level of evidence A).
Drug abuse
Recommendations
Drugs of abuse include khat, cocaine, amphetamines. Drug users should be recommended to a drug-rehabilitation programme, for whom an appropriate detoxification therapy may be effective in primary stroke prevention (class IIa; level of evidence C).
Oral contraceptives
Recommendations
Before using OCs, women should have their BP measured and their risk factors of stroke such as hypertension, diabetes mellitus, hyperlipidaemia, obesity and migraine fully assessed (class I; level of evidence B).
For women ≥35 years of age, OCs are not recommended (class III; level of evidence B).
For women with risk factors such as cigarette smoking, hypertension, diabetes mellitus, migraine, hypercoagulability, OCs are not recommended (class III; level of evidence B).
Genetic screening for mutations associated with hypercoagulability before first administration of oral contraceptives is not recommended (class III; level of evidence B).
Postmenopausal hormone therapy
Recommendations
For postmenopausal women, hormone replacement therapy is not recommended for the primary prevention of stroke (class III; level of evidence A).
Selective estrin receptor modulators such as raloxifen, tamoxifen, tibolone is not recommended for the primary prevention of stroke (class III; level of evidence A).
For women who need HRT for other reasons, transdermal or intravaginal drug delivery can be used instead of oral HRT (class IIa; level of evidence B).
Antiplatelet agents and aspirin
Recommendations
For people aged 40–70 with sufficiently high risk of cardiovascular and cerebrovascular disease (10-year risk ≥10%), the use of aspirin for primary prevention may be considered (class IIb; level of evidence A).11–16
For women with high risks but a relatively low risk of bleeding, the use of aspirin for stroke prevention might be considered (class IIb; level of evidence B).
Aspirin might be considered for the prevention of a first stroke in people with chronic kidney disease (estimated glomerular filtration rate <45 mL/min/1.73 m2, class IIb; level of evidence C). However, for patients with severe kidney disease (stage 4 or 5; estimated glomerular filtration rate <30 mL/min/1.73 m2), aspirin is not recommended anymore.
Aspirin is not recommended for preventing a first ever stroke in low-risk individuals whose 10 year risk is less than 10% (class III; level of evidence A).
Aspirin is not recommended for preventing a first-ever stroke in people over 70 years old (class III; level of evidence B).
Assessing the risk of first stroke
Recommendations
The use of a risk assessment tool is reasonable and can help identify individuals who could benefit from therapeutic interventions. For high-risk individuals, treatment decisions should be individualised according to the overall risk profile of the patient (class IIa; level of evidence B).
Revised Framingham stroke profile, Pooled Cohort Equations, Stroke Riskometer, PREDICT Scaling, the 10-year risk of ICVD scaling in Chinese, China Multiprovincial Cohort Study (CMCS) Scaling, scales from CMCS, Cerebral Vascular Scaling, China-PAR model and other tools can be used to evaluate the risk of first stroke (class IIa; level of evidence B).
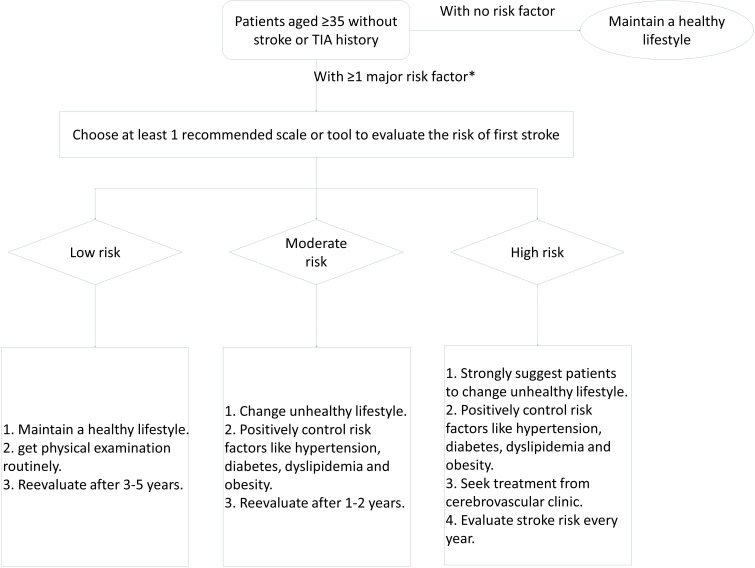
Flow chart for evaluating first stroke (figure 6).
Figure 6.
Flow chart for evaluating first stroke. *Major risk factor includes: age, sex, hypertension, diabetes, dyslipidaemia, atrial fibrillation, cigarette smoking, cerebrovascular or cardiovascular disease family history, BMI. BMI, body mass index; TIA, transient ischaemic attack.
Footnotes
Twitter: @yilong
YW and SH contributed equally.
Correction notice: This paper has been updated since first published to amend some heading levels.
Collaborators: Chinese Stroke Association Stroke Council Guideline Writing Committee. Chairmen: Yongjun Wang, yongjunwang@ncrcnd.org.cn, Department of Neurology, Beijing Tiantan Hospital, Capital Medical University, Beijing, China; Jizong Zhao, zhaojz205@163.com / zhaojz@public.bta.net.cn, Department of Neurosurgery, Beijing Tiantan Hospital, Capital Medical University, Beijing, China. Vice-Chairmen: Qiang Dong, dong_qiang@fudan.edu.cn, Department of Neurology, Huashan Hospital, Fudan University, Shanghai, China; Anding Xu, tlil@jnu.edu.cn, Department of Neurology and Stroke Center, the First Affiliated Hospital, Jinan University, Guangzhou, China. Members of Academic Committee: Kangning Chen, ckn_640827@126.com’ Department of Neurology, The Southwest Hospital, the First Affiliated Hospital of Third Military Medical University, Chongqing, China; Junbo Ge, ge.junbo@zs-hospital.sh.cn’ Shanghai Institute of Cardiovascular Diseases, Department of Cardiology, Zhongshan Hospital, Fudan University, Shanghai, China; Li Guo, guoli6@163.com, Department of Neurology, The Second Hospital of Hebei Medical University, Shijiazhuang, China; Li He, heli2003new@126.com, Department of Neurology, West China Hospital, Sichuan University, Chengdu, China; Bo Hu, hubo@hust.edu.cn, Department of Neurology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology (HUST), Wuhan, China; Yong Huo, huoyong@263.net.cn’ Department of Cardiology, Peking University First Hospital, Beijing, China; Linong Ji, jiln@bjmu.edu.cn, Department of Endocrinology and Metabolism, Peking University People's Hospital, Medicine at Peking University, Beijing, China; Xunming Ji, robertjixm@hotmail.com / jixunming@vip.163.com, Department of Neurosurgery, Xuanwu Hospital, Capital University of Medicine, Beijing, China; Tielin Li, tielin2013@126.com / tielin.li@tom.com, Zhujiang Hospital of Southern Medical University, Guangzhou, China; Liping Liu, lipingsister@gmail.com, Department of Neurology, Beijing Tiantan Hospital, Capital Medical University, Beijing, China; Benyan Luo, luobenyan@zju.edu.cn, Department of Neurology, 1st Affiliated Hospital of Zhejiang University, Hangzhou, China; Zhongrong Miao, zhongrongm@163.com, Department of Interventional Neuroradiology, Beijing Tiantan Hospital, Capital Medical University, Beijing, China; Xiaoyuan Niu, niuxiaoyuan1958@163.com, Department of Neurology, First Hospital of Shanxi Medical University, Taiyuan, China; Bin Peng, pengbin3@hotmail.com; Department of Neurology, Peking Union Medical College Hospital, Peking Union Medical College and Chinese Academy of Medical Sciences, Beijing, China; Dingfeng Su, dfsu@smmu.edu.cn, Department of Pharmacology, the Second Military Medical University (SMMU), Shanghai, China; Beisha Tang, bstang7398@163.com, Department of Neurology, Xiangya Hospital, Central South University, Changsha, China; Chen Wang, wangchen-tr2002@163.com, Beijing Tiantan Hospital, Capital Medical University, Beijing, China; Ning Wang, nwang900@yahoo.com, Department of Neurology and Institute of Neurology, First Affiliated Hospital of Fujian Medical University, Fuzhou, China; Shuo Wang, captain9858@vip.sina.com, Department of Neurosurgery, Beijing Tiantan Hospital, Capital Medical University, Beijing, China; Wei Wang, wwang@vip.126.com / wwang@tjh.tjmu.edu.cn, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China; Xin Wang, wang.xin@zs-hospital.sh.cn, Department of Neurology, Zhongshan Hospital, Fudan University, Shanghai, China; Yilong Wang, yilong528@aliyun.com, Department of Neurology, Beijing Tiantan Hospital, Capital Medical University, Beijing, China; Shizheng Wu, wushizheng2005@hotmail.com, Qinghai Province People's Hospital, Xining, China; Peng Xie, xiepeng@cqmu.edu.cn, Chongqing Medical University (CQMU), Chongqing, China; Yuming Xu, 13903711125@126.com / xym13903711125@126.com, Department of Neurology, the First Affiliated Hospital of Zhengzhou University, Zhengzhou, China; Yun Xu, xuyun20042001@aliyun.com, Department of Neurology, Drum Tower Hospital, Medical School of Nanjing University, Nanjing, China; Yi Yang, doctoryangyi@163.com / doctor_yangyi@hotmail.com, Department of Neurology, the First Hospital of Jilin University, Changchun, China; Jinsheng Zeng, zengjs@pub.guangzhou.gd.cn, Department of Neurology and Stroke Center, the First Affiliated Hospital of Sun Yat-Sen University, Guangdong, China; Chaodong Zhang, scdzhang@163.com, The First affiliated Hospital of China Medical University, Shenyang, China; Tong Zhang, zt61611@sohu.com, Capital Medical University School of Rehabilitation Medicine, China Rehabilitation Research Center, Beijing, China; Zhuo Zhang, zzhuo005@gmail.com, Beijing Anzhen Hospital, Capital Medical University, Beijing, China; Gang Zhao, zhaogang@fmmu.edu.cn, Department of Neurology, Xijing Hospital, The 4th Military Medical University, Xi’an, China; Xingquan Zhao, zxq@vip.163.com, Department of Neurology, Beijing Tiantan Hospital, Capital Medical University, Beijing, China
Contributors: WW, YW, YJ, and ZL designed the protocol and framework. WW, BJ, XR, DS and HS drafted the sections of unmodifiable risk factors, cigarette smoking, alcohol consumption, obesity and body fat distribution, diet and nutrition, and physical inactivity. XX drafted the section of hypertension. QJ drafted the section of diabetes and prediabetes. HZ and YY drafted the sections of dyslipidaemia and elevated Lp(a). SL and XY drafted the sections of atrial fibrillation and other heart diseases. HQ, LZ and YumX drafted the section of asymptomatic extracranial or intracranial stenosis. YC and YJ drafted the section of unruptured intracranial aneurysms. PW and TW drafted the sections of metabolic syndrome and infection and inflammation. HQ and MZ drafted the sections of sleep-disordered breathing and antiplatelet agents and aspirin. LG drafted the section of migraine. WL drafted the section of hyperhomocysteinaemia. HZ and SH drafted the sections of hypercoagulability, drug abuse, oral contraceptives and postmenopausal hormone therapy. YL, YG and YuyX drafted the section of assessing the risk of first stroke. WW, YW, HZ, HQ, BJ and SH discussed and updated the final version. SH and LG did the translation for the supplement material.
Funding: This research received specific funding from Chinese Stroke Association Guidelines Writing Committee.
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Commissioned; externally peer reviewed.
Contributor Information
Chinese Stroke Association Stroke Council Guideline Writing Committee:
Yongjun Wang, Jizong Zhao, Qiang Dong, Anding Xu, Kangning Chen, Junbo Ge, Li He Li Guo, Bo Hu, Yong Huo, Linong Ji, Xunming Ji, Tielin Li, Liping Liu, Benyan Luo, Zhongrong Miao, Xiaoyuan Niu, Bin Peng, Dingfeng Su, Beisha Tang, Chen Wang, Ning Wang, Shuo Wang, Wei Wang, Xin Wang, Yilong Wang, Shizheng Wu, Peng Xie, Yuming Xu, Yun Xu, Yi Yang, Jinsheng Zeng, Chaodong Zhang, Tong Zhang, Zhuo Zhang, Gang Zhao, and Xingquan Zhao
References
- 1. Wang W, Jiang B, Sun H, et al. Prevalence, incidence, and mortality of stroke in China: results from a nationwide population-based Survey of 480 687 adults. Circulation 2017;135:759–71. 10.1161/CIRCULATIONAHA.116.025250 [DOI] [PubMed] [Google Scholar]
- 2. Meschia JF, Bushnell C, Boden-Albala B, et al. Guidelines for the primary prevention of stroke: a statement for healthcare professionals from the American heart Association/American stroke association. Stroke 2014;45:3754–832. 10.1161/STR.0000000000000046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Thompson BG, Brown RD, Amin-Hanjani S, et al. Guidelines for the management of patients with unruptured intracranial aneurysms: a guideline for healthcare professionals from the American heart Association/American stroke association. Stroke 2015;46:2368. 10.1161/STR.0000000000000070 [DOI] [PubMed] [Google Scholar]
- 4. Ference BA, Kastelein JJP, Ray KK, et al. Association of triglyceride-lowering LPL variants and LDL-C–lowering LDLR variants with risk of coronary heart disease. JAMA 2019;321:364–73. 10.1001/jama.2018.20045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. O'Donoghue ML, Fazio S, Giugliano RP, et al. Lipoprotein(a), PCSK9 inhibition, and cardiovascular risk. Circulation 2019;139:1483–92. 10.1161/CIRCULATIONAHA.118.037184 [DOI] [PubMed] [Google Scholar]
- 6. Bhatt DL, Steg PG, Miller M, et al. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med 2019;380:11–22. 10.1056/NEJMoa1812792 [DOI] [PubMed] [Google Scholar]
- 7. Armitage J, Baigent C, Barnes E, et al. Efficacy and safety of statin therapy in older people: a meta-analysis of individual participant data from 28 randomised controlled trials. Lancet 2019;393:407–15. 10.1016/S0140-6736(18)31942-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Manson JE, Cook NR, Lee I-M, et al. Marine n-3 fatty acids and prevention of cardiovascular disease and cancer. N Engl J Med 2019;380:23–32. 10.1056/NEJMoa1811403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ray KK, Bays HE, Catapano AL, et al. Safety and efficacy of bempedoic acid to reduce LDL cholesterol. N Engl J Med 2019;380:1022–32. 10.1056/NEJMoa1803917 [DOI] [PubMed] [Google Scholar]
- 10. Ray KK, Colhoun HM, Szarek M, Souza W K SBD, et al. Effects of alirocumab on cardiovascular and metabolic outcomes after acute coronary syndrome in patients with or without diabetes: a prespecified analysis of the odyssey outcomes randomised controlled trial. Lancet Diabetes Endocrinol 2019;7:618. 10.1016/S2213-8587(19)30158-5 [DOI] [PubMed] [Google Scholar]
- 11. McNeil JJ, Wolfe R, Woods RL, et al. Effect of aspirin on cardiovascular events and bleeding in the healthy elderly. N Engl J Med 2018;379:1509–18. 10.1056/NEJMoa1805819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McNeil JJ, Woods RL, Nelson MR, et al. Effect of aspirin on disability-free survival in the healthy elderly. N Engl J Med 2018;379:1499–508. 10.1056/NEJMoa1800722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. ASCEND Study Collaborative Group, Bowman L, Mafham M, et al. Effects of aspirin for primary prevention in persons with diabetes mellitus. N Engl J Med 2018;379:1529–39. 10.1056/NEJMoa1804988 [DOI] [PubMed] [Google Scholar]
- 14. Gaziano JM, Brotons C, Coppolecchia R, et al. Use of aspirin to reduce risk of initial vascular events in patients at moderate risk of cardiovascular disease (ARRIVE): a randomised, double-blind, placebo-controlled trial. Lancet 2018;392:1036–46. 10.1016/S0140-6736(18)31924-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Abdelaziz HK, Saad M, Pothineni NVK, et al. Aspirin for primary prevention of cardiovascular events. J Am Coll Cardiol 2019;73:2915–29. 10.1016/j.jacc.2019.03.501 [DOI] [PubMed] [Google Scholar]
- 16. Zheng SL, Roddick AJ. Association of aspirin use for primary prevention with cardiovascular events and bleeding events: a systematic review and meta-analysis. JAMA 2019;321:277–87. 10.1001/jama.2018.20578 [DOI] [PMC free article] [PubMed] [Google Scholar]