Abstract
Background
The presence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in saliva has alerted health professionals to the possibility of contamination by aerosols generated in a number of procedures. The indication of preoperative mouthwash containing 1% hydrogen peroxide for reducing the viral load of SARS-CoV-2 in saliva prior to oral procedures has been significantly disseminated through several citations and influenced various dental associations in the elaboration of dental care protocols during this pandemic period, including patients admitted to hospital wards and intensive care units.
Aim
To Our aim was to perform a systematic review to answer the following question: does hydrogen peroxide mouthwash (at any concentration) have a virucidal effect?
Methods
The Cochrane, LILACS, PubMed, Scopus, and Embase databases were searched by using the following key-words: ‘hydrogen peroxide’, ‘mouthwash’, ‘mouth rinse’, ‘rinse’, ‘oral rinse’, ‘mouth bath’, ‘mouth wash’, and ‘mouth washes’. Reviews, letters to the editor, personal opinions, book chapters, case reports, congress abstracts, studies with animals and studies on mouthwash containing other compounds other than hydrogen peroxide were excluded.
Findings
During the initial search 1342 articles were identified on the five electronic databases. After excluding some duplicates, 976 articles remained. Only studies assessing the virucidal effect of hydrogen peroxide mouthwash were selected, regardless of publication date.
Conclusion
After reading titles and abstracts, no article met the eligibility criteria. In conclusion, there is no scientific evidence supporting the indication of hydrogen peroxide mouthwash for control of the viral load regarding SARS-CoV-2 or any other viruses in saliva.
Keywords: Coronavirus, Infection control, Mouthwashes, Saliva, Viral load, Viruses
Introduction
Coronaviruses have been responsible for three major respiratory diseases in recent decades. The third one began in December 2019 in Wuhan, China, giving rise to a pandemic caused by a new virus termed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [1,2]. SARS-CoV-2 is mainly transmitted by direct contact with infected people through respiratory or salivary droplets, and to a lesser extent by indirect contact with contaminated surfaces [3]. The virus has tropism for cells whose membrane contains a specific receptor – the angiotensin converting enzyme-2 (ACE2), which is present in many human tissues other than the lung, which is the main target organ for the virus infection [4,5]. The identification of ACE2 in the oral mucosal epithelial cells, particularly the dorsum of the tongue, and the description of SARS-CoV-2 oral shedding has brought more fear to health professionals, especially those working in close contact with patients and who are exposed to aerosols [[6], [7], [8], [9], [10], [11], [12], [13]].
In an attempt to prevent patient-to-practitioner transmission by means of contaminated saliva, opinions on how to proceed with patients began to emerge in the literature in order to reduce the risk of cross-infection through aerosol generation [[14], [15], [16], [17]]. One of the presented solutions is the use of antiseptic mouthwashes. But it is important to understand that antisepsis (i.e. to destruct or to inhibit micro-organisms in a living tissue) is not the same as disinfection (i.e. to destruct or to inhibit micro-organisms on inanimate objects) [18]. These two processes require types of chemical compounds that must be tested in different ways for approval by the competent agencies according to regulatory norms.
According to Saddik and Pappan, mouthwashes are considered cosmetics in Europe whereas in the USA they can also be registered as drugs, depending on their therapeutic properties (or their intended use declared by the manufacturer) [19]. In the latter case, they should be submitted to a drug approval process involving pre-clinical studies (in vitro with cultured cells and in vivo with laboratory animals) and clinical trials. The last one involves four phases: phase 1: involving fewer than 100 healthy or diseased volunteers; phase 2: several hundreds of volunteers with the majority being patients with the disease; phase 3: with several thousand volunteers, this requires at least a few years for completion; phase 4: post-market phase in which the drug is actively monitored by the consumers [19].
Although it is easier to put a cosmetic product on the European market, those products are also subjected to regulations placed by the local law of all the member countries of the EU. After being made available in the European market, those products are subject to efficiency evaluation in official laboratories [19].
The International Organization for Standardization (ISO) specifies the chemical and physical properties and test methods for oral rinses, but the instructions for microbiological examination are about bacteria, mould, and yeast. There are no instructions about virus (ISO 16408:2015 (en) Dentistry – Oral care products – Oral rinses).
There are some standard tests to determine virucidal activity, but almost all are for surface disinfection and a few are aimed at antiseptic capacity for external surfaces (e.g. skin), which invariably do not have viral replication. None demonstrate how to verify virucidal effect in non-standardized samples from nasopharyngeal or oral cavity [20].
The use of mouthwash containing 1% hydrogen peroxide has been proposed to reduce viral load of SARS-CoV-2 in saliva [18]. This relationship seems to be demonstrated by studies assessing the use of mouthwash for reduction of oral microbiota, especially bacteria, meaning that the oxidative properties of hydrogen peroxide would also enable the degradation of viral particles. Nevertheless, there is no citation of a study supporting such an approach [21].
Because of the increasing number of publications replicating this information (Appendix A) and many institutions in several countries (EU, UK, New Zealand, India, Spain, Portugal, Brazil, Italy) recommending the use of hydrogen peroxide mouthwash as part of a dental care protocol during the coronavirus disease 2019 (COVID-19) pandemic, including patients admitted to hospital wards and intensive care units, the objective of this systematic review was to answer the following question: does hydrogen peroxide mouthwash have a virucidal effect? [[22], [23], [24], [25], [26], [27], [28]].
Methods
This systematic review was performed based on the items of the Systematic Reviews and Meta-Analyses (PRISMA) Checklist. The systematic review protocol was registered at the International Prospective Register of Systematic Reviews (PROSPERO) according to number CRD42020189431.
Eligibility criteria
Inclusion criteria
Only studies assessing the virucidal effect of hydrogen peroxide mouthwash were selected. In order to reduce the publication bias, these studies were included regardless of publication date restrictions.
Exclusion criteria
Reviews, letters to the editor, personal opinions, book chapters, case reports, congress abstracts, studies with animals and studies on mouthwash containing compounds other than hydrogen peroxide were excluded.
Information sources
Individual search strategies were developed for Cochrane, LILACS, PubMed, Scopus and Embase databases. The following keywords were used in the search: ‘hydrogen peroxide’, ‘mouthwash’, ‘mouth rinse’, ‘rinse’, ‘oral rinse’, ‘mouth bath’, ‘mouth wash’ and ‘mouth washes’ (further information is provided in Appendix B). Additionally, the grey literature was also searched by using ProQuest, Open Grey, and Google Scholar, in which the last was limited to the first 120 articles published. The lists of references on this theme were also searched. The above-mentioned databases, including the grey literature, were searched on May 30th, 2020.
Study selection
The studies were selected in two phases. The first phase was conducted by using reference manager software (EndNote® X7; Thomson Reuters, Philadelphia, PA, USA) to collect references and exclude duplicates [29]. In the second phase, two reviewers evaluated independently the titles and abstracts of the articles selected from the search results. Also, in this phase, articles clearly not meeting the inclusion criteria or meeting some of the exclusion criteria were excluded. In case of doubt, a third reviewer was consulted for a final decision.
Results
Study selection
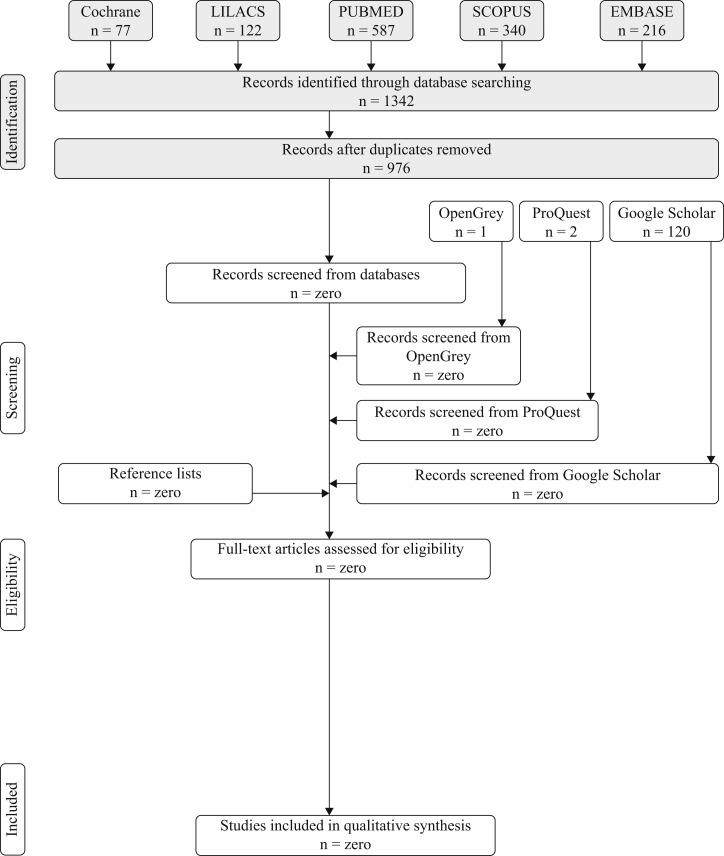
During the initial search, a total of 1342 articles were identified on the five electronic databases (i.e. Cochrane, LILACS, PubMed, Scopus, and Embase) and 976 remained after eliminating duplicate studies. Nevertheless, no article met the eligibility criteria after reading the titles and abstracts. Likewise, no study was found in the grey literature (i.e. Pro Quest, Open Grey, Google Scholar) within the same parameters (Figure 1 ).
Figure 1.
Procedure for searching references and selection criteria.
Discussion
With the recent emergence of the COVID-19 pandemic, the information that mouthwash with 1% hydrogen peroxide would be capable of reducing the viral load of SARS-CoV-2 in the saliva has been disseminated based on this compound's biological action on bacteria or opinions based on its efficacy on surfaces [30,31]. The authors of these publications believed that the reduction of viral load in the saliva would decrease contamination of the dental office environment through aerosol following treatment of an infected patient, or even shorten the patient's hospitalization [30,31].
The routine use of mouthwash prior to dental procedures is aimed at decreasing the formation of biofilm, minimizing transient bacteraemia in the patient undergoing invasive procedures and preventing ventilator-associated pneumonia [32]. The dental practitioner would also benefit from the decreased bacterial load during aerosol generation. However, despite being a well-established routine, there is little scientific evidence on the effectiveness of preoperative mouthwash for control of bacteraemia and contamination through aerosols [[33], [34], [35]]. These analyses considered the action of these products on bacteria only, since nothing is mentioned about viruses.
There are some in-vitro studies demonstrating the virucidal action of Listerine® and chlorhexidine on human immuno-deficiency virus and herpes simplex type 1 (HSV-1) [36,37]. Only one in-vivo study assessed the efficacy of Listerine in decreasing HSV-1 in the saliva of patients with active lip lesions, thus evidencing an effective control for 30 min [38]. It is important to remember that this effect may be related to the replication cycle of the virus itself and/or to the mechanical removal of viral particles with the use of mouthwash, rather than to an alleged virucidal effect of the active principle. A recent publication shows a variable viral load of SARS-CoV-2 in saliva depending on the period of the day analysed [39].
Other in-vitro studies, showing antiviral efficacy by analysing viral titres in cell culture, reported positive results for the efficacy of Listerine against respiratory viruses (e.g. influenza A and rotavirus) and of povidone iodine against influenza H1N1 virus and coronavirus (SARS-CoV, Middle East respiratory syndrome coronavirus, SARS-CoV-2) [20,[40], [41], [42]]. Nevertheless, there is no clinical study demonstrating these findings in vivo.
A limited clinical study with no placebo controls or in-vitro antiviral efficacy data using saline mouthwashes has recently been reported [43].
The small number of published studies on virucidal effect of mouthwashes may be related to different regulations for this product observed worldwide [19]. The current scenario of a pandemic, where transmission also results from contaminated saliva particles, highlights that the classification of mouthwashes as a therapeutic agent is the more appropriate and could provide more robust clinical studies for market registration purposes. Moreover, it is worth pointing out that none of these studies assessed the action of hydrogen peroxide mouthwashes [38,40,43].
Hydrogen peroxide is a substance which is degraded into oxygen and water when in contact with catalase – an enzyme present in almost all living beings, including micro-organisms within the oral microbiota – and this oxidative process would be capable of eliminating bacteria and fungi [44]. Peng et al. assumed that this process of oxidation might also be effective against SARS-CoV-2 by alleging that this virus would be sensitive to oxidation [30]. The work in question has been cited frequently in the literature since its publication (Appendix A), becoming a source of information and basis for attitudes during the COVID-10 pandemic in several dental specialties [30].
Taking into consideration the chemical reaction on which the antimicrobial properties of hydrogen peroxide are based, this degradation occurring almost instantaneously would not be capable of impeding an immediate recontamination of the oral cavity, since the particles of a virus (e.g. SARS-CoV-2) can potentially come from various sources such as respiratory secretions, oropharynx, salivary glands and gingival crevicular fluid [6,10,11,[45], [46], [47]]. Therefore, it would be necessary that any substance to be used as preoperative mouthwash had a high substantivity, that is, the capacity to interact with structures of the oral cavity to allow a slow release of this agent, thus increasing the duration of its virucidal effect.
A systematic review demonstrated that effect of hydrogen peroxide mouthwash in preventing formation of oral biofilm was poor as well as brief, thus showing its low substantivity [48]. Most of the studies included in this review were published between the 1970s and 1990s, which reflects the discontinuation in the indication of hydrogen peroxide as an effective mouthwash for controlling oral microbiota, precisely because of the lack of substantivity [48].
In conclusion, since there is a lack of scientific evidence supporting any virucidal activity of hydrogen peroxide mouthwash, associated with its lack of substantivity, its indication in dental care protocols during the COVID-19 pandemic should be revised.
Acknowledgements
The authors would like to thank J.T. Sales for language correction of the manuscript. This study was supported by grants from the Brazilian National Council for Scientific and Technological Development (CNPq grant no. 44004/2014-5); and from the São Paulo Research Foundation (FAPESP grant no. 2015/07727-9).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhin.2020.10.003.
Conflict of interest statement
None declared.
Funding sources
None.
Appendix C. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Wang C., Horby P.W., Hayden F.G., Gao G.F. A novel coronavirus outbreak of global health concern. Lancet. 2020;395:470–473. doi: 10.1016/S0140-6736(20)30185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li J.Y., You Z., Wang Q., Zhou Z.J., Qiu Y., Luo R. The epidemic of 2019-novel-coronavirus (2019-nCoV) pneumonia and insights for emerging infectious diseases in the future. Microbes Infect. 2020;22:80–85. doi: 10.1016/j.micinf.2020.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization Modes of transmission of virus causing COVID-19: implications for IPC precaution recommendations. https://www.who.int/news-room/commentaries/detail/modes-of-transmission-of-virus-causing-covid-19-implications-for-ipc-precaution-recommendations/ Available at: [last accessed June 2020]
- 4.Nunes Duarte-Neto A., de Almeida Monteiro R.A., da Silva L.F.F., Malheiros D.M.A.C., de Oliveira E.P., Theodoro Filho J. Pulmonary and systemic involvement of COVID-19 assessed by ultrasound-guided minimally invasive autopsy. 2020. Histopathology 2020:0–2. [DOI] [PMC free article] [PubMed]
- 5.Hamming I., Timens W., Bulthuis M.L.C., Lely A.T., Navis G.J., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu J., Li Y., Gan F., Du Y., Yao Y. Salivary glands: potential reservoirs for COVID-19 asymptomatic infection. J Dent Res. 2020;99:989. doi: 10.1177/0022034520918518. [DOI] [PubMed] [Google Scholar]
- 7.Azzi L., Carcano G., Gianfagna F., Grossi P., Gasperina D.D., Genoni A. Saliva is a reliable tool to detect SARS-CoV-2. J Infect. 2020;81:e45–e50. doi: 10.1016/j.jinf.2020.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kojima N., Turner F., Slepnev V., Bacelar A., Deming L., Kodeboyina S. Self-collected oral fluid and nasal swabs demonstrate comparable sensitivity to clinician collected nasopharyngeal swabs for Covid-19 detection. MedRxiv. 2020 doi: 10.1101/2020.04.11.20062372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pasomsub E., Watcharananan S.P., Boonyawat K., Janchompoo P., Wongtabtim G., Suksuwan W. Saliva sample as a non-invasive specimen for the diagnosis of coronavirus disease 2019: a cross-sectional study. Clin Microbiol Infect. 2020 May 15;S1198–743X:30278–30280. doi: 10.1016/j.cmi.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.To K.K.W., Tsang O.T.Y., Chik-Yan Yip C., Chan K.H., Wu T.C., Chan J.M.C. Consistent detection of 2019 novel coronavirus in saliva. Clin Infect Dis. 2020;71:841–843. doi: 10.1093/cid/ciaa149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.To K.K.W., Tsang O.T.Y., Leung W.S., Tam A.R., Wu T.C., Lung D.C. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis. 2020;20:565–574. doi: 10.1016/S1473-3099(20)30196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams E., Bond K., Zhang B., Putland M., Williamson D.A. Saliva as a non-invasive specimen for detection of SARS-CoV-2. J Clin Microbiol. 2020 Jul 23;58 doi: 10.1128/JCM.00776-20. e00776-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wyllie A.L., Fournier J., Casanovas-Massana A., Campbell M., Tokuyama M., Vijayakumar P. Saliva is more sensitive for SARS-CoV-2 detection in COVID-19 patients than nasopharyngeal swabs. MedRxiv. 2020 doi: 10.1101/2020.04.16.20067835. [DOI] [Google Scholar]
- 14.Meng L., Hua F., Bian Z. Coronavirus disease 2019 (COVID-19): emerging and future challenges for dental and oral medicine. J Dent Res. 2020;99:481–487. doi: 10.1177/0022034520914246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Umer F., Haji Z., Zafar K. Role of respirators in controlling the spread of novel coronavirus (Covid-19) among dental health care providers: a review. Int Endod J. 2020;53:1062–1067. doi: 10.1111/iej.13313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baghizadeh Fini M. What dentists need to know about COVID-19. Oral Oncol. 2020;105:104741. doi: 10.1016/j.oraloncology.2020.104741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ge Z yu, Yang L ming, Xia J jia, Fu X hui, Zhang Y Zhen. Possible aerosol transmission of COVID-19 and special precautions in dentistry. J Zhejiang Univ Sci B. 2020;21:361–368. doi: 10.1631/jzus.B2010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention Glossary of terms for infection prevention and control in dental settings. 2020. https://www.cdc.gov/oralhealth/infectioncontrol/glossary.htm/ Available at: [last accessed September 2020]
- 19.Saddik P., Pappan J. Differentiation between the regulatory paths placed on mouthwashes in the US and EU. Int J Drug Regul Aff. 2018;6:8–13. doi: 10.22270/ijdra.v6i2.229. [DOI] [Google Scholar]
- 20.Eggers M., Koburger-Janssen T., Eickmann M., Zorn J. In vitro bactericidal and virucidal efficacy of povidone-iodine gargle/mouthwash against respiratory and oral tract pathogens. Infect Dis Ther. 2018;7:249–259. doi: 10.1007/s40121-018-0200-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ortega K.L., Rodrigues de Camargo A., Bertoldi Franco J., Mano Azul A., Pérez Sayáns M., Braz Silva P.H. SARS-CoV-2 and dentistry. Clin Oral Invest. 2020;24:2541–2542. doi: 10.1007/s00784-020-03381-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jamal M., Shah M., Almarzooqi S.H., Aber H., Khawaja S., El Abed R. Overview of transnational recommendations for COVID-19 transmission control in dental care settings. Oral Dis. 2020 doi: 10.1111/odi.13431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Italian Ministry of Health . 2020. OGGETTO: COVID-2019. Nuove indicazioni e chiarimenti.https://omceopi.org/notizie-utili/1276-oggetto-covid-2019-nuove-indicazioni-e-chiarimenti Available at: [last accessed June 2020] [Google Scholar]
- 24.de España Gobierno, M de trabajo y economia S. 2020. Directrices de buenas prácticas en las clínicas dentales.https://www.insst.es/documents/94886/715218/Directrices+de+buenas+prácticas+en+las+cl%C3%ADnicas+dentales/95b8880e-3a92-4246-b772-abe28f5c2ed5 Available at: [last accessed June 2020] [Google Scholar]
- 25.Ordem dos Médicos Dentistas de Portugal . 2020. COVID-19: procedimentos em clínicas, consultórios ou serviços de saúde oral dos cuidados de saúde primários, setor social e privado.https://covid19.min-saude.pt/orientacoes/ Available at: [last accessed June 2020] [Google Scholar]
- 26.Federal de Odontologia Conselho. 2020. COVID19: manual de Boas Práticas em Biossegurança para Ambientes Odontológicos é lançado com apoio institucional do CFO.https://website.cfo.org.br/covid19-manual-de-boas-praticas-em-biosseguranca-para-ambientes-odontologicos-e-lancado-com-apoio-institucional-do-cfo/ Available at: [last accessed June 2020] [Google Scholar]
- 27.American Dental Association 2020. https://www.ada.org/en/press-room/news-releases/2020-archives/may/as-dental-practices-resume-operations-ada-offers-continued-guidance Available at: [last accessed June 2020]
- 28.British Endodontic Society. 2020. Diagnosis and management of endodontic emergencies, a British endodontic society position paper for primary dental care and other healthcare providers during the COVID-19 pandemic.https://britishendodonticsociety.org.uk/wp-content/uploads/2020/03/BES-Emergency-Protocol-FINAL-DOCUMENT-29-MARCH-2020.pdf Available at: [last accessed June 2020] [Google Scholar]
- 29.Collaborate C. From C. EndNote. 2013;X7 [Google Scholar]
- 30.Peng X., Xu X., Li Y., Cheng L., Zhou X., Ren B. Transmission routes of 2019-nCoV and controls in dental practice. Int J Oral Sci. 2020;12:9. doi: 10.1038/s41368-020-0075–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Caruso A.A., Del Prete A., Lazzarino A.I., Capaldi R., Grumetto L. May hydrogen peroxide reduce the hospitalization rate and complications of SARS-CoV-2 infection? Infect Control Hosp Epidemiol. 2020:1–2. doi: 10.1017/ice.2020.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li J., Xie D., Li A., Yue J. Oral topical decontamination for preventing ventilator-associated pneumonia: a systematic review and meta-analysis of randomized controlled trials. J Hosp Infect. 2013 Aug;84:283–293. doi: 10.1016/j.jhin.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 33.Balejo R.D.P., Cortelli J.R., Costa F.O., Cyrino R.M., Aquino D.R., Cogo-Müller K. Effects of chlorhexidine preprocedural rinse on bacteremia in periodontal patients: a randomized clinical trial. J Appl Oral Sci. 2017;25:586–595. doi: 10.1590/1678-7757-2017-0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Duvall N.B., Fisher T.D., Hensley D., Hancock R.H., Vandewalle K.S. The comparative efficacy of 0.12% chlorhexidine and amoxicillin to reduce the incidence and magnitude of bacteremia during third molar extractions: a prospective, blind, randomized clinical trial. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;115:752–763. doi: 10.1016/j.oooo.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 35.Marui V.C., Souto M.L.S., Rovai E.S., Romito G.A., Chambrone L., Pannuti C.M. Efficacy of preprocedural mouthrinses in the reduction of microorganisms in aerosol: a systematic review. J Am Dent Assoc. 2019;150:1015–1026. doi: 10.1016/j.adaj.2019.06.024. [DOI] [PubMed] [Google Scholar]
- 36.Baqui A.A.M.A., Jabra-Rizk M.A., DePaola L.G., Falkler W.A., Meiller T.F. In vitro effect of oral antiseptics on human immunodeficiency virus-1 and herpes simplex virus type 1. J Clin Periodontol. 2001;28:610–616. doi: 10.1034/j.1600-051x.2001.028007610.x. [DOI] [PubMed] [Google Scholar]
- 37.Bernstein D., Schiff G., Echler G., Prince A., Feller M., Briner W. In vitro virucidal effectiveness of a 0.12%-chlorhexidine gluconate mouthrinse. J Dent Res. 1990;69:874–876. doi: 10.1177/00220345900690030901. [DOI] [PubMed] [Google Scholar]
- 38.Meiller T.F., Silva A., Ferreira S.M., Jabra-Rizk M.A., Kelley J.I., DePaola L.G. Efficacy of Listerine® antiseptic in reducing viral contamination of saliva. J Clin Periodontol. 2005;32:341–346. doi: 10.1111/j.1600-051X.2005.00673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hung D.L.-L., Li X., Chiu K.H.-Y., Yip C.C.-Y., To K.K.-W., Chan J.F.-W. Early morning versus spot posterior oropharyngeal saliva for diagnosis of SARS-CoV-2 infection: implication of timing of specimen collection for community-wide screening. Open Forum Infect Dis. 2020;7 doi: 10.1093/ofid/ofaa210. ofaa210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dennison D.K., Meredith G.M., Shillitoe E.J., Caffesse R.G. The antiviral spectrum of Listerine antiseptic. Oral Surgery. Oral Med Oral Pathol Oral Radiol. 1995;79:442–448. doi: 10.1016/S1079-2104(05)80124-6. [DOI] [PubMed] [Google Scholar]
- 41.Eggers M., Eickmann M., Zorn J. Rapid and effective virucidal activity of povidone-iodine products against Middle East Respiratory Syndrome Coronavirus (MERS-CoV) and Modified Vaccinia Virus Ankara (MVA) Infect Dis Ther. 2015;7:249–259. doi: 10.1007/s40121-015-0091-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bidra A.S., Pelletier J.S., Westover J.B., Frank S., Brown S.M., Tessema B. Rapid in-vitro inactivation of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) using povidone-iodine oral antiseptic rinse. J Prosthodont. 2020;2:1–14. doi: 10.1111/jopr.13209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ramalingam S., Graham C., Dove J., Morrice L., Sheikh A. A pilot, open labelled, randomised controlled trial of hypertonic saline nasal irrigation and gargling for the common cold. Sci Rep. 2019;9:1015. doi: 10.1038/s41598-018-37703-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhu G., Wang Q., Lu S., Niu Y. Hydrogen peroxide: a potential wound therapeutic target? Med Princ Pract. 2017;26:301–308. doi: 10.1159/000475501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pallos D., Ruivo G.F., Ferrari-Junior S.H., Pannuti C.S., Perozini C., Sarmento D.J.S. Periodontal disease and detection of human herpesviruses in saliva and gingival crevicular fluid of chronic kidney disease patients. J Periodontol. 2020 doi: 10.1002/jper.19-0583. [DOI] [PubMed] [Google Scholar]
- 46.Badran Z., Gaudin A., Struillou X., Amador G., Soueidan A. Periodontal pockets: a potential reservoir for SARS-CoV-2? Med Hypoth. 2020;143:42–44. doi: 10.1016/j.mehy.2020.109907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Braz-Silva P.H., Pallos D., Giannecchini S., To K.K.W. SARS-CoV-2: what can saliva tell us? Oral Dis. 2020 doi: 10.1111/odi.13365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hossainian N., Slot D.E., Afennich F., Van Der Weijden G.A. The effects of hydrogen peroxide mouthwashes on the prevention of plaque and gingival inflammation: a systematic review. Int J Dent Hyg. 2011;9:171–181. doi: 10.1111/j.1601-5037.2010.00492.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.