Abstract
There are reports of children COVID-19 or COVID-19 like symptoms with hyperinflammatory multisystem syndrome, ARDS, gastrointestinal and atypical Kawasaki disease presenting to PICU worldwide temporally associated with COVID-19, for which there are important nutrition support considerations. As a result, the European Society of Pediatric and Neonatal Intensive Care – Metabolism, Endocrine and Nutrition group (ESPNIC-MEN) and paediatric nutritionists working in PICUs are being consulted regarding nutrition management of critically ill children with COVID-19 or COVID-19 like symptoms. Therefore, the aim of this short report is to provide a summary of nutrition support recommendations for critically ill children with COVID-19. They are based on the ESPNIC-MEN section recommendations published in January 2020 and surviving sepsis recommendations from February 2020.
Keywords: Paediatric intensive care, Critically ill children, Nutrition, Enteral feeding, COVID-19
1. Introduction
The novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was first identified in Wuhan, China, December 2019 [2]. The World Health Organization (WHO) declared the outbreak of SARS-CoV-2 disease (COVID-19) a pandemic in March 2020, and to date there have been over 3.5 million cases, 250,000 deaths affecting 215 countries around the world [3]. Although children remain largely unaffected and only represent 2% of cases [4], a cumulative pediatric infection proportion model of 50% estimates there would be 37 million children infected with SARS-CoV-2, of which almost 100,000 would require hospitalization for severe pneumonia and nearly 11,000 would require admission to Paediatric Intensive Care unit (PICU) [5]. It is anticipated this current pandemic will last for months, if not years with significant health implications for children with regards to short and longer term nutritional support and recovery.
There are reports of children with COVID-19 or COVID-19 like symptoms with hyper inflammatory multi system syndrome, ARDS, gastrointestinal and atypical Kawasaki disease presenting to PICU worldwide paediatric, for which there are important nutrition support considerations. As a result, the European Society of Pediatric and Neonatal Intensive Care – Metabolism, Endocrine and Nutrition group (ESPNIC-MEN) and paediatric nutritionists working in PICUs are being consulted regarding nutrition management of critically ill children with COVID-19 or COVID-19 like symptoms. Therefore, the aim of this editorial is to provide an adaptation of nutrition support recommendations for the overall population of critically ill children, to provide further refined recommendations for critically ill children presenting with COVID-19 or paediatric hyper-inflammatory syndrome temporally associated with COVID-19. They are based on the ESPNIC-MEN section recommendations published in January 2020 [6] and Surviving Sepsis Campaign recommendations from February 2020 [7]. These recommendations cover the acute, stable and rehabilitation phases (Table 1, Table 2 ).
Table 1.
Summary of nutrition requirements during acute, stable and recovery phase of paediatric critical illness [1,[18], [19], [20], [21]].
| Acute phase | Stable phase | Recovery phase | |
|---|---|---|---|
| Enteral nutrition (Preferred route) | |||
| Energy | It is recommended to commence early enteral nutrition (EN) within 24 h of admission unless contraindicated (e.g. inadequate signs of systemic perfusion and rising lactate) | EN may need to be continued for longer into the recovery phase to support physical and nutritional rehabilitation | |
| Protein (g/kg/day) | 1–2 | 2–3 | 3–4 |
| Parenteral nutrition | |||
| Energy | < resting energy expenditure (REE) | 1.3–1.5xREE | 2xREE |
| Carbohydrates mg/kg/min (g/kg/day) | |||
| Newborn | 2.5–5 (3.6–7.2) | 5-10 (7.2–14) | 5-10 (7.2–14) |
| 28 d-10 kg | 2-4 (2.9–5.8) | 4-6 (5.8–8.6) | 6-10 (8.6–14) |
| 11–30 kg | 1.5–2.5 (1.4–2.2) | 2-4 (2.8–5.8) | 3-6 (4.3–8.6) |
| 31–45 kg | 1–1.5 (1.4–2.2) | 1.5–3 (2.2–4.3) | 3-4 (4.3–5.8) |
| >45 kg | 0.5–1 (0.7–1.4) | 1-2 (1.4–2.9) | 2-3 (2.9–4.3) |
| Protein (g/kg/day) | 0 | 1–2 | 2–3 |
Table 2.
Considerations for nutrition support in critically ill children with COVID-19 and Paediatric inflammatory multisystem syndrome temporal systems (PMS-TS) [7,19].
| Question | Recommendation for critically ill children [7] | COVID-19 adapted recommendations |
|---|---|---|
| In critically ill children, when should enteral nutrition (EN) be commenced and how should it be increased? |
|
|
| In critically ill children on hemodynamic support (vasoactive medications, extracorporeal life support ECLS) does enteral feeding compared to no enteral feeding affect outcomes? |
|
|
| What are critically ill children requirements? |
|
|
| In critically ill children, do different feed formulas (polymeric vs. semi-elemental feed, standard vs. enriched formula) impact on clinical outcomes? |
|
|
| In critically ill children, does continuous feeding compared to intermittent bolus gastric feeding impact on outcomes? |
|
|
| In critically ill children, does gastric feeding compared to post-pyloric feeding impact on clinical outcomes? |
|
|
| In critically ill children does routine Gastric Residual Volume (GRV) to guide enteral feeding impact on outcomes? |
|
|
| In critically ill children, when should Parenteral Nutrition (PN) be started? |
|
|
| In critically ill children, does pharmaconutrition (glutamine, lipids and/or micronutrients) impact on clinical outcomes? |
|
|
2. Clinical presentation of critically ill children with severe SARS-CoV-2 disease
In adults, the severity of COVID-19 disease is postulated to arise from renin-angiotensin system (RAS) and the angiotensin-converting enzyme (ACE)2 receptor on cells which the virus uses as a mode of cell entry. Individuals with high ACE2 levels e.g. diabetes or low levels e.g. hypertension may have a dysregulated response leading to pulmonary inflammation and acute respiratory distress syndrome (ARDS) [8,9]. Although ACE2 receptors are predominantly found in the respiratory mucosa, they have been found to be expressed in the gastrointestinal tract, which may facilitate viral entry into the epithelial cells within the gastrointestinal tract [10,11].
ARDS induced hypoxia, inotrope resistant shock, dehydration from fever, vomiting and diarrhoea, elevated liver enzymes, coagulation dysfunction, rhabdomyolysis including other manifestations suggests injuries to vital organs [12,13]. ARDS remains a rare clinical feature of COVID19 in paediatrics. However, very recently clinicians have been reporting a rise in the number of children of all ages presenting with a paediatric multisystem inflammatory syndrome associated with COVID-19, eventually leading to hyperinflammatory shock and associated with myocarditis; common features of this syndrome are toxic shock syndrome, atypical Kawasaki Disease, macrophage activation syndrome and bacterial sepsis with cardiac inflammation; serum blood measures are similar to severe COVID-19, although many have negative SARS-CoV-2 real-time reverse transcription (rRT-PCR) tests while some present with positive SARS CoV2 serology [[13], [14], [15]].
In children atypical Kawasaki disease symptoms include vasculitis changes in peripheral and visceral arteries including cerebrovascular, renal and gastrointestinal systems [13,15]. Gastrointestinal tract involvement is reported in approximately 20–35% of atypical Kawasaki disease cases with varying clinical manifestations such as vomiting, diarrhoea, abdominal pain, abdominal distension, jaundice, paralytic ileus, hepatomegaly, and ultrasound findings of hydrops of the gallbladder, pancreatitis, gastrointestinal obstruction/pseudo-obstruction [13,16].
3. Considerations for nutrition support in COVID-19 and paediatric inflammatory multisystem syndrome temporally associated with COVID-19
Gastrointestinal symptoms reported amongst children and adults in Wuhan in the later stages of the epidemic with COVID-19 were thought to arise from infected epithelial cells [10]. Although less than 10% of children with COVID-19 develop diarrhea or vomiting, reports of prolonged reverse transcription-polymerase chain reaction (RT-PCR) positivity in the stool has raised the concern of faeco-oral transmission [11]. The newer gastrointestinal and atypical Kawasaki disease presentation may mean that feeding is more delayed than usual in some children, especially if this is indicative of a novel COVID-19 direct intraluminal viral process (PCRs negative in stool to date).
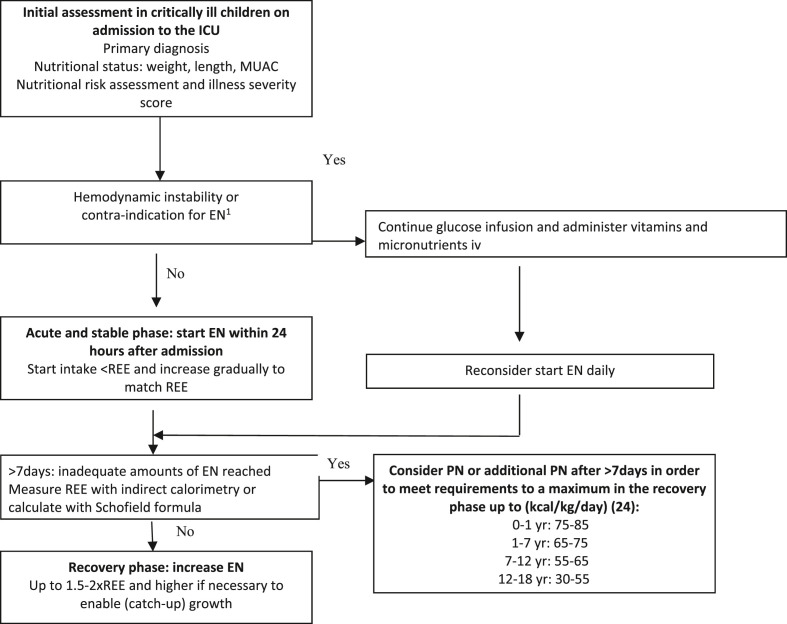
The cornerstones of nutrition recommendations for critically ill children remain early enteral feeding within 24 h of admission, energy requirements not to exceed resting energy expenditure during the acute phase and parenteral nutrition to be withheld during the first 7 days of admission (Fig. 1 , Table 1). For critically ill children with severe COVID-19 and COVID-19 like symptoms there are a number of different nutritional considerations including; 1) severe gastrointestinal or cardiac manifestations and inotrope resistance shock may mean usual early gastric enteral feeding is not be possible, in these instances it may be possible to consider providing enteral feeds via a post-pyloric tube, 2) the placement of naso-enteric tube is considered aerosol producing and as such appropriate personal protective equipment should be worn, 3) children who are nursed prone or in a medically induced coma may tolerated post-pyloric feeds better, 4) measurement of GRV is not recommended unless there is repeated vomiting and no possibility to increase gastric enteral feeding (measured additional care should be taken using personal protective equipment (PPE) as SARS-COV 2 virus has been found in the gastrointestinal lumen, 5) there is no evidence to support supra-physiological doses of micronutrient supplementation, including zinc during the acute phase [17], and 6) as children may have undergone a prolonged admission to PICU, nutrition support may be required well into the recovery period to ensure adequate and appropriate nutrition recovery (Table 2).
Fig. 1.
Nutritional strategy in paediatric intensive care patients.
3.1. Nutritional rehabilitation following PICU
The duration required for recovery is unknown so far. If children have experienced severe disease physical and nutritional rehabilitation may be required for a number of weeks [28]. During recovery higher energy and protein intake may be required up to twice the resting energy expenditure depending on age [18] until nutritional deficits are replete. There is a paucity of information relating to nutritional recovery in children following critical illness. Close monitoring of nutritional status in addition to nutrition support may be required post discharge as longer term functional outcomes have been associated with duration of mechanical ventilation, use of vasoactive medications and duration of PICU stay [29].
In addition, the impact of paediatric critical illness on feeding and feeding difficulties post-discharge remains unknown [30]. Adult survivors of critical care report significant changes to their ability to eat, with reduced appetite, altered taste and food preferences lasting up to 3 months post ICU discharge, which is likely to be an important consideration in children [31]. Adult recommendations post-recovery include the use of enteral and oral nutrition support [32,33]. As such EN support should continue after discharge from PICU until children are able to eat able to consume >75% of their nutrition requirements from food alone. Micronutrient supplementation may also be required to support catch up growth and muscle mass deposition, and serum levels should be measured once the inflammatory response has resolved and CRP is within normal range [26,34].
4. Limitations
Whilst the considerations for nutrition support in critically ill children, are based on a peer review publication [6], the addition of statements pertaining to the nutrition management of children with COVID-19 and paediatric inflammatory multisystem syndrome temporally associated with COVID-19 are extrapolated from other patient groups, as there is a paucity of published evidence based nutrition therapy in critically ill children with COVID-19.
5. Conclusion
Early enteral feeding critically ill children with COVID-19 and COVID-19 like symptoms should be considered. However, in those with significant gastrointestinal issues or inotrope resistance shock this may not be possible for several days. Clinicians should account for gastrointestinal intolerance, taking factors influencing this tolerance into account, and we recommend deciding early about post-pyloric feeding and intuiting this if appropriate. In the acute phase, energy intake provided to critically ill children should not exceed resting energy expenditure and postponing parenteral nutrition for 7 days may be considered. The placement of naso-enteric tubes is considered AGP and as such appropriate personal protective equipment should be worn and there is no evidence to support supra-physiological doses of micronutrient supplementation, including zinc during the acute phase. As children may have had a prolonged admission to PICU, nutrition support may be required well into the recovery period to ensure adequate and appropriate nutrition recovery.
Author contributions
Authors made the following contribution to the manuscript: (1) LVM, FV, SCATV formulated the original idea, (2) LVM drafted the manuscript (3) LVM, KJ, CJC, CM,LL, LT FV and SCATV reviewed and revised the manuscript for important intellectual content, (4) and all authors provided final approval of the version to be submitted.
Conflict of interest
None to declare.
References
- 1.Joosten K.F., Kerklaan D., Verbruggen S.C. Nutritional support and the role of the stress response in critically ill children. Curr Opin Clin Nutr Metab Care. 2016 May;19(3):226–233. doi: 10.1097/MCO.0000000000000268. PubMed PMID: 26963579. [DOI] [PubMed] [Google Scholar]
- 2.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020 Feb 15;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. PubMed PMID: 31986264. Pubmed Central PMCID: PMC7159299. Epub 2020/01/28. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO . World Health Organisation; Geneva: 2020. Coronavirus disease (COVID-19) pandemic Geneva.https://www.who.int/emergencies/diseases/novel-coronavirus-2019 [cited 2020 6 May 2020]. Available from: [Google Scholar]
- 4.Tagarro A., Epalza C., Santos M., Sanz-Santaeufemia F.J., Otheo E., Moraleda C., et al. Screening and severity of coronavirus disease 2019 (COVID-19) in children in madrid, Spain. JAMA Pediatr. 2020 doi: 10.1001/jamapediatrics.2020.1346. PubMed PMID: 32267485. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pathak EB, Salemi JL, Sobers N, Menard J, Hambleton IR. COVID-19 in children in the United States: intensive care admissions, estimated total infected, and projected numbers of severe pediatric cases in 2020. J Publ Health Manag Pract.9000;Publish Ahead of Print. PubMed PMID: 00124784-900000000-99293. [DOI] [PMC free article] [PubMed]
- 6.Tume L.N.V.F., Joosten K., Jotterand Chaparro C., Latten L., Marino L.V., Macleod I., et al. 2020 6 Jan. Nutritional support for children during critical illness: European society of pediatric and neonatal intensive care (ESPNIC) metabolism, endocrine and nutrition section position statement and clinical recommendations paediatric intensive care medicine. 2020. Epub 6 Jan 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tume L.N., Valla F.V., Joosten K., Jotterand Chaparro C., Latten L., Marino L.V., et al. Nutritional support for children during critical illness: European Society of Pediatric and Neonatal Intensive Care (ESPNIC) metabolism, endocrine and nutrition section position statement and clinical recommendations. Intensive Care Med. 2020 Mar;46(3):411–425. doi: 10.1007/s00134-019-05922-5. PubMed PMID: 32077997. Pubmed Central PMCID: PMC7067708. Epub 2020/02/23. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Molloy E.J., Bearer C.F. COVID-19 in children and altered inflammatory responses. Pediatr Res. 2020;88(3):340–341. doi: 10.1038/s41390-020-0881-y. PubMed PMID: 32244248. Epub 2020/04/04. eng. [DOI] [PubMed] [Google Scholar]
- 9.Wong H.R., Freishtat R.J., Monaco M., Odoms K., Shanley T.P. Leukocyte subset-derived genomewide expression profiles in pediatric septic shock. Pediatr Crit Care Med : J Soc Crit Care Med World Feder Pediatr Inten Crit Care Soc. 2010;11(3):349–355. doi: 10.1097/PCC.0b013e3181c519b4. PubMed PMID: 20009785. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tian Y., Rong L., Nian W., He Y. Review article: gastrointestinal features in COVID-19 and the possibility of faecal transmission. Aliment Pharmacol Ther. 2020 May;51(9):843–851. doi: 10.1111/apt.15731. PubMed PMID: 32222988. Pubmed Central PMCID: PMC7161803. Epub 2020/03/31. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matthai J., Shanmugam N., Sobhan P. Coronavirus disease (COVID-19) and the gastrointestinal system in children. Indian Pediatr. 2020;57(6):533–535. doi: 10.1007/s13312-020-1851-5. PubMed PMID: 32279064. Epub 2020/04/13. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Du W., Yu J., Wang H., Zhang X., Zhang S., Li Q., et al. 2020 Apr 16. Clinical characteristics of COVID-19 in children compared with adults in Shandong Province, China. Infection. PubMed PMID: 32301099. Pubmed Central PMCID: PMC7161094. Epub 2020/04/18. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Riphagen S.G.X., Gonzalez-Martinez C., Wilkinson N., Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet. 2020;395(10237):1607–1608. doi: 10.1016/S0140-6736(20)31094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mahase E. Covid-19: concerns grow over inflammatory syndrome emerging in children. BMJ. 2020 Apr 28;369:m1710. doi: 10.1136/bmj.m1710. PubMed PMID: 32345602. Epub 2020/04/30. eng. [DOI] [PubMed] [Google Scholar]
- 15.Health RCoPaC Guidance: paediatric multisystem inflammatory syndrome temporally associated with COVID-192020. 5 May 2020. https://www.rcpch.ac.uk/resources/guidance-paediatric-multisystem-inflammatory-syndrome-temporally-associated-covid-19 Available from:
- 16.Fabi M., Corinaldesi E., Pierantoni L., Mazzoni E., Landini C., Bigucci B., et al. Gastrointestinal presentation of Kawasaki disease: a red flag for severe disease? PloS One. 2018;13(9) doi: 10.1371/journal.pone.0202658. PubMed PMID: 30180185. Pubmed Central PMCID: PMC6122791. Epub 2018/09/05. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Skalny A.V., Rink L., Ajsuvakova O.P., Aschner M., Gritsenko V.A., Alekseenko S.I., et al. Zinc and respiratory tract infections: perspectives for COVID19 (Review) Int J Mol Med. 2020;46(1):17–26. doi: 10.3892/ijmm.2020.4575. PubMed PMID: 32319538. Epub 2020/04/23. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Joosten K.F.M., Eveleens R.D., Verbruggen S. Nutritional support in the recovery phase of critically ill children. Curr Opin Clin Nutr Metab Care. 2019 Mar;22(2):152–158. doi: 10.1097/MCO.0000000000000549. PubMed PMID: 30585805. Epub 2018/12/27. eng. [DOI] [PubMed] [Google Scholar]
- 19.Weiss S.L., Peters M.J., Alhazzani W., Agus M.S.D., Flori H.R., Inwald D.P., et al. Surviving sepsis Campaign international guidelines for the management of septic shock and sepsis-associated organ dysfunction in children. Pediatr Crit Care Med. 2020 Feb;21(2):e52–e106. doi: 10.1097/PCC.0000000000002198. PubMed PMID: 32032273. Epub 2020/02/08. eng. [DOI] [PubMed] [Google Scholar]
- 20.Fivez T., Kerklaan D., Verbruggen S., Vanhorebeek I., Verstraete S., Tibboel D., et al. Impact of withholding early parenteral nutrition completing enteral nutrition in pediatric critically ill patients (PEPaNIC trial): study protocol for a randomized controlled trial. Trials. 2015 May 1;16:202. doi: 10.1186/s13063-015-0728-8. PubMed PMID: 25927936. Pubmed Central PMCID: PMC4422419. Epub 2015/05/01. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mihatsch W.A., Braegger C., Bronsky J., Cai W., Campoy C., Carnielli V., et al. ESPGHAN/ESPEN/ESPR/CSPEN guidelines on pediatric parenteral nutrition. Clin Nutr (Edinburgh, Scotland) 2018 Dec;37(6 Pt B):2303–2305. doi: 10.1016/j.clnu.2018.05.029. PubMed PMID: 30471662. Epub 2018/11/26. eng. [DOI] [PubMed] [Google Scholar]
- 22.Reveiz L., Guerrero-Lozano R., Camacho A., Yara L., Mosquera P.A. Stress ulcer, gastritis, and gastrointestinal bleeding prophylaxis in critically ill pediatric patients: a systematic review. Pediatr Crit Care Med. 2010 Jan;11(1):124–132. doi: 10.1097/PCC.0b013e3181b80e70. PubMed PMID: 19770788. Epub 2009/09/23. eng. [DOI] [PubMed] [Google Scholar]
- 23.Martinez E.E., Ariagno K., Arriola A., Lara K., Mehta N.M. Challenges to nutrition therapy in the pediatric critically ill obese patient. Nutr Clin Pract. 2015 Jun;30(3):432–439. doi: 10.1177/0884533615569887. PubMed PMID: 25667233. Epub 2015/02/11. eng. [DOI] [PubMed] [Google Scholar]
- 24.Organisation W.H. Human energy requirements. 2004 6 May. https://www.who.int/nutrition/publications/nutrientrequirements/9251052123/en/ Report of a Joint FAO/WHO/UNU Expert Consultation [Internet]. 2020. Available from:
- 25.Marino L.V., Eveleens R.D., Morton K., Verbruggen S., Joosten K.F.M. Peptide nutrient-energy dense enteral feeding in critically ill infants: an observational study. J Hum Nutr Diet : Off J Brit Diet Asso. 2019 Jun;32(3):400–408. doi: 10.1111/jhn.12645. PubMed PMID: 30848864. Epub 2019/03/09. eng. [DOI] [PubMed] [Google Scholar]
- 26.Marino L.V., Valla F.V., Beattie R.M., Verbruggen S.C.A.T. Micronutritien requirements in paediatric critical illness: a scoping review. Clin Nutr. 2020;S0261-5614(20):30186. doi: 10.1016/j.clnu.2020.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marino L.V., Pathan N., Meyer R., Wright V.J., Habibi P. The effect of 2 mMol glutamine supplementation on HSP70 and TNF-alpha release by LPS stimulated blood from healthy children. Clin Nutr (Edinburgh, Scotland) 2015 Dec;34(6):1195–1201. doi: 10.1016/j.clnu.2014.12.009. PubMed PMID: 25556350. Epub 2015/01/06. eng. [DOI] [PubMed] [Google Scholar]
- 28.Ong C., Lee J.H., Senna S., Chia A.Z.H., Wong J.J.M., Fortier M.V., et al. Body composition and acquired functional impairment in survivors of pediatric critical illness. Crit Care Med. 2019 Jun;47(6):e445–e453. doi: 10.1097/CCM.0000000000003720. PubMed PMID: 30958426. Epub 2019/04/09. eng. [DOI] [PubMed] [Google Scholar]
- 29.Pinto N.P., Rhinesmith E.W., Kim T.Y., Ladner P.H., Pollack M.M. Long-term function after pediatric critical illness: results from the survivor outcomes study. Pediatr Crit Care Med. 2017 Mar;18(3):e122–e130. doi: 10.1097/PCC.0000000000001070. PubMed PMID: 28107265. Epub 2017/01/21. eng. [DOI] [PubMed] [Google Scholar]
- 30.Morton K., Marino L.V., Pappachan J.V., Darlington A.S. Feeding difficulties in young paediatric intensive care survivors: a scoping review. Clin Nutr ESPEN. 2019 Apr;30:1–9. doi: 10.1016/j.clnesp.2019.01.013. PubMed PMID: 30904206. Epub 2019/03/25. eng. [DOI] [PubMed] [Google Scholar]
- 31.Merriweather J.L., Salisbury L.G., Walsh T.S., Smith P. Nutritional care after critical illness: a qualitative study of patients' experiences. J Hum Nutr Diet : Off J Brit Diet Asso. 2016 Apr;29(2):127–136. doi: 10.1111/jhn.12287. PubMed PMID: 25522771. Epub 2016/04/28. eng. [DOI] [PubMed] [Google Scholar]
- 32.Cintoni M., Rinninella E., Annetta M.G., Mele M.C. Nutritional management in hospital setting during SARS-CoV-2 pandemic: a real-life experience. Eur J Clin Nutr. 2020;74(5):846–847. doi: 10.1038/s41430-020-0625-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Caccialanza R., Laviano A., Lobascio F., Montagna E., Bruno R., Ludovisi S., et al. Early nutritional supplementation in non-critically ill patients hospitalized for the 2019 novel coronavirus disease (COVID-19): rationale and feasibility of a shared pragmatic protocol. Nutrition. 2020 Apr 3:110835. doi: 10.1016/j.nut.2020.110835. PubMed PMID: 32280058. Epub 2020/04/14. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Golden M.H. Proposed recommended nutrient densities for moderately malnourished children. Food Nutr Bull. 2009 Sep;30(3 Suppl):S267–S342. doi: 10.1177/15648265090303S302. PubMed PMID: 19998863. Epub 2009/12/17. eng. [DOI] [PubMed] [Google Scholar]