Abstract
Enhanced processing following a warning cue is thought to be mediated by a phasic alerting response involving the locus coeruleus-noradrenergic (LC-NA) system. We examined the effect of aging on phasic alerting using pupil dilation as a marker of LC-NA activity in conjunction with a novel assessment of task-evoked pupil dilation. While both young and older adults displayed behavioral and pupillary alerting effects, reflected in decreased RT and increased pupillary response under high (tone) versus low (no tone) alerting conditions, older adults displayed a weaker pupillary response that benefited more from the alerting tone. The strong association between dilation and speed displayed by older adults in both alerting conditions was reduced in young adults in the high alerting condition, suggesting that in young (but not older) adults the tone conferred relatively little behavioral benefit beyond that provided by the alerting effect elicited by the target. These findings suggest a functioning but deficient LC-NA alerting system in older adults, and help reconcile previous results concerning the effects of aging on phasic alerting.
Keywords: aging, arousal, attention, pupil dilation, locus coeruleus, noradrenergic system
INTRODUCTION
The presentation of a task-irrelevant warning cue or accessory stimulus just prior to the onset of a task-relevant target stimulus has repeatedly been shown to elicit a reduction in reaction time (RT) to the target stimulus (Bernstein, Clark, & Edelstein, 1969; Bernstein, Rose, & Ashe, 1970; Bertelson & Tisseyre, 1969; Forster, Cavina-Pratesi, Aglioti, & Berlucchi, 2002; Hackley & Valle-Inclan, 1998; Hackley & Valle-Inclán, 1999; Ishigami & Klein, 2010; Jepma, Wagenmakers, Band, & Nieuwenhuis, 2009; Posner, Klein, Summers, & Buggie, 1973; Sanders, 1980; Schröter, Frei, Ulrich, & Miller, 2009; Wang & Munoz, 2015). This enhancement is thought to be mediated by a phasic arousal response within an alerting network (Bernstein, Chu, Briggs, & Schurman, 1973; Bertelson & Tisseyre, 1969; Bueno & Ribeiro-do-valle, 2012; Hackley et al., 2009; Lawrence & Klein, 2013; Posner & Boies, 1971; Posner et al., 1973; Sanders, 1980; Stahl & Rammsayer, 2005) that serves to facilitate perceptual processing and/or motor preparation. This arousal response is often referred to as phasic alerting, and has been found across different studies to increase visual processing speed (Petersen, Petersen, Bundesen, Vangkilde, & Habekost, 2017), increase visual attention capacity (Wiegand, Petersen, Bundesen, & Habekost, 2017), enhance cortical encoding of salient stimuli (Vazey, Moorman, & Aston-Jones, 2018), and increase neural gain in the processing of salient versus non-salient stimuli (Lee et al., 2018). More generally, the alerting network has been conceptualized as one of three components of the attention system along with orienting and executive control networks (Posner & Petersen, 1990). The alerting network functions to maintain an optimal level of arousal for task performance, and is thought to be driven by arousal-related brain stem systems and right hemispheric areas involved in sustained vigilance (Aston-Jones et al. 1991; Carli et al. 1983; Foote et al. 1980; Marrocco & Davidson 1998; Petersen and Posner 2012; Stafford & Jacobs 1990; Song et al. 2017; Witte, Davidson, & Marrocco, 1997). In particular, alertness is modulated by phasic activity within the locus coeruleus noradrenergic (LC-NA) system, from which NA projections can directly modulate target cortical neurons by increasing the gain of neuronal circuits to facilitate task-specific performance (Aston-Jones et al. 1991).
Several recent neuroimaging studies suggest a decline in the effectiveness of the LC-NA system in healthy older adults (Hämmerer et al., 2018; Elman et al., 2017; Lee et al., 2018), consistent with the presence of age-related changes in catecholaminergic function (Arnsten & Goldman-Rakic, 1985; Arnsten, 1993; Mann, 1983; Spokes, 1979; Vijayashankar & Brody, 1979). Moreover, pathological changes associated with the earliest stages of Alzheimer’s disease have been observed in the LC years before clinical symptoms emerge (Braak & Del Tredici, 2011a, 2011b; Mather & Harley, 2016; Theofilas et al., 2017), suggesting that measures of LC-NA integrity in cognitively normal older adults could potentially be useful in identifying those individuals who are at greatest risk for developing the disease (Betts, Kirilina, et al., 2019). Given the proposed role of the LC-NA system as a fundamental driver of the alerting network, one might expect attenuated phasic alerting responses to follow changes in the LC-NA system during aging. However, previous behavioral studies examining the effects of aging on phasic alerting have produced mixed results, with some studies demonstrating impaired alerting (Festa-Martino, Ott, & Heindel, 2004; Gamboz, Zamarian, & Cavallero, 2010; Ishigami et al., 2016; Jennings, Dagenbach, Engle, & Funke, 2007; Wiegand et al., 2017; Zhou, Fan, Lee, Wang, & Wang, 2011) and others demonstrating intact or even increased alerting (Fernandez-Duque & Black, 2006; Nebes & Brady, 1993; Rabbitt, 1984) in cognitively normal older adults. This variability is likely due in part to differences in the methodological approaches and behavioral measures used to assess phasic alerting across studies, including differences in target response requirements (e.g., detection vs. discrimination), response outcome measures (e.g., reaction time vs. accuracy), alerting cue saliency, cue-target time interval, and the inclusion of other task demands (e.g., inhibiting task-irrelevant distractors).
In contrast to purely behavioral measures of phasic alerting, measures of pupil dilation may provide a more sensitive assessment of the effects of aging on phasic alerting through the continuous dynamic monitoring of LC-NA activity during performance of a warning cue task. A number of studies have demonstrated a link between pupil diameter and LC-NA signaling (Aston-Jones & Cohen, 2005; Eckstein, Guerra-carrillo, Singley, & Bunge, 2016; McDougal & Gamlin, 2015; Nieuwenhuis, De Geus, & Aston-Jones, 2011; Samuels & Szabadi, 2008). Single-neuron recoding studies have shown a strong temporal correlation between LC neuronal discharge and pupil dilation in animal models (Joshi, Li, Kalwani, & Gold, 2016; Liu, Rodenkirch, Moskowitz, Schriver, & Wang, 2017; Rajkowski, Kubiak, & Aston-Jones, 1994; Reimer et al., 2016), and covariation of fMRI signals in the LC region with averaged pupil dilation has also been reported in human subjects (Murphy, O’Connell, O’Sullivan, Robertson, & Balsters, 2014). Moreover, previous pupil dilation studies with healthy young adults have confirmed that task-irrelevant warning cues can reliably elicit an increase in pupil dilation (Gabay, Pertzov, & Henik, 2011; Geva, Zivan, Warsha, & Olchik, 2013) that is associated with facilitation in task performance (Tona, Murphy, Brown, & Nieuwenhuis, 2016), and that the magnitude of pupil dilation is associated with the intensity of the alerting cue (Petersen et al., 2017). Taken together, these findings suggest that pupil dilation can be used as an indirect marker of LC-NA signaling mediating phasic alerting.
To our knowledge, however, no study has utilized pupillometry to assess the effects of aging on the functional integrity of the LC-NA system during the performance of a phasic alerting task. To this end, the present study compared the performance of healthy young and older adults on a simple visual target localization task under high or low alerting conditions, as manipulated by the presence or absence of an auditory tone approximately 150ms prior to the onset of the target stimulus. The auditory tone was expected to increase the phasic response within the LC-NA system beyond that elicited by the visual target alone, and to enhance the speed of response to the target stimulus. Pupil diameter measures were recorded continuously throughout the trial window in combination with behavioral measures of performance. In addition to the standard approach of subtracting average baseline pupil diameter from trial pupil diameter, we also assessed task-evoked pupil dilation with a recently adopted approach (Hämmerer et al., 2017; Krishnamurthy, Nassar, Sarode, & Gold, 2016) that uses multiple regression models to predict trial pupil diameter at each time point across the duration of a test trial. This method: a) allows for delineation of the task-evoked pupillary response waveform while controlling for other factors (e.g., baseline pupil diameter, time-on-task) that may obscure the effect of warning cues; b) avoids arbitrary definition of windows for averaging across time points; and c) provides a more thorough assessment of the temporal time course of the relationship between pupil dilation and behavioral performance. We predicted that the magnitude of task-evoked pupil dilation would be positively associated with the speed of behavioral response within a participant, reflecting the modulation of the LC-NA system on task-relevant neural processes. We also predicted that both age groups would display greater task-evoked pupillary dilation in the high alerting (auditory tone) trials compared to the low alerting (no tone) trials, reflecting an increase in the phasic response elicited by the warning tone. However, we also expected the older adults to display a slower time course and a smaller magnitude of the pupillary response to the auditory tone compared to young adults, reflecting a decrease in the effectiveness of the LC-NA system with increasing age.
Consistent with our predictions, we observed distinct patterns of performance across the two age groups that serve to provide a fuller characterization of the effects of aging on the integrity of the alerting network. Similar to previous behavioral studies demonstrating intact phasic alerting in older adults, we found that both groups displayed significant behavioral and pupillary alerting effects as reflected in decreased RTs and increased task-evoked pupillary responses under high versus low alerting conditions. However, we also found age-related differences in the overall magnitude of pupil dilatation, the temporal dynamics of the task-evoked pupillary response, and the strength of association between pupillary response and behavioral performance. Taken together, these findings suggest the presence of a functioning but deficient alerting network, reflecting decreased integrity of the LC-NA system in older adults.
METHODS
Participants
Twenty-eight Brown University young adult (YA) students and thirty-two older adult (OA) individuals recruited from local communities participated in this study. Data from three YA and two OA individuals were excluded due to technical issues with the eye-tracking device. Data from three OA individuals were excluded due to self-report of a neurological diagnosis, and data from two additional OA individuals were excluded due to poor performance (defined as a total score in the lower 25 percentile range) on the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) exam (Randolph, Tierney, Mohr, & Chase, 1998). All analyses were performed on the remaining twenty-five participants in each age group. Informed consent was obtained from all participants, and all study procedures were approved by the Brown University Institutional Review Board in accordance with the Helsinki Declaration. All participants received monetary payment for their participation.
No individuals included in the study reported any active or recent history of psychological or neurological disorders. All included individuals also reported normal hearing and normal or corrected-to-normal vision. Participants’ vision was further assessed using the Tumbling-E Visual Acuity Test and the Mars Letter Contrast Sensitivity Test. While both visual acuity and contrast sensitivity for the YA group were significantly better than for the OA group [Visual Acuity: YA=16.3/20, OA=24.4/20, t(48)=5.50, p<0.001; Contrast Sensitivity: YA=1.83, OA=1.64, t(48)=6.20, p<0.001], all individuals scored in the normal vision range for both tests.
Demographic information and neuropsychological test performance for the YA and OA groups are presented in Table 1. The YA group had significantly fewer years of education than the OA group [t(48)=2.75, p<0.01] (primarily because the YA individuals were still in college), but did not differ on sex composition or measures of sleep quality (ps>0.36). On neuropsychological tests of cognitive status, the YA group performed significantly better than the OA group on working memory measures that required digit manipulation or category switching [ts(48)>3.16, ps<0.01] and on speed of processing tasks [ts(48)>5.34, ps<0.001]. Performance on other measures of cognitive status and emotional/social well-being questionnaires indicated high cognitive functioning and emotional well-being in the OA group.
Table 1.
Demographics and Neuropsychological Test Scores
| YA (n=25) | OA (n=25) | |||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Age (years)** | 20.0 | 2.2 | 74.6 | 6.3 |
| Sex (Male, Female) | 6, 19 | 9, 16 | ||
| Education (years)* | 14.0 | 2.1 | 15.7 | 2.4 |
| PSQI | 3.8 | 2.0 | 3.6 | 2.3 |
| Prior Night Sleep (hours) | 7.75 | 1.11 | 7.81 | 1.10 |
| WAIS-IV Digit Span Forward | 12.2 | 2.2 | 12.2 | 2.3 |
| WAIS-IV Digit Span Back | 10.5 | 2.1 | 9.8 | 2.1 |
| WAIS-IV Digit Sequence* | 10.4 | 1.6 | 9.1 | 1.3 |
| WAIS-IV Scaled | 12.7 | 2.7 | 13.7 | 1.9 |
| DKEFS Letter Fluency | 47.0 | 9.0 | 46.3 | 10.3 |
| DKEFS Category Fluency | 45.0 | 4.1 | 46.4 | 9.6 |
| DKEFS Category Switch** | 16.1 | 2.8 | 13.3 | 3.0 |
| DKEFS Switch Accuracy* | 15.4 | 2.9 | 12.6 | 3.0 |
| Trails A Time (sec)** | 23.2 | 7.4 | 37.9 | 8.7 |
| Trails B Time (sec)** | 45.1 | 11.9 | 79.3 | 29.8 |
| MMSE | -- | -- | 28.9 | 1.0 |
| RBANS Total | -- | -- | 111.5 | 11.5 |
| ANART | -- | -- | 121.1 | 4.3 |
| GDS | -- | -- | 3.8 | 3.4 |
| CRIq Totalab | -- | -- | 133.9 | 16.1 |
p < .01;
p < 0.001;
YA=Young Adults; OA=Older Adults; SD= Standard deviation; PSQI = Pittsburgh Sleep Quality Index; WAIS = Wechsler Adult Intelligence Scale; DKEFS = Delis-Kaplan Executive Function System; MMSE = Mini-Mental State Examination; RBANS = Repeatable Battery for the Assessment of Neuropsychological Status; ANART = American National Adult Reading Test; GDS = Geriatric Depression Scale; CRIq = Cognitive Reserve Index questionnaire;
Low: < 70; Medium Low: 70–84; Medium: 85–114; Medium High: 115–130; High: >130 (Nucci et al., 2012);
Score range: 105–178
Apparatus
Stimuli were displayed on a 19-inch CRT monitor (1152×864 pixel resolution, 72Hz, Viewsonic G90fb) situated 75cm from the participants’ eyes. The task was delivered and controlled on an Intel Core 2 Quad 2.836GHz PC (OS Windows XP SP3 32-bit, 2002) with Nvidia GeForce GT440 graphics card and onboard RealTek/Azalia Audio soundcard using E- Prime v2.0 (Psychology Software Tools, Pittsburgh, PA). An isoluminant color-scheme was created by using modified colors from the Teufel colors set (Teufel & Wehrhahn, 2000). A slate blue (RGB = [55, 123, 170], CIE-xyY = [0.196, 0.199, 25.5]) and a dark orange (RGB = [186, 94, 47], CIE-xyY = [0.234, 0.226, 25.8]) were used for the background and foreground stimuli, respectively. A constant light luminance of 25cd/m2 for both colors was measured with a ColorCAL MKII Colorimeter (Cambridge Research Systems, Rochester, UK).
Pupil diameter and eye position were recorded using an EyeLink®1000 video-based desktop-mounted eye tracker (EyeLink1000, SR Research, Ontario, Canada) with a sampling rate of 250Hz. Participants’ heads were stabilized in a chin rest, and pupil diameter from the left eye was captured using the Centroid Pupil Tracking Algorithm (EyeLink®1000) by an infrared-sensitive camera. The system was calibrated and validated to < 1° average visual angle Cartesian prediction error at the onset of each block using a 9-point grid calibration. Behavioral and pupillary data were post-processed and analyzed using MATLAB R2018b (The MathWorks, Natick, MA).
Phasic Alerting Task
The sequence of events within a trial for each of the four conditions is illustrated in Figure 1. Participants focused on a fixation point at the center of the screen flanked on the left and right by two square boxes that measured 3° of visual angle on each side. The center of each box was 5.3° of visual angle from fixation. Participants were asked to indicate as quickly and as accurately as possible the location of a target stimulus that appeared in one of the two boxes. On 30% of the trials, an auditory tone (white noise, 53 dB, 100ms, Fast Response Weighting C Mode) was presented for 100ms after a variable delay interval between 1,000 and 1,500ms. After a variable inter-stimulus interval (ISI) ranging from 40 to 60ms (mean ISI = 50ms), this warning cue was followed by the presentation of a target at the center of one of the two boxes. This arrangement yielded a mean Stimulus-onset asynchrony (SOA) between the warning cue and the target of 150ms. A short SOA was chosen to maximize exogenous alerting, rather than endogenous expectancy effects. The target was a black circle with a diameter of 0.7° of visual angle. Participants indicated which box contained the target by pressing the response key corresponding to the target’s spatial location. The target disappeared from the screen immediately following a response, but the box frames and fixation cross remained on the screen for an additional 2,000ms. A fixation cross was then displayed for 500ms before the next trial began. For the 70% trials with No Sound, an additional 100ms was added to the delay interval in order to match the timing of events in the Sound trials.
Figure 1.
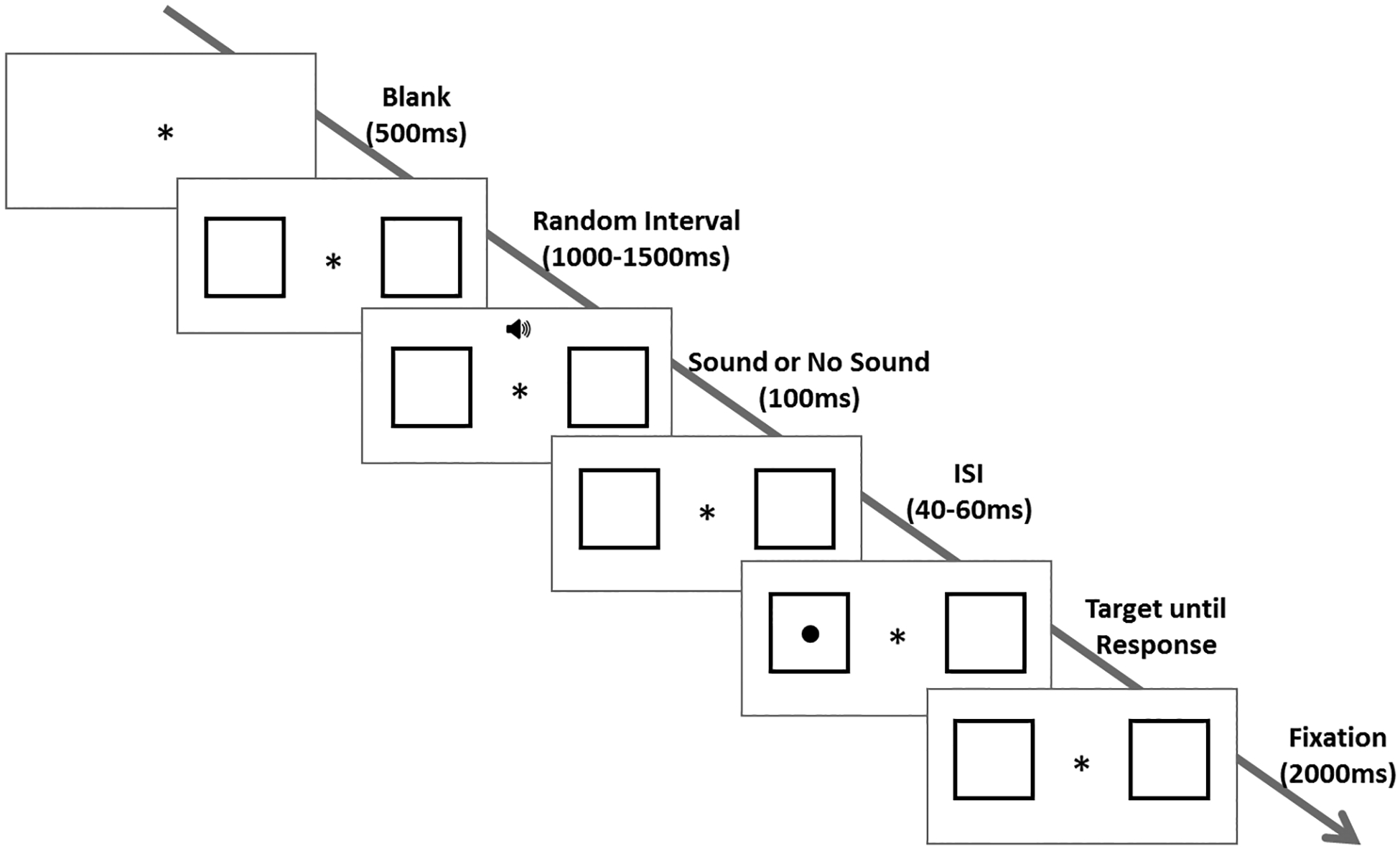
Sequence of events within a trial: participants focused on a fixation point with two flanking square boxes for a variable delay interval. On 30% of the trials, an auditory tone was played followed by a jittered inter-stimulus interval. On the remaining 70% of the trials, no tone was played during the 100ms and ISI period. A target was then presented in one of the two boxes until response. After the response, the target was removed and the boxes remained for 2000ms. The next trial began following a blank screen with central fixation cross, during which blinking is permitted. Participants completed a practice block of 10 trials and two blocks of 100 trials.
Participants completed a practice block of 10 trials (30% Sound trials), followed by two blocks of 100 trials (30% Sound trials) in which the Sound and No Sound trials were randomized both within blocks and across participants. The target appeared equally often in both boxes across trials in each block. Participants were instructed to maintain fixation at the center of screen and to minimize blinking throughout the task. Blinking was encouraged between trials when the fixation cross was presented alone. Performance feedback was given only during the practice trials.
Data Analysis
Behavioral reaction times (RT) and accuracy rates were recorded for each trial. Mean RT of correct trials was computed separately for the Sound and No Sound conditions. A two-way ANOVA with age group as a between-subject factor and sound condition as a within-subject factor was used to investigate age group difference in the behavioral alerting effect (characterized by reduced mean RT in the Sound relative to No Sound conditions). In order to test the unique contribution of age group to sound induced reduction in RT while accounting for overall slowing in the OA group, a follow-up group-level regression model was used to predict the sound effect on RT, with both mean RT and age group as independent variables.
Baseline pupil diameter was defined as the average pupil size during the final 200ms of the delay interval before the onset of each trial. Trial pupil diameter was then measured every 4ms (i.e., at the sampling rate of 250Hz) for a duration of 2000ms beginning with the onset of the target stimulus. Artifacts such as abrupt changes in pupil diameter and blinking (zero pupil diameter) were detected and removed from the pupillary data. Incorrect trials, trials with RTs greater than two-standard deviations from the mean of each participant, and trials with more than 50% of the baseline or trial pupillary data containing artifacts or blinking were excluded (YA: 4.78%; OA: 4.46% of total trials). The remaining pupil diameter measures in pixel units were z-transformed for each participant in order to adjust for inter-individual differences in the range of pupil diameter size. Segments removed due to artifacts and blinking were then interpolated linearly to recover pupillary measurements every 4ms.
Pupil dilation analyses first followed a conventional averaging approach to pupillary data (Beatty & Lucero-Wagoner, 2000) and were then extended to a trial-level regression model approach (Hämmerer et al., 2017; Krishnamurthy et al., 2016). For the conventional analyses, trial pupil dilation was computed by subtracting the average baseline pupil diameter from the trial pupil diameter at each time point. The average pupil dilation across the 2000ms trial time window was used to characterize the task-evoked pupil dilatory response. In order to examine the quickness of the pupillary response in different sound conditions, a half-max time analysis (i.e., the time taken to reach half of the maximal pupil dilation in each trial) was applied to the trace of pupil dilation. This half-max time point takes into account both the rising slope of a pupillary response curve and the peak amplitude of the pupillary response. These time points were then averaged across trials separately for Sound and No Sound conditions in each age group.
While the conventional approach has been employed in numerous studies using either peak dilation (Geva et al., 2013) or average dilation over a defined time window (Gabay et al., 2011; Tona et al., 2016), its power in analyzing pupillary responses to warning cues is limited in at least two respects. First, defining pupil dilation as a relative change from baseline introduces noise in computing pupil dilation, and does not allow for an assessment of the phasic response independent of tonic arousal level (Gilzenrat, Nieuwenhuis, & Cohen, 2010; Murphy, Robertson, Balsters, & O’Connell, 2011; Reimer et al., 2014; Steinhauer, Siegle, Condray, & Pless, 2004; Unsworth & Robison, 2016; Van Den Brink, Murphy, & Nieuwenhuis, 2016). Second, averaging trial pupil dilation across a specified time window for an ANOVA analysis does not allow for an investigation of the time course of pupillary responses to warning cues, and the choice of window used for averaging can bias test statistics in unjustified ways (Sirois & Brisson, 2014).
In order to both address these limitations and better account for other covarying task factors, we adopted a trial-level regression model approach to pupillary data as used in several recent studies (Hämmerer et al., 2017; Krishnamurthy et al., 2016). This approach computes independent regression coefficients for the sound condition and other variables (baseline pupil diameter, behavioral response speed, and time-on-task) that may also be associated with trial pupil diameter. Specifically, the regression model used in this study incorporated the following mean-centered independent variables:
where Speed is the behavioral response speed defined as 1/ RT, Sound is a dummy variable coding the Sound (1) or No Sound (0) condition, Speed X Sound is the interaction between mean-centered Speed and Sound variables, Baseline is the trial baseline pupil diameter, ToT is time-on-task defined as the log of trial number in each block, and Speed X ToT, Sound X ToT, and Baseline X ToT are three interaction terms included to capture any change in associations of Speed, Sound and Baseline with trial pupil diameter as participants went through a trial block.
This regression model was used in trial-level linear regression/correlation to analyze the pupillary data at every sampled time point (i.e., every 4ms) across the 2000ms trial window. For example, the derived regression coefficients for Sound were used to characterize the pupillary response to the auditory tone as a single time-series response curve for each participant. One-sample t-tests were then used to test the significance of the different regression coefficients at each time point; for example, one-sample t-tests were used to test whether the group distributions of the Sound coefficient significantly differed from zero at each time point during the 2000ms window (Sirois et al. 2014). Thus, the temporal plots of the group mean coefficients illustrate the effect of each task variable on trial pupil diameter across the 2000ms window.
To correct for multiple comparisons at multiple time points, cluster-size-based permutation testing was employed for significance testing of regression coefficients along the time-series. A cluster size was calculated by summing the number of time points with p < 0.05 across one-sample t-tests, which was converted to an effect duration in millisecond units. A permutation corrected p-value was then determined by calculating the mean frequency of this cluster size exceeding cluster sizes in a permutation distribution obtained by running one-sample t-tests after randomizing the sign of coefficients among participants (Krishnamurthy et al., 2016; Nichols & Holmes, 2001).
Age group comparisons were implemented by running group-level regressions with derived regression coefficients of each task variable as the dependent variable and age group as the independent variable. Considering that main effects of task variables were consistently detected in the first second after the onset of target stimulus, these group-level regressions were computed at each sampled time point restricted to a time window of the first 1s, whose p-values were corrected using the same permutation method described above.
To examine the inter-individual correlation between the pupillary alerting effect and behavioral performance in the YA and OA groups, we used a group-level regression similar to that in other studies (Hammerer et al. 2017). Specifically, the following regression model tested for the unique correlations between mean pupillary alerting effect in each participant (sound coefficients averaged across the 2000ms time window) and two correlated but distinct behavioral measures (difference in RT between the Sound and No Sound conditions and mean RT), while also controlling for the inter-individual variability in overall pupil dilation magnitude:
where pupil dilation here is quantified by subtracting the mean baseline pupil diameter from the intercept coefficients in the trial-level regression models averaged across the 2000ms time window.
RESULTS
Behavioral Performance - Accuracy and Reaction time
Accuracy rates for the Sound and No Sound conditions are shown in Table 2. Mean accuracy rates were all near ceiling, and did not differ either between the two age groups overall [Mann-Whitney Z=0.90, p=0.37] or between the Sound and No Sound conditions within each age group [paired-sample Wilcoxon Signed Rank tests, YA: Z=1.37, p=0.17; OA: Z=0.18, p=0.86].
Table 2.
Mean Reaction Times and Accuracies
| YA (n=25) | OA (n=25) | |||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Mean Accuracy (%) | ||||
| • Sound Condition | 99.3 | 1.2 | 99.7 | 0.7 |
| • No Sound Condition | 99.6 | 0.6 | 99.7 | 0.6 |
| Reaction Time (ms) | ||||
| • Sound Condition** | 292.0 | 18.9 | 402.2 | 79.0 |
| • No Sound Condition** | 318.4 | 27.1 | 453.5 | 91.7 |
| • RT Difference** | 26.3 | 18.7 | 51.3 | 28.7 |
p < 0.001; YA=Young Adults; OA=Older Adults; SD= Standard deviation
The mean behavioral reaction times (RT) of each age group for the Sound and No Sound conditions are shown in Table 2 and Figure 2. A two-way ANOVA revealed both main effects of Group [F(1,48)=49.64, p<.001, ] and Condition [F(1,48)=128.53, p<.001, ], as well as a significant Group X Condition interaction [F(1,48)=13.27, p<.001, ]. Overall, the YA group displayed significantly faster RT than the OA group, and the RT in the Sound condition were significantly faster than in the No Sound condition. To assess the significant Group X Condition interaction, the difference in mean RT for the Sound and No Sound conditions (i.e., the behavioral alerting effect) was computed for each participant, and a pairwise comparison indicated that the OA group displayed a significantly larger alerting effect than the YA group [t(48)=3.64, p<0.001]. A group-level regression analysis was then performed on this behavioral alerting effect incorporating both age group and mean RT as independent variables; this analysis found that the magnitude of the alerting effect was associated with magnitude of overall RT [p<0.001] with no additional significant contribution of age group [p=0.874], suggesting that the larger behavioral alerting effect observed in the OA than YA group was attributable primarily to overall slowing in the OA group.
Figure 2.
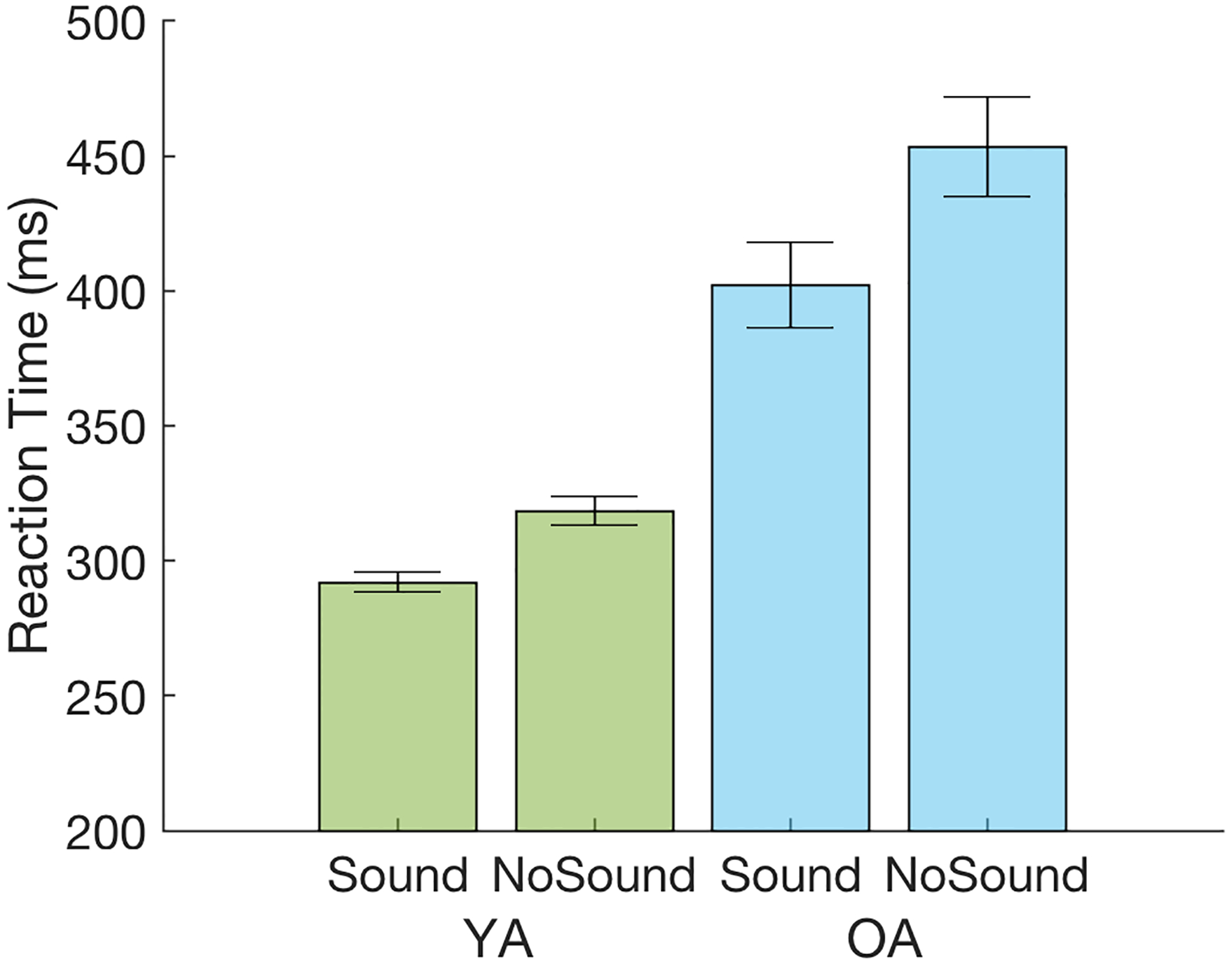
Behavioral mean reaction times (RT) averaged across age groups and conditions. Error bars represent the standard error of the mean. YA=Young Adults (in green); OA=Older Adults (in blue).
A trial-level regression model analysis of RT was also performed in order to match the approach taken to analyze pupillary data, and to assess any time-on-task effects on behavioral performance (Supplementary Material: Section 1). Results from this regression analysis confirmed the findings obtained using ANOVA analysis and identified a significant Sound by time-on-task interaction in the OA group only, where the behavioral alerting effect was smaller later in a block [t(24)=−2.39, p=0.025].
Pupillary Responses - Baseline Subtraction and Averaging
Figure 3 shows the z-scored grand-average pupil dilation (trial pupil diameter minus baseline pupil diameter) waveforms separately for the Sound and No Sound conditions in each age group. A two-way ANOVA on the averaged pupil dilation across the 2000ms revealed main effects of Group [F(1,48)=5.75, p<.05, ] and Condition [F(1,48)=64.24, p<.001, ], but no Group X Condition interaction [F(1,48)=0.27, p<.61, ]. Thus, while the YA group displayed significantly greater overall pupil dilation than the OA group, both groups showed greater pupil dilation in the Sound condition than the No Sound condition, indicating the presence of a pupillary alerting effect in both YA and OA groups.
Figure 3.
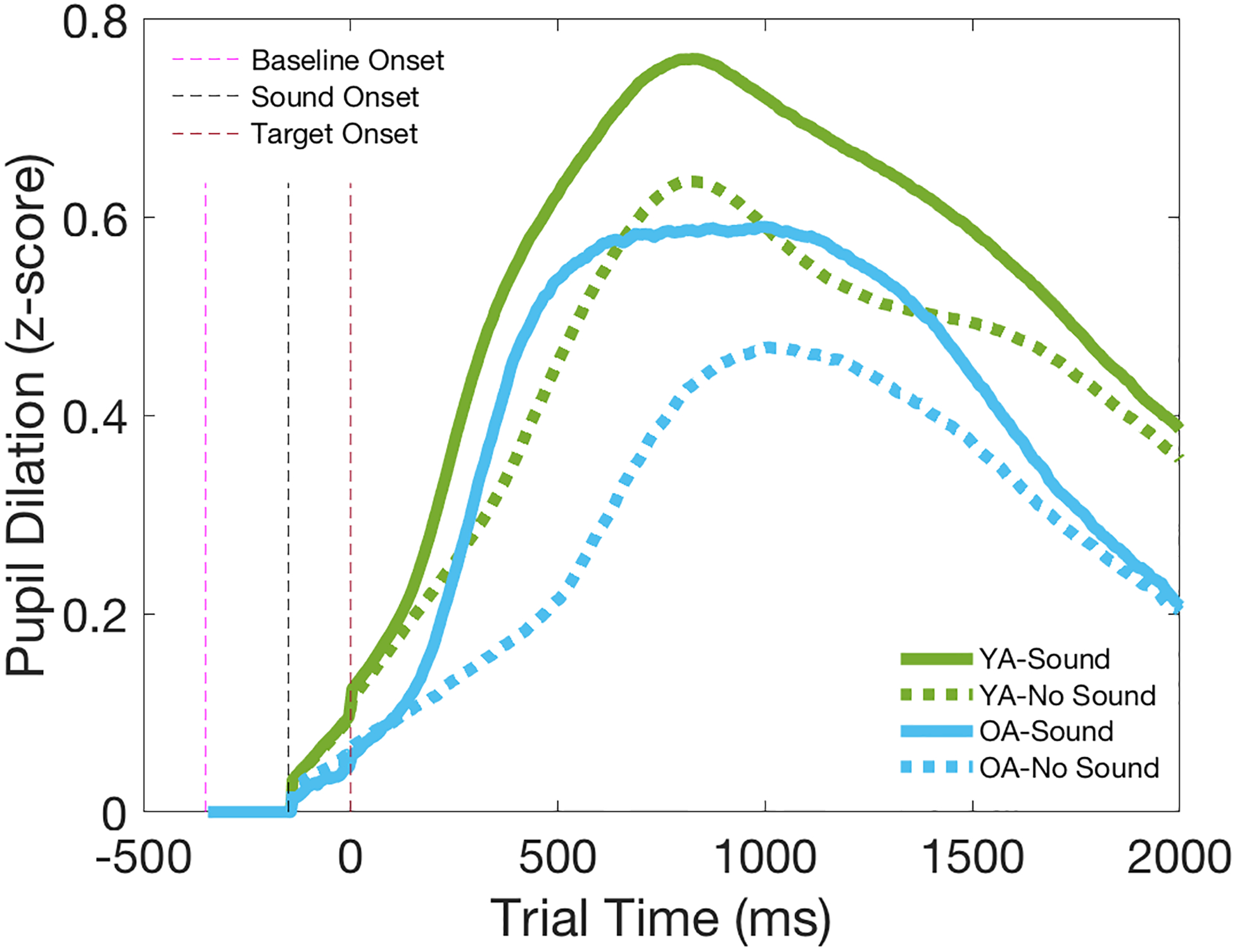
Z-scored grand-average pupil dilation (PD) waveforms for Sound and No Sound conditions computed by subtracting mean baseline pupil diameter from trial pupil diameter at each sampled time point, averaged across age groups and conditions. YA=Young Adults (in green); OA=Older Adults (in blue).
In order to assess age-related differences in the time course of the evoked pupillary response in the Sound and No Sound conditions, a half-max analysis was used to determine the time point at which the pupillary response reached half of the maximal pupil dilation in each condition (Figure 4). A two-way ANOVA revealed both main effects of Group [F(1,48)=9.82, p<.005, ] and Condition [F(1,48)=46.29, p<.001, ], as well as a significant Group X Condition interaction [F(1,48)=5.22, p<.05, ]. The half-max time points were overall significantly faster in the Sound condition than the No Sound condition, and the YA group displayed significantly faster overall half-max time points than the OA group. Pairwise comparisons to evaluate the significant Group X Condition interaction further indicated that the task-evoked pupillary response was significantly slower in the OA group than the YA group in the No Sound condition [t(48)=2.96, p=0.005], but emerged as quickly as that of the YA group in the Sound condition [t(48)=1.79, p=0.079].
Figure 4.
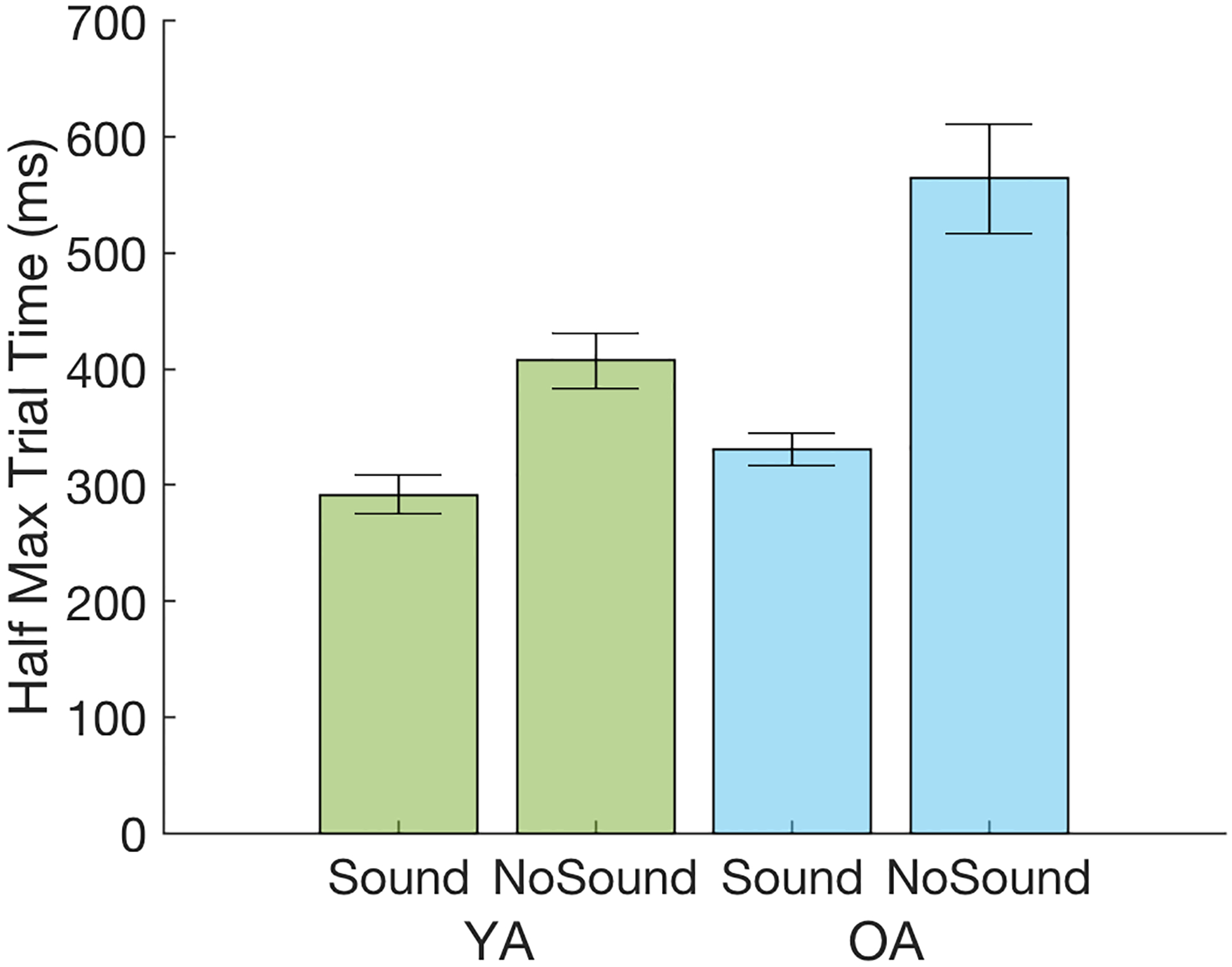
Half max time points for pupil dilation: the time taken for pupil dilation to reach half of the maximum amplitude of dilation in a trial, averaged across age groups and conditions. Error bars represent the standard error of the mean. YA=Young Adults (in green); OA=Older Adults (in blue).
Pupillary Responses - Regression Analyses
A trial-level regression approach to pupillary data (Hämmerer et al., 2017; Krishnamurthy et al., 2016) was used to better characterize the task-evoked pupillary response across conditions and age groups, controlling for other factors which may also influence trial pupil diameter, using the following regression model as described in the Methods section.
Pairwise comparison of the intercept coefficient of the regression models averaged across 2000ms trial period revealed no significant group difference [t(48)=0.23, p=0.82], suggesting no inherent difference in z-scored pupil diameter between groups. However, pairwise comparison of the average intercept subtracting average baseline pupil diameter revealed a larger overall evoked pupil dilation in the YA group than the OA group [t(48)=2.45, p=0.025], confirming the main effect of age group observed in ANOVA analysis of the conventional approach (Figure 3).
As expected, larger trial pupil diameter was significantly associated with larger baseline pupil diameter across the 2000ms trial period for both groups [permutation test of duration: YA: 2000ms, p<0.05; OA: 2000ms, p<0.05], with the strength of this association decreasing across the trial time window (Supplementary Figure S1). Time-on-task (i.e., the log of trial number) was negatively associated with trial pupil diameter in both groups [permutation test of duration: YA: 2000ms, p<0.01; OA: 1976ms, p<0.01], with the strength of this negative association increasing across the trial time window (Supplementary Figure S2). No significant age group difference was identified for these effects (Supplementary material: Section 2). These findings highlight the advantage of the regression approach used in the present study to examine the effects of sound condition and speed on trial pupil diameter independent of effects associated with baseline pupil diameter or time-on-task, since (for example) subtracting the same baseline pupil diameter from every time point would not have captured the decrease in the strength of the association between baseline pupil diameter and trial pupil diameter over the 2000ms time window. The three interaction terms with time-on-task (Speed X ToT, Sound X ToT, and Baseline X ToT) were not significant (Supplementary material: Section 2), suggesting the absence of detectable changes in the effects of these task variables over time in a trial block.
Effect of Sound Condition on Trial Pupil Diameter.
Figure 5 illustrates the time-series response curves for the effect of the sound condition (Sound vs No Sound) on trial pupil diameter for each age group, with the magnitude of the standardized regression coefficient for the sound condition plotted on the y-axis across the 2000ms trial window. Consistent with the pupil dilation waveforms observed in Figure 3, the regression model analyses revealed a strong effect of the auditory warning cue on trial pupil diameter in both age groups as indicated by the red lines (Figure 5). A significant increase in pupil dilation was elicited by the sound tone starting at around 200ms after the target stimulus onset, with the pupillary response curves peaking early in the time window. Permutation testing confirmed that the durations of pupillary alerting effects were significant after correcting for multiple comparisons [permutation test of duration: YA: 1820ms, p<0.01; OA: 1384ms, p<0.01].
Figure 5.
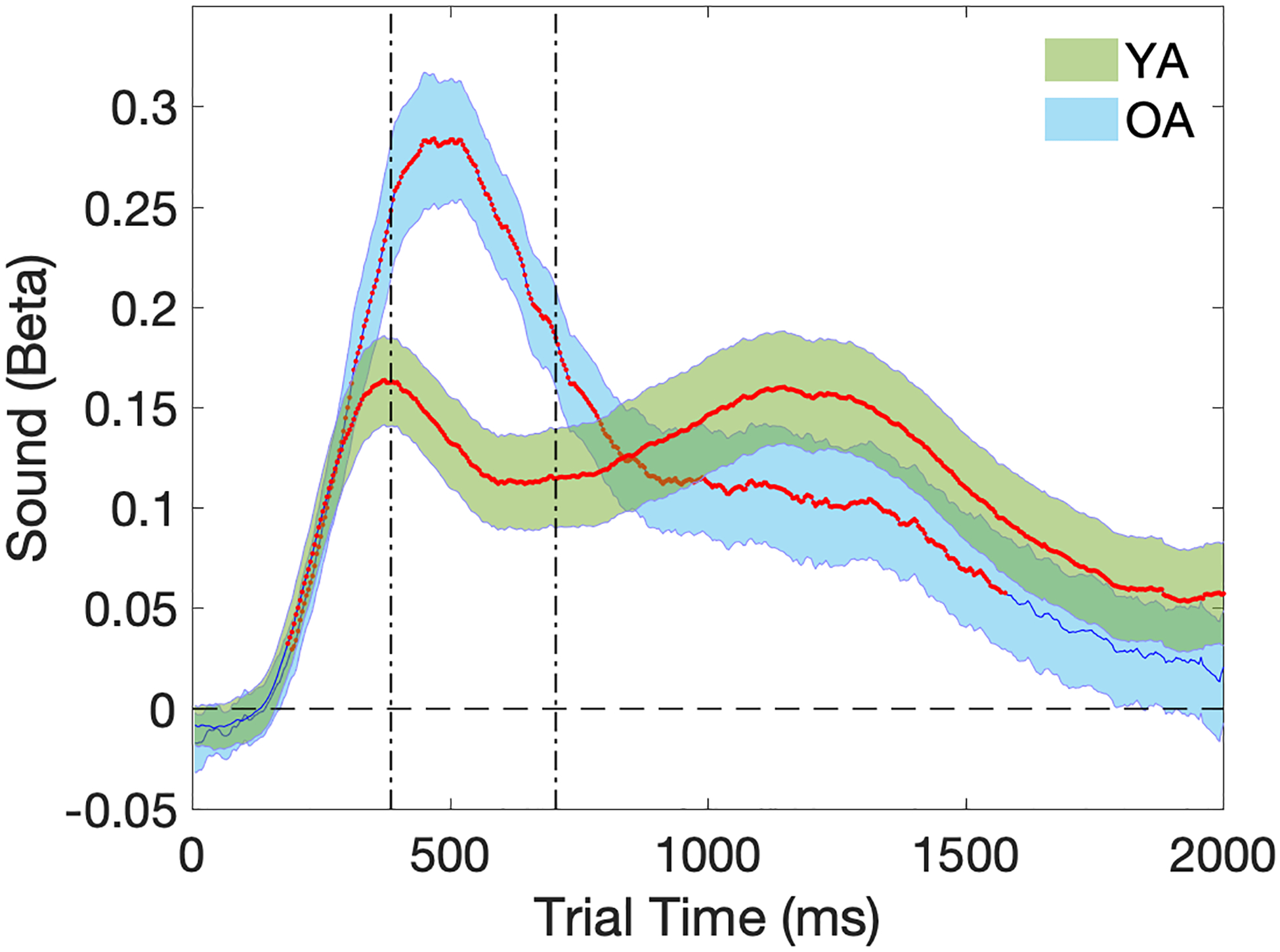
Temporal plots (2000ms) of pupillary response curves for the auditory tone. Beta (Sound) on the y-axis represents the effect size of increase in trial pupil diameter in z-score units in the Sound condition compared to the No Sound condition. Shaded areas represent the standard error of the mean. Time points with significant p-values at one-sample t-tests are marked in red to indicate group distributions of Beta (Sound) that are significantly different from zero. Vertical dotted lines indicate the time window during which a group-level regression revealed significant age group difference. YA=Young Adults (in green); OA=Older Adults (in blue).
Group-level regression analyses on the time series with age group as an independent variable revealed a selective window around 500ms (Figure 5) where the OA group showed a greater pupillary alerting effect than the YA group that was marginally significant [permutation test of duration: 324ms, p=0.052]. This result was surprising, given our a priori hypothesis of reduced pupillary alerting effect in the OA group compared to the YA group. To confirm that the greater pupillary alerting effect in the OA group is not due simply to the presence of a greater temporal delay between pupillary response curves in the Sound and No Sound conditions compared to the YA group (Figures 3 and 4), the group-level regression analysis for age comparison was repeated using 1s pupillary data time-locked to the behavioral RT (160ms before RT and 840ms after RT) in each trial rather than to the onset of target stimuli. Results from this analysis confirmed the increased pupillary alerting effect in the OA group than the YA group (Supplementary material: Section 3), peaking around 50ms after RT in each trial [permutation test duration: 472ms, p=0.037].
To examine whether individual differences in the pupillary alerting effect correspond to individual differences in behavioral performance, we carried a group-level regression analysis on the pupillary alerting effect (Figure 5) in each participant averaged across the 2000ms time window, as detailed in the Methods section. In the OA group, a significant positive correlation was observed for the mean pupillary alerting effect with overall mean behavioral RT [t(24)=3.01, p=0.007], but not with the behavioral alerting effect [t(24)=−1.24, p=0.227], suggesting that older adults with overall slower behavioral responses displayed greater pupillary alerting effects (Figure 6). This association between the mean pupillary alerting effect and mean RT was not significant in the YA group [t(24)=−0.65, p=0.522].
Figure 6.
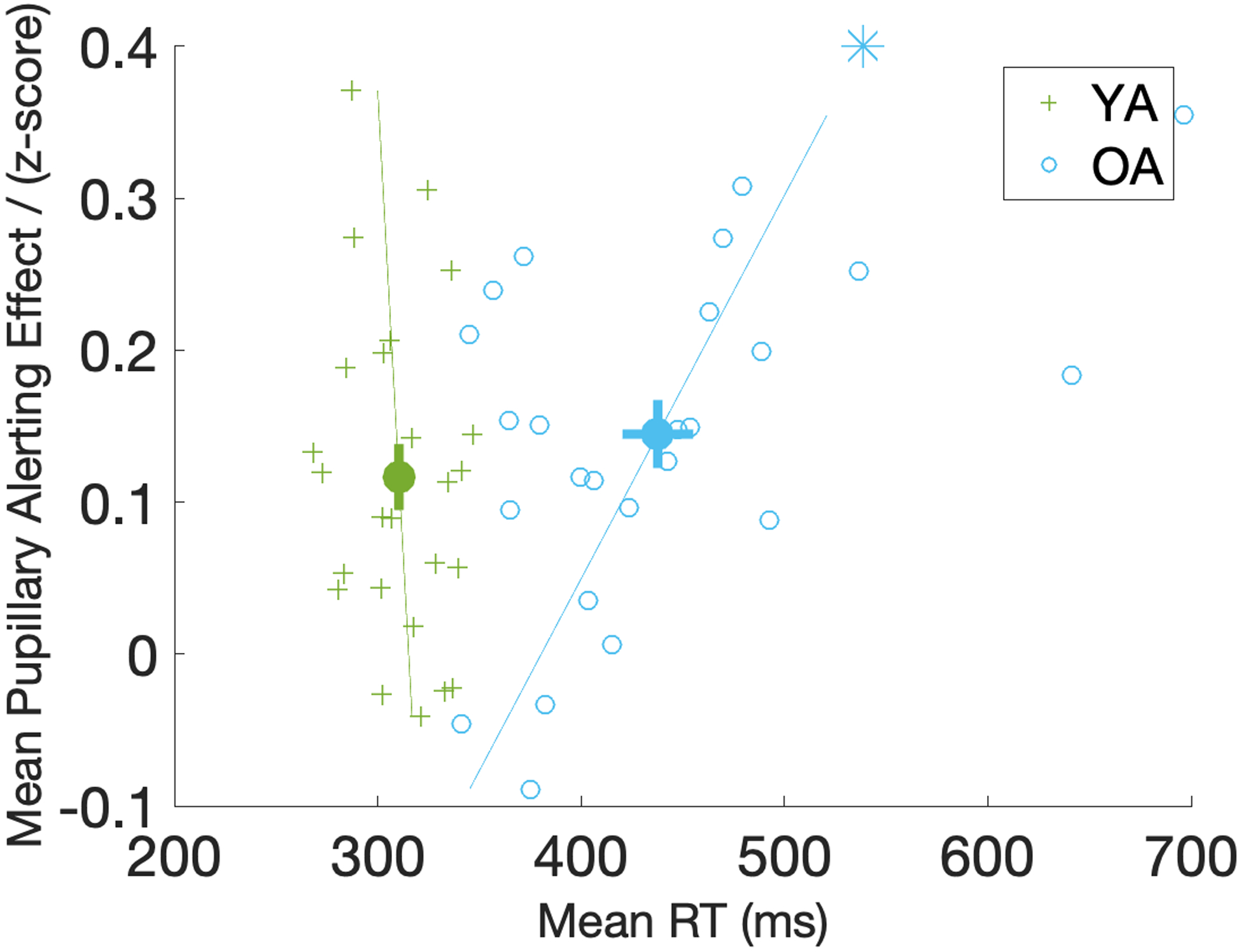
Scatter plot of mean pupillary alerting effect against mean RT in both age groups. Mean RT on the x-axis is the reaction times averaged across the Sound and No Sound conditions. Pupillary alerting effect on the y-axis is calculated by averaging the coefficients of the sound response curves (Figure 5) across the 2000ms time window in each participant. Lines are fitted to the scatter points. Significant correlation in the group-level regression analysis is marked with an asterisk on the fitted line. The same patterns are observed when looking at RT in the Sound and No Sound conditions due to strong correlation between mean RT and RT in both sound conditions in a participant. Error bars around the mean dots represent the standard error of the mean. YA=Young Adults (in green); OA=Older Adults (in blue).
Behavioral Response Speed and Trial Pupil Diameter.
Trial-level regression analyses further revealed a significant positive effect of behavioral response speed on trial pupil diameter in both the YA and OA groups (Figure 7). The positive standardized beta coefficients indicate that, independent of the sound condition, trials with greater trial pupil diameter were significantly associated with faster response speed in both age groups [permutation test of duration: YA: 820ms, p<0.05; OA: 924ms, p<0.05]. This effect remained significant throughout the first half of the 2000ms trial, and peaked at around 550ms. No age group difference on the speed response curves was identified in the group-level regression analyses [ps>0.05].
Figure 7.
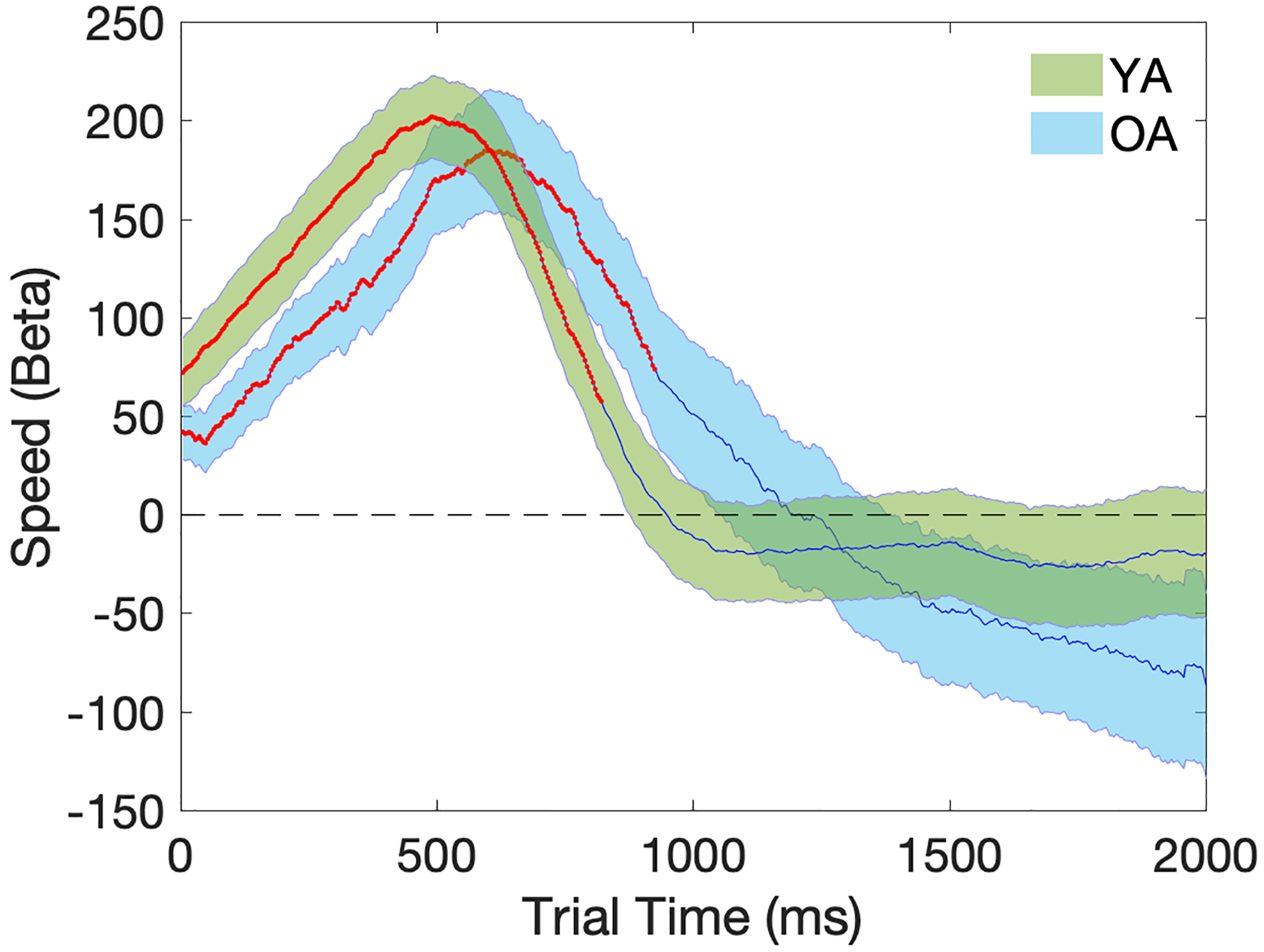
Temporal plots (2000ms) of regression coefficients for the Speed (response speed) term in the trial pupil diameter regression model. Beta (Speed) on the y-axis represents the correlation across trials between response speed and trial pupil diameter after controlling for other independent variables in the same model. Shaded areas represent the standard error of the mean. Time points with significant p-values at one-sample t-tests are marked in red. YA=Young Adults (in green); OA=Older Adults (in blue).
Trial-level regression analyses also revealed a significant interaction between response speed and sound condition in the YA but not the OA group [permutation test of duration: YA: 756ms, p<0.05; OA: 0ms, p>0.05], as shown in Figure 8. To further investigate the significant interaction between response speed and sound condition in the YA but not the OA group, trial-level regression models were performed separately for the Sound and No Sound conditions in each age group. Figure 9 shows that the significant interaction between response speed and sound condition in the YA group was driven by a reduced (but still significant) association between trial pupil diameter and response speed in the Sound condition compared to the No Sound condition. In contrast, there was no change in the magnitude of the association between trial pupil diameter and response speed across the two sound conditions in the OA group, which stayed at a comparable level to the association strength observed in the No Sound condition in the YA group (Figure 9).
Figure 8.
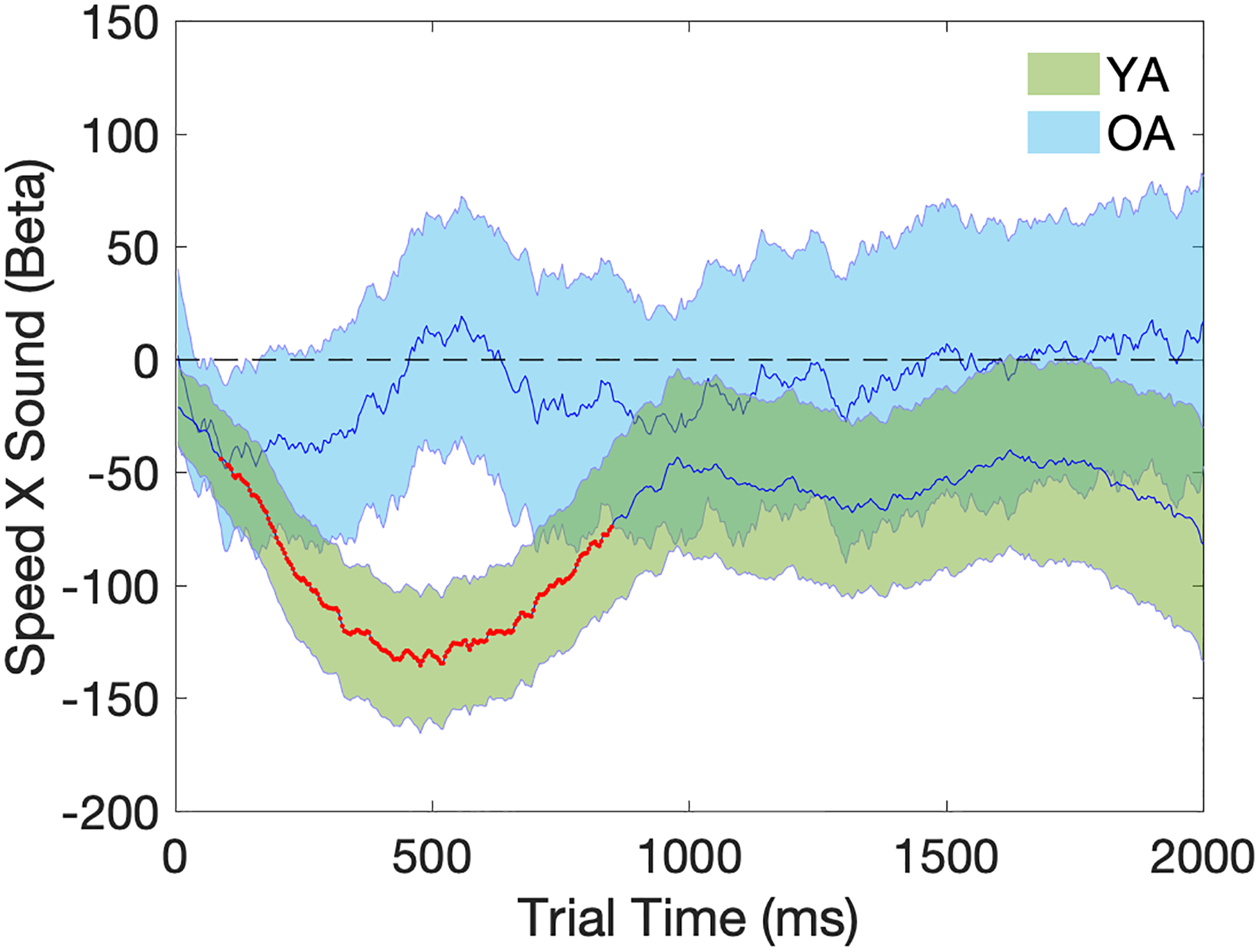
Temporal plots (2000ms) of regression coefficients for the Speed X Sound term in the trial pupil diameter regression model. Beta (Speed X Sound) on the y-axis represents the effect of the interaction between response speed and the sound condition on trial pupil diameter, where a negative value indicates smaller positive correlation between Speed and trial pupil diameter in the Sound condition than the No Sound condition. Shaded areas represent the standard error of the mean. Time points with significant p-values at one-sample t-tests are marked in red. YA=Young Adults (in green); OA=Older Adults (in blue).
Figure 9.
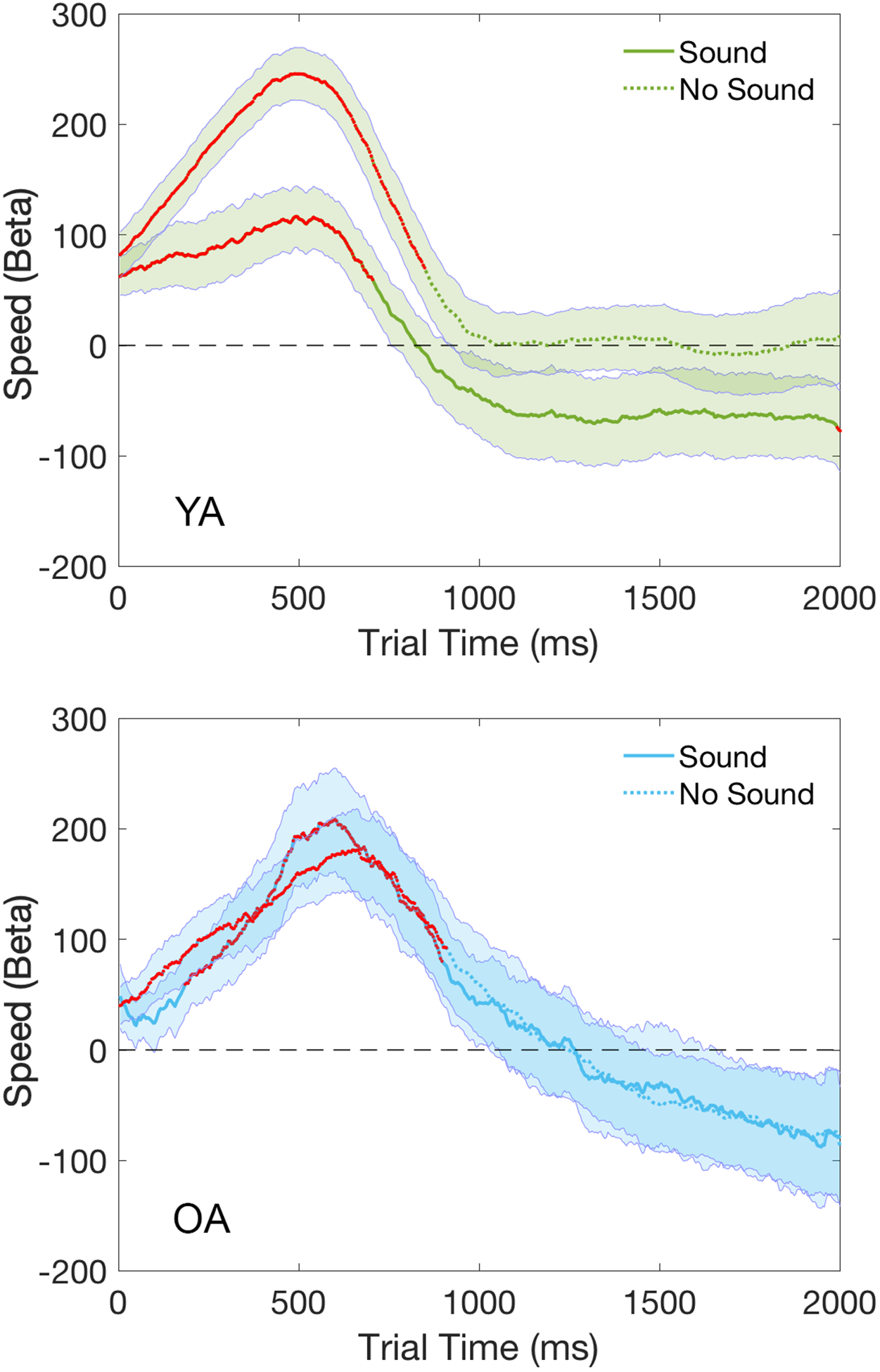
Temporal plots (2000ms) of regression coefficients for the Speed term modeled separately for the Sound and No Sound conditions in each age group. Shaded areas represent the standard error of the mean. Time points with significant p-values at one-sample t-tests are marked in red. YA=Young Adults (in green); OA=Older Adults (in blue).
DISCUSSION
The present study utilized both behavioral and pupillometric measures to assess the effects of aging on the functional integrity of the LC-NA system during the performance of a phasic alerting task. Alerting was manipulated by the presence or absence of an auditory tone prior to the onset of a visual target stimulus, and pupil diameter was recorded continuously throughout the trial window. Task-evoked pupil dilation was analyzed using both the standard approach of subtracting average baseline pupil diameter from trial pupil diameter (e.g., Beatty & Lucero-Wagoner, 2000), as well as a trial-level multiple regression approach (Hämmerer et al., 2017; Krishnamurthy et al., 2016; Sirois & Brisson, 2014) that addresses some of the weaknesses of the standard subtraction approach. In particular, these regression analyses confirmed a positive association between baseline pupil diameter and trial pupil diameter that declined steadily over the course of a trial, as well as decreases in both baseline pupil diameter and trial pupil dilation over the course of the experiment (due possibly to the presence of fatigue or habituating effects); the ability to regress out these confounding factors allows for a clearer characterization of task-evoked pupillary responses than can be seen with the baseline subtraction approach alone. Using converging findings from both approaches, we were able to observe distinct patterns of performance across the two age groups that help clarify the effects of aging on the alerting network mediated by the LC-NA system and that may serve to reconcile previous behavioral findings.
As expected, the healthy young adults in the present study demonstrated a significant behavioral phasic alerting effect, as reflected in faster reaction times to the visual target under high alerting (Sound) than low alerting (No Sound) conditions (Figure 2). Young adults also demonstrated a significant pupillary alerting effect, characterized by both greater amplitude and faster time course of the task-evoked pupil dilation response under Sound than No Sound conditions (Figure 3, 4). The trial-level regression analyses extracted a pupillary response curve that characterized the significant increase in pupil dilation elicited by the alerting tone (Figure 5), which was present throughout the 2000ms trial and peaking early in the time window. Importantly, these analyses also demonstrated a significant association between the magnitude of the task-evoked pupil dilation and the speed of the behavioral response to the visual target within each individual (Figure 7). Taken together, these findings provide strong support for the role of the LC-NA system in mediating the effect of warning cues within a phasic alerting task (Geva et al., 2013; Tona et al., 2016). That is, the increased task-evoked pupil dilation response under the Sound condition suggests that the auditory warning cue triggered a phasic burst of LC-NA signaling reflecting a phasic increase in arousal level, and the positive association between pupil dilation and response speed suggests that LC-NA projections modulate cortical circuits involved in processing of the target stimuli and/or motor preparation (Petersen & Posner, 2012). Hence, at an individual level, a plausible mechanism of the effect of the warning cue in increasing response speed is via the phasic increase in NA signaling that modulates subsequent neuronal processing.
The presence of a warning cue may not only produce a phasic increase in arousal level, but may also provide temporal expectancy regarding the occurrence of the target stimulus (Weinbach & Henik, 2012). While these two processes can be dissociated with careful experimental design (Hackley et al., 2009; Lawrence & Klein, 2013), the effects of alerting and temporal expectancy on response speed may ultimately be mediated through the same LC-NA system. Indeed, temporal expectancy may be explained by a gradual change in the endogenous arousal state (Matthias et al., 2010; Wiegand et al., 2017) associated with the LC-NA system. Pharmacological manipulation of the LC-NA system using clonidine to reduce noradrenaline release has been shown to affect both alerting response and temporal expectancy (Coull, Nobre, & Frith, 2001). Hence, the warning cue could elicit changes in arousal level within the LC-NA system through either bottom-up phasic alerting effects or top-down expectancy effects. Given the short time interval between the warning cue and the target (140 to 160ms), however, it is likely that the effect of the warning cue was driven more by exogenous alerting processes rather than endogenous expectancy processes (Hackley et al., 2009). This interpretation of current findings is consistent with previous studies that demonstrated pupillary alerting effects in young adults that occurred shortly after the presentation of the imperative stimulus (Geva et al., 2013; Tona et al., 2016) and persisted over the course of the entire trial (Geva et al., 2013). While the pupillary effect observed in the study by Tona et al. (2016) diminished over the course of the trial, that study also included a top-down conflict processing component in the behavioral task that may have altered the temporal course of the bottom-up phasic alerting response.
Interestingly, the regression analyses in the present study also demonstrated a significant interaction between alerting condition and the strength of the association between trial pupil dilation and speed of response in the young adults (Figure 8). That is, while response speed was significantly associated with trial pupil dilation in both the Sound and No Sound conditions, the strength of this association was actually weaker in the Sound than the No Sound condition (Figure 9). This interaction was observed despite the fact that a) the task-evoked pupillary response was larger in the Sound than the No Sound condition consistent with a phasic alerting effect, and b) response speed was faster in the Sound than No Sound condition consistent with a facilitatory effect of phasic alerting on neural processing.
One explanation for the reduced association between speed and pupil dilation in the Sound condition is that the LC-NA system is sufficiently robust in young adults that the visual target stimulus alone elicited a task-evoked alerting response in the No Sound condition that mediated an already-fast response speed, so that the addition of the auditory tone in the Sound condition conferred relatively little additional behavioral benefit. Figure 10 shows a schematic curve articulating the relationship between arousal level and speed of response, with young adults positioned near the saturating portion of the curve. In this view, the reduced association between speed and pupil dilation in the Sound condition is due to an upper-bounded increase in response speed elicited by the combined auditory cue and visual target compared to that elicited by the visual target alone. Thus, the visual target elicited a pupillary response that was associated with increased response speed, and the additional warning cue elicited an incrementally larger pupillary response that further increased response speed, consistent with the role of the LC-NA system in phasic alerting. However, the relative strengths of the positive association between response speed and pupil dilation in these two conditions will depend on their relative positions along the arousal/response curve for any individual. It is worth noting that this schematic saturating curve may be viewed as a special case of the classic Yerkes-Dodson curve, an inverted U-shaped relationship more recently articulated within a theory of LC-NA function (Aston-Jones & Cohen, 2005). Consistent with this viewpoint, a recent study found that young adults with fast response speeds did not benefit from cues that elicited a phasic alerting effect in healthy older adults (Haupt, Sorg, Napiórkowski, & Finke, 2018), suggesting that the young adults were in the asymptotic phase of the arousal curve for that task. In the present study, the young adults were likely still within the left half of the inverted U-shaped curve since they exhibited a robust behavioral alerting effect, but they were closer to the saturating region of the curve than the older adults given their reduced association between response speed and phasic arousal (as indexed by pupil dilation).
Figure 10.
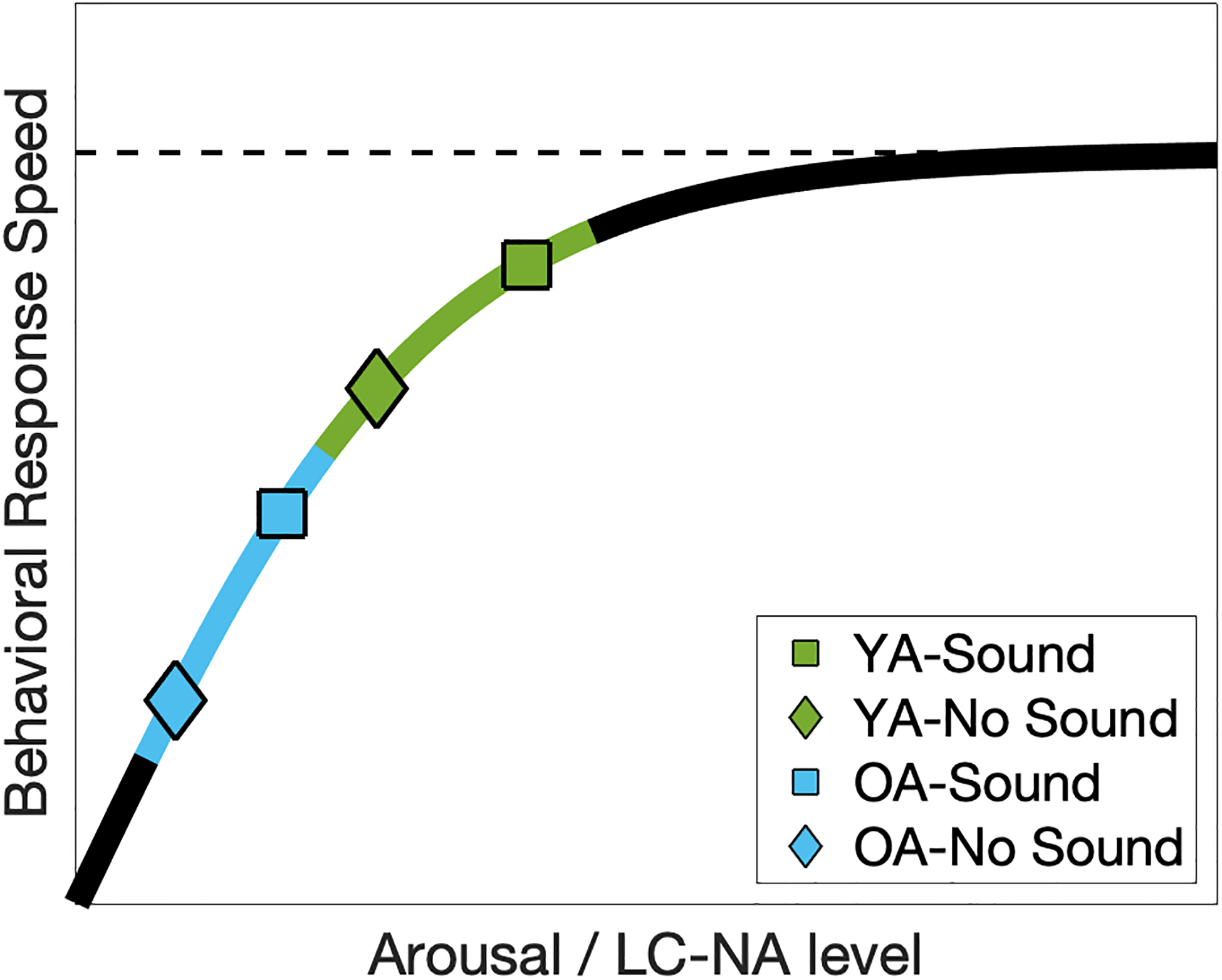
Schematic curve of the association between behavioral response speed and trial pupil diameter in different sound conditions. Observed reduction in the association strength in the Sound condition in the YA group can be explained by a saturating effect of LC-NA signaling on behavioral performance when the arousal level is sufficiently high. In contrast, the auditory warning tone increases arousal level in the OA group to a higher level but with similar slope in relating to behavioral performance as in the No Sound condition. In addition, at the lower end of this curve, slower responses are more likely to benefit from a greater increase in LC-NA in the Sound condition, explaining the observed group-level correlation between pupillary alerting effect and mean reaction times in the OA group (Figure 6). YA=Young Adults (in green); OA=Older Adults (in blue).
The healthy older adults in the present study displayed behavioral and pupillary alerting effects that were as large as, if not larger than, those observed in the young adults, suggesting that the alerting network remains functionally intact in older adults. Thus, older adults, like young adults, responded faster to the visual target stimulus under Sound than No Sound conditions (Figure 2), and demonstrated a task-evoked pupil dilation response that was larger and faster under Sound than No Sound conditions (Figure 3,4). Trial-level regression analyses further confirmed that, like young adults, older adults displayed a significant increase in pupil dilation elicited by the sound tone that persisted throughout the trial window and peaked near the time of their mean behavioral reaction time (Figure 5). As with young adults, older adults also displayed a significant association between the magnitude of their task-evoked pupil dilation and the speed of their behavioral response to the visual target (Figure 7), consistent with a link between LC-NA activity and facilitation in processing. Both the behavioral alerting effect and the pupillary alerting effect were also found to be significantly larger in the older adults than the young adults (Figure 2,5) due primarily to the increase in overall mean reaction time in the older adults (Figure 6). Taken together, these results suggest that the older adults in the present study demonstrated a preserved phasic burst of LC-NA signaling in response to the auditory warning cues that is consistent with a largely intact alerting network, and that this phasic burst facilitated subsequent neural processing related to the target stimulus.
The demonstration of a relatively intact phasic response within the LC-NA system in older adults, while consistent with some behavioral findings of intact phasic alerting in older adults (Haupt et al., 2018; Nebes & Brady, 1993), appears to be in contrast to neurobiological evidence of aging effects on LC-NA signaling. Previous studies have reported age-related degradation of LC nuclei during normal aging, from early work showing reduced LC cell counts (Mann, 1983; Spokes, 1979; Vijayashankar & Brody, 1979) to more recent studies showing increased LC pathology in the aging population (Braak & Del Tredici, 2011b; Braak, Thal, Ghebremedhin, & Del Tredici, 2011; Grudzien et al., 2007). Moreover, studies using MR neuroimaging of neuromelanin (a metabolic byproduct of NA) found that LC integrity as estimated from signal contrasts to adjacent pontine tegmentum region was significantly decreased in older adults (Betts, Cardenas-Blanco, et al., 2019; Betts, Kirilina, et al., 2019; Shibata et al., 2006). It is possible, however, that despite an overall degradation of LC integrity with normal aging, the functional responsiveness of the LC-NA system (as reflected in the pupillary response) remains largely intact until a critical proportion of cells have been damaged either by age-related changes or by early Alzheimer’s disease pathology (Grudzien et al., 2007). On the other hand, recent neuroimaging findings suggest that even in the presence of intact functional LC signaling, older adults display changes in attentional mechanisms associated with disruptions in the effective transformation of this signal within target cortical regions (Lee et al., 2018).
Indeed, the present study identified distinct age-related differences in the effectiveness of the alerting network despite the presence of an intact pupillary alerting response in older adults. First, while both groups displayed greater pupil dilation under Sound than No Sound conditions, the young adults demonstrated greater overall pupil dilation than older adults under both conditions (Figure 3), indicating that the visual target elicited a more robust response within the LC-NA system in young adults. This age-related difference in pupil dilation was observed despite first z-transforming pupil dilation pixel measures for each participant in order to adjust for any age-related differences in the effective range of pupil diameter size (Gilzenrat et al., 2010; Moloney et al., 2006; Peysakhovich, Vachon, & Dehais, 2017; Van Gerven, Paas, Van Merriënboer, & Schmidt, 2004). Second, in older adults but not younger adults, the magnitude of the pupillary alerting response was larger in those individuals with slower mean reaction times (Figure 6). Consistent with this pattern, older adults overall were slower in behavioral performance but showed a larger pupillary alerting effect than young adults (Figure 5).
One explanation for these findings is that the level of stimulus-driven phasic activity within the LC-NA system is generally lower in older adults than young adults (Hammerer et al., 2017). Given this decreased activity, the presentation of a weak alerting cue (i.e., the visual target stimulus alone) elicits a small pupillary response in older adults rather than the robust response observed in young adults. In contrast, the presentation of a strong alerting cue (i.e. the auditory warning cue) is able to elicit a strong pupillary response in older adults that can compensate for the diminished regular phasic activity within the LC-NA system, and particularly in those older adults with slower overall RT. That is: a) In young adults with a robust LC-NA system, the visual target elicited a strong pupillary response that was incrementally increased with the addition of the auditory warning cue; whereas b) In older adults with a functioning but deficient LC-NA system, the visual target elicited a weak pupillary response that was rescued (i.e., increased to a level comparable to that displayed by young adults) by the addition of the strong auditory alerting cue.
Consistent with this interpretation, the time course of the task-evoked pupillary response displayed by the older adults was significantly slower than the young adults in the No Sound condition (i.e., the visual target alone), yet was comparable to that of young adults in the Sound condition (Figure 4). Thus, older adults appeared to display a weaker task-evoked phasic response within the LC-NA system in the low alerting condition that then benefited more from the addition of the stronger auditory warning cue in the high alerting condition. Also consistent with this interpretation, trial-level regression analyses revealed that older adults individually displayed a strong association between task-evoked pupil dilation and speed of response in both the low and high alerting conditions, whereas this association was reduced in young adults in the high alerting condition (Figure 9). On the schematic curve of the relation between arousal level and speed of response (Figure 10), these results suggest that older adults are positioned on the lower arousal end of the curve such that they remained in the steep portion of the curve under both low and high alerting conditions, whereas the higher arousal level of young adults positions them closer to the asymptotic portion of the curve during which further increases in arousal level result in attenuated benefit for behavioral response speed. Older adults, but not young adults, were also found to display a significant Sound × Time-on-Task interaction on the magnitude of behavioral alerting, suggesting a detectable decrease in the potency of the warning cue over the course of a trial block in the older but not young adults.
Taken together, these converging findings suggest the presence of a functioning but deficient alerting network associated with a decrease in the integrity of the LC-NA system in older adults. Overall, findings in this study demonstrate some preserved patterns of LC-NA signaling in the aging population while also identifying other age-related changes in the LC-NA system within the context of cognitive performance. Previous behavioral studies examining the effects of aging on phasic alerting have produced discrepant results (Fernandez-Duque & Black, 2006; Festa-Martino et al., 2004; Gamboz et al., 2010; Ishigami et al., 2016; Jennings et al., 2007; Nebes & Brady, 1993; Rabbitt, 1984; Zhou et al., 2011) that could be attributable to a number of methodological differences across studies (Gamboz et al., 2010). For example, tasks with longer cue-target intervals, greater response complexity (e.g., target discrimination vs. simple target detection), and additional processing demands (e.g., inclusion of a flanker interference task) may have encouraged the recruitment of top-down attentional and other processes that altered the time-course and behavioral effects of the bottom-up phasic alerting processes (Tales et al., 2011; Wiegand & Sander, 2019). Differences in the strength or saliency of the alerting cue across studies could also lead to different effects of aging on the magnitude of behavioral alerting, given our finding that the visual target elicited a weak pupillary response in older adults that increased to a level comparable to that displayed by young adults with the addition of the strong auditory alerting cue. Indeed, it is not clear that a larger behavioral alerting effect (defined as the difference in performance between high and low alerting conditions) can be unambiguously interpreted as reflecting enhanced alerting, since it may actually reflect the presence of deficient alerting under low alerting conditions that is then recovered under high alerting conditions (thereby creating a larger behavioral difference between the high and low alerting conditions).
The difficulty in interpreting purely behavioral studies highlights the importance of incorporating additional modalities, such as pupillometry and EEG (e.g., Wiegand & Sander, 2019) to provide a fuller understanding of the mechanisms underlying age-related changes in phasic alerting. Moreover, the ability to characterize age-related changes in the associations between trial pupil dilation and behavior also highlights the benefits of using partial pupil dilatory response curves to characterize pupil dilation during cognitive task performance. This approach may be particularly fruitful in identifying functional signatures of LC-NA system activity that can be used to distinguish healthy aging from neuropathological changes within the LC-NA system associated with the earliest stages of Alzheimer’s disease (Braak & Del Tredici, 2011a, 2011b; Clewett et al., 2016; Mather & Harley, 2016; Theofilas et al., 2017), and that could therefore be useful in identifying those individuals who are at greatest risk for developing the disease.
Supplementary Material
ACKNOWLEDGEMENTS
This research is based on work conducted as part of the first author’s master’s thesis. This work was supported by Brown University. We would like to thank Milena Rmus and Charlotte Jeong for help in data collection.
Footnotes
DISCLOSURE
None of the authors have financial or nonfinancial conflicts of interest to disclose.
REFERENCES
- Arnsten AFT (1993). Catecholamine mechanisms in age-related cognitive decline. Neurobiology of Aging, 14(6), 639–641. [DOI] [PubMed] [Google Scholar]
- Arnsten AFT, & Goldman-Rakic PS (1985). Alpha 2-adrenergic mechanisms in prefrontal cortex associated with cognitive decline in aged nonhuman primates. Science, 230(4731), 1273–1276. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Chiang C, & Alexinsky T (1991). Discharge of noradrenergic locus coeruleus neurons in behaving rats and monkeys suggests a role in vigilance In Progress in Brain Research (Vol. 88, pp. 501–520). Elsevier. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, & Cohen JD (2005). An integrative theory of locus coeruleus-norepinephrine function: Adaptive Gain and Optimal Performance. Annual Review of Neuroscience, 28(1), 403–450. [DOI] [PubMed] [Google Scholar]
- Beatty J, & Lucero-Wagoner B (2000). The pupillary system Handbook of Psychophysiology (2nd Ed.). [Google Scholar]
- Bernstein IH, Chu PK, Briggs P, & Schurman DL (1973). Stimulus intensity and foreperiod effects in intersensory facilitation. The Quarterly Journal of Experimental Psychology, 25(2), 171–181. [DOI] [PubMed] [Google Scholar]
- Bernstein IH, Clark MH, & Edelstein BA (1969). Effects of an auditory signal on visual reaction time. Journal of Experimental Psychology, 80(3p1), 567–569. [DOI] [PubMed] [Google Scholar]
- Bernstein IH, Rose R, & Ashe V (1970). Preparatory state effects in intersensory facilitation. Psychonomic Science, 19(2), 113–114. [Google Scholar]
- Bertelson P, & Tisseyre F (1969). The time course of preparation: Confirmatory results with visual and auditory warning signals. Acta Psychologica, 30, 145–154. [Google Scholar]
- Betts MJ, Kirilina E, Otaduy MCG, Ivanov D, Acosta-Cabronero J, Callaghan MF, … Passamonti L (2019). Locus coeruleus imaging as a biomarker for noradrenergic dysfunction in neurodegenerative diseases. Oxford University Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, & Del Tredici K (2011a). Alzheimer’s pathogenesis: Is there neuron-to-neuron propagation? Acta Neuropathologica, 121(5), 589–595. [DOI] [PubMed] [Google Scholar]
- Braak H, & Del Tredici K (2011b). The pathological process underlying Alzheimer’s disease in individuals under thirty. Acta Neuropathologica, 121(2), 171–181. [DOI] [PubMed] [Google Scholar]
- Braak H, Thal DR, Ghebremedhin E, & Del Tredici K (2011). Stages of the pathologic process in Alzheimer disease: age categories from 1 to 100 years. Journal of Neuropathology & Experimental Neurology, 70(11), 960–969. [DOI] [PubMed] [Google Scholar]
- Bueno VF, & Ribeiro-do-valle LE (2012). Effects of visual and auditory stimuli in a choice reaction time task. Psychology & Neuroscience, 5(2), 199–205. [Google Scholar]
- Carli M, Robbins TW, Evenden JL, & Everitt BJ (1983). Effects of lesions to ascending noradrenergic neurones on performance of a 5-choice serial reaction task in rats; implications for theories of dorsal noradrenergic bundle function based on selective attention and arousal. Behavioural Brain Research, 9(3), 361–380. [DOI] [PubMed] [Google Scholar]
- Clewett DV, Lee T-H, Greening S, Ponzio A, Margalit E, & Mather M (2016). Neuromelanin marks the spot: identifying a locus coeruleus biomarker of cognitive reserve in healthy aging. Neurobiology of Aging, 37, 117–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coull JT, Nobre AC, & Frith CD (2001). The noradrenergic α2 agonist clonidine modulates behavioural and neuroanatomical correlates of human attentional orienting and alerting. Cerebral Cortex, 11(1), 73–84. [DOI] [PubMed] [Google Scholar]
- Eckstein MK, Guerra-Carrillo B, Singley ATM, & Bunge SA (2017). Beyond eye gaze: What else can eyetracking reveal about cognition and cognitive development? Developmental Cognitive Neuroscience, 25, 69–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elman JA, Panizzon MS, Hagler DJ Jr, Eyler LT, Granholm EL, Fennema-Notestine C, … & Kremen WS (2017). Task-evoked pupil dilation and BOLD variance as indicators of locus coeruleus dysfunction. Cortex, 97, 60–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Duque D, & Black SE (2006). Attentional networks in normal aging and Alzheimer’s disease. Neuropsychology, 20(2), 133–143. [DOI] [PubMed] [Google Scholar]
- Festa-Martino E, Ott BR, & Heindel WC (2004). Interactions between phasic alerting and spatial orienting: effects of normal aging and Alzheimer’s disease. Neuropsychology, 18(2), 258–268. [DOI] [PubMed] [Google Scholar]
- Foote SL, Aston-Jones G, & Bloom FE (1980). Impulse activity of locus coeruleus neurons in awake rats and monkeys is a function of sensory stimulation and arousal. Proceedings of the National Academy of Sciences, 77(5), 3033–3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster B, Cavina-Pratesi C, Aglioti SM, & Berlucchi G (2002). Redundant target effect and intersensory facilitation from visual-tactile interactions in simple reaction time. Experimental Brain Research, 143(4), 480–487. [DOI] [PubMed] [Google Scholar]
- Gabay S, Pertzov Y, & Henik A (2011). Orienting of attention, pupil size, and the norepinephrine system. Attention, Perception & Psychophysics, 73(1), 123–9. [DOI] [PubMed] [Google Scholar]
- Gamboz N, Zamarian S, & Cavallero C (2010). Age-related differences in the attention network test (ANT). Experimental Aging Research, 36(3), 287–305. [DOI] [PubMed] [Google Scholar]
- Geva R, Zivan M, Warsha A, & Olchik D (2013). Alerting, orienting or executive attention networks: Differential patters of pupil dilations. Frontiers in Behavioral Neuroscience, 7(October), 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilzenrat MS, Nieuwenhuis S, & Cohen JD (2010). Pupil diameter tracks changes in control state predicted by the adaptive gain theory of locus coeruleus function. Cognitive, Affective, & Behavioral Neuroscience, 10(2), 252–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grudzien A, Shaw P, Weintraub S, Bigio E, Mash DC, & Mesulam MM (2007). Locus coeruleus neurofibrillary degeneration in aging, mild cognitive impairment and early Alzheimer’s disease. Neurobiology of Aging, 28(3), 327–335. [DOI] [PubMed] [Google Scholar]
- Hackley SA, Langner R, Rolke B, Erb M, Grodd W, & Ulrich R (2009). Separation of phasic arousal and expectancy effects in a speeded reaction time task via fMRI. Psychophysiology, 46(1), 163–171. [DOI] [PubMed] [Google Scholar]
- Hackley SA, & Valle-Inclan F (1998). Automatic alerting does not speed late motoric processes in a reaction-time task. Nature, 391(February), 786–788. [DOI] [PubMed] [Google Scholar]
- Hackley S. a, & Valle-Inclán F (1999). Accessory stimulus effects on response selection: Does arousal speed decision making? Journal of Cognitive Neuroscience, 11(3), 321–329. [DOI] [PubMed] [Google Scholar]
- Hackley SA, Langner R, Rolke B, Erb M, Grodd W, & Ulrich R (2009). Separation of phasic arousal and expectancy effects in a speeded reaction time task via fMRI. Psychophysiology, 46(1), 163–171. [DOI] [PubMed] [Google Scholar]
- Hämmerer D, Callaghan MF, Hopkins A, Kosciessa J, Betts M, Cardenas-Blanco A, … Dolan RJ (2018). Locus coeruleus integrity in old age is selectively related to memories linked with salient negative events. Proceedings of the National Academy of Sciences, 115(9), 2228–2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hämmerer D, Hopkins A, Betts MJ, Maaß A, Dolan RJ, & Düzel E (2017). Emotional arousal and recognition memory are differentially reflected in pupil diameter responses during emotional memory for negative events in younger and older adults. Neurobiology of Aging, 58, 129–139. [DOI] [PubMed] [Google Scholar]
- Haupt M, Sorg C, Napiórkowski N, & Finke K (2018). Phasic alertness cues modulate visual processing speed in healthy aging. Neurobiology of Aging, 70, 30–39. [DOI] [PubMed] [Google Scholar]
- Ishigami Y, Eskes GA, Tyndall AV, Longman RS, Drogos LL, & Poulin MJ (2016). The Attention Network Test-Interaction (ANT-I): Reliability and validity in healthy older adults. Experimental Brain Research, 234(3), 815–827. [DOI] [PubMed] [Google Scholar]
- Ishigami Y, & Klein RM (2010). Repeated measurement of the components of attention using two versions of the Attention Network Test (ANT): Stability, isolability, robustness, and reliability. Journal of Neuroscience Methods, 190(1), 117–128. [DOI] [PubMed] [Google Scholar]
- Jennings JM, Dagenbach D, Engle CM, & Funke LJ (2007). Age-related changes and the attention network task: An examination of alerting, orienting, and executive function. Neuropsychology, Development, and Cognition. Section B, Aging, Neuropsychology and Cognition, 14(July 2013), 353–369. [DOI] [PubMed] [Google Scholar]
- Jepma M, Wagenmakers E, Band GPH, & Nieuwenhuis S (2009). The effects of accessory stimuli on information processing: Evidence from electrophysiology and a diffusion model analysis. Journal of Cognitive Neuroscience, 21(5), 847–864. [DOI] [PubMed] [Google Scholar]
- Joshi S, Li Y, Kalwani RM, & Gold JI (2016). Relationships between pupil diameter and neuronal activity in the locus coeruleus, colliculi, and cingulate cortex. Neuron, 89(1), 221–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamurthy K, Nassar MR, Sarode S, & Gold JI (2016). Adaptive, arousal-related adjustments of perceptual biases optimize perception in a dynamic environment. BioRxiv, 83766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence MA, & Klein RM (2013). Isolating exogenous and endogenous modes of temporal attention. J Exp Psychol Gen, 142(2), 560–572. [DOI] [PubMed] [Google Scholar]
- Lee TH, Greening SG, Ueno T, Clewett D, Ponzio A, Sakaki M, & Mather M (2018). Arousal increases neural gain via the locus coeruleus–noradrenaline system in younger adults but not in older adults. Nature Human Behaviour, 2(5), 356–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Rodenkirch C, Moskowitz N, Schriver B, & Wang Q (2017). Dynamic lateralization of pupil dilation evoked by locus coeruleus activation results from sympathetic, not parasympathetic, contributions. Cell Reports, 20(13), 3099–3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann DMA (1983). The locus coeruleus and its possible role in ageing and degenerative disease of the human central nervous system. Mechanisms of Ageing and Development, 23(1), 73–94. [DOI] [PubMed] [Google Scholar]
- Marrocco RT, & Davidson MC (1998). Neurochemistry of attention In Parasuraman R (Ed.), The Attentive Brain (pp. 35–50). Cambridge, MA, US: The MIT Press. [Google Scholar]
- Mather M, & Harley CW (2016). The locus coeruleus: Essential for maintaining cognitive function and the aging brain. Trends in Cognitive Sciences, 20(3), 214–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthias E, Bublak P, Müller HJ, Schneider WX, Krummenacher J, & Finke K (2010). The influence of alertness on spatial and nonspatial components of visual attention. Journal of Experimental Psychology. Human Perception and Performance, 36(1), 38–56. [DOI] [PubMed] [Google Scholar]
- McDougal DH, & Gamlin PD (2015). Autonomic control of the eye. Comprehensive Physiology, 5(1), 439–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moloney KP, Jacko JA, Vidakovic B, Sainfort F, Leonard VK, & Shi B (2006). Leveraging data complexity: Pupillary behavior of older adults with visual impairment during HCI. ACM Transactions on Computer-Human Interaction (TOCHI), 13(3), 376–402. [Google Scholar]
- Murphy PR, O’Connell RG, O’Sullivan M, Robertson IH, & Balsters JH (2014). Pupil diameter covaries with BOLD activity in human locus coeruleus. Human Brain Mapping, 35(8), 4140–4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy PR, Robertson IH, Balsters JH, & O’Connell RG (2011). Pupillometry and P3 index the locus coeruleus-noradrenergic arousal function in humans. Psychophysiology, 48(11), 1532–1543. [DOI] [PubMed] [Google Scholar]
- Nebes RD, & Brady CB (1993). Phasic and tonic alertness in Alzheimer’s disease. Cortex, 29(1), 77–90. [DOI] [PubMed] [Google Scholar]
- Nichols TE, & Holmes AP (2001). Nonparametric Permutation Tests for Functional Neuroimaging Experiments: A Primer with examples. Human Brain Mapping, 15(1), 1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuis S, De Geus EJ, & Aston-Jones G (2011). The anatomical and functional relationship between the P3 and autonomic components of the orienting response. Psychophysiology, 48(2), 162–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen A, Petersen AH, Bundesen C, Vangkilde S, & Habekost T (2017). The effect of phasic auditory alerting on visual perception. Cognition, 165, 73–81. [DOI] [PubMed] [Google Scholar]
- Petersen SE, & Posner MI (2012). The Attention System of the Human Brain: 20 Years After. Annual Review of Neuroscience, 35(1), 73–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peysakhovich V, Vachon F, & Dehais F (2017). The impact of luminance on tonic and phasic pupillary responses to sustained cognitive load. International Journal of Psychophysiology, 112, 40–45. [DOI] [PubMed] [Google Scholar]
- Posner MI, & Boies SJ (1971). Components of attention. Psychological Review, 78(5), 391–408. [Google Scholar]
- Posner MI, Klein R, Summers J, & Buggie S (1973). On the selection of signals. Memory & Cognition, 1(1), 2–12. [DOI] [PubMed] [Google Scholar]
- Posner MI, & Petersen SE (1990). The attention system of the human brain. Annual Review of Neuroscience, 13(1), 25–42. [DOI] [PubMed] [Google Scholar]
- Rabbitt P (1984). How old-people prepare themselves for events which they expect. Attention and Performance, 10, 515–527. [Google Scholar]
- Rajkowski J, Kubiak P, & Aston-Jones G (1994). Locus Coeruleus Activity in Monkey : Phasic and Tonic Changes Are Associated With Altered Vigilance. Brain Research Bulletin, 35, 607–616. [DOI] [PubMed] [Google Scholar]
- Randolph C, Tierney MC, Mohr E, & Chase TN (1998). The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): preliminary clinical validity. Journal of Clinical and Experimental Neuropsychology, 20(3), 310–319. [DOI] [PubMed] [Google Scholar]
- Reimer J, Froudarakis E, Cadwell CR, Yatsenko D, Denfield GH, & Tolias AS (2014). Pupil fluctuations track fast switching of cortical states during quiet wakefulness. Neuron, 84(2), 355–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimer J, McGinley MJ, Liu Y, Rodenkirch C, Wang Q, McCormick DA, & Tolias AS (2016). Pupil fluctuations track rapid changes in adrenergic and cholinergic activity in cortex. Nature Communications, 7, 13289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels ER, & Szabadi E (2008). Functional neuroanatomy of the noradrenergic locus coeruleus: its roles in the regulation of arousal and autonomic function part I: principles of functional organisation. Current Neuropharmacology, 6(3), 235–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders AF (1980). 20 Stage analysis of reaction processes. Advances in Psychology, 1, 331–354. [Google Scholar]
- Schröter H, Frei LS, Ulrich R, & Miller J (2009). The auditory redundant signals effect: An influence of number of stimuli or number of percepts? Attention, Perception, & Psychophysics, 71(6), 1375–1384. [DOI] [PubMed] [Google Scholar]
- Shibata E, Sasaki M, Tohyama K, Kanbara Y, Otsuka K, Ehara S, & Sakai A (2006). Age-related changes in locus ceruleus on neuromelanin magnetic resonance imaging at 3 Tesla. Magn Reson Med Sci, 5(4), 197–200. [DOI] [PubMed] [Google Scholar]
- Sirois S, & Brisson J (2014). Pupillometry. Wiley Interdisciplinary Reviews: Cognitive Science, 5(6), 679–692. [DOI] [PubMed] [Google Scholar]
- Song AH, Kucyi A, Napadow V, Brown EN, Loggia ML, & Akeju O (2017). Pharmacological modulation of noradrenergic arousal circuitry disrupts functional connectivity of the locus coeruleus in humans. Journal of Neuroscience, 0446–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spokes EGS (1979). An analysis of factors influencing measurements of dopamine, noradrenaline, glutamate decarboxylase and choline acetylase in human post-mortem brain tissue. Brain: A Journal of Neurology, 102(2), 333–346. [DOI] [PubMed] [Google Scholar]
- Stafford IL, & Jacobs BL (1990). Noradrenergic modulation of the masseteric reflex in behaving cats. II. Physiological studies. Journal of Neuroscience, 10(1), 99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl J, & Rammsayer TH (2005). Accessory stimulation in the time course of visuomotor information processing: Stimulus intensity effects on reaction time and response force. Acta Psychologica, 120(1), 1–18. [DOI] [PubMed] [Google Scholar]
- Steinhauer SR, Siegle GJ, Condray R, & Pless M (2004). Sympathetic and parasympathetic innervation of pupillary dilation during sustained processing. International Journal of Psychophysiology, 52(1), 77–86. [DOI] [PubMed] [Google Scholar]
- Tales A, Snowden RJ, Phillips M, Haworth J, Porter G, Wilcock G, & Bayer A (2011). Exogenous phasic alerting and spatial orienting in mild cognitive impairment compared to healthy ageing: Study outcome is related to target response. Cortex, 47(2), 180–190. [DOI] [PubMed] [Google Scholar]
- Teufel HJ, & Wehrhahn C (2000). Evidence for the contribution of S cones to the detection of flicker brightness and red-green. J Opt Soc Am A Opt Image Sci Vis, 17(6), 994–1006. [DOI] [PubMed] [Google Scholar]
- Theofilas P, Ehrenberg AJ, Dunlop S, Alho ATDL, Nguy A, Leite REP, … Ferretti-Rebustini REDL (2017). Locus coeruleus volume and cell population changes during Alzheimer’s disease progression: a stereological study in human postmortem brains with potential implication for early-stage biomarker discovery. Alzheimer’s & Dementia, 13(3), 236–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tona KD, Murphy PR, Brown SBRE, & Nieuwenhuis S (2016). The accessory stimulus effect is mediated by phasic arousal: A pupillometry study. Psychophysiology, 53(7), 1108–1113. [DOI] [PubMed] [Google Scholar]
- Unsworth N, & Robison MK (2016). Pupillary correlates of lapses of sustained attention. Cognitive, Affective, & Behavioral Neuroscience, 16(4), 601–615. [DOI] [PubMed] [Google Scholar]
- Van Den Brink RL, Murphy PR, & Nieuwenhuis S (2016). Pupil diameter tracks lapses of attention. PLoS ONE, 11(10), 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Gerven PWM, Paas F, Van Merriënboer JJG, & Schmidt HG (2004). Memory load and the cognitive pupillary response in aging. Psychophysiology, 41(2), 167–174. [DOI] [PubMed] [Google Scholar]
- Vazey EM, Moorman DE, & Aston-Jones G (2018). Phasic locus coeruleus activity regulates cortical encoding of salience information. Proceedings of the National Academy of Sciences, 115(40), E9439–E9448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayashankar N, & Brody H (1979). A quantitative study of the pigmented neurons in the nuclei locus coeruleus and subcoeruleus in man as related to aging. Journal of Neuropathology & Experimental Neurology, 38(5), 490–497. [DOI] [PubMed] [Google Scholar]
- Wang CA, & Munoz DP (2015). A circuit for pupil orienting responses: Implications for cognitive modulation of pupil size. Current Opinion in Neurobiology, 33, 134–140. [DOI] [PubMed] [Google Scholar]
- Weinbach N, & Henik A (2012). Temporal orienting and alerting - the same or different? Frontiers in Psychology, 3(JUL), 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiegand I, Petersen A, Bundesen C, & Habekost T (2017). Phasic alerting increases visual attention capacity in younger but not in older individuals. Visual Cognition, 25(1–3), 343–357. [Google Scholar]
- Wiegand I, Petersen A, Finke K, Bundesen C, Lansner J, & Habekost T (2017). Behavioral and brain measures of phasic alerting effects on visual attention. Frontiers in Human Neuroscience, 11, 176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiegand I, & Sander MC (2019). Cue-related processing accounts for age differences in phasic alerting. Neurobiology of aging, 79, 93–100. [DOI] [PubMed] [Google Scholar]
- Witte EA, Davidson MC, & Marrocco RT (1997). Effects of altering brain cholinergic activity on covert orienting of attention: comparison of monkey and human performance. Psychopharmacology, 132(4), 324–334. [DOI] [PubMed] [Google Scholar]
- Zhou S, Fan J, Lee TMC, Wang C, & Wang K (2011). Age-related differences in attentional networks of alerting and executive control in young, middle-aged, and older Chinese adults. Brain and Cognition, 75(2), 205–210. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


