Abstract
Anthelmintic resistance in equine cyathostomins is both widespread and highly prevalent in the benzimidazole and tetrahydropyrimidine classes; however, reports of resistance to macrocyclic lactone (ML) drugs are sparse and sporadic. This study reports a case of clear ML resistance in a group of Thoroughbred yearlings imported from Ireland to the US in 2019. Fecal egg count reduction (FECR) following ivermectin administered in February 2020 demonstrated 100% reduction in the US bred yearlings, but 93.5%, 70.5%, and 74.5% reduction in three groups of the imported yearlings. The two former groups were then retreated with ivermectin, yielding FECRs of 33.8% and 23.5%, respectively. Horses from these two groups were then assigned randomly to two possible treatments; moxidectin or a triple combination of moxidectin, oxibendazole, and pyrantel pamoate. The groups treated with moxidectin had FECRs of 90.2%, 57.3%, and 50.0%, while the triple combination had a 100% FECR in all treated groups. Subsequently, the efficacy of ivermectin was reassessed in June 2020 yielding FECRs of 99.8%, 87.7%, and 62.0% in the three imported groups. The FECRs of the US bred yearlings all remained in the 99–100% range. This is the first study to clearly demonstrate ML resistance in cyathostomins and to confirm the suspicion through reassessment. These data demonstrate that ML-resistant cyathostomins were imported from Ireland and serve to illustrate that the global movement of horses has the potential to quickly spread ML-resistant parasite isolates around the world. The equine industry is strongly encouraged to routinely monitor anthelmintic efficacy, so occurrence of ML resistant cyathostomins can be detected and appropriate interventions implemented as early as possible.
Keywords: Cyathostomins, Ivermectin, Moxidectin, Resistance, Import
Graphical abstract
Highlights
-
•
Reduced macrocyclic lactone efficacy in yearlings imported from Ireland.
-
•
Ivermectin efficacy was retested twice and was reduced on all occasions.
-
•
100% strongyle fecal egg count reduction in US bred yearlings on all occasions.
-
•
A combination of moxidectin, oxibendazole and pyrantel pamoate was 100% effective.
-
•
Global movement of horses likely to spread macrocyclic lactone resistance quickly.
1. Introduction
Equine cyathostomin parasites are ubiquitous in grazing horses across the world, and anthelmintic resistance is widely documented to two of three available drug classes (Kaplan 2002; Peregrine et al., 2014). Despite early predictions of resistance developing to macrocyclic lactone (ML) anthelmintics (e.g., ivermectin, moxidectin) in cyathostomins (Sangster, 1999), to date there have been very few reports published. The first report of ML resistance was a conference abstract reporting reduced efficacy of moxidectin in donkeys kept at a donkey sanctuary (Trawford et al., 2005). However, the donkeys were treated in an extra-label manner using oral administration of an injectable formulation registered for ruminants, leading to questions as to the level of evidence and the clinical significance to horses. The next report was in a herd of Thoroughbred horses in Brazil (Molento et al., 2008), where treatment with ivermectin, abamectin and moxidectin all yielded reduced efficacies. Since then, a very limited number of studies, all of which were multiple-farm surveys, have reported macrocyclic lactone resistance in the form of reduced fecal egg count reduction at two weeks following administration (Traversa et al., 2009; Milillo et al., 2009; Näreaho et al., 2011; Canever et al., 2013; Relf et al., 2014). This is remarkable given that ML anthelmintics have been the most widely administered drugs in horses by far over the past several decades (Stratford et al., 2014; Becher et al., 2018). It is also noteworthy that the reports published, thus far, are from Europe (4) and Brazil (1), but so far none have been published from North America, despite a remarkably high anthelmintic treatment intensity, especially in the Thoroughbred industry (Robert et al., 2015). Furthermore, these reports were all limited to single farms/populations, most often due to a single or very few horses within the treated groups exhibiting a decrease in fecal egg count reduction, and in none of these studies were horses re-treated to confirm the suspicion of ivermectin resistance. Thus, the currently available data on ML resistance in cyathostomin populations is scarce and mostly inconclusive. Truly convincing evidence with follow-up confirmation of full-fledged resistance to ML anthelmintics in cyathostomins has yet to be presented.
Despite the limited evidence, it is generally believed that ML resistance is emerging in cyathostomin populations. This is supported by several reports of shortened egg reappearance periods (ERPs) following administration of this drug class. Originally, ERPs were reported in the 9–13 week range for ivermectin (Borgsteede et al., 1993; Boersema et al., 1996; Demeulenaere et al., 1997), while moxidectin was capable of suppressing strongyle egg shedding for 16–22 weeks (Jacobs et al., 1995; DiPietro et al., 1997; Demeulenaere et al., 1997). However, in the past fifteen years, there have been multiple reports of significantly shortened ERPs (von Samson-Himmelstjerna et al., 2007; Relf et al., 2014; Geurden et al., 2014; Molena et al., 2018) and in some cases as short as four weeks for both MLs (Lyons et al., 2008, 2011; Bellaw et al., 2018). Terminal studies performed at the University of Kentucky have demonstrated that for ivermectin, the shortened ERP appears to be due to resistance at the luminal L4 stage with efficacy levels often well below 50% (Lyons et al., 2009; Lyons and Tolliver, 2013). However, with moxidectin the pattern is less clear as the efficacy against luminal L4s was still above 96% in cyathostomin populations with 4-week ERPs (Lyons et al., 2010; Bellaw et al., 2018). Taken together, while the multiple reports of shortened ERP may be indicative of emerging resistance, more data are needed to fully understand the possible mechanisms.
In this report, we present a case of unequivocal ML treatment failure in a group of Thoroughbred yearlings imported to the US from a farm in Ireland. We describe how the lack of efficacy was confirmed through rigorous testing, and describe the treatment and management decisions made on the farm to mitigate the situation.
2. Materials and methods
2.1. Horses
The data presented herein were obtained from a Thoroughbred operation in Central Kentucky. This farm breeds a large number of foals every year, but also imports a contingency of weanlings from Europe every autumn. Following current recommendations, the farm has implemented routine monitoring of parasite egg count levels and anthelmintic treatment efficacy.
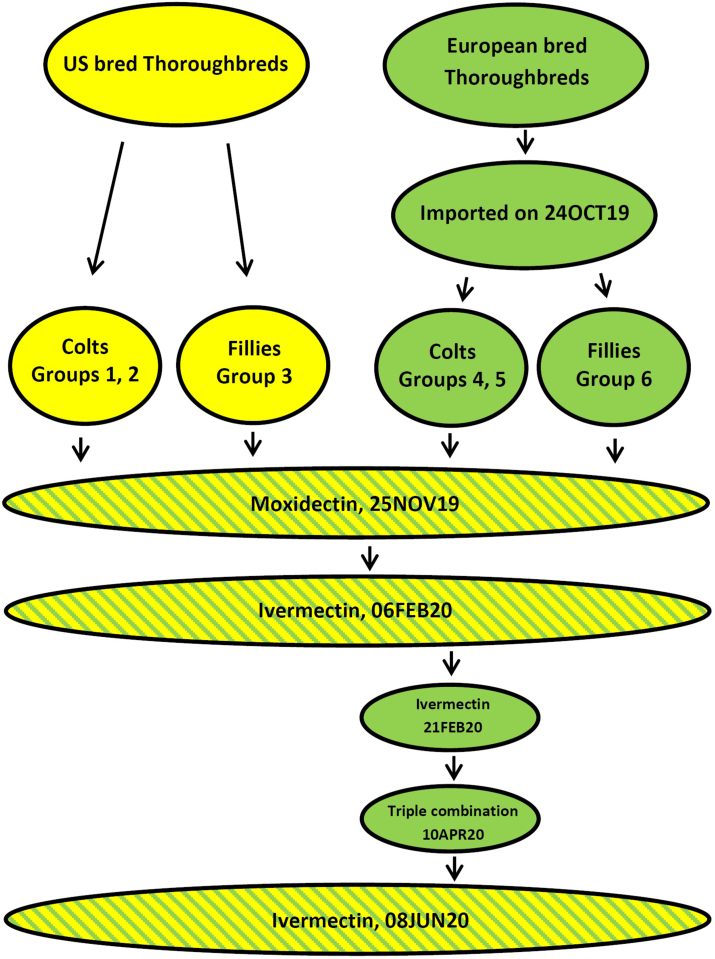
The study population consisted of two groups of Thoroughbred yearlings born in 2019 and all kept at the same farm in Kentucky, USA. One group (25 fillies and 33 colts) were born in Ireland and were imported into the US on October 24, 2019. The other group (26 fillies and 29 colts) were born on the US farm. The horses were organized in six groups (Fig. 1), which were kept separate from each other with no overlap in use of paddocks, pastures or barns. As per the usual procedure on Kentucky Thoroughbred farms, many of the yearlings were sold in the traditional yearling sales in September 2020. Regardless of ownership, the horses were then placed into training for the 2021 season.
Fig. 1.
Flow diagram describing the series of events for the study population consisting of yearlings born in either Kentucky, USA (yellow) or Ireland (green) in 2019. The Irish yearlings were imported to the US in October 2019. The flowchart indicates when horses received anthelmintic treatment and what they were treated with. The triple anthelmintic treatment administered in April 2020 consisted of moxidectin, oxibendazole, and pyrantel pamoate. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
2.2. Treatment history
Prior to weaning, foals at the farm in Ireland were dewormed with fenbendazole at 2 and 5 months of age. Post weaning, they were treated with ivermectin at 6 months and moxidectin at 7 months of age prior to exporting them to the US. The US born population was dewormed with a combination of piperazine and fenbendazole at 2 and 4 months of age, followed by ivermectin at 5 and 7 months of age.
2.3. Treatments administered
The course of events and the timing of anthelmintic treatments are outlined in the flow diagram in Fig. 1. All horses were weighed on electronic scales on a monthly basis and anthelmintic products were administered orally according to weight.
All horses were administered moxidectin (400 μg/kg, Quest Plus, Zoetis, New Jersey, USA) on November 25, 2019, and ivermectin (200 μg/kg, Equimax, Bimeda, Illinois, USA) was administered to all yearlings on February 6, 2020. Given a lack of fecal egg count reduction observed among the imported yearlings following these moxidectin and ivermectin treatments, Groups 4 and 5 were re-treated 21 days later on February 28, 2020 and FEC repeated 14 days following treatment. Subsequent to this, the colts in Groups 4 and 5 were allocated randomly to two treatment groups; one group was administered moxidectin (400 μg/kg, Quest, Zoetis, New Jersey, USA) and the other was administered a triple combination of oxibendazole (10 mg/kg, Anthelcide, Zoetis, New Jersey, USA), pyrantel pamoate (6.6 mg/kg, Strongid P, Zoetis, New Jersey, USA), and moxidectin (400 μg/kg, Quest, Zoetis, New Jersey, USA). Following this, all yearlings were treated again with ivermectin (200 μg/kg, Zimecterin, Boehringer-Ingelheim, Ingelheim, Germany) on June 8, 2020 to verify the reduced efficacy observed in February.
2.4. Fecal egg counts
Fecal samples were collected either rectally or from samples freshly deposited on stall floors, were put in airtight containers, kept refrigerated, and analyzed within one working week. All post-treatment samples were collected at 14 days following anthelmintic administration.
Strongyle fecal egg counts (FEC) were performed by farm personnel using the OvaTector system (BG Medical Products Inc, Venice, Florida, USA) with a multiplication factor of 10 eggs per gram (EPG) and sodium nitrate (specific gravity 1.25) as flotation medium.
2.5. Statistical analysis
Fecal egg count reductions (FECRs) were calculated for each treatment and group using an online web interface providing a Bayesian hierarchical model analysis of the data with estimation of mean FECR and 95% Credible Interval (Torgerson et al., 2014; Wang et al., 2018). Resistance was suspected whenever the group mean FECR fell below 95% and the lower Credible Interval fell below 90%.
The FECR data were analyzed using SAS version 9.4 (Cary, NC, USA). Data were evaluated for normal distribution with Shapiro-Wilk and Kolmogorov-Smirnov statistics as well as normal probability plots, and were log-transformed to achieve normal distribution. A mixed linear model was constructed for the ivermectin FECR data (treatments A and C) with time point and group as class variables. The ‘mixed’ procedure was used with repeated measures across the two time points and horse ID as random effect. Analyses analyzed for differences between groups and time points as well as the effect of the interaction term between group and time point. In one set of analyses, the group variable was replaced by country of origin (USA or Ireland) to analyze for associated trends. Whenever any of the covariates was found significant, a Tukey's pair-wise comparison of the adjusted least squares means was performed. All analyses were interpreted at the α = 0.05 level.
3. Results
Results are presented for each of the six groups, three of which were US bred and three that were imported from Ireland.
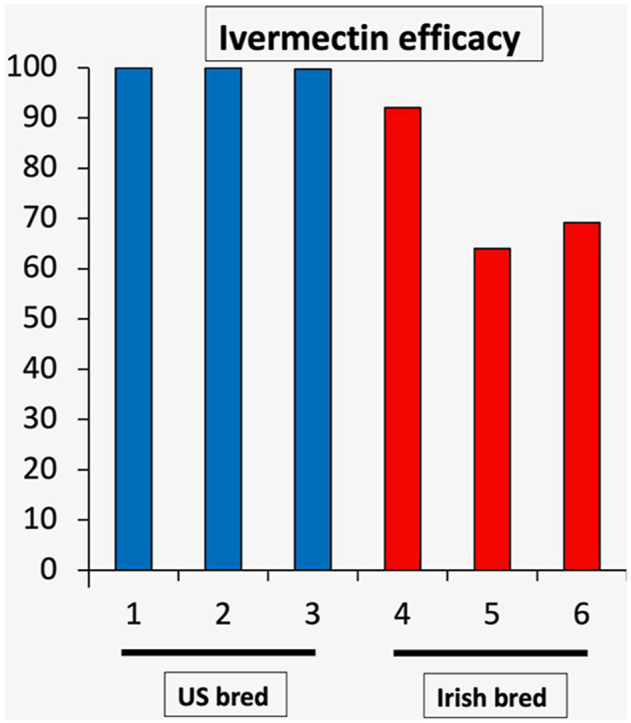
The three US bred groups (1, 2, 3) were each dewormed with ivermectin on two occasions four months apart, and the FECRs were all in the 99–100% range (Table 1). The ivermectin FECRs from the imported yearlings (groups 4, 5, 6) are reported in Table 2. All three imported groups demonstrated reduced ivermectin efficacy. Additionally, group 6, which was the only group where efficacy was monitored following the November 2019 moxidectin treatment, yielded reduced efficacy there as well (Table 3). Overall, there were no significant differences in FECR between the February and June ivermectin treatments (p = 0.839), but there were significant differences between groups (p < 0.001) with group 6 demonstrating a significantly lower FECR than groups 1–4. There was a significant association between FECR and the interaction term between group and time point (p < 0.001) with group 6 having a significantly lower FECR than all other groups for the June treatment. The analysis with the country of origin variable revealed significantly lower FECRs in the Irish yearlings (p < 0.001) but no difference between the two treatment time points (p = 0.893). The follow-up treatments administered on February 28 in Groups 3 and 4 both demonstrated a pronounced lack of reduction, and the June 8 ivermectin treatment yielded reduced efficacy in two of the three groups. Table 3 presents the results of treating the imported yearlings with either moxidectin alone or the triple combination. Moxidectin efficacy was reduced in all three groups, but the triple combination demonstrated a 100% FECR.
Table 1.
Strongyle fecal egg counts presented in eggs per gram of feces (EPG) in three groups of US-bred Thoroughbred yearlings and the percent reduction with 95% credible intervals (CI) following oral administration of ivermectin (200 μg/kg) on two separate occasions.a
| Datea | N | Pre-treatment (EPG) |
Post-treatment (EPG) |
FECR (95% CI) | ||
|---|---|---|---|---|---|---|
| Mean | Range | Mean | Range | |||
| Group 1 | ||||||
| A | 12 | 1142.5 | 530–2070 | 0 | - | 100% (99.8–100) |
| C |
16 |
868.2 |
310–1910 |
0 |
- |
100% (99.7–100) |
| Group 2 | ||||||
| A | 10 | 1347.0 | 720–2640 | 0 | - | 100% (99.8–100) |
| C |
12 |
1116.7 |
320–1840 |
10.8 |
0–60 |
99.4% (97.6–100) |
| Group 3 | ||||||
| A | 24 | 1236.0 | 610–1720 | 0.5 | 0–60 | 100% (99.8–100) |
| C | 26 | 1239.2 | 170–2090 | 0 | - | 100% (99.9–100) |
Ivermectin treatments administered on A: February 6 and C: June 8, 2020.
Table 2.
Strongyle fecal egg counts presented in eggs per gram of feces (EPG) and percent reduction with 95% credible intervals (CI) in three groups of yearling Thoroughbreds imported from Ireland following oral ivermectin administration (200 μg/kg) on three separate occasions.a
| Datea | N | Pre-treatment (EPG) |
Post-treatment (EPG) |
FECR (95% CI) | ||
|---|---|---|---|---|---|---|
| Mean | Range | Mean | Range | |||
| Group 4 | ||||||
| A | 10 | 1317.0 | 620–1970 | 105.0 | 0–320 | 93.5% (85.6–97.6) |
| B | 8 | 131.3 | 10–320 | 118.9 | 0–350 | 33.8% (13.4–61.9) |
| C |
15 |
1605.3 |
10–2680 |
4.0 |
0–20 |
99.8% (99.4–99.9) |
| Group 5 | ||||||
| A | 16 | 968.1 | 550–1720 | 348.8 | 20–1170 | 70.5% (55.5–88.9) |
| B | 16 | 348.8 | 20–1170 | 358.1 | 20–760 | 23.5% (12.6–45.0) |
| C |
18 |
1278.9 |
210–2460 |
174.1 |
0–670 |
87.7% (79.6–93.1) |
| Group 6 | ||||||
| A | 19 | 1080.0 | 510–1980 | 332.6 | 20–880 | 74.5% (62.4–83.2) |
| C | 25 | 1246.3 | 470–2490 | 570.0 | 10–1490 | 62.0% (48.3–72.4) |
Ivermectin treatments administered on A: February 6, B: February 28, and C: June 8, 2020.
Table 3.
Strongyle fecal egg counts and percent reduction with 95% credible intervals (CI) in three groups of yearling Thoroughbreds imported from Ireland following either an oral administration of a triple combination (T) consisting of moxidectin (400 μg/kg), pyrantel pamoate (6.6 mg/kg) and oxibendazole (10 mg/kg) or a single dose of moxidectin (M, 400 μg/kg).
| N | Pre-treatment EPG |
Post-treatment EPG |
FECR (95% CI) | |||
|---|---|---|---|---|---|---|
| Mean | Range | Mean | Range | |||
| Group 4 | ||||||
| Ta | 2 | 336.0 | 320–350 | 0 | - | 100% (76.2–100) |
| Ma |
5 |
80.0 |
10–160 |
5.0 |
0–20 |
90.2% (62.6–98.2) |
| Group 5 | ||||||
| Ta | 9 | 313.3 | 20–760 | 0 | - | 100% (98.6–100) |
| Ma |
7 |
385.7 |
180–720 |
250.0 |
20–760 |
57.3% (26.1–78.6) |
| Group 6 | ||||||
| Mb | 16 | 1229.4 | 570–2060 | 638.8 | 180–1180 | 50.0% (30.7–64.2) |
Treatments administered on April 10, 2020.
Treatment administered on November 25, 2019.
4. Discussion
The data presented herein are remarkable in at least three ways. First, this is the most comprehensive data set demonstrating reduced ML efficacy against equine cyathostomins reported to date. Second, reduced efficacy was demonstrated to both ivermectin and moxidectin, and the suspicion of resistance was confirmed through re-testing. Third, the high efficacy of the ivermectin treatments in all three groups of US bred horses measured concomitantly with the reduced efficacy in the Irish bred horses demonstrates that the drugs were of high quality, administered correctly by farm personnel, and that the reduced efficacy was not a result of laboratory error. These data present clear evidence that the cyathostomin population harbored by the US bred horses were susceptible to ivermectin, whereas the parasites of the Irish imports were resistant to both ivermectin and moxidectin. As these populations were kept completely separate throughout the study, this is clear evidence that the ML resistant parasites were imported from the Irish farm.
An earlier equine anthelmintic resistance survey indicated that anthelmintic resistance levels were more pronounced in Kentucky than other southern states, and these levels were among the highest reported anywhere in the world (Kaplan et al., 2004). The use of anthelmintics on Kentucky Thoroughbred farms is very intense, with the large majority of farms deworming their yearlings six times a year or more (Robert et al., 2015). Thus, it was always the expectation that ML-resistant cyathostomins were very likely to eventually occur in the heart of Thoroughbred country in Kentucky. In light of this, it is a genuine surprise that the first case of full-fledged ML resistance was diagnosed in horses imported from Ireland. A recent survey conducted in Ireland documented that parasite control programs adopted by the equine industry there (including Thoroughbred farms) were not following current recommendations, with little or no diagnostic monitoring of parasite presence and treatment efficacy, and frequent administration of anthelmintic treatments with heavy reliance on ML products (Elghryani et al., 2019). Thus, several similar risk factors for development of ML resistance were also present in Ireland. Nonetheless, it is important to note that the Kentucky farm has imported weanlings from Ireland every year for the past several years, and that routine testing in previous years had always demonstrated full ML efficacy. Furthermore, the anthelmintic treatment regimen practiced on the Ireland facility with two benzimidazole treatments targeting ascarid parasites followed by two ML treatments directed at the strongyle parasites is in line with general recommendations, and cannot be considered overly excessive compared with general trends within the Thoroughbred industry. Thus, it is not possible to identify what set of conditions and circumstances led this particular cyathostomin population to become resistant, or why it occurred now.
The heavy reliance on ML anthelmintics for equine strongyle control is obviously problematic, but the industry is left with little choice. In cyathostomins, anthelmintic resistance is widespread to both the benzimidazoles and pyrimidines (Peregrine et al., 2014), often leaving the MLs as the only viable treatment option. This is reflected by current guidelines for equine parasite control, wherein it is recommended to primarily use MLs for strongyle control and only use other drug classes if pertinent testing has demonstrated good efficacy (Nielsen et al., 2019; ESCCAP 2019; Rendle et al., 2019). While ML resistance has been remarkably slow to progress in cyathostomins, the data presented herein clearly demonstrate that a resistance break-through can happen, and likely is occurring elsewhere as well. It is noteworthy that the degree of parasite egg count monitoring adopted by this farm is well above industry norms. As is most common with cyathostomin infections, the horses infected with the ML-resistant parasites were all clinically normal; consequently, the ML resistance was discovered here only due to this monitoring program. Other equine operations in the US, Ireland, and elsewhere, most likely already have ML resistant cyathostomins without being aware due to a lack of testing and the relatively low pathogenicity of cyathostomins. This case clearly illustrates the importance of quality routine FECR testing, which immediately informed the farm manager of the situation and allowed him to react in time by keeping the populations completely separate, thereby avoiding an introduction of the resistant parasites to the entire facility.
The triple combination of oxibendazole, pyrantel pamoate and moxidectin was administered in an attempt to achieve the desired 100% reduction in FEC. We expected that the moxidectin efficacy would be somewhat higher than that for ivermectin, given the higher dose and lipophilic properties of this molecule. However, this was not observed, as the 95% CI for FECR overlapped for both drugs. Although the efficacies of oxibendazole and pyrantel pamoate were not tested separately in these horses, the 100% reduction in FEC of the combination suggests that one or both of these drugs were reasonably or highly effective. However, high efficacy for these two drugs is unlikely on Kentucky Thoroughbred farms, as resistance to benzimidazoles and pyrimidines is highly prevalent (Kaplan et al., 2004). Consequently, using the triple combination to treat ML resistant cyathostomins in Kentucky is unlikely to be a sustainable treatment choice over time. We recently demonstrated that use of a combination of oxibendazole and pyrantel pamoate against a cyathostomin population with documented resistance to both classes was not efficacious (Scare et al., 2018). Thus, if a triple combination treatment is used, the efficacy will need to be closely monitored; once this approach loses efficacy we will have run out of treatment options. The pharmaceutical industry has not introduced any new anthelmintic classes with new modes of action for equine usage since ivermectin almost 40 years ago, and there are no indications that any new anthelmintic classes will be introduced for horses in the foreseeable future. The data presented herein strongly demonstrate that the portfolio of equine anthelmintics is woefully unsatisfactory, leaving end-users with few viable treatment options and almost no alternatives, when current options fail.
Some of these imported yearlings were sold at the 2020 September yearling sales, while the majority were retained by the owner and sent away for training. Thus, they likely brought their ML resistant cyathostomins with them and would almost certainly be introducing these to the new facilities. With a high degree of national and international movement of horses and the general lack of quarantine testing, this population of ML resistant worms will quickly spread to numerous locations, and given the heavy reliance on ML anthelmintics across the world, resistant populations will be quickly selected for. While this outlook is scary, the fact that these yearlings are destined for a career as race horses should mitigate the risk of spread, since they will go into training and have little or no access to pasture for the next couple years. Exceptions will be injured horses sent home for rehabilitation, situations where horses are given a break from training to allow additional maturation and growth, or horses that are retired from racing to become off-the-track-Thoroughbreds in search of new careers as riding horses. Thus, in a many of these horses, the cyathostomin infection may be somewhat self-limiting. However, it should be kept in mind that encysted cyathostomin larvae undergo arrested development at the early third stage, which means that it can take several years for the infection to be eliminated, even if effective adulticidal anthelmintics are administered (Smith, 1976a, 1976b). Taken together, these yearlings are likely to introduce ML resistant cyathostomins to their new facilities, and despite the reduced levels of parasite transmission that are typical of the management used for Thoroughbred horses in training, spread of infection to other horses and properties is likely. Thus, it would be pertinent to recommend quarantine testing of ML efficacy for any yearlings newly acquired at a sale.
In conclusion, this study is the first in the world to confirm ML resistance in a population of equine cyathostomins through a series of repeated testing. We have presented data from a large study population (58 and 55 horses in each group), and followed this population over an eight-month period, conducting 19 different FECRTs for evaluation of ivermectin, moxidectin, and the triple combination of oxibendazole, pyrantel pamoate, and moxidectin. Furthermore, the study demonstrated that the isolate was imported to the US from Ireland, demonstrating how resistant parasites can quickly traverse continents and quickly be spread globally. Equine operations are strongly encouraged to heed this threat to equine health, and routinely monitor anthelmintic efficacy on a yearly basis. Only by taking such actions will it be possible to detect cases like these as early as possible and institute appropriate interventions.
Declaration of competing interest
The authors declare no conflicts of interest.
Acknowledgements
The staff at Godolphin, USA are warmly thanked for their cooperation while handling this case. Chris Yoakam is especially acknowledged for a tremendous effort with determining all the fecal egg counts.
References
- Becher A.M., van Doorn D.C., Pfister K., Kaplan R.M., Reist M., Nielsen M.K. Equine parasite control and the role of national legislation - a multinational questionnaire survey. Vet. Parasitol. 2018;259:6–12. doi: 10.1016/j.vetpar.2018.07.001. [DOI] [PubMed] [Google Scholar]
- Bellaw J.L., Krebs K., Reinemeyer C.R., Norris J.K., Scare J.A., Pagano S., Nielsen M.K. Anthelmintic therapy of equine cyathostomin nematodes – larvicidal efficacy, egg reappearance period, and drug resistance. Int. J. Parasitol. 2018;48:97–105. doi: 10.1016/j.ijpara.2017.08.009. [DOI] [PubMed] [Google Scholar]
- Boersema J.H., Eysker M., Maas J., van der Aar W.M. Comparison of the reappearance of strongyle eggs in foals, yearlings, and adult horses after treatment with ivermectin or pyrantel. Vet. Q. 1996;18:7–9. doi: 10.1080/01652176.1996.9694602. [DOI] [PubMed] [Google Scholar]
- Borgsteede F.H.M., Boersma J.H., Gaasenbeek C.P.H., Vanderburg W.P.J. The reappearance of eggs in feces of horses after treatment with ivermectin. Vet. Q. 1993;15:24–26. doi: 10.1080/01652176.1993.9694363. [DOI] [PubMed] [Google Scholar]
- Canever R.J., Braga P.R.C., Boeckh A., Grycajuck M., Bier D., Molento M.B. Lack of Cyathostomin sp. reduction after anthelmintic treatment in horses in Brazil. Vet. Parasitol. 2013;194:35–39. doi: 10.1016/j.vetpar.2012.12.020. [DOI] [PubMed] [Google Scholar]
- Demeulenaere D., Vercruysse J., Dorny P., Claerebout E. Comparative studies of ivermectin and moxidectin in the control of naturally acquired cyathostome infections in horses. Vet. Rec. 1997;15:383–386. doi: 10.1136/vr.141.15.383. [DOI] [PubMed] [Google Scholar]
- DiPietro J.A., Hutchens D.E., Lock T.F., Walker K., Paul A.J., Shipley C., Rulli D. Clinical trial of moxidectin oral gel in horses. Vet. Parasitol. 1997;72:167–177. doi: 10.1016/s0304-4017(97)01108-4. [DOI] [PubMed] [Google Scholar]
- Elghryani N., Duggan V., Relf V., de Waal T. Questionnaire survey on helminth control practices in horse farms in Ireland. Parasitology. 2019;146:873–882. doi: 10.1017/S0031182019000271. [DOI] [PubMed] [Google Scholar]
- ESCCAP . second ed. European Scientific Counsel Compantion Animal Parasites; 2019. A Guide to the Treatment and Control of Equine Gastrointestinal Parasite Infections.https://www.esccap.org/uploads/docs/rtjqmu6t_0796_ESCCAP_Guideline_GL8_v7_1p.pdf Online at. [Google Scholar]
- Geurden T., van Doorn D., Claerebout E., Kooyman F., De Keersmaecker S., Vercruysse J., Besognet B., Vanimisetti B., di Regalbono A.F., Beraldo P., Di Cesare A., Traversa D. Decreased strongyle egg re-appearance period after treatment with ivermectin and moxidectin in horses in Belgium, Italy and The Netherlands. Vet. Parasitol. 2014;204:291–296. doi: 10.1016/j.vetpar.2014.04.013. [DOI] [PubMed] [Google Scholar]
- Jacobs D.E., Hutchinson M.J., Parker L., Gibbons L.M. Equine cyathostome infection – suppression of faecal egg output with moxidectin. Vet. Rec. 1995;137:545. doi: 10.1136/vr.137.21.545. [DOI] [PubMed] [Google Scholar]
- Kaplan R.M. Anthelmintic resistance in nematodes of horses. Vet. Res. 2002;33:491–507. doi: 10.1051/vetres:2002035. [DOI] [PubMed] [Google Scholar]
- Kaplan R.M., Klei T.R., Lyons E.T., Lester G., Courtney C.H., French D.D., Tolliver S.C., Vidyashankar A.N., Zhao Y. Prevalence of anthelmintic resistant cyathostomes on horse farms. J. Am. Vet. Med. Assoc. 2004;225:903–910. doi: 10.2460/javma.2004.225.903. [DOI] [PubMed] [Google Scholar]
- Lyons E.T., Tolliver S.C. Further indication of lowered activity of ivermectin on immature small strongyles in the intestinal lumen of horses on a farm in Central Kentucky. Parasitol. Res. 2013;112:889–891. doi: 10.1007/s00436-012-3098-0. [DOI] [PubMed] [Google Scholar]
- Lyons E.T., Tolliver S.C., Ionita M., Lewellen A., Collins S.S. Field studies indicating reduced activity of ivermectin on small strongyles in horses on a farm in Central Kentucky. Parasitol. Res. 2008;103:209–215. doi: 10.1007/s00436-008-0959-7. [DOI] [PubMed] [Google Scholar]
- Lyons E.T., Tolliver S.C., Collins S.S. Probable reason why small strongyle EPG counts are returning "early" after ivermectin treatment of horses on a farm in Central Kentucky. Parasitol. Res. 2009;104:569–574. doi: 10.1007/s00436-008-1231-x. [DOI] [PubMed] [Google Scholar]
- Lyons E.T., Tolliver S.C., Kuzmina T.A., Collins S.S. Critical tests evaluating efficacy of moxidectin against small strongyles in horses from a herd for which reduced activity had been found in field tests in Central Kentucky. Parasitol. Res. 2010;107:1495–1498. doi: 10.1007/s00436-010-2025-5. [DOI] [PubMed] [Google Scholar]
- Lyons E.T., Tolliver S.C., Collins S.S., Ionita M., Kuzmina T.A., Rossano M. Field tests demonstrating reduced activity of ivermectin and moxidectin against small strongyles in horses on 14 farms in Central Kentucky in 2007–2009. Parasitol. Res. 2011;108:355–360. doi: 10.1007/s00436-010-2068-7. [DOI] [PubMed] [Google Scholar]
- Milillo P., Boeckh A., Cobb R., Otranto D., Lia R.P., Perrucci S., di Regalbono A.F., Beraldo P., von Samson-Himmelstjerna G., Demeler J., Bartolini R., Traversa D. Faecal cyathostomin egg count distribution and efficacy of anthelmintics against cyathostomins in Italy: a matter of geography? Parasites Vectors. 2009;2(Suppl. 2):S4. doi: 10.1186/1756-3305-2-S2-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molena R.A., Peachey L.E., Di Cesare A., Traversa D., Cantacessi C. Cyathostomine egg reappearance period following ivermectin treatment in a cohort of UK Thoroughbreds. Parasites Vectors. 2018;11:61. doi: 10.1186/s13071-018-2638-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molento M.B., Antunes J., Bentes R.N., Coles G.C. Anthelmintic resistant nematodes in Brazilian horses. Vet. Rec. 2008;162:384–385. doi: 10.1136/vr.162.12.384. [DOI] [PubMed] [Google Scholar]
- Näreaho A., Vainio K., Oksanen A. Impaired efficacy of ivermectin against Parascaris equorum, and both ivermectin and pyrantel against strongyle infections in trotter foals in Finland. Vet. Parasitol. 2011;182:372–377. doi: 10.1016/j.vetpar.2011.05.045. [DOI] [PubMed] [Google Scholar]
- Nielsen M.K., Mittel L., Grice A., Erskine M., Graves E., Vaala W., Tully R.C., French D.D., Bowman R., Kaplan R.M. American Association of Equine Practitioners; 2019. AAEP Parasite Control Guidelines.www.aaep.org Online at. on 10/14/2019. [Google Scholar]
- Peregrine A.S., Molento M.B., Kaplan R.M., Nielsen M.K. Anthelmintic resistance in important parasites of horses: does it really matter? Vet. Parasitol. 2014;201:1–8. doi: 10.1016/j.vetpar.2014.01.004. [DOI] [PubMed] [Google Scholar]
- Relf V., Lester H., Morgan E., Hodgkinson J., Matthews J.B. Anthelmintic efficacy on UK thoroughbred stud farms. Int. J. Parasitol. 2014;44:507–514. doi: 10.1016/j.ijpara.2014.03.006. [DOI] [PubMed] [Google Scholar]
- Rendle D., Austin C., Bowen M., Cameron I., Furtado T., Hodgkinson J., McGorum B., Matthews Jacqueline. Equine de-worming: a consensus on current best practice. UK-Vet Equine. 2019;3(1):1–14. [Google Scholar]
- Robert M., Hu W., Nielsen M.K., Stowe C.J. Attitudes towards implementation of surveillance-based parasite control on Kentucky Thoroughbred farms – current strategies, awareness, and willingness-to-pay. Equine Vet. J. 2015;47:694–700. doi: 10.1111/evj.12344. [DOI] [PubMed] [Google Scholar]
- Samson-Himmelstjerna G.v., Fritzen B., Demeler J., Schürmann S., Rohn K., Schnieder T., Epe C. Cases of reduced cyathostomin egg-reappearance period and failure of Parascaris equorum egg count reduction following ivermectin treatment as well as survey on pyrantel efficacy on German horse farms. Vet. Parasitol. 2007;144:74–80. doi: 10.1016/j.vetpar.2006.09.036. [DOI] [PubMed] [Google Scholar]
- Sangster N.C. Pharmacology of anthelmintic resistance in cyathostomes: will it occur with the avermectin/milbemycins? Vet. Parasitol. 1999;85:189–204. doi: 10.1016/s0304-4017(99)00099-0. [DOI] [PubMed] [Google Scholar]
- Scare J.A., Lyons E.T., Wielgus K.M., Nielsen M.K. Combination deworming for the control of double-resistant cyathostomin parasites – short and long term consequences. Vet. Parasitol. 2018;251:112–118. doi: 10.1016/j.vetpar.2018.01.010. [DOI] [PubMed] [Google Scholar]
- Smith H.J. Strongyle infections in ponies. I. Response to intermittent thiabendazole treatments. Can. J. Comp. Med. 1976;40:327–333. [PMC free article] [PubMed] [Google Scholar]
- Smith H.J. Strongyle infections in ponies. II. Reinfection of treated animals. Can. J. Comp. Med. 1976;40:334–340. [PMC free article] [PubMed] [Google Scholar]
- Stratford C.H., Lester H.E., Morgan E.R., Pickles K.J., Relf V., McGorum B.C., Matthews J.B. A questionnaire study of equine gastrointestinal parasite control in Scotland. Equine Vet. J. 2014;46:25–31. doi: 10.1111/evj.12101. [DOI] [PubMed] [Google Scholar]
- Torgerson P.R., Paul M., Furrer R. Evaluating faecal egg count reduction using a specifically designed package “eggCounts” in R and a user friendly web interface. Int. J. Parasitol. 2014;44:299–303. doi: 10.1016/j.ijpara.2014.01.005. [DOI] [PubMed] [Google Scholar]
- Traversa D., von Samson-Himmelstjerna G., Demeler J., Milillo P., Schurmann S., Barnes H., Otranto D., Perrucci S., di Regalbono A.F., Beraldo P., Boeckh A., Cobb R. Anthelmintic resistance in cyathostomin populations from horse yards in Italy, United Kingdom and Germany. Parasites Vectors. 2009;2(Suppl. 2):S2. doi: 10.1186/1756-3305-2-S2-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trawford A.F., Burden F., Hodgkinson J.E. Proc 20th Int. Conf. World Assoc. Adv. Vet. Parasitol., Christchurch, NZ, 16–20 October. 2005. Suspected moxidectin resistance in cyathostomes in two donkey herds at the Donkey Sanctuary, UK; p. 196. [Google Scholar]
- Wang C., Torgerson P.R., Kaplan R.M., George M.M., Furrer R. Modelling anthelmintic resistance by extending eggCounts package to allow individual efficacy. Int. J. Parasit. Drugs Drug Resist. 2018;8:386–393. doi: 10.1016/j.ijpddr.2018.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]