Abstract
Paromomycin is an aminoglycoside antibiotic approved in 2006 for the treatment of visceral leishmaniasis caused by Leishmania donovani in Southeast Asia. Although this drug is not approved for the treatment of visceral and cutaneous leishmaniasis in Brazil, it is urgent and necessary to evaluate the potential of this drug as alternative for the treatment against species responsible for these clinical forms of the disease. In Brazil, Leishmania amazonensis is responsible for cutaneous and diffuse cutaneous leishmaniasis. The diffuse cutaneous form of the disease is difficult to treat and frequent relapses are reported, mainly when the treatment is interrupted. Here, we evaluated paromomycin susceptibility in vitro of a L. amazonensis clinical isolate from a patient with cutaneous leishmaniasis and the reference strain L. amazonensis M2269, as well as its in vivo efficacy in a murine experimental model. Although never exposed to paromomycin, a significant differential susceptibility between these two lines was found. Paromomycin was highly active in vitro against the clinical isolate in both forms of the parasite, while its activity against the reference strain was less active. In vivo studies in mice infected with each one of these lines demonstrated that paromomycin reduces lesion size and parasite burden and a direct correlation between the susceptibility in vitro and the effectiveness of this drug in vivo was found. Our findings indicate that paromomycin efficacy in vivo is dependent on intrinsic susceptibility of the parasite. Beyond that, this study contributes for the evaluation of the potential use of paromomycin in chemotherapy of cutaneous leishmaniasis in Brazil caused by L. amazonensis.
Keywords: Paromomycin, Drug susceptibility, Leishmaniasis, Leishmania amazonensis
Graphical abstract
Abbreviations
- AST
aspartate transaminase
- ALT
alanine transaminase
- BMDM
bone-marrow derived macrophages; bp, base pair
- CL
cutaneous leishmaniasis
- DCL
diffuse cutaneous leishmaniasis
- PBS
phosphate-buffered saline;
- PCR
polymerase chain reaction
- PM
paromomycin
- VL
visceral leishmaniasis
1. Introduction
Leishmaniasis is a neglected disease endemic in more than 98 countries located in tropical and subtropical areas. This parasitic disease is caused by flagellate protozoa belonging to the genus Leishmania that are transmitted through the bite of hematophagous insects known as phlebotomines (Burza et al., 2018). The clinical manifestations of leishmaniasis in humans are related to the species of the infecting parasite and the susceptibility of the host ranging from a visceral form to a tegumentary disease (Murray et al., 2005). The latter form may be classified in different forms of disease: localized, disseminated, diffuse and mucocutaneous leishmaniasis (Reithinger et al., 2007). The estimated global prevalence of the disease is 1.3 million cases per year. In Brazil, about 25,000 new cases of tegumentary leishmaniasis have been reported annually (Alvar et al., 2012). Different clinical forms of the tegumentary disease in Brazil are caused mainly by Leishmania braziliensis, the most prevalent species, followed by L. amazonensis and L. guyanensis (Alvar et al., 2012; Reithinger et al., 2007). Beyond cutaneous leishmaniasis (CL), L. amazonensis may also cause diffuse cutaneous leishmaniasis (DCL), a severe form of the disease associated with a strong inhibition of the T-cell immune response (Silveira, 2019). This form of the disease is mainly associated with this species in Brazil. In other regions beyond Brazil, DCL may also be caused by L. mexicana and L. aethiopica (Burza et al., 2018). While DCL is characterized by multiple non-ulcerative nodules and papules that disseminate in the body of the patient; in the CL, an erythema develops at the site of bite of sandfly, followed by a nodule that ulcerates after a variable period of some weeks up to 6 months (Burza et al., 2018; Reithinger et al., 2007).
Chemotherapy of leishmaniasis in Brazil is limited to pentavalent antimonials and amphotericin B (available in deoxycholate and liposomal formulations), drugs that have several limitations related to toxicity, efficacy and parenteral administration (Uliana et al., 2018). In addition, a failure rate of approximately 50% has been described in patients with CL treated with pentavalent antimonials due to different species of the parasite (Chrusciak-Talhari et al., 2011; Machado et al., 2010), indicating the urgency for alternative drugs for treatment in Brazil. Paromomycin (PM) is a broad-spectrum aminoglycoside antibiotic that has been shown to be an effective oral agent for a large number of infectious agents, from bacteria to intestinal protozoa (Davidson et al., 2009). Clinical studies have demonstrated high efficacy of PM in the treatment of visceral leishmaniasis (VL) in India and Bangladesh, with cure rates higher than 90% when administered intramuscularly (Jamil et al., 2015; Sundar et al., 2007). PM has a short half-life (around 2–3 h in patients) and have been also proposed in combination therapies against VL (van Griensven et al., 2010). Clinical studies in Southeast Asia using PM in combination with miltefosine or amphotericin B demonstrated that treatments were effective and safe, with shorter duration of the treatment and lower dose of drugs administered compared to the monotherapy using amphotericin B (Rahman et al., 2017; Sundar et al., 2011). Cases of clinical resistance have not been described so far, which demonstrates its potential for use in treating of leishmaniasis (Croft et al., 2006). There are no reports of clinical studies using PM in the treatment of patients with CL, caused by species that are endemic in Brazil. Topical treatment using PM plus methylbenzethonium chloride had a final clinical response of 85.7% in patients from Guatemala with CL (Arana et al., 2001). Recently, a topical formulation of PM in combination with gentamicin was clinically tested against L. panamensis, with a cure rate of approximately 80% (Sosa et al., 2019).
Although the susceptibility of a L. amazonensis reference strain has already been determined (de Morais-Teixeira et al., 2014), reports about the susceptibility of Brazilian isolates of this species are limited. Here, we evaluate the activity of PM in vitro against a reference strain and a clinical isolate from a patient with CL caused by L. amazonensis. A significant variation in drug susceptibility between these lines was found, with a direct correlation between PM susceptibility in vitro and clinical efficacy using a BALB/c mouse model.
2. Materials and methods
2.1. Ethics statement
Animal experiments were approved by the Ethics Committee for Animal Experimentation of UNICAMP (Protocol: 4797-1/2018), according to the guidelines of the Sociedade Brasileira de Ciência de Animais de Laboratório (SBCAL) and of the Conselho Nacional de Controle da Experimentação Animal (CONCEA). The procedures involving the patient were approved by Human Research Ethics Committee of Instituto de Infectologia Emílio Ribas and it was registered at Plataforma Brasil (http://plataformabrasil.saude.gov.br) under the number of CAAE: 07801112.1.0000.0061. The patient signed a term informed consent, previously to the procedures.
2.2. Drug
For in vitro drug assays, stocks of PM sulfate (100 mM [aq]) (Sigma-Aldrich) were prepared and kept at −20 °C until use. For in vivo experiments, PM was prepared daily in PBS, in concentrations of 75, 150, 300 and 600 mg/kg PM, considering an average weight per mouse of 20 g.
2.3. Parasites and animals
Promastigotes of the reference strain L. amazonensis (MHOM/BR/1973/M2269) and ER256 clinical isolate (MHOM/BR/2012/ER256) were grown at 25 °C in M199 medium (Sigma-Aldrich) supplemented with HEPES 40 mM [pH 7,4], adenine 0,1 mM, hemin 0,25%, 10% heat-inactivated fetal bovine serum (Thermo Scientific), 50 U/mL penicillin and 50 μg/mL streptomycin (Kapler et al., 1990). The ER256 clinical isolate (MHOM/BR/2012/2506) was obtained from a woman patient with CL from Instituto de Infectologia Emílio Ribas, São Paulo. Parasites were obtained from an aspiration of skin lesions performed as part of the follow up procedure. Material of the skin lesion was subjected to initial cultivation in the diphasic agar medium NNN (Novy, Mac Neal, Nicolle), followed by cultivation in M199 medium, both incubated at 25 °C. Once isolated in culture, the isolate was typed as L. amazonensis through PCR sequencing of the internal transcribed ribosomal DNA (Cupolillo et al., 1995). The GenBank accession number is MT523027.
Female BALB/c mice (aged 4–6 weeks) were obtained from CEMIB (Centro Multidisciplinar para Investigação Biológica) of UNICAMP. Animals were kept in mini-isolators and received food and water ad libitum.
2.4. Drug susceptibility in promastigotes and intracellular amastigotes of L. amazonensis and cytotoxicity assays
The susceptibility of L. amazonensis promastigotes to PM was determined using the MTT colorimetric assay, after incubation of 2 × 106 parasites per well in a 96-well plate in M199 medium for 24 h at 25 °C in the presence of increasing concentrations of PM as previously described (Espada et al., 2017). Promastigotes were counted in a Neubauer haemocytometer and experiments were carried out in triplicate.
For intracellular amastigotes, we first determined the cytotoxicity of PM against bone-marrow derived macrophages (BMDM) from BALB/c mice as described (Zamboni and Rabinovitch, 2003). BMDM were plated in 24-well culture dishes in 300 μL of RPMI 1640 (Thermo Scientific) supplemented with 10% heat-inactivated fetal bovine serum (around 3 × 105 macrophages per well) in a 5% CO2 atmosphere for 24 h at 37 °C. Later, BMDM were incubated in complete RPMI 1640 medium in increasing concentrations of PM (25-3,000 μM) for 48 h or 72 h. After this period, RPMI medium containing PM was removed and macrophages were washed with warmed PBS, followed by addition of 100 μL of trypsin solution (2,5 μg/μL) (Vitrocell, Brazil) for 10–15 min for detaching macrophages. Then, approximately 400 μL of PBS and 10 μL of 0.4% Trypan Blue (Sigma Aldrich) were added for each well and viable and non-viable macrophages were counted in a Neubauer haemocytometer in three independent experiments performed in duplicate.
To determine PM susceptibility of intracellular amastigotes, BMDM were plated at a density of 3 × 105 macrophages per well in complete RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum on round glass coverslips 24-well culture dishes in a 5% CO2 atmosphere for 24 h at 37 °C allowing macrophages to adhere. Stationary-phase promastigotes of L. amazonensis were used to infect macrophages in a ratio of 5:1 (parasites per macrophages) in a 5% CO2 atmosphere at 34 °C. After 4 h, non-internalized parasites were removed by washing with warmed PBS and infected macrophages were cultivated in fresh RPMI 1640 medium containing increasing PM concentrations (0.1–200 μM) for 72 h. Infected macrophages were then fixed in methanol (Sigma-Aldrich) and stained with a panoptic haematological method (Laborclin, Brazil). The percentage of infected macrophages and the number of intracellular amastigotes were determined by counting 100 macrophages in three independent experiments in duplicate.
2.5. Mice infections and treatment with PM
Groups of five female BALB/c mice were infected in their footpads using 106 stationary-phase promastigotes of both lines of L. amazonensis. Parasites were injected subcutaneously in the right hind footpad in a final volume of 30 μL. After five weeks of infection, PM was administered intraperitoneally in doses of 75, 150, 300, and 600 mg/kg per day for 14 consecutive days. As control, an untreated group for each line was used. Effectiveness of the treatment was evaluated by weekly measurements of lesion size and by quantification of parasite loads in the infected footpad at the end of treatment by quantitative real-time PCR (see below). A caliper was used for measuring the difference in the thickness between the infected and contralateral uninfected footpad. In addition, infected footpad of animals infected with both lines of the parasite were submitted to histopathological examination of infected tissues at the end of the treatment. Body weight of the animals was also recorded weekly and levels of aspartate transaminase (AST), alanine transaminase (ALT) and creatinine in the blood of infected and treated animals were evaluated at the end of the treatment.
2.6. Evaluation of parasite burden in treated mice by quantitative real-time PCR
At the end of the treatment, each group of treated and untreated animals were euthanized and about 25–50 mg of tissue from the infected footpad was obtained. Genomic DNA was extracted using the PureLink Genomic DNA kit (Thermo Fisher Scientific) according to the protocol provided by the manufacturer. Total genomic DNA was quantified in a NanoDrop 2000 spectrophotometer (Thermo Fischer Scientific) and then used for evaluation of parasite burden by quantitative real-time PCR. The construction of standard curves was performed with genomic DNA of L. amazonensis previously isolated from promastigotes and serial dilutions of genomic DNA ranging from 2 × 107 to 2 parasites were used, considering that each diploid genome of L. amazonensis has approximately 0.1 pg (Nicolas et al., 2002). Ultrapure water and uninfected mouse DNA were used as negative control. For each reaction, 4 μL DNA extract samples (diluted 1/100) was amplified in 10 μL of SYBR Green, 1.2 mM MgCl2 and 500 nM of each primer in a total volume of 20 μL. For the quantitative PCR reaction, an initial cycle of 3 min at 95 °C for denaturation were used, followed by 40 cycles of amplification. The steps of each cycle were: 15 s at 95 °C and 30 s at 60 °C. The pair of primers used were G6PDH-F (5′-CGYCTYCCAGACGCYTACGA-3′) and G6PDH-R (5′-AGCGGYGTGAAGATGCGCCA-3′) which amplifies a 110 bp fragment of the glucose-6-phosphate dehydrogenase gene (g6pdh), a single copy gene in L. amazonensis (Castilho et al., 2008). Triplicates of each dilution corresponding to genomic DNA of 2 × 107 to 2 parasites and duplicates of each sample were included. The equipment used was StepOnePlus Real-Time PCR System (Thermo Fisher Scientific).
2.7. Statistical analysis
The determination of CC50, EC50 and ED50 values were determined by sigmoidal regression curves. Data on lesion size, parasite burden by quantitative real-time PCR, body weight and biochemical parameters were analyzed for statistical significance by One Way ANOVA, followed by Tukey's post-test. The value of p < 0.05 was considered statistically significant. All analyzes were performed using GraphPad Prism 7 software.
3. Results
3.1. Susceptibility of promastigotes and intracellular amastigotes of Leishmania amazonensis to PM
A previous screening of PM susceptibility in vitro against a panel of clinical isolates from Instituto de Infectologia Emílio Ribas, a reference center for treatment of leishmaniasis in the city Sao Paulo, allowed us identify a clinical isolate, ER256, highly susceptible to PM. This isolate was typed as L. amazonensis by sequencing the internal transcribed ribosomal DNA (ITS), as previously described (Gosch et al., 2018). Sequence analysis of full sequence of ITS indicated 99% identity with the reference strain L. amazonensis (MHOM/BR/73/M2269) (GenBank accession number AJ000316.1), a strain isolated from a patient of the Amazon region (Miles et al., 1980).
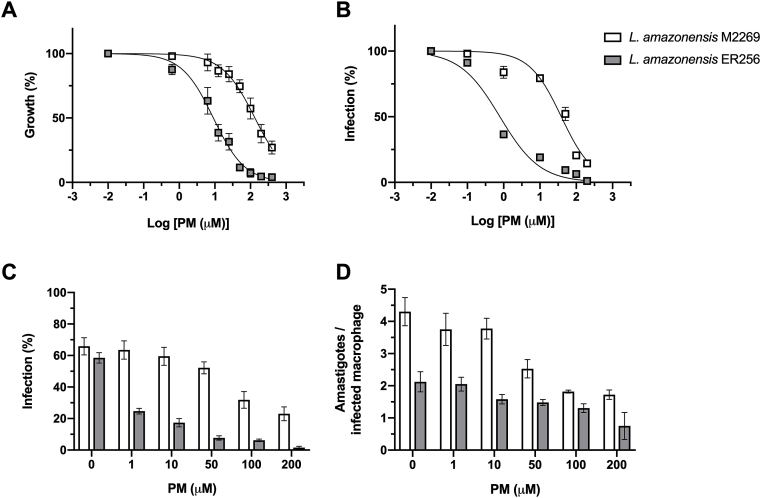
The EC50 of PM against the ER256 clinical isolate was around 14.5-fold lower than the reference strain of L. amazonensis M2269 in promastigote form of the parasite (Table 1 and Fig. 1). We extend the analysis of PM activity against intracellular amastigotes, the form responsible for the disease in humans. Initially, we determined the toxicity of PM against macrophages after 48 and 72 h in presence of the drug. The cytotoxicity assay showed that PM has low toxicity to macrophages with a CC50 value of 962.4 ± 65.1 μM after 48 h with an increase of cytotoxicity after 72 h of incubation (CC50 = 536.6 ± 27.1 μM) (Supplementary Fig. 1). The activity of PM against intracellular amastigotes in infected macrophages was significantly different between the clinical isolate and the reference strain M2269. While the EC50 value for the clinical isolate was 0.54 μM, the EC50 for M2269 strain was 61 μM, indicating that this isolate was more than 100-fold more susceptible than the reference strain, and reflecting a significant difference in the selectivity index (SI) for these two lines of parasites (Table 1 and Fig. 1B). The SI value for PM in each line of parasite was calculated by the ratio between the macrophage cytotoxicity corresponding to 72 h (CC50 = 536.6 ± 27.1 μM) and the activity against intracellular amastigotes (EC50) of each strain (Table 1). Although a lower number of amastigotes per infected macrophage was found for ER256 isolate, a similar percentage of infected macrophages in absence of PM was observed for the clinical isolate and the reference strain, indicating that the high susceptibility to PM of the clinical isolate is not due to its infectivity in vitro (Table 1 and Fig. 1C–D). Finally, a concentration dependent effect was observed in infected BMDM treated with PM for both lines (Fig. 1B–D).
Table 1.
Activity of PM against promastigotes and intracellular amastigotes of L. amazonensis reference strain M2269 and ER256 clinical isolate.
| Promastigotea |
Amastigoteb |
SI | Infection (%)d | |||
|---|---|---|---|---|---|---|
| EC50c | EC90 | EC50 | EC90 | |||
| L. amazonensis M2269 | 145.23 ± 23.04 | 730.4 | 61 ± 9.48 | 111.9 | 8.6 | 66% |
| L. amazonensis ER256 | 9.98 ± 2.97 | 101.8 | 0.54 ± 0.11 | 4.86 | 993.7 | 59% |
SI: Selectivity Index, which corresponds to the ratio between the CC50 and EC50 values of intracellular amastigotes. The CC50 value for BMDM was 536.6 ± 27.1 μM.
Three independent experiments carried out in triplicate per strain/isolate.
Three independent experiments carried out in duplicate per strain/isolate.
EC50 ± standard deviation in μM.
Percentage of infected BMDM.
Fig. 1.
Activity in vitro of PM against promastigotes (A) and intracellular amastigotes (B-D) of L. amazonensis M2269 strain and ER256 isolate. (A) Promastigotes were growth in increasing concentrations of PM and viability of the parasites was determined by the MTT assay after 24 h. (B) BMDM were infected with stationary-phase promastigotes and exposed to increasing concentrations of PM for 72 h. The percentage of infection was determined by counting 100 macrophages per coverslip. (C) PM susceptibility of intracellular amastigotes. Each bar represents the percentage of infected BMDM in different concentrations of PM (1–200 μM). The average ± standard deviation of three independent experiments is shown. (D) Number of amastigotes per infected macrophage treated with different concentrations of PM.
3.2. Effectiveness of PM against L. amazonensis lines in vivo
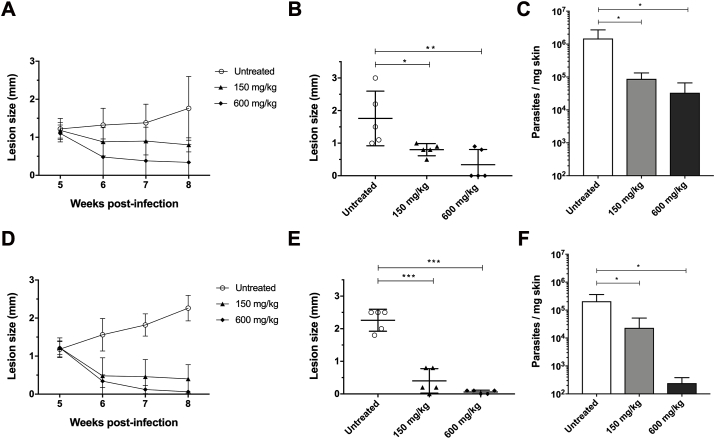
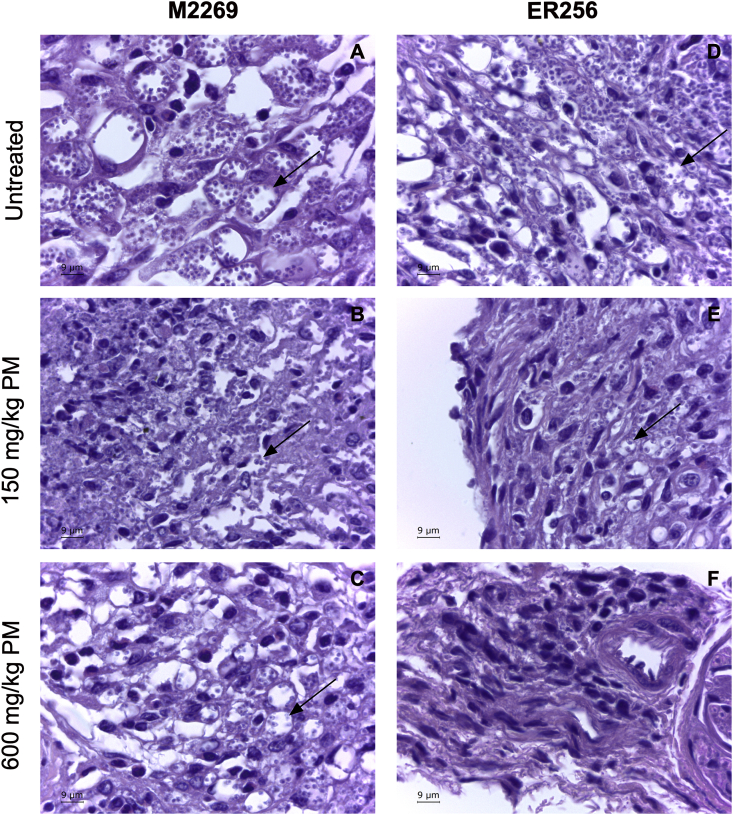
In humans, PM is administered by intramuscular route, in doses of 11–20 mg/kg/day (Zulfiqar et al., 2017). Preliminary findings using doses of up to 75 mg/kg PM by intramuscular route in mice infected with L. amazonesis strain M2269 showed a reduction in lesion size of only 40–50% (data not show). The maximum volume to be administered intramuscularly in mice is 30 μL (Flecknell, 2009) and the PM solubility is 50 mg/mL, which means that the maximum dosage that could be administered by intramuscular route in mice would be 75 mg/kg. Initially, efficacy of PM was assessed in BALB/c mice infected with L. amazonesis strain M2269 treated intraperitoneally with four doses of PM (75, 150, 300 and 600 mg/kg) over 14 days. In infected animals treated with PM, lesions were significantly smaller when compared to the untreated animals (Supplementary Fig. 2). In the group of animals treated with 600 mg/kg PM, lesions were significantly reduced and two animals fully resolved their lesion (Supplementary Fig. 2). At the end of the treatment, body weight of animals of each group were determined due the high dosages of PM administered in the treated animals, particularly dosages of 300 and 600 mg/kg. There was no significant difference in the average body weight of the groups of untreated and treated animals with PM, indicating that there was no toxicity in the dosages administered (Supplementary Fig. 3). After determining the activity of PM against L. amazonensis in vivo, we evaluated if the high susceptibility to PM in intracellular amastigotes of clinical isolate ER256 would affect the treatment outcome and parasite burden when compared to the reference strain M2269. BALB/c mice were infected with each one of the lines and treated with two different dosages of PM (150 and 600 mg/kg PM) for 14 days. Untreated animals of both lines had similar disease and no significant difference in the progression of disease and in the quantification of parasite burden was found (Fig. 2). When compared to untreated animals, lesions size in animals infected with both lines and treated with doses of 150 and 600 mg/kg were significantly smaller (Fig. 2). Infections with M2269 strain partially resolved the lesions (Fig. 2A–B), while infections with ER256 isolate responded better in the highest dose of PM, with animals clinically cured at the end of the treatment (Fig. 2D–E). Reduction of the parasite burden was dose dependent in animals infected with both lines (Fig. 2C and 2F). The number of ER256 parasites was lower when compared to the untreated animal (more than 100-fold), while animals infected with M2269 strain, an approximately 10-fold reduction was found in animals treated with 600 mg/kg PM (Fig. 2C and 2F). The effective doses that eliminated 50% of the parasites in the lesions (ED50), according to the values obtained by quantitative real-time PCR, were calculated and corresponded to 200 mg/kg for the M2269 strain and 60 mg/kg for ER256 clinical isolate. Histopathological analysis of tissues of animals treated and untreated with PM indicated a direct correlation between parasite burden and PM dosage, corroborating the data of lesion size and parasite burden (Figs. 2 and 3). Although parasites persist in animals treated with 600 mg/kg PM in both lines of the parasites, a significant reduction in the number of intracellular amastigotes was found in infections with ER256 when compared to infections with M2269, where parasites were easily identified (Fig. 3C and 3F).
Fig. 2.
Evaluation of PM efficacy in mice infected with L. amazonensis M2269 and ER256 isolate. Evolution of lesion size in infected animals with L. amazonensis M2269 or ER256 over the weeks (A and D respectively). Lesion size represents the average difference between infected and contralateral non-infected hind footpads (five mice per group). Animals were treated with 150 and 600 mg/kg/day of PM intraperitoneally after five weeks post-infection for 14 days. Lesion size at the end of the treatment (8th week post-infection) of five animals infected per group with each line of parasite is indicated (M2269 and ER256 lines are indicated by B and E respectively). Parasite burden was determined by quantitative real-time PCR of animals infected with L. amazonensis M2269 strain (C) and L. amazonensis ER256 isolate (F) treated or not with PM. At the end of the treatment (8th week post-infection), animals were euthanized and DNA of the lesion of the infected hind footpad was isolated. Statistical analysis was performed with One Way ANOVA, followed by the Tukey post-test, *p < 0.05; **p < 0.01; ***p < 0.001. Untreated, group of infected animals not treated with PM.
Fig. 3.
Histological analysis of the infected mice with L. amazonensis M2269 or ER256 isolate. Animals were euthanized at the end of the treatment with PM (8th week post-infection) and then infected hind footpad fragments were isolated, washed with PBS, fixed with formalin and processed with paraffin. Sections were stained with haematoxylin-eosin and then visualized in a light microscope. Images of untreated and treated animals with 150 and 600 mg/kg PM infected with L. amazonensis M2269 strain (A, B and C respectively) or L. amazonensis ER256 clinical isolate (D, E and F respectively). Arrows in the Figure indicate amastigotes inside parasitophorous vacuoles. Bar: 9 μm.
Finally, at the end of the treatment with PM, no significant change in body weight was found among animals and biochemical analysis of ALT, AST and creatinine showed no statistical difference among the groups animals (uninfected, infected and infected and treated with 150 and 600 mg/kg PM) (Supplementary Fig. 4), indicating no adverse effect due the high dosage of PM used, particularly 600 mg/kg.
4. Discussion
This study investigated the activity of PM against the reference strain of L. amazonensis M2269 and a clinical isolate isolated from a patient with CL. PM has been used in Southeast Asia in the treatment of VL caused by L. donovani (Jamil et al., 2015; Sundar et al., 2007; Sundar and Rai, 2005). Although some authors have argued against the use of PM as monotherapy and the emergence of drug resistance was already reported after selection in vitro (den Boer and Davidson, 2006; Jhingran et al., 2009; Rastrojo et al., 2018), clinical studies using PM against CL are still limited. PM was already used in combination with sodium stibogluconate against DCL due to L. aethiopica. Lesions in treated patients were completely cured, with minimal side effects and no relapse was reported after 21 months after the end of the treatment (Teklemariam et al., 1994). PM was also effective against L. panamensis using a topical formulation (PM plus gentamicin) in skin lesions, with a cure rate of approximately 80% (Sosa et al., 2019).
Here, we first evaluated the in vitro susceptibility of PM in two lines of L. amazonensis and then its efficacy in the treatment of CL was analyzed through in vivo assays using infected BALB/c mice. In Brazil, only pentavalent antimonials and amphotericin B are available for treatment of leishmaniaisis, and alternatives for treatment are urgent needed. The clinical isolate ER256 was highly susceptible in vitro when compared to the reference strain of the same species. The EC50 values for the reference strain were 14.5 and 113-fold higher in promastigotes and intracellular amastigotes when compared with the clinical isolate. Previous reports have already shown variable PM susceptibility in species and isolates of the parasites of the genus Leishmania (de Morais-Teixeira et al., 2014; Utaile et al., 2013). These observations confirm our findings with these two lines of the parasite that were never exposed to PM and therefore confirm that this variation in susceptibility is intrinsic to these lines. To the best of our knowledge, this is the first report that describes a significant variation in PM susceptibility between two or more isolates from the same species of the parasite (more than 100-fold in intracellular amastigotes). PM resistant mutants selected in vitro by drug pressure or even chemical mutagenesis, for example, reach levels of resistance not higher than 5 to 10-fold (Bhattacharya et al., 2019; Jhingran et al., 2009; Rastrojo et al., 2018). Even an in vitro selection protocol using intracellular amastigotes was not able to select PM resistant parasites lines higher than 4-fold, when compared to the EC50 of untreated parasites (Hendrickx et al., 2012, 2014).
Paromomycin resistance studies in Leishmania have demonstrated that translation machinery is the main target of the PM, as revealed by proteomics of susceptible and resistant lines of L. donovani and by structural analysis of the Leishmania ribosome in complex with the drug through of an atomic resolution electron cryo-microscopy (Chawla et al., 2011; Jhingran et al., 2009; Shalev-Benami et al., 2017). Interestingly, whole genome sequencing of L. infantum resistant lines identified the presence of the mutations in genes that code proteins involved in translation, corroborating these findings (Bhattacharya et al., 2019). Among these proteins, the most relevant was CDPK1, a protein kinase involved in the control of translation (Bhattacharya et al., 2019). A reduction in the uptake of the drug and a less pronounced reduction in the membrane potential were also observed in PM resistant lines (Jhingran et al., 2009). It would be interesting to investigate whether the molecular basis of differential susceptibility found in M2269 and ER256 lines are related to those described in selected resistant lines or whether other mechanisms/targets are involved.
Regarding in vitro susceptibility, both lines of L. amazonensis were more susceptible to PM in intracellular amastigotes than promastigotes. These findings corroborate previous reports that show higher activity of PM against the form of the parasite responsible for human disease (Rastrojo et al., 2018; Utaile et al., 2013). Similarly, miltefosine and amphotericin B are also more active against intracellular amastigotes when compared to promastigotes of L. amazonensis (Coelho et al., 2014; Reimao et al., 2013).
Considering the differential PM activity in vitro in these two lines, the effectiveness of this drug in vivo was evaluated in a murine experimental model, in order to investigate whether the PM susceptibility in vitro would affect the clinical outcome. After a previous assay using four different dosages of PM by intraperitoneal route, animals infected with each line were treated with two dosages of PM (150 and 600 mg/kg). At the end of the treatment, a significant reduction in the lesion size and parasite burden in mice infected with the clinical isolate was found, but not with the reference strain M2269. In this case, a significant decrease in lesion size was found in animals infected with strain, but the parasite burden was not significantly reduced and parasites persist at the highest dosage used. On the other hand, PM activity in vivo against the isolate ER256 demonstrated a direct correlation with the lesion size and correlated well with the parasite burden. In this case, although genomic DNA of the parasite was still detected at the highest dosage used, parasites in the infected footpad were scarce in histological analyses. Our findings demonstrate a direct correlation of activity of PM in vitro and the clinical outcome in mice infected with these lines of L. amazonensis and that the treatment outcome with PM is dependent on intrinsic susceptibility of the parasites. Previous studies did not report similar correlation in isolates with differential susceptibility in vitro to antimonials or miltefosine for example (Coelho et al., 2014; Rijal et al., 2007; Yardley et al., 2006), although the levels of differential susceptibility in these studies were not so high, as described here.
Recently, PM was investigated in vivo, using BALB/c mice as a model, against two species responsible for tegumentary disease, L. major and L. mexicana. PM showed antiparasitic activity against L. major when administered at dosage of 50 mg/kg, with significant reduction in the lesion size and parasite burden (Wijnant et al., 2017, 2018). On the other hand, the same dosage was used to treat animals infected with L. mexicana and no clinical and parasitological cure was found (Wijnant et al., 2017). One possible explanation for low efficacy against L. mexicana could be the low dosage used (50 mg/kg for 10 days); here the ED50 values for M2269 strain and ER256 clinical isolate were 200 mg/kg and 60 mg/kg respectively and therefore higher than used against L. mexicana. Another possibility, according to our findings, would be the low intrinsic susceptibility of this strain of L. mexicana, in this case (>360 μM) (Wijnant et al., 2017). The EC50 values of the reference strain M2269 and the clinical isolate ER256 for intracellular amastigotes were 61 μM and 0.54 μM respectively. Finally, it is important to state that despite the high dosages of PM used in this study, they did not affect the body weight of treated animals or cause adverse effects (measured by the blood levels of the liver enzymes, ALT and AST, and creatinine). Increased levels of liver enzymes ALT and AST may be caused by liver damage (McGill, 2016), while increased levels of creatinine indicate renal failure (Srisawat and Kellum, 2011).
In a BALB/c model infected with L. major, L. mexicana or L. amazonensis, a topical formulation of PM containing gentamicin was tested. In these models, this formulation was effective against all species, with lesions completely healed and no relapse was reported after the end of the treatment (Grogl et al., 1999). Here, the intraperitoneal route was used to treat animals infected with L. amazonensis, with a significant reduction in the size of the lesion. In this case, the animals were not followed up after the end of the treatment and a possible relapse would be expected even at the highest dosage used, since parasites were detected at the end of the treatment.
Taken together, our findings indicate that PM effectiveness in vivo is dependent on intrinsic activity against L. amazonensis and that susceptibility in vitro may be useful to evaluate the potential of PM against the parasite in vivo. In a scenario where PM is highly active against the parasite in vitro, this drug may be considered as potential partner in drug combination studies against CL.
Funding
This work was supported by the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) (grant number 2016/21171-6). ACC has also, in part, received funding from UK Research and Innovation via the Global Challenges Research Fund under grant agreement ‘A Global Network for Neglected Tropical Diseases’ (grant number MR/P027989/1). EMC and BAF were fellows supported by FAPESP (2018/03299-0 and 2017/18488-4 respectively). BAF is currently supported by a FAPESP fellowship (2020/01948-1).
Declaration of competing interest
The authors of this study declare no conflict of interest.
Acknowledgments
We thank Dr. Silvia R. B. Uliana from Universidade de São Paulo for providing the M2269 strain. We are also grateful to Mussya M. C. Rocha and Camilo C. Janeri for technical assistance.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijpddr.2020.08.001.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Alvar J., Velez I.D., Bern C., Herrero M., Desjeux P., Cano J., Jannin J., den Boer M. Leishmaniasis worldwide and global estimates of its incidence. PloS One. 2012;7 doi: 10.1371/journal.pone.0035671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arana B.A., Mendoza C.E., Rizzo N.R., Kroeger A. Randomized, controlled, double-blind trial of topical treatment of cutaneous leishmaniasis with paromomycin plus methylbenzethonium chloride ointment in Guatemala. Am. J. Trop. Med. Hyg. 2001;65:466–470. doi: 10.4269/ajtmh.2001.65.466. [DOI] [PubMed] [Google Scholar]
- Bhattacharya A., Leprohon P., Bigot S., Padmanabhan P.K., Mukherjee A., Roy G., Gingras H., Mestdagh A., Papadopoulou B., Ouellette M. Coupling chemical mutagenesis to next generation sequencing for the identification of drug resistance mutations in Leishmania. Nat. Commun. 2019;10:5627. doi: 10.1038/s41467-019-13344-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burza S., Croft S.L., Boelaert M. Leishmaniasis. Lancet. 2018;392:951–970. doi: 10.1016/S0140-6736(18)31204-2. [DOI] [PubMed] [Google Scholar]
- Castilho T.M., Camargo L.M., McMahon-Pratt D., Shaw J.J., Floeter-Winter L.M. A real-time polymerase chain reaction assay for the identification and quantification of American Leishmania species on the basis of glucose-6-phosphate dehydrogenase. Am. J. Trop. Med. Hyg. 2008;78:122–132. [PubMed] [Google Scholar]
- Chawla B., Jhingran A., Panigrahi A., Stuart K.D., Madhubala R. Paromomycin affects translation and vesicle-mediated trafficking as revealed by proteomics of paromomycin -susceptible -resistant Leishmania donovani. PloS One. 2011;6 doi: 10.1371/journal.pone.0026660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrusciak-Talhari A., Dietze R., Chrusciak Talhari C., da Silva R.M., Gadelha Yamashita E.P., de Oliveira Penna G., Lima Machado P.R., Talhari S. Randomized controlled clinical trial to access efficacy and safety of miltefosine in the treatment of cutaneous leishmaniasis Caused by Leishmania (Viannia) guyanensis in Manaus, Brazil. Am. J. Trop. Med. Hyg. 2011;84:255–260. doi: 10.4269/ajtmh.2011.10-0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho A.C., Trinconi C.T., Costa C.H., Uliana S.R. In vitro and in vivo miltefosine susceptibility of a Leishmania amazonensis isolate from a patient with diffuse cutaneous leishmaniasis. PLoS Neglected Trop. Dis. 2014;8:e2999. doi: 10.1371/journal.pntd.0002999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croft S.L., Sundar S., Fairlamb A.H. Drug resistance in leishmaniasis. Clin. Microbiol. Rev. 2006;19:111–126. doi: 10.1128/CMR.19.1.111-126.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cupolillo E., Grimaldi G., Jr., Momen H. Discrimination of Leishmania isolates using a limited set of enzymatic loci. Ann. Trop. Med. Parasitol. 1995;89:17–23. doi: 10.1080/00034983.1995.11812924. [DOI] [PubMed] [Google Scholar]
- Davidson R.N., den Boer M., Ritmeijer K. Paromomycin. Trans. R. Soc. Trop. Med. Hyg. 2009;103:653–660. doi: 10.1016/j.trstmh.2008.09.008. [DOI] [PubMed] [Google Scholar]
- de Morais-Teixeira E., Gallupo M.K., Rodrigues L.F., Romanha A.J., Rabello A. In vitro interaction between paromomycin sulphate and four drugs with leishmanicidal activity against three New World Leishmania species. J. Antimicrob. Chemother. 2014;69:150–154. doi: 10.1093/jac/dkt318. [DOI] [PubMed] [Google Scholar]
- den Boer M., Davidson R.N. Treatment options for visceral leishmaniasis. Expert Rev. Anti-infect. Ther. 2006;4:187–197. doi: 10.1586/14787210.4.2.187. [DOI] [PubMed] [Google Scholar]
- Espada C.R., Ribeiro-Dias F., Dorta M.L., Pereira L.I.A., Carvalho E.M., Machado P.R., Schriefer A., Yokoyama-Yasunaka J.K.U., Coelho A.C., Uliana S.R.B. Susceptibility to miltefosine in Brazilian clinical isolates of Leishmania (Viannia) braziliensis. Am. J. Trop. Med. Hyg. 2017;96:656–659. doi: 10.4269/ajtmh.16-0811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flecknell P. Academic Press; 2009. Laboratory Animal Anaesthesia. [Google Scholar]
- Gosch C.S., Resende B.S., Amorim C.B., Marques C.P., Pereira L.I.A., Pinto S.A., Uliana S.R.B., Coelho A.C., Ribeiro-Dias F., Dorta M.L. Case report: atypical cutaneous leishmaniasis in a patient with mixed Leishmania guyanensis and Leishmania amazonensis infection. Am. J. Trop. Med. Hyg. 2018;99:1165–1169. doi: 10.4269/ajtmh.17-0760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grogl M., Schuster B.G., Ellis W.Y., Berman J.D. Successful topical treatment of murine cutaneous leishmaniasis with a combination of paromomycin (Aminosidine) and gentamicin. J. Parasitol. 1999;85:354–359. [PubMed] [Google Scholar]
- Hendrickx S., Boulet G., Mondelaers A., Dujardin J.C., Rijal S., Lachaud L., Cos P., Delputte P., Maes L. Experimental selection of paromomycin and miltefosine resistance in intracellular amastigotes of Leishmania donovani and L. infantum. Parasitol. Res. 2014;113:1875–1881. doi: 10.1007/s00436-014-3835-7. [DOI] [PubMed] [Google Scholar]
- Hendrickx S., Inocencio da Luz R.A., Bhandari V., Kuypers K., Shaw C.D., Lonchamp J., Salotra P., Carter K., Sundar S., Rijal S., Dujardin J.C., Cos P., Maes L. Experimental induction of paromomycin resistance in antimony-resistant strains of L. donovani: outcome dependent on in vitro selection protocol. PLoS Neglected Trop. Dis. 2012;6:e1664. doi: 10.1371/journal.pntd.0001664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamil K.M., Haque R., Rahman R., Faiz M.A., Bhuiyan A.T., Kumar A., Hassan S.M., Kelly H., Dhalaria P., Kochhar S., Desjeux P., Bhuiyan M.A., Khan M.M., Ghosh R.S. Effectiveness study of paromomycin IM injection (PMIM) for the treatment of visceral leishmaniasis (VL) in Bangladesh. PLoS Neglected Trop. Dis. 2015;9 doi: 10.1371/journal.pntd.0004118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhingran A., Chawla B., Saxena S., Barrett M.P., Madhubala R. Paromomycin: uptake and resistance in Leishmania donovani. Mol. Biochem. Parasitol. 2009;164:111–117. doi: 10.1016/j.molbiopara.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapler G.M., Coburn C.M., Beverley S.M. Stable transfection of the human parasite Leishmania major delineates a 30-kilobase region sufficient for extrachromosomal replication and expression. Mol. Cell Biol. 1990;10:1084–1094. doi: 10.1128/mcb.10.3.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado P.R., Ampuero J., Guimaraes L.H., Villasboas L., Rocha A.T., Schriefer A., Sousa R.S., Talhari A., Penna G., Carvalho E.M. Miltefosine in the treatment of cutaneous leishmaniasis caused by Leishmania braziliensis in Brazil: a randomized and controlled trial. PLoS Neglected Trop. Dis. 2010;4:e912. doi: 10.1371/journal.pntd.0000912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill M.R. The past and present of serum aminotransferases and the future of liver injury biomarkers. Excli J. 2016;15:817–828. doi: 10.17179/excli2016-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles M.A., Povoa M.M., de Souza A.A., Lainson R., Shaw J.J. Some methods for the enzymic characterization of Latin-American Leishmania with particular reference to Leishmania mexicana amazonensis and subspecies of Leishmania hertigi. Trans. R. Soc. Trop. Med. Hyg. 1980;74:243–252. doi: 10.1016/0035-9203(80)90253-9. [DOI] [PubMed] [Google Scholar]
- Murray H.W., Berman J.D., Davies C.R., Saravia N.G. Advances in leishmaniasis. Lancet. 2005;366:1561–1577. doi: 10.1016/S0140-6736(05)67629-5. [DOI] [PubMed] [Google Scholar]
- Nicolas L., Prina E., Lang T., Milon G. Real-time PCR for detection and quantitation of Leishmania in mouse tissues. J. Clin. Microbiol. 2002;40:1666–1669. doi: 10.1128/JCM.40.5.1666-1669.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman R., Goyal V., Haque R., Jamil K., Faiz A., Samad R., Ellis S., Balasegaram M., Boer M.D., Rijal S., Strub-Wourgaft N., Alves F., Alvar J., Sharma B. Safety and efficacy of short course combination regimens with AmBisome, miltefosine and paromomycin for the treatment of visceral leishmaniasis (VL) in Bangladesh. PLoS Neglected Trop. Dis. 2017;11 doi: 10.1371/journal.pntd.0005635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastrojo A., Garcia-Hernandez R., Vargas P., Camacho E., Corvo L., Imamura H., Dujardin J.C., Castanys S., Aguado B., Gamarro F., Requena J.M. Genomic and transcriptomic alterations in Leishmania donovani lines experimentally resistant to antileishmanial drugs. Int. J. Parasitol. Drugs Drug Resist. 2018;8:246–264. doi: 10.1016/j.ijpddr.2018.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimao J.Q., Trinconi C.T., Yokoyama-Yasunaka J.K., Miguel D.C., Kalil S.P., Uliana S.R. Parasite burden in Leishmania (Leishmania) amazonensis-infected mice: validation of luciferase as a quantitative tool. J. Microbiol. Methods. 2013;93:95–101. doi: 10.1016/j.mimet.2013.02.007. [DOI] [PubMed] [Google Scholar]
- Reithinger R., Dujardin J.C., Louzir H., Pirmez C., Alexander B., Brooker S. Cutaneous leishmaniasis. Lancet Infect. Dis. 2007;7:581–596. doi: 10.1016/S1473-3099(07)70209-8. [DOI] [PubMed] [Google Scholar]
- Rijal S., Yardley V., Chappuis F., Decuypere S., Khanal B., Singh R., Boelaert M., De Doncker S., Croft S., Dujardin J.C. Antimonial treatment of visceral leishmaniasis: are current in vitro susceptibility assays adequate for prognosis of in vivo therapy outcome? Microb. Infect. 2007;9:529–535. doi: 10.1016/j.micinf.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Shalev-Benami M., Zhang Y., Rozenberg H., Nobe Y., Taoka M., Matzov D., Zimmerman E., Bashan A., Isobe T., Jaffe C.L., Yonath A., Skiniotis G. Atomic resolution snapshot of Leishmania ribosome inhibition by the aminoglycoside paromomycin. Nat. Commun. 2017;8:1589. doi: 10.1038/s41467-017-01664-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silveira F.T. What makes mucosal and anergic diffuse cutaneous leishmaniases so clinically and immunopathogically different? A review in Brazil. Trans. R. Soc. Trop. Med. Hyg. 2019 doi: 10.1093/trstmh/trz037. [DOI] [PubMed] [Google Scholar]
- Sosa N., Pascale J.M., Jiménez A.I., Norwood J.A., Kreishman-Detrick M., Weina P.J., Lawrence K., McCarthy W.F., Adams R.C., Scott C., Ransom J., Tang D., Grogl M. Topical paromomycin for New World cutaneous leishmaniasis. PLoS Neglected Trop. Dis. 2019;13 doi: 10.1371/journal.pntd.0007253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srisawat N., Kellum J.A. Acute kidney injury: definition, epidemiology, and outcome. Curr. Opin. Crit. Care. 2011;17:548–555. doi: 10.1097/MCC.0b013e32834cd349. [DOI] [PubMed] [Google Scholar]
- Sundar S., Jha T.K., Thakur C.P., Sinha P.K., Bhattacharya S.K. Injectable paromomycin for Visceral leishmaniasis in India. N. Engl. J. Med. 2007;356:2571–2581. doi: 10.1056/NEJMoa066536. [DOI] [PubMed] [Google Scholar]
- Sundar S., Rai M. Treatment of visceral leishmaniasis. Expet Opin. Pharmacother. 2005;6:2821–2829. doi: 10.1517/14656566.6.16.2821. [DOI] [PubMed] [Google Scholar]
- Sundar S., Sinha P.K., Rai M., Verma D.K., Nawin K., Alam S., Chakravarty J., Vaillant M., Verma N., Pandey K., Kumari P., Lal C.S., Arora R., Sharma B., Ellis S., Strub-Wourgaft N., Balasegaram M., Olliaro P., Das P., Modabber F. Comparison of short-course multidrug treatment with standard therapy for visceral leishmaniasis in India: an open-label, non-inferiority, randomised controlled trial. Lancet. 2011;377:477–486. doi: 10.1016/S0140-6736(10)62050-8. [DOI] [PubMed] [Google Scholar]
- Teklemariam S., Hiwot A.G., Frommel D., Miko T.L., Ganlov G., Bryceson A. Aminosidine and its combination with sodium stibogluconate in the treatment of diffuse cutaneous leishmaniasis caused by Leishmania aethiopica. Trans. R. Soc. Trop. Med. Hyg. 1994;88:334–339. doi: 10.1016/0035-9203(94)90106-6. [DOI] [PubMed] [Google Scholar]
- Uliana S.R.B., Trinconi C.T., Coelho A.C. Chemotherapy of leishmaniasis: present challenges. Parasitology. 2018;145:464–480. doi: 10.1017/S0031182016002523. [DOI] [PubMed] [Google Scholar]
- Utaile M., Kassahun A., Abebe T., Hailu A. Susceptibility of clinical isolates of Leishmania aethiopica to miltefosine, paromomycin, amphotericin B and sodium stibogluconate using amastigote-macrophage in vitro model. Exp. Parasitol. 2013;134:68–75. doi: 10.1016/j.exppara.2013.01.022. [DOI] [PubMed] [Google Scholar]
- van Griensven J., Balasegaram M., Meheus F., Alvar J., Lynen L., Boelaert M. Combination therapy for visceral leishmaniasis. Lancet Infect. Dis. 2010;10:184–194. doi: 10.1016/S1473-3099(10)70011-6. [DOI] [PubMed] [Google Scholar]
- Wijnant G.J., Van Bocxlaer K., Yardley V., Harris A., Alavijeh M., Silva-Pedrosa R., Antunes S., Mauricio I., Murdan S., Croft S.L. Comparative efficacy, toxicity and biodistribution of the liposomal amphotericin B formulations Fungisome((R)) and AmBisome((R)) in murine cutaneous leishmaniasis. Int. J. Parasitol. Drugs Drug Resist. 2018;8:223–228. doi: 10.1016/j.ijpddr.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijnant G.J., Van Bocxlaer K., Yardley V., Murdan S., Croft S.L. Efficacy of paromomycin-chloroquine combination therapy in experimental cutaneous leishmaniasis. Antimicrob. Agents Chemother. 2017;61 doi: 10.1128/AAC.00358-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yardley V., Ortuno N., Llanos-Cuentas A., Chappuis F., Doncker S.D., Ramirez L., Croft S., Arevalo J., Adaui V., Bermudez H., Decuypere S., Dujardin J.C. American tegumentary leishmaniasis: is antimonial treatment outcome related to parasite drug susceptibility? J. Infect. Dis. 2006;194:1168–1175. doi: 10.1086/507710. [DOI] [PubMed] [Google Scholar]
- Zamboni D.S., Rabinovitch M. Nitric oxide partially controls Coxiella burnetii phase II infection in mouse primary macrophages. Infect. Immun. 2003;71:1225–1233. doi: 10.1128/IAI.71.3.1225-1233.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zulfiqar B., Shelper T.B., Avery V.M. Leishmaniasis drug discovery: recent progress and challenges in assay development. Drug Discov. Today. 2017;22:1516–1531. doi: 10.1016/j.drudis.2017.06.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.