Abstract
Despite the success of antiretroviral therapy (ART), ART fails to eradicate the virus and HIV cure has remained beyond the reach of current treatments. ART targets replicating virally infected but not latently infected cells, which have limited expression of factors important for proliferation and cellular activity, including positive transcription elongation factor b (P-TEFb) and nuclear factor κB (NF-κB). Levels of the cyclin T1 (CycT1) subunit of P-TEFb are low to absent in resting T cells, and treatment with proteasome inhibitors (PIs) increases CycT1 protein levels to those of proliferating T cells. In this study, the clinically approved PI bortezomib reactivated latent HIV in latently infected primary CD4+ T cells. Bortezomib not only increased levels of CycT1 but also activated NF-κB. Strikingly, as opposed to most currently researched latency reversing agents (LRAs), bortezomib did not require a second LRA to potently reactivate latent HIV. Effects of bortezomib on resting T cells and reactivation of HIV suggest a possible direction for future attempts to diminish the viral reservoir in HIV+ individuals.
Keywords: HIV latency, proteasome inhibitor, bortezomib, P-TEFb, cyclin T1, NF-κB, latency reversing agent
Introduction
Although HIV + individuals survive on maintenance antiretroviral regimens, with viral titers below the limits of detection, these drugs are unable to fully eradicate the virus. Individuals must remain on lifelong antiretroviral therapy (ART), as even a brief break in adherence leads to viral rebound.1,2 Therefore, despite increased life span and quality of life, ART does not result in HIV cure.3 Soon after infection, a subset of virally infected cells becomes quiescent and harbors transcriptionally silent HIV.4,5 These cells are a major contributor to the HIV latent reservoir.4 This reservoir remains one of the last hurdles to HIV cure efforts.3 Latently infected cells fail to express HIV proteins, allowing them to escape detection and elimination by the immune system and ART.4
In recent years, research efforts have focused on targeting the latent HIV reservoir.6 Two main arms of research to target this reservoir are “shock and kill” and “block and lock.”7,8 In an effort to eliminate the need for daily ART, new compounds which “block” further HIV transcription and “lock” the virus in a state of deeper latency have been explored, thereby limiting HIV transcription and potential reactivation.8–10 Latency reversing agents (LRAs) have been widely studied, which “shock” the latent virus out of its transcriptionally repressed state, making it accessible to cellular “kill” mechanisms.7 Both strategies aim to eliminate the need for daily ART and the potential resurgence of viral titers when treatment is interrupted.
The earliest efforts involving latency reversal included histone deacetylase inhibitors (HDACis) and BET bromodomain inhibitors (BETis), both of which reactivate latent virus using chromatin stress and activation of positive transcription elongation factor b (P-TEFb).11,12 P-TEFb is a complex of proteins, including cyclin T1 (CycT1) and cyclin dependent kinase 9 (CDK9), which is present in inactive or free forms that are recruited to gene promoters in proliferating cells.4,13 It phosphorylates RNA polymerase II and negative elongation factors associated with the polymerase, to facilitate transcriptional elongation. Resting CD4+ T cells, including memory T cells, have limited CycT1, while transformed, long-lived cell lines have abundant CycT1.14,15 This finding is the primary reason that promising early results using HDACis and BETis in cell line models of HIV latency did not translate to reactivation efforts in primary T cells or in vivo.16,17 More promising research involved LRAs, which activate T cells and increase cellular pools of P-TEFb, most notably protein kinase C (PKC) agonists.18,19 One promising PKC agonist has been ingenol.20–22 Even a crude extract of the Euphorbia kansui (kansui), a natural plant source of ingenol, is effective at reactivating latent HIV.14 While cellular activation potently increases levels of P-TEFb, global inflammation and cytokine storm are a risk of using PKC agonists in patients.23 Therefore, combinations of PKC agonists and HDACis/BETis administered sequentially at less toxic concentrations have been investigated,14,23,24 which increase the efficacy of both compounds while mitigating the toxic effects of each. In this manner, several pathways are activated, which when combined result in potent HIV reactivation. First, PKC agonists increase levels of cellular P-TEFb. They are followed by compounds which activate P-TEFb, such as HDACis or BETis.11,12,14 Nonetheless, potential global immune activation when administered in vivo is a continued concern and has resulted in the search for LRAs, which can be used safely in a clinical context.
CycT1 protein expression in resting CD4+ T cells is increased upon treatment with the general proteasomal inhibitor MG132, suggesting inhibition of the proteasome as a means to reactivate latent HIV.25 MG132 is too toxic for clinical use, but a number of other proteasome inhibitors (PIs) are clinically approved for cancer therapy.26 The inhibitor bortezomib (Velcade, Millennium Pharmaceuticals) targets the 26s subunit of the proteasome. Bortezomib has become a standard of care treatment for multiple myeloma, and the most frequently reported adverse clinical side effect is a 30% incidence of reversible neuropathy.27 This neuropathy is reversed in most patients by a decrease in dosage or cessation of the drug.28,29 Despite this side effect, bortezomib is widely used and has been successful in increasing progression-free survival in multiple myeloma patients. Bortezomib also increases levels of the super elongation complex protein elongation factor for RNA Polymerase II (ELL2) in cell lines and reactivates latent HIV in CD4+ T cells and HIV+ patient cells.30 In addition, PR-957, an inhibitor of the immunoproteasome, also reactivates latent HIV in patient cells and activates heat shock factor 1 (HSF-1) in cell lines.31 Inhibition of the proteasome has the potential to limit further viral replication, since viral accessory proteins target host cell restriction factors, leading to their degradation by proteasomal machinery.32,33 Taken together, proteasomal inhibition has the potential to reactivate latent HIV and limit viral replication. Preventing CycT1 degradation suggests that it could be an effective mode of reactivation of latent HIV.
In this study, we extend findings of previous studies using PI treatment of latently infected T cells and demonstrate that bortezomib could be used to target latent HIV at relatively low concentrations. Bortezomib potently reactivated latent HIV in a primary CD4+ T cell model of latency and proved sufficient to reactivate latent HIV without activating T cells. Bortezomib not only increased the expression of P-TEFb but concurrently activated the nuclear factor-κB (NF-κB) pathway in primary cells. Taken together, these results suggest that bortezomib, which is approved for the clinical treatment of multiple myeloma, efficiently reactivates latent HIV by increasing cellular P-TEFb levels and activating NF-κB.
Materials and Methods
Primary human peripheral blood mononuclear cells and CD4+ T cells
Trima residuals from healthy donors, from Trima apheresis collection and enriched for peripheral blood mononuclear cells (PBMCs), were obtained from Vitalant (San Francisco, CA). Bulk PBMCs were cultured for 3 days. Nonadherent PBMCs were negatively selected for purified CD4+ T cells or used directly in PBMC experiments. CD4+ T cells were selected from bulk PBMCs using negative bead selection (Dynal CD4+ untouched beads, Invitrogen). Primary CD4+ T cells were activated and expanded using CD3/CD28 beads (Invitrogen) and 30 U/mL interleukin 2 (IL-2) for 5 days.
Cell culture and reactivation conditions
Cells were maintained in RPMI 1640 supplemented with 10% fetal bovine serum and penicillin/streptomycin at 37°C with 5% carbon dioxide. Cells were seeded in triplicate wells of a 24-well plate and stimulated for 24 h. Approximately 5–10 × 106 cells/5–10 mL on 10 cm plates were stimulated to obtain lysates for protein expression analysis. Cells were treated with indicated concentrations of: bortezomib (Millipore), dimethyl sulfoxide (DMSO), phorbol myristate acetate (PMA) (Sigma Aldrich) and phytohemagglutinin (PHA) (Sigma Aldrich), tumor necrosis factor alpha (TNFα) (eBiosciences), MG132, kansui extract (Baoji F.S. Biological Development Co. Ltd., Shanxi, China), suberanilohydroxamic acid (SAHA) [Martin Delaney Collaboratory of AIDS Researchers for Eradication (CARE)], or JQ1 (CARE).
Generation of HIV-1 infectious titers and infections
Infectious stocks of HIV-1 were generated by transfecting 293T cells with 15 μg of pNL4.3-Nef(+)-heat stable antigen (HSA) (National Institutes of Health AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases (NIAID), National Institute of Health (NIH): pNL4-3.HSA.R+.E- from Dr. Nathaniel Landau at the NYU School of Medicine) and 3 μg of vesticular stomatitus virus G protein (VSV-6) using calcium phosphate. Viral supernatants were harvested and filtered through a 0.45 μm filter at 48 h post-transfection. Approximately 0.5 × 106 pg p24 of infectious virus, as determined by HIV-1 P24 antigen capture assay (Advanced Biosciences Laboratories), was added per 1 × 106 activated CD4+ T cells. Cells were spinoculated for 90 min at 1200× g with polybrene (2 μg/mL). Twenty-four hours postinfection, cells were washed twice to thoroughly remove initial infectious virus.
Human CD4+ T cell model of HIV latency
Primary CD4+ T cells were activated, infected with HIV-1 NL4.3 HSA, and given decreasing concentrations of IL-2 over 12 days in culture to induce quiescence and HIV latency, as previously described.14 Latently infected and uninfected control cells were reactivated for 24 h, and samples were collected for flow cytometry analysis (FACS).
Flow cytometry analysis
Cells were harvested 24 h postreactivation and washed in cold phosphate buffered saline (PBS), and 0.5 × 106 cells were allotted to each tube. Cells were stained with PE mouse anti-human CD69 (555531; BD Biosciences) or fluorescein isothiocyanate (FITC) rat anti-mouse HSA (553261; BD Biosciences). Cells were fixed in 2% paraformaldehyde and analyzed using the BD Biosciences FACSCalibur and CellQuest Pro software at the University of California at San Francisco Parnassus Flow Cytometry Core. Cells were gated on the live lymphocyte gate using the forward and side scatter plot, and the percentage of live lymphocytes in 10,000 collected total cells was used as an estimate for cell viability.
Western blotting analysis
Whole cell lysates were generated using 2 × laemmli buffer (Bio-Rad) in the presence of proteinase inhibitor cocktail. Nuclei were extracted using nuclear extract lysis buffer for hematopoietic cells (Antalis and Godbolt 1991),34 and lysates of these extracts were generated using laemmli buffer in the presence of protease inhibitor cocktail. Lysates were run on 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto a nitrocellulose membrane. Whole cell lysate membranes were probed for phospho-(p-) (42 kDa), total IκBα (40 kDa), and β-actin (42 kDa). Nuclear extract membranes were probed for CycT1 (75 kDa), p-p65 (65 kDa), total p65 (65 kDa), and β-actin (42 kDa). Membranes were blocked in 5% nonfat milk (NFM) for at least 1 h and blotted overnight with mouse anti-human CycT1 antibody (sc-271348; Santa Cruz Biotechnology), rabbit anti-human p-p65 (3033S; Cell Signaling), rabbit anti-human p65 (8242S; Cell Signaling), mouse anti-human p-IκBα (9246S; Cell Signaling), mouse anti-human IκBα (48145S; Cell Signaling), and rabbit anti-human β-actin (ab8227; Abcam) antibodies in 5% NFM. Membranes were washed 3 × with PBS with 0.05% Tween 20 and then blotted for 1 h with HRP anti-rabbit or -mouse Immunoglobulin G (IgG) (Sigma Aldrich), in 5% NFM. After washing 3 × with PBS with 0.05% Tween 20, membranes were treated with enhanced luminol-based chemiluminescent (ECL) Plus chemiluminescence reagent (Promega) for 5 min and imaged using Odyssey Fc imaging system and Image Studio software (LI-COR). Reprobed membranes were stripped with NewBlot Stripping Buffer (LI-COR) and then washed 3 × with PBS.
Statistical analysis
Statistical analysis was performed using a Student's t-test, two-tailed distribution, and assuming equal variances.
Results
Bortezomib reactivates latent HIV in a primary CD4+ T cell model of HIV latency
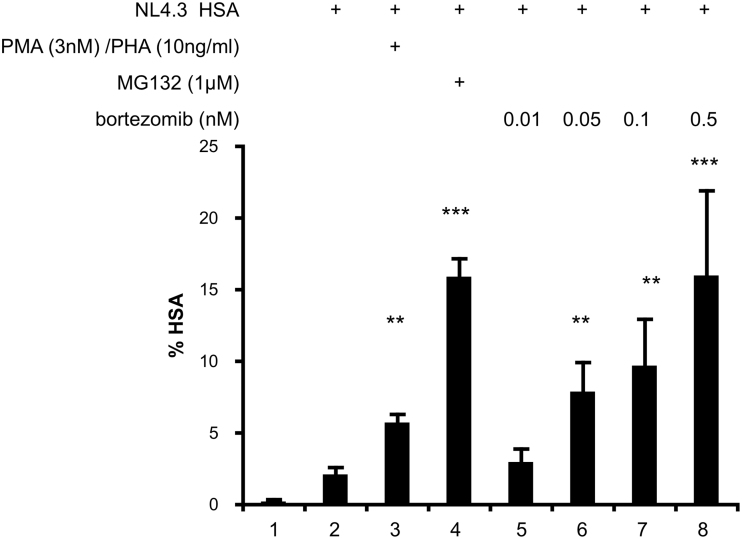
Using our established HIV latency model in human CD4+ T cells,14 we sought to determine if bortezomib would reactivate latent HIV at concentrations well below the recommended clinical doses for the treatment of multiple myeloma.35 T cells were activated and infected with VSV-G-pseudotyped NL4.3 HSA R+E- (NL4.3 HSA), HIV virus which expresses the murine HSA and allows reactivated cells to be identified using flow cytometry. Following infection, IL-2 concentrations were reduced over the course of 12 days from 30 to 2 U/mL, which drives the cells into quiescence and HIV into a latent, transcriptionally silent state.14 HSA on the surface of infected cells was minimal (Fig. 1, lane 2). Cells treated with the positive control PMA and PHA expressed 2.4-fold increased levels of HSA (Fig. 1, lane 3). Furthermore, cells treated with a potent PI, MG132, expressed 6.5-fold increased levels of HSA (Fig. 1, lane 4). Cells treated with bortezomib responded in a dose-dependent manner (Fig. 1, lanes 5–8), and 0.5 nM bortezomib resulted in fivefold increased levels of HSA, similar to the MG132 control (Fig. 1, lane 8). Overall, we conclude that bortezomib robustly reactivated latent HIV at relatively low concentrations.
FIG. 1.
Bortezomib reactivates latent HIV in a primary CD4+ T cell model of HIV latency. Human primary CD4+ T cells were infected with VSV-G-pseudotyped HIV NL4.3 HSA and maintained over 12 days with decreasing concentrations of IL-2 to establish latency as previously described.14 Uninfected cells were maintained in the same conditions. At 12 days postinfection, cells were stimulated for 24 h with DMSO, PMA and PHA, MG132, and bortezomib at the indicated concentrations. Cells were stained with anti-HSA antibody and measured by FACS. Data are presented as the percentage of cells positive for HSA expression. Experiments were repeated with three independent donors, each with three technical repeats. A representative experiment from a single donor is presented. Error bars represent standard error of the mean (***p < .001, **p < .01). DMSO, dimethyl sulfoxide; FACS, flow cytometry analysis; HSA, heat stable antigen; IL-2, interleukin 2; PHA, phytohemagglutinin; PMA, phorbol myristate acetate; VSV-G, vesticular stomatitus virus G protein.
Bortezomib does not activate uninfected CD + T cells
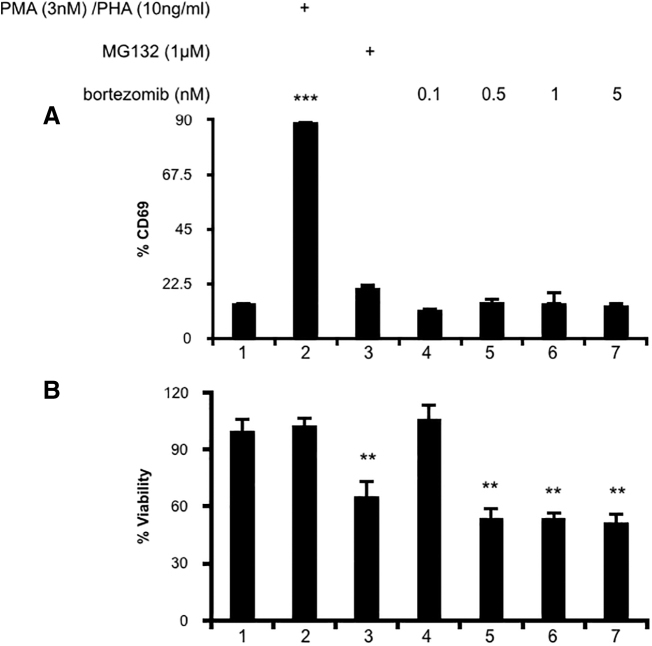
There is a risk that compounds which reactivate latent HIV might also induce global T cell activation. The most successful LRAs have been PKC agonists, such as ingenol, which at their effective concentrations also potently activate T cells.14,20,21 A previous study reported no activation of T cells when treated with bortezomib.30 It was important to verify that expression of CD69 was not elevated in our system. While uninfected T cells treated with the positive control PMA and PHA had 6.3-fold increased CD69 levels (Fig. 2A, lane 2), cells treated with MG132 or increasing concentrations of bortezomib did not (Fig. 2A, lanes 3–7). Even cells treated with a maximal dose of 5 nM bortezomib did not increase the expression of CD69 over unstimulated controls (Fig. 2A, lane 1 compared to lane 7). PI treatment was mildly toxic to cells, as MG132 and all concentrations of bortezomib tested reduced cell viability (Fig. 2B, lanes 3–7). We concluded that bortezomib treatment does not stimulate T cell activation, which suggests that it will not induce systemic T cell activation.
FIG. 2.
(A) Bortezomib does not activate uninfected CD + T cells. Uninfected human primary CD4+ T cells were stimulated for 24 h with DMSO, PMA and PHA, MG132, or bortezomib at the indicated concentrations. Cells were stained with anti-CD69 antibody and measured by FACS. (B) Percent viability was estimated using forward/side scatter and the percentage of live lymphocytes in 10,000 total cells analyzed. Data are presented with viability of untreated cells set to 100%. Experiments were repeated with three independent donors, each with three technical repeats. A representative experiment, from a single donor, is presented. Error bars represent standard error of the mean (***p < .001, **p < .01).
Combination therapy with SAHA, JQ1, PMA, or kansui does not increase latent HIV reactivation by bortezomib in a primary CD4+ T cell model of HIV latency
Combinatorial strategies, combining LRAs that function through different cellular mechanisms, were successful for ingenol and kansui.14,36 PKC agonists increase levels of active and inactive P-TEFb. Thus a PKC agonist can be combined with compounds that activate P-TEFb. In this way, lower concentrations of both compounds can be used to reactivate latent HIV to levels previously only achieved by high concentrations of a single agent.
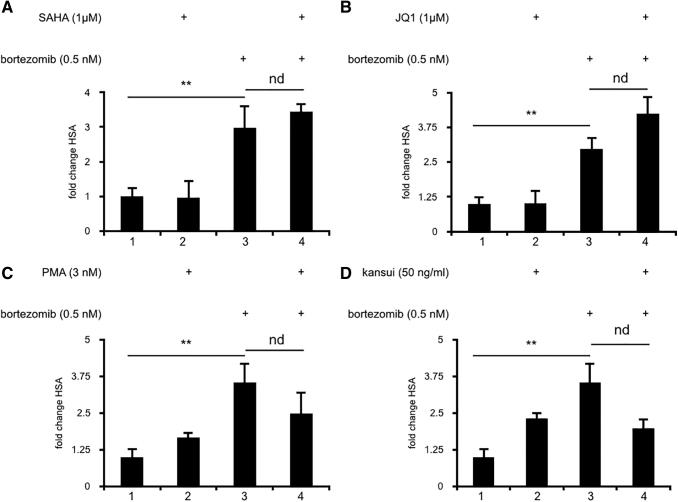
Preliminary studies to test suboptimal concentrations of bortezomib with low concentrations of SAHA (0.5 μM) or JQ1 (0.1 μM) yielded no increase in HSA expression over cells treated with bortezomib alone (data not shown). Concentrations of SAHA and JQ1 were thus increased to normally effective concentrations (1 μM). Alone neither SAHA (Fig. 3A, lane 2) nor JQ1 (Fig. 3B, lane 2) induced significant expression of HSA. Furthermore, neither the addition of SAHA (Fig. 3A, lane 4) nor JQ1 (Fig. 3B, lane 4) increased HSA expression induced over bortezomib alone (Fig. 3A, B, lane 3).
FIG. 3.
Combination therapies with SAHA, JQ1, PMA, or kansui do not increase latent HIV reactivation by bortezomib. Human primary CD4+ T cells were infected with VSV-G-pseudotyped HIV-1 NL4.3 HSA and maintained over 12 days with decreasing concentrations of IL-2 to establish latency as previously described.14 Uninfected cells were maintained in the same conditions. At 12 days postinfection, cells were stimulated for 24 h with: (A) bortezomib and SAHA, (B) bortezomib and JQ1, (C) bortezomib and PMA, and (D) bortezomib and kansui. Cells were stained with anti-HSA antibody and measured by FACS. Data are presented as a fold change in HSA expression over uninfected control cells. Experiments were repeated with three independent donors, each with three technical repeats. A representative experiment, from a single donor, is presented. Error bars represent standard error of the mean (**p < .01). nd, no difference.
Synergistic combinations with PKC agonists have been well described.23,24 Since bortezomib had no obvious synergy with compounds that activate P-TEFb, we next tested whether compounds that increase P-TEFb expression synergized with bortezomib. High concentrations of PKC agonists are known to robustly activate CD4+ T cells without requiring further stimulation. Thus, suboptimal concentrations of PMA and kansui were tested with bortezomib to determine if combination therapy would enhance bortezomib's activity. Alone, neither lower concentrations of PMA (Fig. 3C, lane 2) nor kansui (Fig. 3D, lane 2) induced significant expression of HSA. Furthermore, neither the addition of PMA (Fig. 3C, lane 4) nor kansui (Fig. 3D, lane 4) increased HSA expression over that induced by bortezomib alone (Fig. 3C, D, lane 3). Taken together, these results indicate that bortezomib alone is sufficient to reactivate latent HIV.
Bortezomib increases levels of CycT1 and activates NF-κB
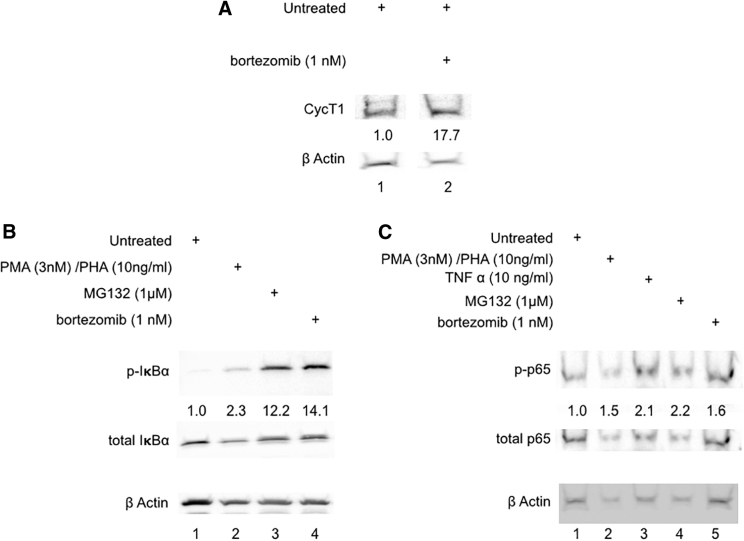
While it has been previously reported that treatment with PIs increases cellular expression of CycT1, its additional cellular effects are less well established.25 In primary human PBMCs, bortezomib increased cellular P-TEFb levels, as determined by a 17.7-fold increase in the expression of CycT1, when normalized to the differences in β-actin expression (Fig. 4A, lane 2).
FIG. 4.
Bortezomib increases Cyclin T1 and activates NF-κB. (A) Human PBMCs were stimulated for 24 h with DMSO and bortezomib. Nuclear extracts were prepared and run on 10% SDS-PAGE. Membranes were probed for anti-human CycT1 and β-actin. Densitometry was performed and normalized to β-actin, and the expression in untreated controls was set to 1. All samples represented were run on the same gel (space indicates lanes omitted from the figure). (B) Human PBMCs were stimulated for 9 h with DMSO, PMA and PHA, MG132, and bortezomib at indicated concentrations. Nuclear extracts were prepared and run on 10% SDS-PAGE. Membranes were probed for anti-human ph-IκBα, total IκBα, and β-actin. Densitometry was performed and normalized to total IκBα and β-actin, and the expression in untreated controls was set to 1. (C) Human PBMCs were stimulated for 1 h with DMSO, PMA and PHA, MG132, TNFα, and bortezomib at indicated concentrations. Nuclear extracts were prepared and run on 10% SDS-PAGE. Membranes were probed for ph-p65, total p65, and β-actin. Densitometry was performed and normalized to total p65 and β-actin, and the expression in untreated controls was set to 1. Results are representative of Western blots from three healthy donors. NF-κB, nuclear factor κB; PBMCs, peripheral blood mononuclear cells; SDS-PAGE, sodium dodecyl sulfate–polyacrylamide gel electrophoresis; TNFα, tumor necrosis factor alpha.
NF-κB activation occurs through phosphorylation of its inhibitory IκBα, which releases p65-p50 NF-κB heterodimers, which are then phosphorylated and translocated into the nucleus.37 Treatment of PBMCs with the positive control PMA and PHA induced a 2.3-fold increase in phosphorylated IκBα (Fig. 4B, lane 2) and a 1.5-fold increase in phosphorylated p65 (Fig. 4C, lane 2). An additional positive control TNFα induced a 2.1-fold increase in phosphorylated p65 (Fig. 4C, lane 3). Treatment with MG132 resulted in a 12.2-fold increase in phosphorylated IκBα (Fig. 4B, lane 3) and a 2.2-fold increase in phosphorylated p65 (Fig. 4C, lane 4). Bortezomib treated cells had a 14.1-fold increase in phosphorylated IκBα (Fig. 4B, lane 4), as well as a 1.5-fold increase in phosphorylated p65 (Fig. 4C, lane 5). Therefore, in addition to increasing levels of P-TEFb, bortezomib activates NF-κB. This finding provides two mechanisms by which bortezomib activates HIV transcription and reactivates latent HIV.
Discussion
In this study, we determined that the PI bortezomib reactivates latent HIV in CD4+ T cells. Although bortezomib reactivated HIV potently, it did not increase expression of CD69, indicating that it does not activate T cells. While most LRAs benefit from therapeutic combinations of compounds which are mechanistically distinct, bortezomib was sufficient to reactivate latent HIV as a solo treatment. The efficacy of bortezomib was unaffected by the addition of SAHA, JQ1, PMA, or kansui. Bortezomib treatment increased levels of P-TEFb and activated the NF-κB pathway. Taken together, our results indicate that bortezomib is an ideal candidate to reactivate latent HIV.
Many compounds tested for their latency reversing potential have proven insufficient to reactivate HIV alone.16,17 A subset of LRAs, such as SAHA and JQ1, activates HIV by inducing chromatin stress, which releases and activates P-TEFb.11,12 These compounds are unable to function if there is insufficient P-TEFb in cells, as is the case with resting T cells.14,25 Therefore, these compounds require a second stimulation with compounds that increase levels of P-TEFb to mediate their effects. In sharp contrast, bortezomib did not require a second compound to reactivate HIV fully. When combined with SAHA, JQ1, PMA, or kansui, there was no additional activation observed, indicating that bortezomib is sufficient to reactivate latent HIV on its own. This may arise from the dual mechanisms that bortezomib treatment induces. It increases protein expression of the CycT1 subunit of P-TEFb and also activates the NF-κB pathway, which itself requires active P-TEFb. Thus, bortezomib is capable of increasing and activating P-TEFb without the addition of a second LRA. Despite the robust activation suggested by these two mechanisms, cells treated with bortezomib did not increase expression of the early T cell activation marker, CD69. This finding is supported by other studies of PI in CD4+ T cells, which also showed no induction of CD69 or CD25 following PI treatment.30,31 Thus, although bortezomib activates cells by multiple mechanisms, it does not result in global T cell activation and suggests that treatment with bortezomib will not induce systemic inflammation or cytokine storm.
The results of this study support and build upon recent work by others on the role of PIs and HIV.25,30,31 Li et al. described effects of bortezomib treatment on ELL2, a protein subunit of the super elongation complex.30 In this report, bortezomib treatment did not affect P-TEFb levels. While these data appear to conflict with our results (Fig. 4A), it is important to note that this study used immortalized Jurkat T cell lines, which already express abundant NF-κB and P-TEFb without additional stimulation,15 and they did not test P-TEFb levels in primary cells. While our study utilized primary T cell models of HIV latency,14 we did not test lower concentrations of bortezomib on patient samples. In all of our previous studies, we found that conditions that reactivated latent HIV in this model were equally effective in PBMCs isolated from HIV+ ART suppressed patients.14 In addition, Li et al. demonstrated that 10- to 100-fold higher concentrations of bortezomib reactivated latent HIV in HIV+ ART suppressed patient samples.30 Thus, our in vitro primary cell latency model provides an appropriate proxy for responses in patient samples.
Although bortezomib affected cell viability (Fig. 2B), this finding is less concerning given the clinical use of this drug. Indeed, bortezomib has proven successful in the treatment of multiple myeloma.26 The most frequently reported severe side effect of bortezomib affects the nervous system.27 A subset of patients experience adverse neuropathic symptoms and respond well to bortezomib dose reduction. However, roughly 5% of patients are forced to halt therapy.28,29 Following bortezomib cessation, this neuropathy is reversible and is resolved after a median of 2–3 months. Despite these side effects, the efficacy of bortezomib against multiple myeloma has led to its continued use.26 Next generations of similar PIs, including carfilzomib and ixazomib, have been equally effective as bortezomib without the associated neuropathy.27,38 In fact, Li et al., demonstrated that carfilzomib had similar efficacy reactivating latent HIV as bortezomib.30 As the prospect of proteasomal inhibition for the treatment latent HIV is further explored, it will be important to consider alternate PIs, which reduce unwanted clinical side effects and toxicity.
A full picture of the regulation of P-TEFb and CycT1 expression in resting T cells is still lacking. While the mechanisms which degrade cell cycle dependent kinases (CDKs) are well established, the regulation of P-TEFb is less well understood. CDKs are stabilized by binding to their partner cyclins. When these proteins dissociate, they are quickly degraded.39,40 CDK9 expression is maintained by heat shock proteins and is less regulated by cell activation status.41 CycT1 protein expression is dependent on cellular activation.25 Further understanding of the mechanisms that regulate P-TEFb, as well as the specific E3 ubiquitin ligases which target CycT1 for degradation, is important for the discovery of compounds, which can therapeutically target this complex.
The final barrier to HIV cure is the persistence of the latent reservoir, and one strategy to target this reservoir is through carefully controlled therapeutic reactivation.7 Strategies to target cancer have been adapted to target HIV. Currently, gene therapy and chimeric antigen receptor (CAR)-T cell approaches that have yielded success in cancer models are being modified to target HIV,42,43 and the aforementioned ingenol, SAHA, and JQ1 have proven successful in combination latency reversing strategies in vitro.11,12,21 The potential benefit of a PI, such as bortezomib, is the dual mechanisms of therapeutic HIV reactivation and inhibition of further replication of reactivated viruses.31 To persist in cells, HIV accessory proteins target host restriction factors for proteasome mediated degradation.32,33 In this way, use of PIs to reactivate latent HIV also prevents the ability of HIV accessory proteins to degrade host restriction factors.31 The effects of bortezomib on CycT1 expression in resting T cells and reactivation HIV in vitro suggest a possible direction for clinical research to include targeting the proteasomal machinery.
Acknowledgments
The authors thank the Peterlin laboratory, in particular Zeping Luo, Koh Fujinaga, Fang Huang, and Rachad Nasr for technical expertise, discussions, and careful reading of the article.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
This work was supported by National Institutes of Health grants: R01 AI049104 (Peterlin, PI) and P50AI150476 (HARC grant, Krogan, PI).
References
- 1. Finzi D, Hermankova M, Pierson T, et al. : Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science (New York, NY) 1997;278:1295–1300 [DOI] [PubMed] [Google Scholar]
- 2. Wong JK, Hezareh M, Gunthard HF, et al. : Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science (New York, NY) 1997;278:1291–1295 [DOI] [PubMed] [Google Scholar]
- 3. Ho YC, Shan L, Hosmane NN, et al. : Replication-competent noninduced proviruses in the latent reservoir increase barrier to HIV-1 cure. Cell 2013;155:540–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cary DC, Fujinaga K, Peterlin BM: Molecular mechanisms of HIV latency. J Clin Investig 2016;126:448–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schiralli Lester GM, Henderson AJ: Mechanisms of HIV transcriptional regulation and their contribution to latency. Mol Biol Int 2012;2012:614120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cary DC, Peterlin BM: Targeting the latent reservoir to achieve functional HIV cure. F1000Res 2016;5:F1000 Faculty Rev-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Deeks SG: HIV: Shock and kill. Nature 2012;487:439–440 [DOI] [PubMed] [Google Scholar]
- 8. Vansant G, Bruggemans A, Janssens J, et al. : Block-and-lock strategies to cure HIV infection. Viruses 2020;12:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Leoz M, Kukanja P, Luo Z, et al. : HEXIM1-Tat chimera inhibits HIV-1 replication. PLoS Pathogens 2018;14:e1007402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kessing CF, Nixon CC, Li C, et al. : In vivo suppression of HIV rebound by Didehydro-Cortistatin A, a “Block-and-Lock” strategy for HIV-1 treatment. Cell Rep 2017;21:600–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bartholomeeusen K, Xiang Y, Fujinaga K, et al. : Bromodomain and extra-terminal (BET) bromodomain inhibition activate transcription via transient release of positive transcription elongation factor b (P-TEFb) from 7SK small nuclear ribonucleoprotein. J Biol Chem 2012;287:36609–36616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bartholomeeusen K, Fujinaga K, Xiang Y, et al. : Histone deacetylase inhibitors (HDACis) that release the positive transcription elongation factor b (P-TEFb) from its inhibitory complex also activate HIV transcription. J Biol Chem 2013;288:14400–14407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Budhiraja S, Rice AP: Reactivation of latent HIV: Do all roads go through P-TEFb? Fut Virol 2013;8:10..2217/fvl.13.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cary DC, Fujinaga K, Peterlin BM: Euphorbia Kansui Reactivates Latent HIV. PLoS One 2016;11:e0168027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Couturier J, Orozco AF, Liu H, et al. : Regulation of cyclin T1 during HIV replication and latency establishment in human memory CD4 T cells. Virol J 2019;16:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Spina CA, Anderson J, Archin NM, et al. : An in-depth comparison of latent HIV-1 reactivation in multiple cell model systems and resting CD4+ T cells from aviremic patients. PLoS Pathogens 2013;9:e1003834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Blazkova J, Chun TW, Belay BW, et al. : Effect of histone deacetylase inhibitors on HIV production in latently infected, resting CD4(+) T cells from infected individuals receiving effective antiretroviral therapy. J Infect Dis 2012;206:765–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Korin YD, Brooks DG, Brown S, et al. : Effects of prostratin on T-cell activation and human immunodeficiency virus latency. J Virol 2002;76:8118–8123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Perez M, de Vinuesa AG, Sanchez-Duffhues G, et al. : Bryostatin-1 synergizes with histone deacetylase inhibitors to reactivate HIV-1 from latency. Curr HIV Res 2010;8:418–429 [DOI] [PubMed] [Google Scholar]
- 20. Spivak AM, Bosque A, Balch AH, et al. : Ex vivo bioactivity and HIV-1 latency reversal by ingenol dibenzoate and panobinostat in resting CD4+ T cells from aviremic patients. Antimicrob Agents Chemother 2015;59:5984–5991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pandelo Jose D, Bartholomeeusen K, da Cunha RD, et al. : Reactivation of latent HIV-1 by new semi-synthetic ingenol esters. Virology 2014;462–463:328–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gama L, Abreu CM, Shirk EN, et al. : Reactivation of SIV reservoirs in the brain of virally suppressed macaques. AIDS (London, England) 2017;31:5–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Laird GM, Bullen CK, Rosenbloom DI, et al. : Ex vivo analysis identifies effective HIV-1 latency-reversing drug combinations. J Clin Invest 2015;125:1901–1912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bullen CK, Laird GM, Durand CM, et al. : New ex vivo approaches distinguish effective and ineffective single agents for reversing HIV-1 latency in vivo. Nat Med 2014;20:425–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Budhiraja S, Famiglietti M, Bosque A, et al. : Cyclin T1 and CDK9 T-loop phosphorylation are downregulated during establishment of HIV-1 latency in primary resting memory CD4+ T cells. J Virol 2013;87:1211–1220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Manasanch EE, Orlowski RZ: Proteasome inhibitors in cancer therapy. Nat Rev Clin Oncol 2017;14:417–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Arastu-Kapur S, Anderl JL, Kraus M, et al. : Nonproteasomal targets of the proteasome inhibitors bortezomib and carfilzomib: A link to clinical adverse events. Clinical cancer research: an official journal of the American Association for Cancer Research 2011;17:2734–2743 [DOI] [PubMed] [Google Scholar]
- 28. Grammatico S, Cesini L, Petrucci MT: Managing treatment-related peripheral neuropathy in patients with multiple myeloma. Blood Lymphat Cancer 2016;6:37–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Richardson PG, Sonneveld P, Schuster MW, et al. : Reversibility of symptomatic peripheral neuropathy with bortezomib in the phase III APEX trial in relapsed multiple myeloma: impact of a dose-modification guideline. Br J Haematol 2009;144:895–903 [DOI] [PubMed] [Google Scholar]
- 30. Li Z, Wu J, Chavez L, et al. : Reiterative enrichment and authentication of CRISPRi Targets (REACT) identifies the proteasome as a key contributor to HIV-1 latency. PLoS Pathogens 2019;15:e1007498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lin J, Zhang X, Lu W, et al. : PR-957, a selective immunoproteasome inhibitor, reactivates latent HIV-1 through p-TEFb activation mediated by HSF-1. Biochem Pharmacol 2018;156:511–523 [DOI] [PubMed] [Google Scholar]
- 32. Rojas VK, Park IW: Role of the Ubiquitin Proteasome System (UPS) in the HIV-1 Life Cycle. Int J Mol Sci 2019;20:2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Seissler T, Marquet R, Paillart JC: Hijacking of the Ubiquitin/Proteasome Pathway by the HIV Auxiliary Proteins. Viruses 2017;9:322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Antas TM, Godbot D: Isolation of intact nuclei from hematopoietic cell types. Nucleic Acid Res 1991; 19:4301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Palumbo A, Chanan-Khan A, Weisel K, et al. : Daratumumab, bortezomib, and dexamethasone for multiple myeloma. N Engl J Med 2016;375:754–766 [DOI] [PubMed] [Google Scholar]
- 36. Jiang G, Mendes EA, Kaiser P, et al. : Synergistic reactivation of latent HIV expression by ingenol-3-angelate, PEP005, targeted NF-kB signaling in combination with JQ1 induced p-TEFb activation. PLoS Pathogens 2015;11:e1005066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hayden MS, Ghosh S: Signaling to NF-kappaB. Genes Dev 2004;18:2195–2224 [DOI] [PubMed] [Google Scholar]
- 38. Offidani M, Corvatta L, Caraffa P, et al. : An evidence-based review of ixazomib citrate and its potential in the treatment of newly diagnosed multiple myeloma. OncoTargets Ther 2014;7:1793–1800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ekholm SV, Reed SI: Regulation of G(1) cyclin-dependent kinases in the mammalian cell cycle. Curr Opin Cell Biol 2000;12:676–684 [DOI] [PubMed] [Google Scholar]
- 40. Winston JT, Chu C, Harper JW: Culprits in the degradation of cyclin E apprehended. Genes Dev 1999;13:2751–2757 [DOI] [PubMed] [Google Scholar]
- 41. O'Keeffe B, Fong Y, Chen D, et al. : Requirement for a kinase-specific chaperone pathway in the production of a Cdk9/cyclin T1 heterodimer responsible for P-TEFb-mediated tat stimulation of HIV-1 transcription. J Biol Chem 2000;275:279–287 [DOI] [PubMed] [Google Scholar]
- 42. Seif M, Einsele H, Loffler J: CAR T cells beyond cancer: Hope for immunomodulatory therapy of infectious diseases. Front Immunol 2019;10:2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Peterson CW, Kiem HP: Cell and gene therapy for HIV cure. Curr Top Microbiol Immunol 2018;417:211–248 [DOI] [PubMed] [Google Scholar]