Abstract
Artificial light at night (LAN) is a pervasive phenomenon in today’s society, and the detrimental consequences of LAN exposure are becoming apparent. LAN is associated with the increased incidence of metabolic disorders, cancers, mood alterations, and immune dysfunction in mammals. Consequently, we examined the effects of dim LAN (DLAN) on wound healing. Female C57BL/6 mice were housed for 3 weeks in DLAN or LD conditions prior to wounding. Following wounding, mice were maintained in either their previous light conditions or switched to the opposite lighting conditions for 3 weeks. DLAN prior to wounding impaired healing; specifically, mice in DLAN/DLAN had significantly larger wounds on day 8. Additionally, mice in DLAN/LD had significantly larger wounds on days 5, 7, 8, and 9, and increased average time to closure. These data demonstrate a potential harmful effect of DLAN on wound healing that should be considered and may represent a target for therapeutic intervention.
Keywords: Dim light at night, Wound healing, Immune function, Circadian rhythms
Introduction
Light at night has become a pervasive phenomenon, with 80% of the world’s population and 99% of people in the United States and Europe living above the threshold for light pollution [6], essentially making completely dark nights a relic of the past. This alteration to natural illumination coincides with increased disturbances to human health; specifically, increased nighttime illumination is associated with the elevated incidence of metabolic dysfunction [7], mood disorders, and certain cancers [5]. Studies examining the effects of dim light at night (DLAN) exposure in rodents recapitulate these effects, demonstrating increased depression-like behaviors, impaired memory, increased weight gain, disrupted glucose metabolism, delayed recovery after ischemic injury, reduced hippocampal dendritic spines, and altered inflammatory responses [4].
Proper immune system function is integral to wound healing. Immune cells regulate the wound healing process via the secretion of cytokines, chemokines, and growth factors [11]. Innate immune cells are prominent during the inflammatory stage of wound healing, and crucial for the appropriate response to injury, by preventing the spread of infection, initiating repair, and recruiting other cell types to the wound. During the resolution of inflammatory phase and the transition into the proliferative phase (~ 5 days following wounding), adaptive immune cells, primarily T- and B-cells, migrate into the wound. Adequate lymphocyte function is necessary as these cells are important for the prevention of immunosuppression and proper tissue repair via their actions on fibroblasts; specifically, T-cells are capable of modulating collagen synthesis, fibroblast migration, and fibroblast replication [12]. Thus, proper functioning on both the innate and adaptive immune systems is necessary for appropriate wound healing.
Inflammatory diseases present with differential severity throughout the day, suggesting circadian-modulated immune function [4]. Numerous studies have demonstrated the importance of dark nights on immune function; nighttime light exposure suppressed the activity of splenic natural killer cells, independent of stress [10]. Additionally, administration of melatonin or housing in long nights demonstrated elevation in splenic mass, lymphocytes, and macrophages, suggesting that dark nights, and the concomitant melatonin release, enhance immune system function [9]. Furthermore, DLAN has been demonstrated to disrupt both innate and adaptive immune responses [1–3], suggesting that the immune system is under circadian control in mammals. Therefore, we sought to examine the effects of DLAN on wound healing. We predicted that housing mice in DLAN would alter wound healing.
Materials and methods
Sixty-three adult (> 8 weeks) female C57Bl/6 mice were obtained from Charles River Laboratories. Upon arrival in the vivarium, the mice were acclimated to LD conditions (14 h 150 lux light:10 h dark 0 lux) for 1 week prior to any experimental manipulation. Following 1 week of acclimation, mice were randomly assigned groups and singly housed in either dark nights (14 h 150 lux light:10 h dark 0 lux) or DLAN (14 h 150 lux light:10 h dark 5 lux) for 3 weeks. DLAN was supplied using standard LUMA5 LED light strips (Hitlights Inc, Baton Rouge, LA; 1.5 W/ft, 5000 K “cool white”, 1200 lumens). Cages were placed equidistant from the light strip and light levels were measured inside each cage, from the center, with the light meter facing upward to ensure ~ 5 lux of light exposure. After 3 weeks, mice were anesthetized via 2% isoflurane and a region of dorsal fur approximately 3 × 3 cm was shaved using electric clippers. The shaved region was cleaned using three alternating betadine and 70% ethanol scrubs. A single 3.5-mm circular wound was made in the dorsal skin using a sterile, disposable 3.5-mm biopsy punch (Integra Miltex, York, PA). Wounds were imaged daily using a Canon EOS Rebel T3 camera that was held 23 cm above the surface of the mouse. Each photograph included a standard-sized circle (3.5-mm ID) placed near the wound. The wound size for each mouse was determined using ImageJ—Fiji software and expressed as the ratio of wound area to the area of the standard circle in the photograph (in pixels). Healing was defined as complete wound closure. Throughout the experiment, mice were allowed ad libitum access to food and reverse osmosis purified water. Additionally, all the experiments were performed in accordance with NIH Animal Welfare guidelines and were approved by the West Virginia University Institutional Animal Care and Use Committee.
Statistical analyses
Average time to healing and area under the curve were analyzed using a two-way ANOVA, with lighting conditions prior to wounding and lighting conditions after wounding as independent factors. Post-hoc comparisons were made using Fisher’s LSD tests. To determine changes in relative wound size across days, multiple t tests were run within each day. Mean differences were considered statistically significant when p ≤ 0.05. All statistical analyses were performed using GraphPad Prism 8 software (San Diego, CA).
Results
Exposure to DLAN prior to wounding impaired wound healing (F1,1099 = 4.839; p < 0.01; Fig. 1a, b); mice housed in DLAN/LD demonstrated significantly delayed wound healing relative to LD/LD and LD/DLAN house mice (p < 0.05 Fisher’s LSD). Specifically, mice housed in DLAN/LD had greater wound sizes on days 7, 8, and 9 when compared to LD/DLAN-housed mice (t = 2.178, p < 0.05; t = 2.080, p < 0.05; t = 2.273, p < 0.05). Additionally, DLAN/LD mice had significantly greater wound sizes on days 5 and 9 compared to LD/LD-housed mice (t = 2.261, p < 0.05; t = 2.033, p = 0.05). Moreover, DLAN/DLAN mice displayed a significantly greater wound size on day 8 relative to LD/DLAN-housed mice (t = 1.916, p = 0.05). DLAN prior to wound healing increased the time to wound closure (F1,58 = 8.935; p < 0.01; Fig. 1c, d); specifically, mice housed in DLAN/LD demonstrated significantly increased average time to wound healing relative to LD/LD or LD/DLAN mice (p < 0.05 Fisher’s LSD).
Fig. 1.
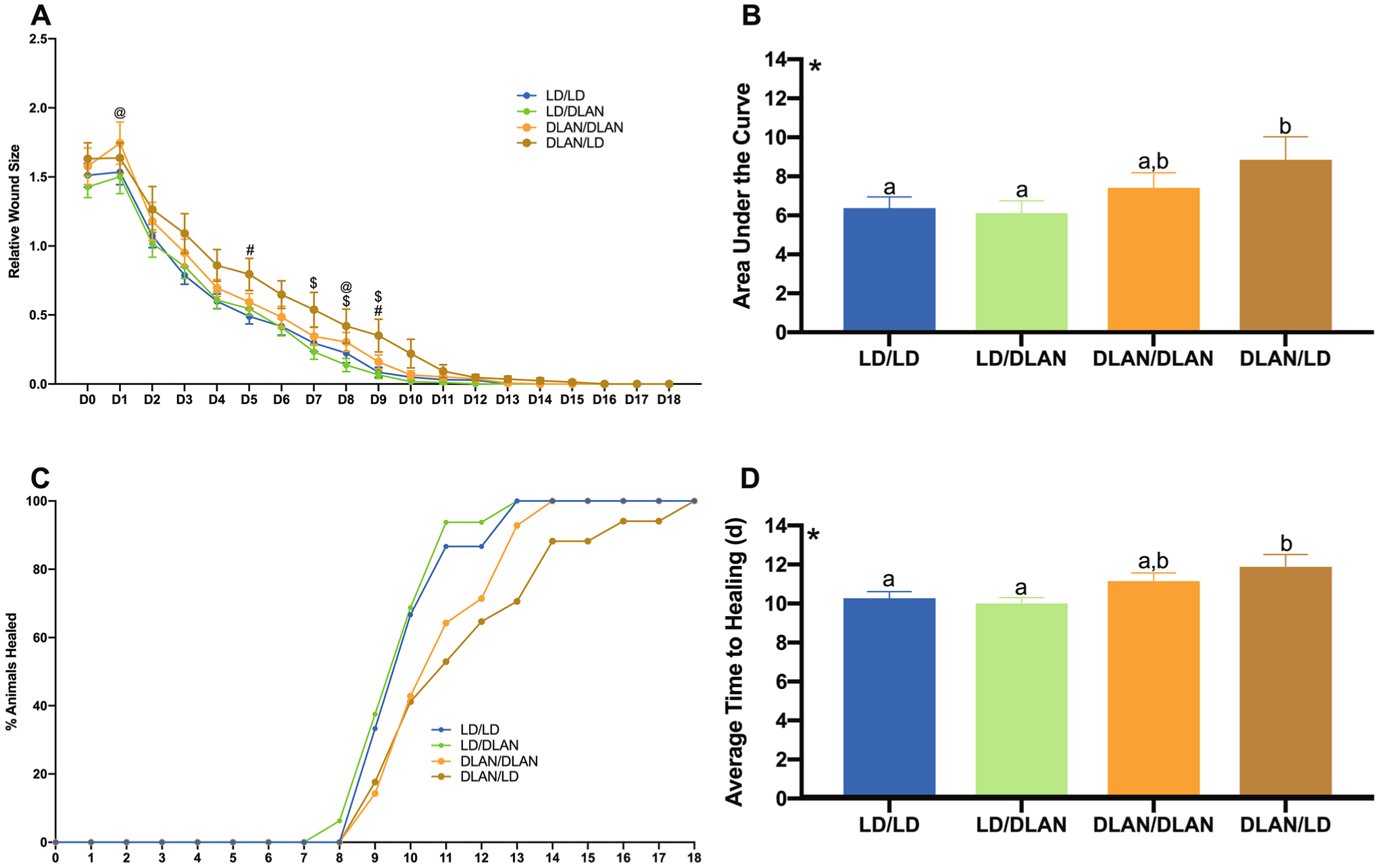
Dim light at night prior to wounding impairs wound healing (a–d). a Relative wound size; DLAN/LD mice displayed significantly larger wound sizes when compared to LD/LD(days 5 and 9)- or LD/DLAN (days 7, 8, and 9)-housed mice. Additionally, DLAN/DLAN-housed mice had significantly larger wound sizes compared to LD/DLAN mice (days 1 and 8). b Quantification of relative wound size (AUC); main effect of lighting prior to wounding (F1,1099 = 4.839; p < 0.01); DLAN/LD mice had significantly impaired wound healing relative to LD/LD and LD/DLAN-housed mice. c Percentage of animals healed across each day. d Average time to healing (days); main effect of lighting prior to wounding (F1,58 = 8.935; p < 0.01); DLAN/LD mice had significantly increased average time to wound healing relative to LD/LD or LD/DLAN mice. Error bars represent SEM; a @significant t test LD/DLAN vs DLAN/DLAN, #significant t test LD/LD vs DLAN/LD, $significant t test LD/DLAN vs DLAN/LD. b, d *Main effect of lighting prior to wounding, bars that do not share a letter represent multiple comparisons at p < 0.05, two-way ANOVA, Fisher’s LSD multiple comparisons test. n = 14–17 per group
Discussion
The current study sought to examine the effects of DLAN (5 lux) on wound healing. Taken together, these data demonstrate that DLAN exposure prior to wounding significantly impairs healing and increases the time until wound closure (Fig. 1). Considering that 99% of the population in the United States and Europe are exposed to LAN, these data demonstrate a potential harmful effect of LAN exposure on wound healing that should be considered in clinical populations such as diabetics and chronic wound sufferers, and may represent a potential target for therapeutic intervention.
Given that DLAN prior to an immune challenge impairs both the innate and adaptive immune responses [1–3], which are necessary for proper wound healing [11], this likely represents the mechanism by which DLAN prior to wounding impairs subsequent healing. Indeed, one such study examined the effects of DLAN prior to acute stressor and subsequent immune challenge in Siberian hamsters [11]. The authors reported that DLAN prior to an acute stressor impairs the delayed-type hypersensitivity response (adaptive immune response) following the acute stressor. Notably, the aforementioned study is relevant to the current study as the process of wounding is itself a stressor. When examining wound healing across days, mice housed in DLAN prior to wounding demonstrated enlarged wound sizes precisely between days 5 and 9. These days correspond to the initiation of lymphocyte trafficking into the wound (day 5), the typical peak of lymphocytes within the wound (day 7), and the resolution of lymphocytes (~ day 10) [11]. Whereas the role of lymphocytes in wound healing is an area of current research, there are numerous studies demonstrating that enhancement of thymic and T lymphocyte function improves wound healing; whereas, suppression of T lymphocyte function impairs wound healing [8, 12]. Thus, it reasonable that DLAN prior to wounding likely impairs wound healing via alterations in immune function, particularly adaptive immune function.
There are limitations to this study. For example, the current study does not demonstrate a causal mechanism that would elucidate how DLAN impairs wound healing. Therefore, future studies should examine the effect of DLAN on the adaptive immune response following a wound challenge. Additionally, DLAN exposure following wounding had no effect on healing, which likely represents an interaction with DLAN and immune function before and after wounding that remains unspecified. Thus, future studies should examine the effect of DLAN exposure before and after wounding on immune function.
In sum, our study is the first to demonstrate that DLAN exposure prior to wounding impairs wound healing and we propose that this effect is likely due to impaired adaptive immune function. Additionally, these data have potential broad clinical implications on diabetics and chronic wound sufferers, as DLAN in today’s society is extremely pervasive.
Footnotes
Conflict of interest The authors declare no conflict of interests.
References
- 1.Aubrecht TG, Weil ZM, Nelson RJ (2014) Dim light at night interferes with the development of the short-day phenotype and impairs cell-mediated immunity in Siberian hamsters (Phodopus sungorus). J Exp Zool A Ecol Genet Physiol 321(8):450–456 [DOI] [PubMed] [Google Scholar]
- 2.Bedrosian TA, Fonken LK, Walton JC, Nelson RJ (2011) Chronic exposure to dim light at night suppresses immune responses in Siberian hamsters. Biol Lett 7(3):468–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bedrosian TA, Aubrecht TG, Kaugars KE, Weil ZM, Nelson RJ (2013) Artificial light at night alters delayed-type hypersensitivity reaction in response to acute stress in Siberian hamsters. Brain Behav Immun 34:39–42 [DOI] [PubMed] [Google Scholar]
- 4.Bedrosian TA, Fonken LK, Nelson RJ (2016) Endocrine effects of circadian disruption. Annu Rev Physiol 78:109–131 [DOI] [PubMed] [Google Scholar]
- 5.Bedrosian TA, Nelson RJ (2017) Timing of light exposure affects mood and brain circuits. Transl Psychiatry 7(1):e1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Falchi F, Cinzano P, Duriscoe D, Kyba CC, Elvidge CD, Baugh K, Furgoni R (2016) The new world atlas of artificial night sky brightness. Sci Adv 2(6):e1600377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fonken LK, Nelson RJ (2014) The effects of light at night on circadian clocks and metabolism. Endocr Rev 35(4):648–670 [DOI] [PubMed] [Google Scholar]
- 8.Ghatak S, Maytin EV, Mack JA, Hascall VC, Atanelishvili I, Moreno Rodriguez R, Markwald RR, Misra S (2015) Roles of proteoglycans and glycosaminoglycans in wound healing and fibrosis. Int J Cell Biol 2015:1–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Navara KJ, Nelson RJ (2007) The dark side of light at night: physiological, epidemiological, and ecological consequences. J Pineal Res 43(3):215–224 [DOI] [PubMed] [Google Scholar]
- 10.Oishi K, Shibusawa K, Kakazu H, Kuriyama T, Ohkura N, Machida K (2006) Extended light exposure suppresses nocturnal increases in cytotoxic activity of splenic natural killer cells in rats. Biol Rhythm Res 37(01):21–35 [Google Scholar]
- 11.Park JE, Barbul A (2004) Understanding the role of immune regulation in wound healing. Am J Surg 187(5):S11–S16 [DOI] [PubMed] [Google Scholar]
- 12.Schäffer M, Barbul A (1998) Lymphocyte function in wound healing and following injury. Br J Surg 85(4):444–460 [DOI] [PubMed] [Google Scholar]


