Abstract
Programmed cell death promotes homeostatic cell turnover in the epithelium but is dysregulated in cancer. The glycosyltransferase ST6Gal-I is known to block homeostatic apoptosis through α2,6-linked sialylation of the death receptor TNFR1 in many cell types. However, its role has not been investigated in gastric epithelial cells or gastric tumorigenesis. We determined that human gastric antral epithelium rarely expressed ST6Gal-I, but the number of ST6Gal-I–expressing epithelial cells increased significantly with advancing premalignancy leading to cancer. The mRNA expression levels of ST6GAL-I and SOX9 in human gastric epithelial cells correlated positively with one another through the premalignancy cascade, indicating that increased epithelial cell expression of ST6Gal-I is associated with premalignant progression. To determine the functional impact of increased ST6Gal-I, we generated human gastric antral organoids from epithelial stem cells and differentiated epithelial monolayers from gastric organoids. Gastric epithelial stem cells strongly expressed ST6Gal-I, suggesting a novel biomarker of stemness. In contrast, organoid-derived epithelial monolayers expressed markedly reduced ST6Gal-I and underwent TNF-induced, caspase-mediated apoptosis, consistent with homeostasis. Conversely, epithelial monolayers generated from gastric cancer stem cells retained high levels of ST6Gal-I and resisted TNF-induced apoptosis, supporting prolonged survival. Protection from TNF-induced apoptosis depended on ST6Gal-I overexpression, because forced ST6Gal-I overexpression in normal gastric stem cell–differentiated monolayers inhibited TNF-induced apoptosis, and cleavage of α2,6-linked sialic acids from gastric cancer organoid-derived monolayers restored susceptibility to TNF-induced apoptosis. These findings implicate up-regulated ST6Gal-I expression in blocking homeostatic epithelial cell apoptosis in gastric cancer pathogenesis, suggesting a mechanism for prolonged epithelioid tumor cell survival.
Keywords: apoptosis; cancer biology; epithelial cell; gastric cancer; glycosylation; sialic acid; stem cellsα-26-sialyltransferase 1 (ST6Gal-I); stem cells; β-galactoside α2,6-sialyltransferase 1 (ST6GAL1)
Epithelial cell homeostasis in the gastrointestinal mucosa is maintained by the finely tuned balance between cell proliferation and apoptosis. This balance is disrupted transiently in mucosal injury and permanently in mucosal cancer cells, which acquire ineffective apoptosis. The latter leads to increased survival and proliferation with the dysregulated apoptosis unable to eliminate cancer cells and restrict progressive tumor cell development (1–4). In the stomach, impaired epithelial apoptosis has been detected in tissue sections of premalignant gastric lesions (5), suggesting that dysregulated apoptosis may contribute to the histological changes leading to gastric cancer. Gastric cancer cells and epithelial stem cells have many features in common, including prolonged survival. Thus, elucidation of the cellular pathways involved in prolonging epithelial stem cell survival could inform our understanding of gastric cancer pathogenesis. However, the mechanism of dysregulated apoptosis during the malignant transformation of gastric epithelial cells, especially at the stem cell level, has received insufficient investigative attention.
The glycosyltransferase ST6Gal-I has been shown to contribute to multiple cell processes, including apoptosis, differentiation, and survival (6, 7). ST6Gal-I is located in the Golgi, where it catalyzes the addition of α2,6-linked sialic acids to N-glycans on glycoproteins destined via the trans-Golgi network for the plasma membrane. Homeostatic epithelial cell death receptors tumor necrosis receptor 1 (TNFR1) and Fas are key glycoproteins targeted by ST6Gal-I for addition of α2,6 sialic acids (α2,6 sialylation). The α2,6 sialylation of TNFR1 does not block ligand binding but prevents TNFR1 internalization, thereby blocking TNF-induced apoptosis and subsequent cell turnover (8, 9). ST6Gal-I is typically expressed at low levels in differentiated epithelium, but its overexpression occurs in several epithelioid cancers, including colonic, pancreatic, and ovarian adenocarcinomas (7, 8, 10–13). The role of ST6Gal-I in gastric epithelial cell homeostasis and the pathogenesis of gastric adenocarcinoma is not known, despite gastric cancer being the fifth most common cancer and the third leading cause of cancer-related mortality worldwide (14).
Here we determined the expression of ST6Gal-I in normal human gastric antrum and in the gastric antrum of subjects throughout the premalignant histological cascade leading to, and including, gastric adenocarcinoma. We used Lgr5+ stem cells (15, 16) from human normal gastric antrum and gastric adenocarcinoma to generate epithelial stem cell organoids and establish dynamic in vitro models of normal antral epithelium and gastric tumor epithelium (17). We employed these model systems, together with ST6Gal-I overexpression and knockdown, to elucidate the role of ST6Gal-I in normal epithelial homeostasis and gastric adenocarcinoma.
Results
Epithelial ST6Gal-I expression is increased during gastric premalignancy and adenocarcinoma
Helicobacter pylori infection is the primary risk factor for gastric cancer (18–21). Usually aquired during early childhood and persisting for decades if untreated (22, 23), H. pylori colonizes the stomach in 50% of the world's population (19). Over time, H. pylori–induced histopathology progresses through chronic gastritis, atrophy, complete metaplasia, incomplete metaplasia, and dysplasia (the Correa cascade) to adenocarcinoma in 2–3% of infected persons (24, 25). Notably, the premalignancy cascade may progress even after loss of H. pylori in later stages of the cascade because of inflammation-induced destruction of the ecological niche of the bacterium (26). Using gastric biopsy tissue from subjects in Chile, where H. pylori is endemic, we show that epithelial cell expression of ST6Gal-I increased progressively with each advancing histological stage (no dysplasia tissues have been available to us to date) of the Correa cascade, leading to and including gastric cancer (Fig. 1A). Notably, gastric tissue from uninfected children and children with H. pylori chronic gastritis, who rarely develop gastric cancer (27–29), contained very few ST6Gal-I–expressing gastric epithelial cells, in contrast to the progressively increased expression in the epithelium from adult gastric tissue (Fig. 1A). We next analyzed ST6Gal-I expression in gastric adenocarcinoma tissue sections. The number of ST6Gal-I–expressing cells was strikingly increased in gastric adenocarcinoma epithelium compared with noninvolved regions of gastric tissue from the same donors, confirming ST6Gal-I overexpression in gastric cancer (Fig. 1, B and C). Further, we correlated ST6GAL-I expression with the expression of SOX9, a transcription factor whose expression is associated with the development and progression of malignancies such as colon cancer (30) and induced by H. pylori, as shown in a mouse model of the infection (31). Many of the gastric epithelial cells that expressed ST6Gal-I also expressed Sox9 (Fig. 1D). Furthermore, the mRNA expression level of ST6GAL1 and SOX9 in gastric epithelial cells correlated positively with one another through the premalignancy cascade (Fig. 1E), indicating that increased epithelial cell expression of ST6Gal-I is associated with premalignant progression in the human stomach. These findings extend the epithelioid tumors that express ST6Gal-I to gastric adenocarcinoma.
Figure 1.

Epithelial ST6Gal-I expression is progressively up-regulated in the successive stages of gastric premalignancy and gastric cancer. A, gastric antral biopsies from 39 subjects with the indicated histological finding were analyzed for the number of ST6Gal-I+ cells per HPF in 5 randomly selected fields per donor tissue. B and C, gastric adenocarcinoma and noninvolved tissue from the same donor (confirmed by pathologist L. N. C.) were (B) analyzed for ST6Gal-I by immunohistochemistry (representative image, 20×; n = 6; scale bar, 50 μm) and (C) enumerated for ST6Gal-I+ cells per HPF in 10 randomly selected fields per donor tissue. D, sequential sections from gastric adenocarcinoma (representative image, 20×; n = 3; scale bar, 50 μm) were stained for ST6Gal-I–positive and Sox9-positive cells using immunohistochemistry. E, 42 gastric antral biopsies were examined for mRNA expression of ST6GAL1 and SOX9 by quantitative real-time PCR and expressed as -fold change relative to GAPDH expression. (A and C, significance: *, p < 0.05; **, p < 0.01; ***, p < 0.001; and ****, p < 0.0001.
ST6Gal-I is a novel biomarker of gastric epithelial stem cells
To determine the role of ST6Gal-I in gastric epithelial cell biology and gastric adenocarcinoma pathogenesis, we established gastric epithelial stem cell organoids and organoid-derived monolayers to model antral stem cells and epithelium, respectively. Biopsies of normal human gastric antrum were digested and enriched for stem cells through culture in the presence of 50% l-WRN conditioned media (BASIC-CM) (32). Upon passaging, individual epithelial stem cells proliferated to form spheroid organoids lined by a single layer of cells with the epithelial apical surface directed toward the lumen (Fig. 2A). The stem cell organoids expressed high levels of mRNA for the canonical stem cell biomarker LGR5 and decreased levels of the mucin gene MUC5AC, present in differentiated goblet cells, compared with the biopsy tissue from which the organoids were derived (Fig. 2B). The gastric epithelial stem cell organoids also expressed both transmembrane tight junction protein zonula occludens 1 (ZO-1) and epithelial cell adhesion/signaling molecule E-cadherin (Fig. 2C). Epithelial stem cells generated from normal gastric antrum stained strongly for ST6Gal-I (Fig. 2D) and potently bound Sambucus nigra agglutinin (SNA) (Fig. 2E), which binds specifically to the sialic acid attached to a terminal galactose in an α2,6 linkage, confirming functional ST6Gal-I protein activity. To determine whether ST6Gal-I is associated with stemness, we examined the expression of ST6Gal-I in decreasing concentrations of stem cell factor–rich media. Gastric epithelial organoid expression of LGR5 and ST6GAL1 mRNA progressively decreased in the presence of diminishing levels of stem cell growth factor availability (Fig. 2, F and G), indicating that stem cell expression of LGR5 and ST6GAL1 was dependent, at least in part, on Wnt factor signaling.
Figure 2.
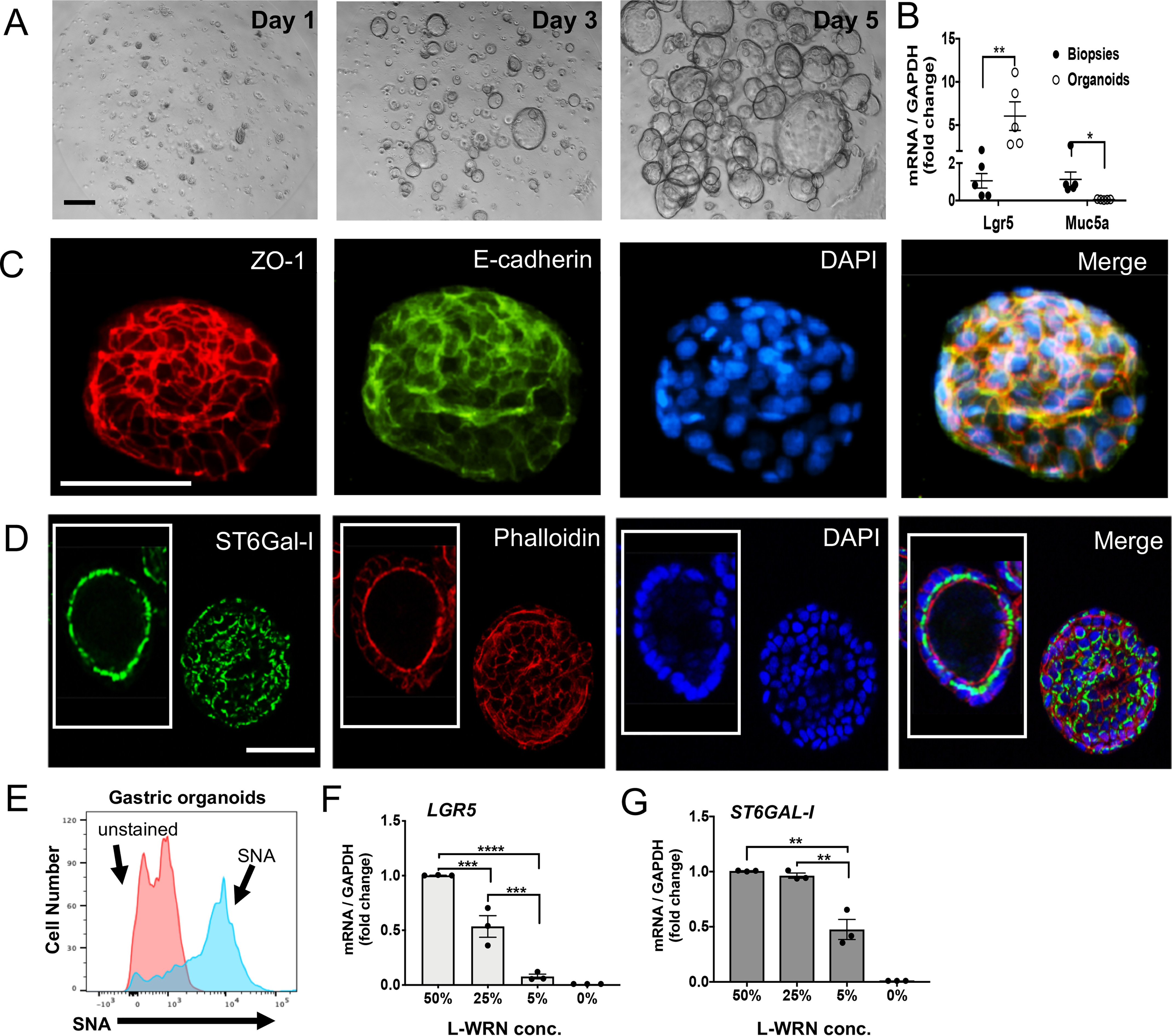
ST6Gal-I is a novel biomarker of gastric epithelial stem cells. A, epithelial stem cells derived from normal gastric antrum (gastric organoids) cultured in Matrigel supplemented with BASIC-CM for 5 days (see “Experimental procedures”) showed a progressive increase in the formation, number and size of stem cell organoids (representative images, 10×; n = 6; scale bar, 200 μm). B, matching gastric biopsies and organoids were analyzed for LGR5 and MUC5AC expression by real time qPCR. C, gastric organoids were isolated on day 3 and examined by immunofluorescence after staining with antibodies to ZO-1 (Alexa Fluor 594) or E-cadherin (FITC) and counterstained with DAPI (representative organoids, 20×; n = 3; scale bar, 50 μm). D, gastric organoids were stained with an antibody to ST6Gal-I (FITC) in addition to phalloidin (Alexa Fluor 594) and DAPI (representative donor; n = 3) (40×; scale bar, 100 μm). E, gastric organoids were stained with SNA lectin (blue) (see “Experimental procedures”) using flow cytometry. F and G, organoids were cultured 3–4 days in decreasing concentrations of l-WRN CM in BASIC-CM and analyzed for (F) LGR5 and (G) ST6GAL1 gene expression (n = 3). B, F, and G, significance: *, p < 0.05; **, p < 0.01; ***, p < 0.001; and ****, p < 0.0001.
To generate differentiated gastric epithelium, gastric organoids were trypsinized and the individual stem cells plated on glass slides thinly coated with Matrigel in the presence of reduced Wnt availability (5% l-WRN CM, monolayer media) (Fig. 3A). The epithelial stem cells formed monolayers that up-regulated MUC5AC mRNA compared with the organoids (Fig. 3B), indicating differentiated gastric epithelium. Epithelial stem cell monolayers expressed ZO-1 and E-cadherin (Fig. 3C) but exhibited very low levels of ST6Gal-I protein (Fig. 3D). Indeed, the differentiation of gastric organoids into epithelial monolayers resulted in sharp decreases in mRNA expression of both LGR5 and ST6GAL1 (Fig. 3, E and F). Further, re-culturing established monolayers in stem cell growth factor complete media (50% l-WRN CM) did not induce significant up-regulation of either LGR5 or ST6GAL1 (Fig. 3G). These data suggest ST6Gal-I is a potential biomarker of human antral gastric epithelial stem cells but not differentiated epithelium and that Wnt factor signaling contributes to ST6Gal-I expression.
Figure 3.

ST6Gal-I is not expressed in normal differentiated gastric epithelium. A, epithelial cell monolayers derived from gastric organoids (see “Experimental procedures”) were examined for confluence by microscopy on days 1, 2, and 4 (representative images, 10×; n = 3; scale bar, 100 μm, 20 μm for inset). B, mRNA from day 0 (organoids) and day 2 (monolayers) was analyzed for MUC5AC expression by qPCR (n = 3). C, confluent epithelial cell monolayers derived from gastric organoids were stained for ZO-1 (Alexa Fluor 594) and E-cadherin (FITC) using antibodies and nuclei (DAPI) using immunofluorescence (representative staining, 40×; n = 3; scale bar, 50 μm). D, gastric organoid–derived monolayers (day 2) were stained with an antibody for ST6Gal-I (FITC) as well as phalloidin (Alexa Fluor 594) and DAPI (representative donor; n = 3) (20×; scale bar, 50 μm). E and F, gastric organoids (day 0) and organoid-derived epithelial monolayers (days 1–4) from a representative donor were analyzed for (E) LGR5 and (F) ST6GAL1 gene expression and (E and F, insets) from three donors analyzed on day 2. G, monolayers were generated as in (A) and on day 2 the media was kept at 5% l-WRN (monolayer media) or changed to 50% l-WRN (organoid media, BASIC-CM) for 24 h and analyzed for LGR5 and ST6GAL1 gene expression (n = 3–5). B, E, and F, significance: *, p < 0.05; **, p < 0.01; ***, p < 0.001; and ****, p < 0.0001.
Gastric epithelial cell ST6Gal-I overexpression enhances resistance to TNF-induced epithelial cell apoptosis
We next investigated the function of epithelial ST6Gal-I on homeostatic apoptosis. Epithelial stem cell organoids from healthy antral mucosa were transfected with an shRNA interference vector (7) targeting ST6GAL1 to knock down ST6Gal-I or transduced with an ST6GAL1 lentiviral vector (7) to overexpress ST6Gal-I. ST6GAL1 mRNA knockdown and overexpression were confirmed in the organoids at both the mRNA and protein level (Fig. 4, A and B). Epithelial stem cells successfully formed organoids in the presence of ST6Gal-I knockdown (Fig. 4B), indicating ST6Gal-I was not essential for organoid formation. Importantly, forced overexpression of ST6Gal-I in gastric organoids resulted in sustained ST6Gal-I expression in the organoid-derived epithelial monolayers at both the mRNA and protein level (Fig. 4, C and D).
Figure 4.

ST6Gal-I overexpression in gastric epithelial organoids is maintained in organoid-derived monolayers and inhibits TNF-mediated apoptosis. A, epithelial stem cell organoids derived from normal gastric antrum (Normal) with ST6Gal-I knockdown (KD) or ST6Gal-I overexpressed (OE) or empty vehicle control (EV) (see “Experimental procedures”) were analyzed on days 3–4 for ST6GAL1 gene expression (n = 4). B, organoids were stained with an ST6Gal-I antibody (FITC), phalloidin (Alexa Fluor 594) and DAPI (representative images; n = 3; scale bar, 50 μm). C, normal, KD, and OE gastric organoids (day 0) and their derived epithelial monolayers (days 1–4) were analyzed for ST6GAL1 gene expression. D, normal, KD and OE epithelial monolayers (day 2) were stained with an ST6Gal-I antibody (FITC) as well as phalloidin (Alexa Fluor 594) and DAPI (monolayers from a representative donor (n = 3; 20×; scale bar, 50 μm). E, ST6Gal-I normal, KD or OE organoid-derived monolayers were analyzed for surface TNFR1 (FITC) by flow cytometry (representative data shown, n = 4). F, mRNA from day 2 monolayers was analyzed for TNFRSF1 expression by qPCR (n = 7). G, ST6Gal-I normal, OE, and KD organoid-derived monolayers (n = 5–10 in four independent experiments) were treated with TNF 50 ng/ml for 24 h or media and evaluated for caspase 3/7 activity by CaspaseGlo 3/7 luminescence assay. A–C significance: *, p < 0.05; **, p < 0.01; and ***, p < 0.001.
TNFR1 participates in a wide variety of downstream signaling pathways, including homeostatic apoptosis, and is ubiquitously expressed in most tissues (33). TNFR1 is packaged in the Golgi and trafficked via the trans-Golgi network to the cell surface, where it engages with its ligand TNF (34, 35). TNF ligation of TNFR1 initiates cell surface receptor aggregation that induces TNFR1 internalization, activating downstream caspases 3/7 to initiate apoptosis (9, 36–39). ST6Gal-I–mediated α2,6-linked sialylation of TNFR1 N-glycans blocks internalization of the receptor, thereby protecting against caspase-activated apoptosis (8, 9). Interrogating this key function of ST6Gal-I, we next determined the impact of ST6Gal-I overexpression on the ability of epithelial monolayers derived from healthy gastric antrum to undergo apoptosis. Stable transduction of the organoids with a lentiviral vector containing the ST6GAL1 gene caused increased levels of surface TNFR1 (p = 0.03) in the epithelial cell monolayers (Fig. 4E), consistent with impaired receptor internalization and continued TNFR1 trafficking to the epithelial cell surface (40, 41). Importantly, the levels of TNFRSF1 gene expression in ST6Gal-I overexpressing and knockdown monolayers, as well as monolayers derived from normal gastric organoids, were similar (Fig. 4F), indicating the increased surface expression of TNFR1 in the ST6Gal-I overexpressing monolayers was indeed because of reduced receptor internalization and not increased TNFRSF1 mRNA expression.
To determine the impact of increased ST6Gal-I expression on TNF-induced epithelial cell apoptosis, we treated epithelial monolayers generated from normal and ST6Gal-I knockdown or overexpressing gastric stem cell organoids with TNF for 24 h and measured caspase activity. Caspase activity was increased in both the normal monolayers and the monolayers derived from ST6Gal-I knockdown organoids (Fig. 4G), indicating susceptibility to TNF-induced apoptosis. In contrast, caspase activity was not significantly augmented by TNF treatment of ST6Gal-I overexpressing monolayers (Fig. 4G). Thus, increased epithelial ST6Gal-I expression leads to the suppression of TNF-mediated apoptosis, likely contributing to enhanced epithelial cell longevity in vivo.
ST6Gal-I overexpression dysregulates apoptosis in gastric cancer
To determine the impact of high-level ST6Gal-I expression on epithelial cell function in gastric cancer, we generated epithelial organoids from gastric adenocarcinoma, derived differentiated epithelial cell monolayers, and assessed monolayer ST6Gal-I expression and function. The gastric cancer monolayers displayed diminished LGR5 expression, similar to monolayers derived from normal antral organoids (Fig. 5A). However, in sharp contrast to normal epithelial monolayers, tumor organoid–derived monolayers retained high levels of ST6GAL1 expression throughout the entire culture period (4 days) (Fig. 5B). Moreover, gastric cancer–derived and normal antral–derived organoids both expressed SOX9, but only monolayers generated from tumor organoids maintained this expression (Fig. 5C), consistent with the contribution of Sox9 in tumorigenesis (30, 42, 43) and unfavorable patient prognosis (44). The elevated ST6GAL1 expression in tumor organoid–derived monolayers was confirmed at the protein level using immunofluorescence (Fig. 5D). Importantly, monolayers generated from tumor organoids did not display significantly increased caspase activity in the presence of TNF (Fig. 5E), indicating resistance to TNF-inducible apoptosis. We therefore treated tumor-derived monolayers for 30 min with Arthrobacter ureafaciens neuraminidase to preferentially cleave α2,6-linked sialic acids, in accordance with previously described protocols (45–47). Although A. ureafaciens can cleave both α2,6 and α2,3 sialic acids, α2,6 sialic acids are cleaved more efficiently than α2,3 sialic acids upon short-term treatments with A. ureafaciens (46, 47). Removal of α2,6 sialic acids was confirmed by SNA staining using flow cytometry (Fig. 5F). Neuraminidase removal of the sialic acids from tumor organoid–derived monolayers induced an increase in caspase activity (Fig. 5E), indicating susceptibility to TNF-induced apoptosis. Taken together, our results demonstrate that ST6Gal-I is strongly up-regulated in gastric cancer–derived epithelial cells, where the enzyme dysregulates homeostatic apoptosis to promote epithelial cell survival.
Figure 5.
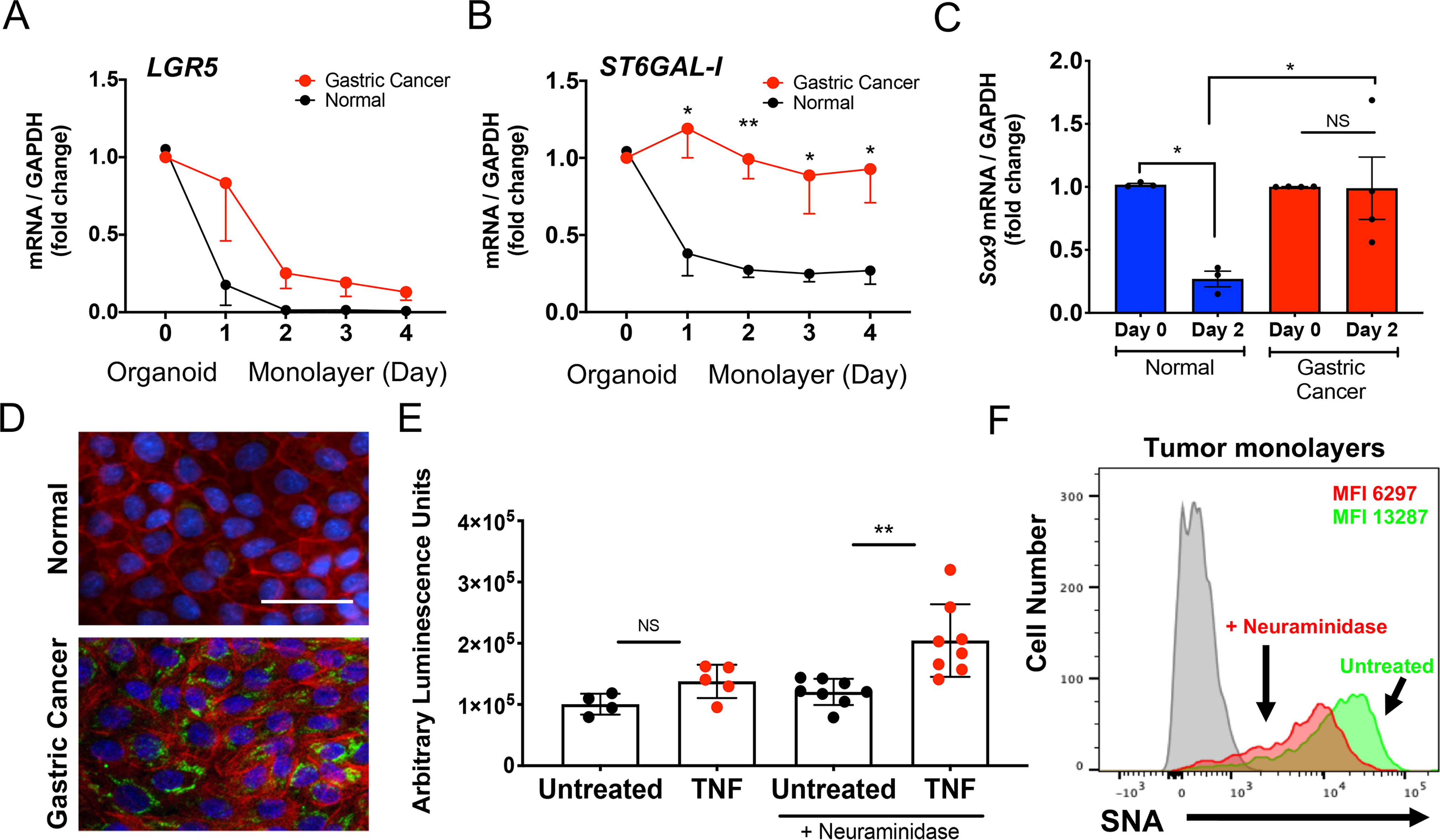
Impact of ST6Gal-I expression on gastric cancer cell apoptosis. A and B, gastric organoids and organoid-derived epithelial monolayers from gastric adenocarcinoma (red line) and normal gastric mucosa (black line) were analyzed on day 0 (organoids) and days 1–4 (epithelial monolayers) for (A) LGR5 and (B) ST6GAL1 gene expression (each n = 4). C, gastric organoids (day 0) and organoid-derived epithelial monolayers (day 2) from normal gastric tissue or gastric cancer were analyzed for SOX9 gene expression (each n = 3). D, epithelial cell monolayers generated from normal antrum-derived (top panel) and gastric adenocarcinoma–derived (bottom panel) organoids were analyzed on day 2 by immunofluorescence by staining with an ST6Gal-I antibody (FITC), phalloidin (Alexa Fluor 594) and DAPI (representative donor, 20×; n = 4; scale bar, 50 μm). E, gastric cancer organoid–derived epithelial monolayers were pretreated with or without A. ureafaciens neuraminidase and assayed for caspase 3/7 activity (n = 4–8 in three separate gastric cancer-derived monolayers). F, gastric organoid–derived epithelial monolayers were analyzed for SNA expression using flow cytometry with (red) or without (green) A. ureafaciens neuraminidase pretreatment. A–C, E, significance: *, p < 0.05; **, p < 0.01; ***, p < 0.001; and ****, p < 0.0001.
Discussion
To date, epithelial cell glycosylation in the gastric mucosa has not been rigorously explored, particularly at the stem cell level and in malignant transformation. Here, we show that epithelial stem cells derived from normal antrum expressed high levels of the glycosyltransferase ST6Gal-I in a Wnt factor–dependent manner, but presence of the enzyme was sharply reduced during and after stem cell differentiation into epithelial cell monolayers, suggesting ST6Gal-I expression reflects epithelial stemness. This finding agrees with a report of the low ST6Gal-I expression in healthy, terminally differentiated epithelium in the pancreas and ovary (7). Although stemness has not been fully defined (48), our study indicates that ST6Gal-I is a candidate biomarker of epithelial stem cells in the antral mucosa of the stomach.
In sharp contrast to the near absence of ST6Gal-I in normal antral epithelium, we show that the number of epithelial cells that express ST6Gal-I increases progressively in advancing stages of H. pylori–associated gastric premalignancy leading to, and including, gastric adenocarcinoma. This finding extends the repertoire of mucosal epithelioid tumors in which the enzyme is overexpressed to gastric cancer and suggests ST6Gal-I plays a role in the pathogenesis of malignant transformation of gastric epithelium (7, 49, 50). Infection with H. pylori initiates the premalignant changes in the stomach and is the primary risk factor for the development of gastric cancer. Although the incidence of H. pylori infection has decreased in North America and Europe recently, infection rates in Asia and Latin America have remained constant since the 1970s (19). Notably, gastric biopsies from pediatric subjects, who very rarely develop gastric cancer, displayed low levels of ST6Gal-I–expressing epithelial cells. The high-level expression of ST6Gal-I in gastric tumor epithelium from adults and the resistance of tumor organoid–derived epithelium to TNF-induced apoptosis is recapitulated in normal epithelial monolayers with forced ST6Gal-I overexpression.
Consistent with ST6Gal-I–mediated inhibition of apoptosis, gastric tumor organoid–derived epithelium was resistant to TNF-induced apoptosis, and cleavage of sialic acids implicate increased expression of ST6Gal-I inhibiting homeostatic apoptosis, thereby contributing to gastric cancer cell longevity. In this connection, the correlation between high-level ST6Gal-I expression in ovarian cancer and reduced overall survival (7) and increased resistance to chemotherapeutic agents in ST6Gal-I–expressing pancreatic cancer cell lines (47), suggest the possibility that ST6Gal-I expression in gastric cancer could similarly contribute to the poor survival and chemotherapeutic response in subjects with this malignancy.
The generation and analysis of gastric stem cell organoids as described here provides an emerging opportunity to investigate the premalignant stages of gastric cancer at the stem cell level. Studies of the human gastric epithelium and its interaction with H. pylori have been limited because of the lack of relevant models. The gastric epithelium is highly dynamic, turning over every 2–6 days (51), constantly replenished by stem cells in response to the regenerative requirements of homeostasis and injury. The progressive expression of markers of stemness, including ST6Gal-I, in epithelial monolayers derived from stage-specific organoids could contribute to more informed surveillance for the prevention of gastric cancer. In addition to enhancing prevention surveillance, gastric tissue–derived organoids might be used therapeutically, as reported in mouse (52, 53) and human (54–56) studies, to restore defective or injured epithelium.
Relevant to our study, human colon cancer organoids have been cultured with autologous peripheral blood T cells to generate tumor-reactive T cells capable of killing the organoid cells (54). Further, neuraminidase cleavage of sialic acids from TNFR1 raises the intriguing possibility of tissue-specific neuraminidase delivery to enhance TNF-induced apoptosis in ST6Gal-I overexpressing gastric cancer cells, thereby expanding the treatment options for gastric adenocarcinoma. Thus, elucidating key cellular events in gastric adenocarcinoma stem cells will enhance our understanding of gastric cancer pathogenesis and potentially inform novel treatment modalities utilizing stem cell organogenesis. In summary, our findings indicate that ST6Gal-I is a novel biomarker of gastric antral epithelial stem cells, is strongly overexpressed in gastric cancer epithelial cells, and mediates dysregulated homeostatic apoptosis, implicating a role for ST6Gal-I in epithelial cell longevity in gastric adenocarcinoma.
Experimental procedures
Human tissue samples
Biopsy specimens were obtained with Institutional Review Board for Human Use approval from the gastric antrum of healthy adults without mucosal pathology and from gastric cancer lesions from subjects with histologically confirmed gastric adenocarcinoma undergoing clinically indicated endoscopy in Birmingham, Alabama, and Santiago, Chile.
Stem cell organogenesis
Epithelial stem cell organoids were generated from gastric tissue specimens in the University of Alabama at Birmingham Stem Cell Organogenesis Unit using previously described protocols (57, 58). To generate epithelial stem cell organoids, biopsies were finely minced and digested for up to 50 min at 37°C in collagenase (2 mg/ml collagenase type 1; Corning), pipetting every 10 min to release the crypts. The collagenase then was neutralized with washing buffer (9 ml) (32), and the cell suspension filtered through a 70-μm screen and centrifuged (200 × g for 5 min at room temperature). Pelleted cells were either washed and pelleted again, or immediately suspended in Matrigel (Corning) in an ice-cold 24-well plate (15 μl/well; Corning). The Matrigel was allowed to polymerize (15 min, 37°C) with the plate upside down to prevent the epithelial cells from attaching to the plate surface, to which BASIC culture medium (450 μl/well; BASIC-CM) (32) containing a 50% mix of l-WRN CM (32) and primary culture medium (Advanced DMEM/F-12; Sigma), both supplemented with 20% fetal bovine serum (Atlanta Biologicals), l-glutamine 2 mm (Fisher Scientific), penicillin 100 units/ml, and streptomycin 0.1 mg/ml (Fisher Scientific). BASIC-CM was further supplemented with TGF-β R1 inhibitor (10 μm SB-431542; Selleck Chemicals), ROCK inhibitor (10 μm Y-27632; R&D Systems), gentamycin (50 μg/ml; Corning), and fungizone (2.5 μg/ml; Fisher Scientific) before addition to the wells. Organoids were maintained at 37°C and 5% CO2 and passaged every 4–7 days. In some experiments, the percentage of l-WRN CM in BASIC-CM was reduced from 50% to 25, 5, or 0% by dilution with additional primary culture medium. Stable organoid lines overexpressing ST6Gal-I were generated by transduction with a lentiviral vector containing the ST6GAL-I gene (Genecopoeia) or with knocked-down ST6GAL-I using shRNA (Sigma), as described previously (7). ST6Gal-I expression in the organoids was confirmed using flow cytometry. Briefly, organoids were dissociated and trypsinized, washed, and then stained with SNA (1:200 dilution; Vector Laboratories, FL1301), a lectin specific for α2,6 sialic acids.
Epithelial cell monolayers
Human epithelial stem cell organoid cultures were dissociated in trypsin, washed (washing buffer 10 ml) (32), passed through a 40-μm screen (Corning) to remove cell clusters, resuspended in 5% l-WRN BASIC medium, counted and seeded onto an 8-well glass chamber slide (200 μl/well) or 48-well cell culture plate (350 μl/well; both Corning), which was previously coated with Matrigel (1:30 dilution in PBS), polymerized for 20–30 min at 37°C, and supernatant was removed immediately prior to the addition of epithelial stem cells. The individual stem cells were allowed to differentiate, reaching confluence within 2–3 days to form a gastric epithelial cell monolayer. Monolayers typically survived at 100% confluency for 2–4 days, after which the monolayers lost viability beginning in the peripheral region of the monolayer. Each day of the culture, 50% of the media was replaced with fresh media.
Quantitative PCR
Quantitative PCR (qPCR) was performed using a QuantStudio 3 (Thermo Fisher Scientific) instrument. Briefly, organoids or monolayers were lysed in RLT buffer (Qiagen), and RNA was extracted using the RNeasy kit (Qiagen). Equal amounts of RNA were used to generate cDNA with the High Capacity cDNA Reverse Transcription Kit (Fisher). qPCR experiments used TaqMan gene expression assay primers (Thermo Fisher) and TaqMan Fast Advanced Master Mix (Thermo Fisher) to generate reactions, and the comparative CT method was used to analyze the corresponding data.
Immunofluorescence analysis
Organoids and organoid-derived epithelial cell monolayers were fixed in 2% paraformaldehyde and stained with phalloidin-Alexa Fluor 594 (Fisher A12381), DAPI (Fisher D1306), and antibodies to ST6Gal-I (goat polyclonal, R&D Systems AF5924), ZO-1 (mouse monoclonal, BD Biosciences 610967), and E-cadherin (goat polyclonal, R&D Systems AF648). Images were obtained using 10×, 20× or 40× objectives with a Nikon Eclipse TE2000-U fluorescent microscope and analyzed with NIS Elements imaging software. Bright field images of organoids and epithelial cell monolayers were taken using 4× and 10× objectives with a Nikon Eclipse TE2000-U microscope and analyzed with NIS Elements imaging software.
TNFR1 detection
Epithelial cell monolayers were allowed to reach confluence, dissociated in PBS/EDTA to isolate the individual cells, washed in PBS, and stained with TNFR1-FITC (Santa Cruz Biotechnology, catalog no. sc-731195) for 15 min at room temperature in the dark. Cells then were washed in PBS and analyzed on an LSRII flow cytometer for fluorescence.
Immunohistochemistry
Tissue sections from healthy donors and subjects through the Correa cascade, including gastric cancer, were confirmed by a pathologist (L. N. C.). Parallel sections of the paraffin-embedded tissues were incubated with ST6Gal-I (1 μg/ml; R&D Systems) or Sox9 (1 μg/ml Abcam) antibody or for 1 h at room temperature, and the number of positive cells per high power field (HPF) in 5–10 randomly selected fields per tissue section per donor, according to our published protocol (7).
Caspase assay
Epithelial cell monolayers were plated in opaque 96-well culture plates (Corning), cultured until confluent and treated with 50 ng/ml TNF for 24 h. Monolayers were brought to room temperature and 100 μl of Cell Caspase-Glo® 3/7 Reagent (Promega) was added to 100 μl of the treated cells and shaken at 500 rpm for 30 s. The plate was incubated for 30 min at room temperature and then analyzed for luminescence (Gen5 Software, BioTek plate reader). In some cases, confluent epithelial cell monolayers were treated with 0.2 units/ml neuraminidase from A. ureafaciens (Millipore Sigma) for 30 min to selectively remove α2,6-linked sialic acids (45–47). Removal of sialic acids was confirmed by flow cytometry after staining with SNA (1:200 dilution, Vector Laboratories, FL1301), a lectin specific for α2,6 sialic acids (47).
Statistical analysis
Student's t test or 1-way analysis of variance followed by Tukey's post test was performed, as indicated in the figure legends, using Prism GraphPad software with p < 0.05 considered to be statistically significant. Significance is indicated as *, p < 0.05; **, p < 0.01; ***, p < 0.001; and ****, p < 0.0001.
Data availability
All data are available in the manuscript or upon request to the corresponding author.
Author contributions—K. L. A., C. A. S., A. R., S. L. B., L. E. S., and P. D. S. conceptualization; K. L. A., C. A. S., L. E. S., and P. D. S. data curation; K. L. A., A. C., M. N., L. N. C., A. R., M. G., and S. L. B. formal analysis; K. L. A., C. A. S., A. C., M. N., and L. N. C. validation; K. L. A., C. A. S., M. N., A. R., and M. G. investigation; K. L. A., C. A. S., A. C., and P. D. S. visualization; K. L. A., C. A. S., A. C., M. N., L. N. C., M. G., and L. E. S. methodology; K. L. A. writing-original draft; K. L. A., S. L. B., L. E. S., and P. D. S. writing-review and editing; A. R., M. G., and P. D. S. resources; S. L. B., L. E. S., and P. D. S. supervision; S. L. B., L. E. S., and P. D. S. funding acquisition; L. E. S. and P. D. S. project administration.
Funding and additional information—This work was supported by National Institutes of Health Grants HD088954 (to P. D. S.), CA233581 (to S. L. B.), CA225177 (to S. L. B.), and RR20136 (to P. D. S.); University of Alabama at Birmingham Training Program in Immunologic Diseases and Basic Immunology 2T32AI007051-41 (K. L. A.); University of Alabama at Birmingham School of Medicine and Health Services Foundation GEF Awards (to L. E. S., P. D. S., Stem Cell Organogenesis Program); Broad Foundation, Broad Medical Research Program (to L. E. S.); Crohn's and Colitis Foundation of America Grant (to L. E. S.); and The DeGregorio Family Foundation Grant (to P. D. S.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. All human specimens were obtained after written informed consent approved by the University of Alabama at Birmingham Institutional Review Board and abide by the Declaration of Helsinki Principles.
Conflict of interest—The authors declare that they have no conflicts of interest with the contents of this article.
- TNF
- tumor necrosis factor
- SNA
- Sambucus nigra agglutinin
- qPCR
- quantitative PCR
- DAPI
- 4′,6-diamidino-2-phenylindole.
References
- 1. Hanahan D., and Weinberg R. A. (2000) The hallmarks of cancer. Cell 100, 57–70 10.1016/S0092-8674(00)81683-9 [DOI] [PubMed] [Google Scholar]
- 2. Plati J., Bucur O., and Khosravi-Far R. (2008) Dysregulation of apoptotic signaling in cancer: Molecular mechanisms and therapeutic opportunities. J. Cell. Biochem. 104, 1124–1149 10.1002/jcb.21707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Evan G. I., and Vousden K. H. (2001) Proliferation, cell cycle and apoptosis in cancer. Nature 411, 342–348 10.1038/35077213 [DOI] [PubMed] [Google Scholar]
- 4. Fernald K., and Kurokawa M. (2013) Evading apoptosis in cancer. Trends Cell Biol. 23, 620–633 10.1016/j.tcb.2013.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rosania R., Varbanova M., Wex T., Langner C., Bornschein J., Giorgio F., Ierardi E., and Malfertheiner P. (2017) Regulation of apoptosis is impaired in atrophic gastritis associated with gastric cancer. BMC Gastroenterol. 17, 84 10.1186/s12876-017-0640-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schultz M. J., Swindall A. F., and Bellis S. L. (2012) Regulation of the metastatic cell phenotype by sialylated glycans. Cancer Metastasis Rev. 31, 501–518 10.1007/s10555-012-9359-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schultz M. J., Holdbrooks A. T., Chakraborty A., Grizzle W. E., Landen C. N., Buchsbaum D. J., Conner M. G., Arend R. C., Yoon K. J., Klug C. A., Bullard D. C., Kesterson R. A., Oliver P. G., O'Connor A. K., Yoder B. K., et al. (2016) The tumor-associated glycosyltransferase ST6Gal-I regulates stem cell transcription factors and confers a cancer stem cell phenotype. Cancer Res. 76, 3978–3988 10.1158/0008-5472.CAN-15-2834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liu Z., Swindall A. F., Kesterson R. A., Schoeb T. R., Bullard D. C., and Bellis S. L. (2011) ST6Gal-I regulates macrophage apoptosis via α2-6 sialylation of the TNFR1 death receptor. J. Biol. Chem. 286, 39654–39662 10.1074/jbc.M111.276063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Holdbrooks A. T., Britain C. M., and Bellis S. L. (2018) ST6Gal-I sialyltransferase promotes tumor necrosis factor (TNF)-mediated cancer cell survival via sialylation of the TNF receptor 1 (TNFR1) death receptor. J. Biol. Chem. 293, 1610–1622 10.1074/jbc.M117.801480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Martin L. T., Marth J. D., Varki A., and Varki N. M. (2002) Genetically altered mice with different sialyltransferase deficiencies show tissue-specific alterations in sialylation and sialic acid 9-O-acetylation. J. Biol. Chem. 277, 32930–32938 10.1074/jbc.M203362200 [DOI] [PubMed] [Google Scholar]
- 11. Hasehira K., Tateno H., Onuma Y., Ito Y., Asashima M., and Hirabayashi J. (2012) Structural and quantitative evidence for dynamic glycome shift on production of induced pluripotent stem cells. Mol. Cell. Proteomics 11, 1913–1923 10.1074/mcp.M112.020586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Costa-Nogueira C., Villar-Portela S., Cuevas E., Gil-Martín E., and Fernández-Briera A. (2009) Synthesis and expression of CDw75 antigen in human colorectal cancer. BMC Cancer 9, 431 10.1186/1471-2407-9-431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vazquez-Martin C., Gil-Martin E., and Fernandez-Briera A. (2005) Elevation of ST6Gal I activity in malignant and transitional tissue in human colorectal cancer. Oncology 69, 436–444 10.1159/000089999 [DOI] [PubMed] [Google Scholar]
- 14. Bray F., Ferlay J., Soerjomataram I., Siegel R. L., Torre L. A., and Jemal A. (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 68, 394–424 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 15. Barker N., Huch M., Kujala P., van de Wetering M., Snippert H. J., van Es J. H., Sato T., Stange D. E., Begthel H., van den Born M., Danenberg E., van den Brink S., Korving J., Abo A., Peters P. J., et al. (2010) Lgr5+ve stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell 6, 25–36 10.1016/j.stem.2009.11.013 [DOI] [PubMed] [Google Scholar]
- 16. Sigal M., Logan C. Y., Kapalczynska M., Mollenkopf H. J., Berger H., Wiedenmann B., Nusse R., Amieva M. R., and Meyer T. F. (2017) Stromal R-spondin orchestrates gastric epithelial stem cells and gland homeostasis. Nature 548, 451–455 10.1038/nature23642 [DOI] [PubMed] [Google Scholar]
- 17. Blutt S. E., Broughman J. R., Zou W., Zeng X. L., Karandikar U. C., In J., Zachos N. C., Kovbasnjuk O., Donowitz M., and Estes M. K. (2017) Gastrointestinal microphysiological systems. Exp. Biol. Med. (Maywood) 242, 1633–1642 10.1177/1535370217710638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rugge M., Genta R. M., Di Mario F., El-Omar E. M., El-Serag H. B., Fassan M., Hunt R. H., Kuipers E. J., Malfertheiner P., Sugano K., and Graham D. Y. (2017) Gastric cancer as preventable disease. Clin. Gastroenterol. Hepatol. 15, 1833–1843 10.1016/j.cgh.2017.05.023 [DOI] [PubMed] [Google Scholar]
- 19. Hooi J. K. Y., Lai W. Y., Ng W. K., Suen M. M. Y., Underwood F. E., Tanyingoh D., Malfertheiner P., Graham D. Y., Wong V. W. S., Wu J. C. Y., Chan F. K. L., Sung J. J. Y., Kaplan G. G., and Ng S. C. (2017) Global prevalence of helicobacter pylori infection: Systematic review and meta-analysis. Gastroenterology 153, 420–429 10.1053/j.gastro.2017.04.022 [DOI] [PubMed] [Google Scholar]
- 20. Ferro A., Peleteiro B., Malvezzi M., Bosetti C., Bertuccio P., Levi F., Negri E., La Vecchia C., and Lunet N. (2014) Worldwide trends in gastric cancer mortality (1980–2011), with predictions to 2015, and incidence by subtype. Eur. J. Cancer 50, 1330–1344 10.1016/j.ejca.2014.01.029 [DOI] [PubMed] [Google Scholar]
- 21. Parkin D. M. (2006) The global health burden of infection-associated cancers in the year 2002. Int. J. Cancer 118, 3030–3044 10.1002/ijc.21731 [DOI] [PubMed] [Google Scholar]
- 22. Blaser M. J., and Kirschner D. (1999) Dynamics of Helicobacter pylori colonization in relation to the host response. Proc. Natl. Acad. Sci. U. S. A. 96, 8359–8364 10.1073/pnas.96.15.8359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Blaser M. J., and Atherton J. C. (2004) Helicobacter pylori persistence: Biology and disease. J. Clin. Invest. 113, 321–333 10.1172/JCI20925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Correa P., and Houghton J. (2007) Carcinogenesis of Helicobacter pylori. Gastroenterology 133, 659–672 10.1053/j.gastro.2007.06.026 [DOI] [PubMed] [Google Scholar]
- 25. Salama N. R., Hartung M. L., and Müller A. (2013) Life in the human stomach: Persistence strategies of the bacterial pathogen Helicobacter pylori. Nat. Rev. Microbiol. 11, 385–399 10.1038/nrmicro3016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ohata H., Kitauchi S., Yoshimura N., Mugitani K., Iwane M., Nakamura H., Yoshikawa A., Yanaoka K., Arii K., Tamai H., Shimizu Y., Takeshita T., Mohara O., and Ichinose M. (2004) Progression of chronic atrophic gastritis associated with Helicobacter pylori infection increases risk of gastric cancer. Int. J. Cancer 109, 138–143 10.1002/ijc.11680 [DOI] [PubMed] [Google Scholar]
- 27. Harting M. T., Blakely M. L., Herzog C. E., Lally K. P., Ajani J. A., and Andrassy R. J. (2004) Treatment issues in pediatric gastric adenocarcinoma. J. Pediatr. Surg. 39, e8–e10 10.1016/j.jpedsurg.2004.04.043 [DOI] [PubMed] [Google Scholar]
- 28. Harris P. R., Wright S. W., Serrano C., Riera F., Duarte I., Torres J., Peña A., Rollán A., Viviani P., Guiraldes E., Schmitz J. M., Lorenz R. G., Novak L., Smythies L. E., and Smith P. D. (2008) Helicobacter pylori gastritis in children is associated with a regulatory T-cell response. Gastroenterology 134, 491–499 10.1053/j.gastro.2007.11.006 [DOI] [PubMed] [Google Scholar]
- 29. Subbiah V., Varadhachary G., Herzog C. E., and Huh W. W. (2011) Gastric adenocarcinoma in children and adolescents. Pediatr. Blood Cancer 57, 524–527 10.1002/pbc.23051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Aguilar-Medina M., Avendaño-Félix M., Lizárraga-Verdugo E., Bermúdez M., Romero-Quintana J. G., Ramos-Payan R., Ruíz-García E., and López-Camarillo C. (2019) SOX9 stem-cell factor: Clinical and functional relevance in cancer. J. Oncol. 2019, 6754040 10.1155/2019/6754040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Serizawa T., Hirata Y., Hayakawa Y., Suzuki N., Sakitani K., Hikiba Y., Ihara S., Kinoshita H., Nakagawa H., Tateishi K., and Koike K. (2016) Gastric metaplasia induced by Helicobacter pylori is associated with enhanced SOX9 expression via interleukin-1 signaling. Infect. Immun. 84, 562–572 10.1128/IAI.01437-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Miyoshi H., and Stappenbeck T. S. (2013) In vitro expansion and genetic modification of gastrointestinal stem cells in spheroid culture. Nat. Protoc. 8, 2471–2482 10.1038/nprot.2013.153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wajant H., Pfizenmaier K., and Scheurich P. (2003) Tumor necrosis factor signaling. Cell Death Differ. 10, 45–65 10.1038/sj.cdd.4401189 [DOI] [PubMed] [Google Scholar]
- 34. Bradley J. R., Thiru S., and Pober J. S. (1995) Disparate localization of 55-kd and 75-kd tumor necrosis factor receptors in human endothelial cells. Am. J. Pathol. 146, 27–32 [PMC free article] [PubMed] [Google Scholar]
- 35. Jones S. J., Ledgerwood E. C., Prins J. B., Galbraith J., Johnson D. R., Pober J. S., and Bradley J. R. (1999) TNF recruits TRADD to the plasma membrane but not the trans-Golgi network, the principal subcellular location of TNF-R1. J. Immunol. 162, 1042–1048 [PubMed] [Google Scholar]
- 36. Schütze S., Tchikov V., and Schneider-Brachert W. (2008) Regulation of TNFR1 and CD95 signalling by receptor compartmentalization. Nat. Rev. Mol. Cell Biol. 9, 655–662 10.1038/nrm2430 [DOI] [PubMed] [Google Scholar]
- 37. Schneider-Brachert W., Tchikov V., Neumeyer J., Jakob M., Winoto-Morbach S., Held-Feindt J., Heinrich M., Merkel O., Ehrenschwender M., Adam D., Mentlein R., Kabelitz D., and Schütze S. (2004) Compartmentalization of TNF receptor 1 signaling: internalized TNF receptosomes as death signaling vesicles. Immunity 21, 415–428 10.1016/j.immuni.2004.08.017 [DOI] [PubMed] [Google Scholar]
- 38. Schütze S., Machleidt T., Adam D., Schwandner R., Wiegmann K., Kruse M. L., Heinrich M., Wickel M., and Krönke M. (1999) Inhibition of receptor internalization by monodansylcadaverine selectively blocks p55 tumor necrosis factor receptor death domain signaling. J. Biol. Chem. 274, 10203–10212 10.1074/jbc.274.15.10203 [DOI] [PubMed] [Google Scholar]
- 39. Micheau O., and Tschopp J. (2003) Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell 114, 181–190 10.1016/S0092-8674(03)00521-X [DOI] [PubMed] [Google Scholar]
- 40. Schneider-Brachert W., Heigl U., and Ehrenschwender M. (2013) Membrane trafficking of death receptors: Implications on signalling. Int. J. Mol. Sci. 14, 14475–14503 10.3390/ijms140714475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cendrowski J., Mamińska A., and Miaczynska M. (2016) Endocytic regulation of cytokine receptor signaling. Cytokine Growth Factor Rev. 32, 63–73 10.1016/j.cytogfr.2016.07.002 [DOI] [PubMed] [Google Scholar]
- 42. Matheu A., Collado M., Wise C., Manterola L., Cekaite L., Tye A. J., Canamero M., Bujanda L., Schedl A., Cheah K. S. E., Skotheim R. I., Lothe R. A., López de Munain A., Briscoe J., Serrano M., et al. (2012) Oncogenicity of the developmental transcription factor Sox9. Cancer Res. 72, 1301–1315 10.1158/0008-5472.CAN-11-3660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Elena E., Roopma W., Hironori S., Kazuki S., Jeannelyn S. E., Brian D. B., Prajnan D., Aurelio M., Shumei S., and Jaffer A. A. (2015) Molecular biomarkers in gastric cancer. J. Natl. Compr. Canc. Netw. 13, e19–e29 10.6004/jnccn.2015.0064 [DOI] [PubMed] [Google Scholar]
- 44. Ruan H., Hu S., Zhang H., Du G., Li X., Li X., and Li X. (2017) Upregulated SOX9 expression indicates worse prognosis in solid tumors: A systematic review and meta-analysis. Oncotarget 8, 113163–113173 10.18632/oncotarget.22635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Suzuki O., and Abe M. (2014) Galectin-1-mediated cell adhesion, invasion and cell death in human anaplastic large cell lymphoma: Regulatory roles of cell surface glycans. Int. J. Oncol. 44, 1433–1442 10.3892/ijo.2014.2319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Uchida Y., Tsukada Y., and Sugimori T. (1979) Enzymatic properties of neuraminidases from Arthrobacter ureafaciens. J. Biochem. 86, 1573–1585 10.1093/oxfordjournals.jbchem.a132675 [DOI] [PubMed] [Google Scholar]
- 47. Chakraborty A., Dorsett K. A., Trummell H. Q., Yang E. S., Oliver P. G., Bonner J. A., Buchsbaum D. J., and Bellis S. L. (2018) ST6Gal-I sialyltransferase promotes chemoresistance in pancreatic ductal adenocarcinoma by abrogating gemcitabine-mediated DNA damage. J. Biol. Chem. 293, 984–994 10.1074/jbc.M117.808584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Farin H. F., Jordens I., Mosa M. H., Basak O., Korving J., Tauriello D. V., de Punder K., Angers S., Peters P. J., Maurice M. M., and Clevers H. (2016) Visualization of a short-range Wnt gradient in the intestinal stem-cell niche. Nature 530, 340–343 10.1038/nature16937 [DOI] [PubMed] [Google Scholar]
- 49. Swindall A. F., and Bellis S. L. (2011) Sialylation of the Fas death receptor by ST6Gal-I provides protection against Fas-mediated apoptosis in colon carcinoma cells. J. Biol. Chem. 286, 22982–22990 10.1074/jbc.M110.211375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Swindall A. F., Londoño-Joshi A. I., Schultz M. J., Fineberg N., Buchsbaum D. J., and Bellis S. L. (2013) ST6Gal-I protein expression is upregulated in human epithelial tumors and correlates with stem cell markers in normal tissues and colon cancer cell lines. Cancer Res. 73, 2368–2378 10.1158/0008-5472.CAN-12-3424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Eastwood G. L. (1977) Gastrointestinal epithelial renewal. Gastroenterology 72, 962–975 10.1016/S0016-5085(77)80221-7 [DOI] [PubMed] [Google Scholar]
- 52. Roper J., Tammela T., Akkad A., Almeqdadi M., Santos S. B., Jacks T., and Yilmaz O. H. (2018) Colonoscopy-based colorectal cancer modeling in mice with CRISPR-Cas9 genome editing and organoid transplantation. Nat. Protoc. 13, 217–234 10.1038/nprot.2017.136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Miyoshi H., Ajima R., Luo C. T., Yamaguchi T. P., and Stappenbeck T. S. (2012) Wnt5a potentiates TGF-β signaling to promote colonic crypt regeneration after tissue injury. Science 338, 108–113 10.1126/science.1223821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Dijkstra K. K., Cattaneo C. M., Weeber F., Chalabi M., van de Haar J., Fanchi L. F., Slagter M., van der Velden D. L., Kaing S., Kelderman S., van Rooij N., van Leerdam M. E., Depla A., Smit E. F., Hartemink K. J., et al. (2018) Generation of tumor-reactive T cells by co-culture of peripheral blood lymphocytes and tumor organoids. Cell 174, 1586–1598.e12 10.1016/j.cell.2018.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Molendijk I., Bonsing B. A., Roelofs H., Peeters K. C., Wasser M. N., Dijkstra G., van der Woude C. J., Duijvestein M., Veenendaal R. A., Zwaginga J. J., Verspaget H. W., Fibbe W. E., van der Meulen-de Jong A. E., and Hommes D. W. (2015) Allogeneic bone marrow-derived mesenchymal stromal cells promote healing of refractory perianal fistulas in patients with Crohn's disease. Gastroenterology 149, 918–927.e6 10.1053/j.gastro.2015.06.014 [DOI] [PubMed] [Google Scholar]
- 56. Fox I. J., Daley G. Q., Goldman S. A., Huard J., Kamp T. J., and Trucco M. (2014) Stem cell therapy. Use of differentiated pluripotent stem cells as replacement therapy for treating disease. Science 345, 1247391 10.1126/science.1247391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. VanDussen K. L., Sonnek N. M., and Stappenbeck T. S. (2019) L-WRN conditioned medium for gastrointestinal epithelial stem cell culture shows replicable batch-to-batch activity levels across multiple research teams. Stem Cell Res. 37, 101430 10.1016/j.scr.2019.101430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. VanDussen K. L., Marinshaw J. M., Shaikh N., Miyoshi H., Moon C., Tarr P. I., Ciorba M. A., and Stappenbeck T. S. (2015) Development of an enhanced human gastrointestinal epithelial culture system to facilitate patient-based assays. Gut 64, 911–920 10.1136/gutjnl-2013-306651 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are available in the manuscript or upon request to the corresponding author.


