Abstract
Actin's interactions with myosin and other actin-binding proteins are essential for cellular viability in numerous cell types, including muscle. In a previous high-throughput time-resolved FRET (TR-FRET) screen, we identified a class of compounds that bind to actin and affect actomyosin structure and function. For clinical utility, it is highly desirable to identify compounds that affect skeletal and cardiac muscle differently. Because actin is more highly conserved than myosin and most other muscle proteins, most such efforts have not targeted actin. Nevertheless, in the current study, we tested the specificity of the previously discovered actin-binding compounds for effects on skeletal and cardiac α-actins as well as on skeletal and cardiac myofibrils. We found that a majority of these compounds affected the transition of monomeric G-actin to filamentous F-actin, and that several of these effects were different for skeletal and cardiac actin isoforms. We also found that several of these compounds affected ATPase activity differently in skeletal and cardiac myofibrils. We conclude that these structural and biochemical assays can be used to identify actin-binding compounds that differentially affect skeletal and cardiac muscles. The results of this study set the stage for screening of large chemical libraries for discovery of novel compounds that act therapeutically and specifically on cardiac or skeletal muscle.
Keywords: Actin, skeletal muscle, cardiac muscle, compound, myofibril, ATPase, actin, myosin
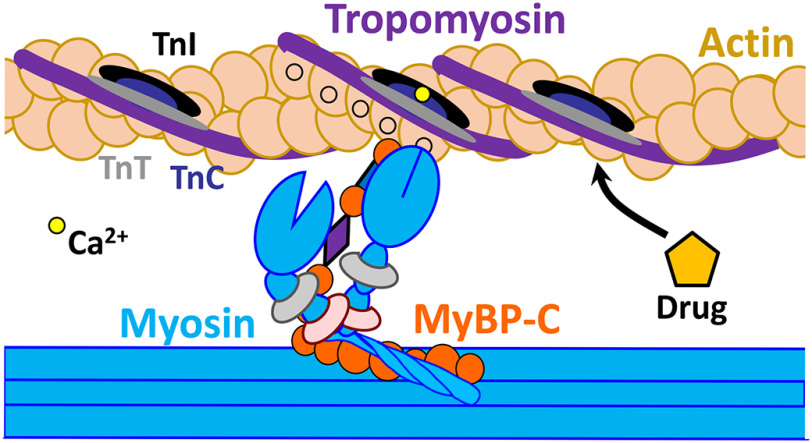
Muscle contraction results from interaction between myosin and actin, in which the transition from relaxation to contraction depends on both (a) calcium-mediated changes in the actin-bound regulatory proteins in the troponin complex (TnI, TnC, TnT) and tropomyosin (Tm), and (b) additional cooperative activation due to the strong binding of myosin to actin (1). The sarcomere is in a dynamic equilibrium between relaxed and contracted states, with calcium concentrations shifting the balance. Under resting conditions with low calcium, the troponin (Tn) complex maintains actin–tropomyosin in a “blocked” state that prevents actin–myosin interaction (2). When the intracellular calcium concentration increases, a conformational change in the Tn complex and weak binding of myosin to actin allows Tm to shift into the “open” position that promotes strong actin–myosin binding (2), thereby triggering muscle contraction mediated by the chemical energy generated from myosin ATP hydrolysis (Fig. 1). Myosin-binding protein C (MyBP-C) is a sarcomeric regulatory protein (3) (Fig. 1). The C-terminal domains of MyBP-C are anchored to titin (4) and myosin light meromyosin (5), whereas the N-terminal domains interact with myosin subfragment 2 and probably also with actin (6). Genetic mutation or a post-translational modification (e.g. oxidation, glycation) of a sarcomeric protein can alter its structure and function and have a detrimental effect on the process of contractility (7, 8). The binding of a small molecule to that altered protein can restore normal contractility (9). Such therapeutic molecules or drugs can work indirectly by intervening in signaling pathways, or directly through interaction with contractile proteins (10). Discovery of such modulators presents a novel approach to treating any condition in which striated muscle function is compromised, including a wide range of cardiomyopathies (9, 11), skeletal muscle myopathies (12–16), and neuromuscular conditions (17).
Figure 1.
Schematic representation of thin (actin, tropomyosin, and troponins) and thick filaments (myosin and MyBP-C) substructures, key participants in Ca2+-dependent muscle contraction and relaxation. Actin-binding compounds can affect interaction between actin and these actin-binding partners and play a crucial role in regulation of these processes.
Sarcomeric modulators identified through small-molecule screening have been shown to act at three different levels: (a) signaling pathways that affect protein phosphorylation, (b) direct interaction with myosin, and (c) interaction with the troponin complex (10). Small-molecule effectors designed to target and modulate striated and smooth muscle myosin isoforms for the treatment of diseases are showing some promising results in preclinical and clinical trials. A cardiac myosin effector, omecamtiv mecarbil, identified via a low-throughput functional assay, has so far only marginally improved the condition of heart failure patients compared with placebo controls (18). Another cardiac myosin modulator, mavacamten, was found to decrease cardiac contractility; and in the future may be applied to treat diseases in which hypercontractility is an issue (19). Several troponin-specific modulators have been identified. Tirasemtiv and CK-2066260, the most advanced of these agents, binds specifically to fast skeletal troponin C (20, 21) and has no observable affinity for cardiac troponin C. In drug development for muscle disorders, it is critical to determine a compound's specificity for muscle type in a high-throughput manner, to maximize its advantages and minimize its disadvantages in clinical application.
None of the previous studies attempted to target striated muscle actin, the main binding companion of striated muscle myosin. Actin is a highly conserved protein that interacts with multiple proteins in both muscle and nonmuscle cells. Actin is flexible and dynamic, and its different structural states can be sensed by its binding partners. Higher eukaryotes have six different actins, expressed from separate genes (22), with most sequence variability occurring in the N-terminal region. In this work, we focus on skeletal and cardiac α-actin. These two isoforms are 99% homologous, with only 4 amino acid substitutions from skeletal to cardiac: Glu2-Asp, Asp3-Glu, Met299-Leu, and Thr358-Ser (23); nevertheless, the two isoforms show significant differences in their functional properties (23, 24). The rates of actin polymerization and activation of myosin ATPase are essentially the same among these actin isoforms (25), but myosin cross-bridge formation and myofiber force production are significantly different (26). In their respective muscles, skeletal and cardiac actin filaments interact with myosin and regulatory proteins such as Tn, Tm, and MyBP-C, at different stages of contraction and relaxation. These interactions are finely tuned, and we expect that a subtle change induced by actin-binding modulators can play an important role in therapeutic treatment for muscle disorders (Fig. 1). Recent studies from our group utilized a high-precision time-resolved FRET approach for high-throughput screening of a small-molecule library and detected several compounds that bind to actin and affect actomyosin structure and function (27) (Table 1). In the current study, we have established reliable biochemical and structural assays to test whether these compounds are specific for skeletal or cardiac muscle protein isoforms. In particular, we focus on the effects on polymerization of both actin isoforms, as well as interaction with myosin in the presence of regulatory proteins, as detected by myofibrillar ATPase activity. We found that these compounds have subtle but differential effects on different isoforms of actin, which impact function of the corresponding muscle type. Thus, our results set the stage to screen large chemical libraries for discovery of novel actin-binding drugs that act specifically on cardiac or skeletal muscle.
Table 1.
Actin-binding compounds detected from high-throughput FRET assay (27)
| Compound | Clinical use |
|---|---|
| 1. Honokiol | Anticancer |
| 2. Fluphenazine | Antipsychotic |
| 3. Phenothiazine | Antipsychotic |
| 4. Tegaserod | Irritable bowel syndrome |
| 5. Carvedilol | β Blocker, treats high BP |
| 6. Novantrone | Multiple sclerosis |
| 7. Thioridazine | Antipsychotic |
| 8. Flutamide | Antiandrogen, anticancer |
| 9. Mefloquine | Anti-malaria |
| 10. Dantrolene | Postsynaptic muscle relaxant |
Results
Effects of compounds on actin polymerization and depolymerization
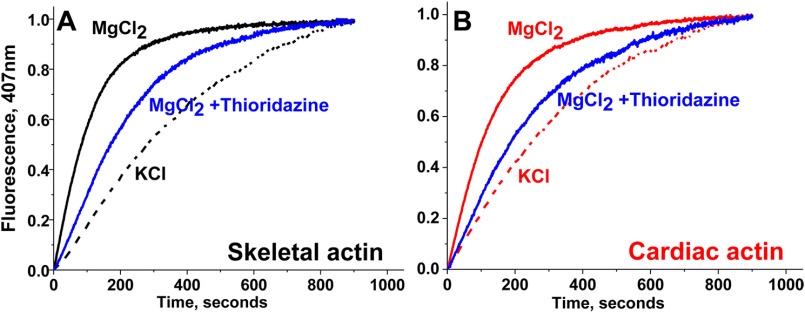
The time course of polymerization of rabbit skeletal and bovine cardiac G-actin was quantified by the increase in fluorescence intensity of pyrene-labeled actin with excitation at 350 nm and emission at 407 nm (Fig. 2). When pyrene–G-actin polymerizes to form F-actin, the pyrene fluorescence intensity increases 7-10–fold, in proportion to the fraction of G-actin incorporated into F-actin (28). Control experiments showed that the compounds in Table 1 did not have significant effects on the fluorescence of G-actin. The polymerization kinetics of skeletal and cardiac actin, measured in the presence of either 50 mm KCl or 2 mm MgCl2, shows that polymerization is faster in the presence of MgCl2 for both isoforms (Fig. 2), as reported previously (28). Polymerization behavior of skeletal and cardiac actin isoforms in response to the different salts (cations) confirms that both proteins are sensitive to different conditions and are adequate to detect compound-specific effects. The effects of the compounds (listed in Table 1) on polymerization of both actin isoforms were then determined in the presence of 50 μm compound (Figs. 2 and 3). Prior to the experiment, skeletal and cardiac G-actin containing 5% pyrene–G-actin were incubated with compounds for 10 min at 23°C. After incubation, 2 mm MgCl2 was added, and polymerization was monitored for at least 15 min, until the saturation of fluorescence intensity was achieved.
Figure 2.
Salt and compound sensitivity of actin polymerization. A, rabbit skeletal actin. B, bovine cardiac actin. In both cases polymerization in faster in the presence of MgCl2 (2 mm) than in the presence of KCl (50 mm). Addition of 50 μm thioridazine slows polymerization of both actin isoforms.
Figure 3.
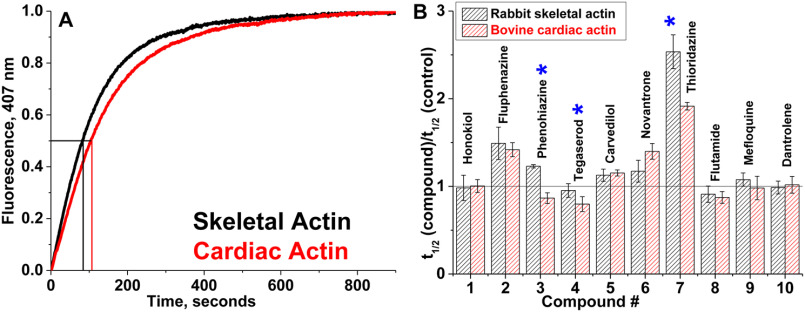
Actin polymerization. A, time courses of polymerization of skeletal and cardiac actin in the absence of compounds. The increase in fluorescence of skeletal and cardiac actin, containing 5% pyrene actin, was monitored at 407 nm. Polymerization half-times (vertical lines) denote the times required for actin to reach half-maximal fluorescence (horizontal line). For each actin species, at least three independent preparations were used. B, relative change in apparent half-times (t½) of polymerization of actin isoforms in the presence of 50 μm compound. Errors are reported as standard deviation, n = 3. Significant difference (*) in drug effect on skeletal versus cardiac actin was established with Student's t test, p < 0.05.
Skeletal and cardiac actin show similar polymerization rates in the absence of compounds (Fig. 3A). The effect of compound on each actin isoform was determined from the ratio of polymerization half-time (t½) in the presence and absence of compound (Fig. 3B). Fluphenazine, carvedilol, novantrone, and thioridazine reduce the rate of polymerization (higher t½) of both actin isoforms. However, with thioridazine, the decrease is more pronounced for skeletal compared with cardiac. Phenothiazine increases the rate of polymerization of cardiac actin and reduces the rate for skeletal actin. Tegaserod and flutamide increase the rate of polymerization of both actin isoforms, and the effect of tegaserod is more pronounced on cardiac actin. Honokiol, dantrolene, and mefloquine did not have a significant effect on the rate of polymerization of either skeletal or cardiac actin. Thus phenothiazine, tegaserod, and thioridazine affect actin polymerization in a isoform-specific manner.
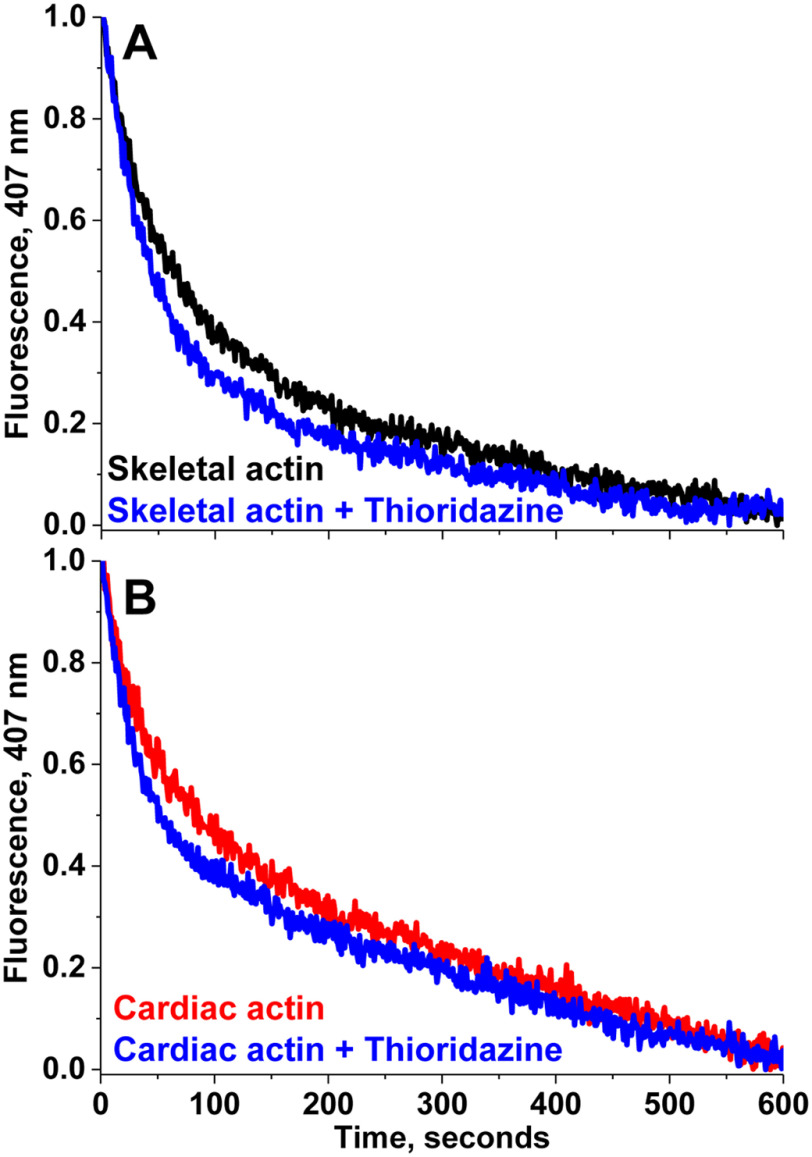
The effects of compounds on actin depolymerization were evaluated with a similar fluorescence technique. The time course of depolymerization of rabbit skeletal and bovine cardiac G-actin was quantified by the decrease in fluorescence intensity of F-actin, diluted into G-buffer. G-actin (20 μm) containing 5% pyrene actin was first polymerized with 2 mm MgCl2 for at least 2 h at 23 °C. This F-actin was diluted 1:20 into G-buffer and the decrease in fluorescence intensity in the presence and absence of drug was monitored at 407 nm wavelength. Fig. 4 shows an example of depolymerization of skeletal and cardiac actin in the presence and absence of a representative drug, thioridazine. The data indicates no change in depolymerization rate. Compounds from Table 1 did not have a significant effect on the rate of depolymerization of either actin isoform. These data suggest that both isoforms of actin are able to maintain filament integrity in the presence of compounds. Any observed functional alterations in muscle due to these compounds is probably not due to the depolymerization of actin. The significant effects on polymerization (Fig. 3) indicate perturbation of actin structure.
Figure 4.
Effect of thioridazine on depolymerization of F-actin. A, skeletal and B, cardiac. Time course of depolymerization of skeletal and cardiac was monitored by decrease in pyrene fluorescence at 407 nm in the presence and absence of a representative compound, thioridazine (50 μm).
Effects of compounds on skeletal and cardiac myofibril ATPase
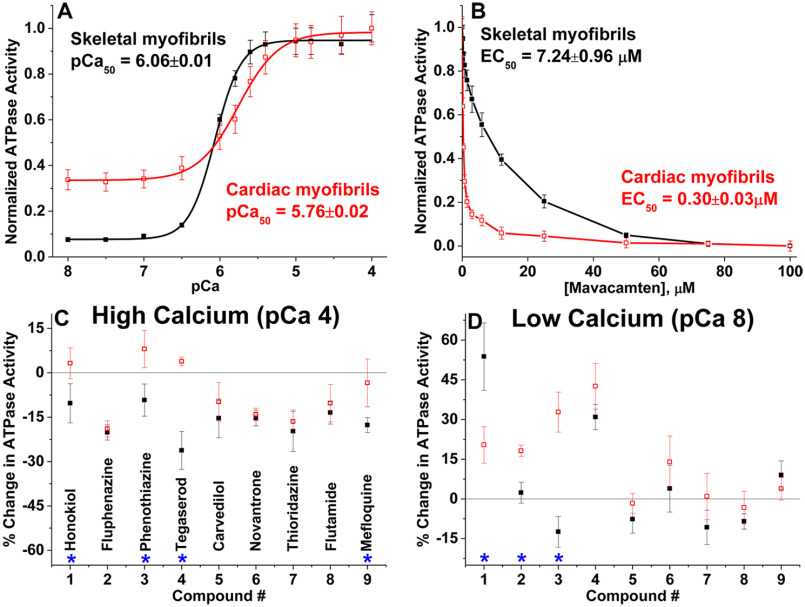
To further determine the effects of compounds' actin isoform specificity on muscle function, we examined the Ca2+-dependent ATPase activity of rabbit fast skeletal (psoas) and bovine cardiac (left ventricle) myofibrils in a high-throughput manner, varying the free calcium concentration from pCa 8 (relaxation) to pCa 4 (full activation). Fig. 5A confirms that skeletal myofibrils have a 10-fold activation in response to calcium (21, 29, 30), whereas activation of cardiac myofibrils is 4-fold (31). At pCa 4, skeletal and cardiac myofibril ATPase show a Vmax of 0.4488 ± 0.0264 and 0.0.0372 ± 0.0024 μmol of ATP/mg of protein/min, respectively. At pCa 8, their corresponding basal ATPase rates (V0) are 0.0336 ± 0.0012 and 0.0096 ± 0.0004 μmol of ATP/mg of protein/min. The pCa50 values of skeletal and cardiac myofibrils are 6.06 ± 0.01 and 5.76 ± 0.02. These values are consistent with previous reports (21, 27, 29, 31–33). The myofibril ATPase activities of both muscle systems were not affected either by the presence of 1% DMSO (data not shown) or by 500 nm thapsigargin, a SERCA (sarcoendoplasmic reticulum calcium transport ATPase pump) modulator (data not shown). Lack of any significant changes in the maximal ATPase activity or pCa50 in either of the myofibril types indicates that the measured Ca2+-dependent activities are determined by myofilament-based calcium regulation, and are not affected by contaminating residual SERCA in the preparation.
Figure 5.
Effects of compounds on skeletal and cardiac myofibrillar ATPase activity at high and low calcium concentrations. A, Ca2+-dependent ATPase activation of rabbit fast skeletal myofibrils (black filled squares, n = 8 independent preparations) and bovine cardiac myofibrils (red open squares, n = 10 independent preparations), without compounds. ATPase activities are normalized to the respective maximum ATP turnover rate at saturating Ca2+ concentration. B, concentration-dependent effects of mavacamten, a known cardiac-specific myosin inhibitor. C, effect of 50 μm compounds on ATPase activities at pCa 4, and D, at pCa 8, expressed as percent change in ATPase activity. Error bars indicate standard deviation (n ≥ 8). Significant difference (*) in drug effects on skeletal versus cardiac myofibril was established with Student's t test, p < 0.05.
To determine the effect of each compound, the ATPase rate of myofibrils in the presence of each compound was normalized that of the 1% DMSO-only control. The high-throughput plate reader ATPase assay was first validated with mavacamten, a known cardiac-specific myosin-binding compound (19). The effective concentration of mavacamten that gives half-maximal response (EC50) is 0.30 ± 0.03 μm for cardiac myofibrils and 7.24 ± 0.96 μm for skeletal (Fig. 5B). Thus, mavacamten selectively inhibits the ATPase activity of cardiac myofibrils compared with skeletal myofibrils, as reported previously (34). Using the same assay, we tested the effect of actin-binding compounds (Table 1) on both types of myofibrils at pCa 4 and 8. Fig. 5, C and D, summarize the comparative percent change in ATPase activities of respective skeletal and cardiac myofibrils in the presence of 50 μm actin-binding compound (same concentration as used in actin polymerization). At this concentration, dantrolene was unstable and caused aggregation of myofibrils, and thus was omitted from the ATPase measurement.
At pCa 4 (high calcium), all of the tested compounds act as inhibitors of ATPase for skeletal myofibrils and some compounds had ∼20% inhibitory effects on cardiac myofibrils (Fig. 5C). Four compounds (honokiol, phenothiazine, tegaserod, and mefloquine) show significantly different effects on ATPase of cardiac and skeletal myofibrils under activating conditions. The aforementioned drugs have minimal effect on cardiac myofibril ATPase activity, but they inhibit skeletal myofibrils. Tegaserod exhibits the most significantly different effects on the two muscle systems; it slightly activates cardiac myofibrils and strongly inhibits (30 ± 7%) skeletal myofibrils. The difference between the two muscle systems for honokiol (13 ± 8%), phenothiazine (17 ± 8%), and mefloquine (14 ± 8%) were also statistically significant (p < 0.05).
Isoform-specific differences in myofibril ATPase activity were also observed at pCa 8 (relaxation). Four compounds (honokiol, fluphenazine, phenothiazine, and tegaserod) activated cardiac myofibril ATPase. Of those four, only two also activated skeletal myofibrils: honokiol and tegaserod. Only honokiol, fluphenazine, and phenothiazine showed significantly different effects on the two muscle systems (Fig. 5D). The percent activation for honokiol is 54 ± 13% for cardiac and 20 ± 7% for skeletal. Fluphenazine's differential effect was less pronounced where cardiac myofibril ATPase is activated by 18 ± 2%, whereas it does not have an effect on skeletal myofibril. Another significant difference in effect on myofibrils ATPase activity at pCa 8 is from phenothiazine. This drug had minimal inhibitory effect on skeletal myofibrils in relaxing conditions, but it increased basal ATPase activity of cardiac myofibrils by 33 ± 8%. Thus, these drugs may play an important role in the calcium sensitization or desensitization of a specific muscle type.
Because tegaserod, phenothiazine, and fluphenazine showed significant effects on either actin polymerization or myofibrillar ATPase activity, they were further tested for their effects on actin-activated myosin ATPase activity of purified actin and myosin subfragment 1 (S1) from skeletal and cardiac muscles. The purified skeletal and cardiac acto-S1 ATPase activities provide insights on the drugs' possible effects on actomyosin (AM) interactions, independent of thin filament regulatory proteins. The respective skeletal and cardiac myofibrillar Ca2+-dependent ATPase activities were also examined to determine shifts in pCa50 (pCa at half-maximal ATPase activity) in response to drugs (Table 2, Fig. 6). pCa50 is a measure of myofibrillar calcium sensitivity and thus provides insight into drugs' effects on the actin-regulatory protein (AR) interaction.
Table 2.
Effect of selected drugs on purified actomyosin and on myofibrils
Values are mean ± S.D. for n = 3 to 8 preparations.
| Compound | Acto-S1 ATPase (% change) |
Myofibril ATPase (% change) |
Ca2+ sensitivity pCa50 |
Possible affected interaction |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Skeletal | Cardiac | Skeletal | Cardiac | Skeletal | Cardiac | Skeletal | Cardiac | |||
| pCa 4 | pCa 8 | pCa 4 | pCa 8 | |||||||
| Phenothiazine | −6.4 ± 3.3 | −29.8 ± 8.1 | −9.2 ± 5.4 | −12.4 ± 5.8 | 8.0 ± 6.0 | 32.7 ± 7.5 | Desensitizer | No effect | AM | AM, AR |
| Tegaserod | −2.3 ± 0.3 | −18.4 ± 7.1 | −26.0 ± 6.4 | 30.9 ± 4.8 | 3.9 ± 1.5 | 42.5 ± 8.6 | Sensitizer | Sensitizer | AM, AR | AM, AR |
| Fluphenazine | −45.4 ± 1.8 | −20.4 ± 3.4 | −20.2 ± 2.6 | 2.3 ± 3.9 | −18.9 ± 2.7 | 18.2 ± 2.2 | Sensitizer | Sensitizer | AM | AM, AR |
Figure 6.
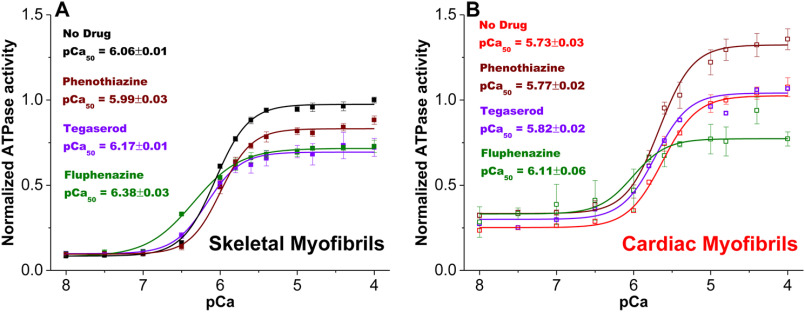
Effect of phenothiazine, tegaserod, and fluphenazine on myofibrillar calcium-dependent ATPase activity and sensitivity. A, ATPase activity of rabbit skeletal myofibrils was measured in the absence (black) and presence of 50 μm tegaserod (purple), phenothiazine (brown), or fluphenazine (green) across a 12-point Ca gradient, pCa 4-8, in a 96-well–plate. Error bars are standard deviation (n = 4).
Tegaserod, phenothiazine, and fluphenazine inhibited skeletal and cardiac acto-S1 ATPases (Table 2), mostly consistent with their effects on myofibrils at activating conditions (Fig. 5C). This indicates that these compounds are effectors of actomyosin interaction. The addition of tegaserod to rabbit skeletal myofibrils resulted in a slight leftward shift of the calcium-dependent ATPase curve (Fig. 6A), with pCa50 shifting from 6.06 ± 0.01 before treatment (control) to 6.17 ± 0.01 after addition of 50 μm compound. The slight leftward shift in pCa50 with tegaserod was also observed in cardiac myofibrils, going from 5.73 ± 0.03 to 5.82 ± 0.02 (Fig. 6B). 50 μm phenothiazine produced a slight rightward shift in pCa50 to 5.99 ± 0.03 for skeletal myofibrils (Fig. 6A). No effect on pCa50 (5.77 ± 0.02) was observed for cardiac myofibrils (Fig. 6B). Fluphenazine resulted a leftward shift in pCa50 for skeletal (6.38 ± 0.03) as well as cardiac (6.11 ± 0.06) myofibril. Thus, tegaserod and fluphenazine act as sensitizers and phenothiazine acts as a desensitizer for skeletal muscle to calcium. In cardiac muscle, tegaserod and fluphenazine act as calcium sensitizers and phenothiazine has no effect on calcium sensitivity.
Discussion
We report a comparative study of a new group of actin-binding compounds, previously detected by our high-throughput and high-precision FRET assay (27), on the polymerization of skeletal and cardiac actin as well as on the ATPase activities of skeletal and cardiac myofibrils, with the goal of testing specificity for actin and muscle isoforms. Our results show that some of these compounds induce isoform-specific effects on actin structure, some of which are reflected in the functional activity of the corresponding muscle type. We conclude that amino acid substitutions between skeletal and cardiac actin induce localized structural changes in actin that affect each actin's susceptibility to the effects of bound compounds. Each compound's mechanism of action probably depends on the structures of the compound and its binding site on actin, resulting in specific changes in the functional interaction of actin and myosin.
Specificity of compounds for actin isoforms
Actin polymerization is a useful tool to monitor effects of structural changes in actin induced by amino acid substitutions, mutations, or binding of chemical compounds. Amino acid substitutions in highly conserved actin isoforms result in different polymerization properties of muscle and nonmuscle actins (23, 35). Numerous naturally obtained compounds have been found to affect the properties of actin filaments (36–38). Most of these compounds fit into one of two groups: (a) those that destabilize actin filaments (usually by decreasing the rate of polymerization), and (b) those that stabilize actin filaments (usually by increasing the rate of polymerization). In skeletal as well cardiac muscle, polymerized actin is a major component of thin filament. In healthy developed muscle, polymerization-depolymerization is not involved in the mechanism of contractility. However, it has been reported that some mutations affecting polymerization are associated with skeletal and cardiac muscle myopathies. For example, several mutations in cardiac actin that affect polymerization rates are associated with cardiomyopathy (39), and several mutations in skeletal actin that alter its polymerization are associated with skeletal muscle myopathies (40, 41). It has been suggested that some of these mutations could produce structurally altered actin filaments, affecting their interaction with multiple actin-binding proteins, and some could enhance polymerization, leading to an excess of thin filaments in muscle, all with detrimental effects on contractility (40, 41).
In the present study, we used actin polymerization kinetics to indicate a significant change in actin's physical properties. Actin polymerization assays (Figs. 2 and 3) showed that skeletal and cardiac muscle actin have very similar rates of polymerization, as reported earlier (25, 42), indicating that the four amino acid substitutions (Glu2-Asp, Asp3-Glu, Met299-Leu, and Thr358-Ser) between these two isoforms do not have a significant effect on the formation of intermonomer bonds. However, polymerization rates were significantly affected by several of these compounds. Most pronounced changes were observed in the presence of fluphenazine, novantrone, and thioriadazine. These compounds also have pronounced effects on skeletal actin filament dynamics and on the environment of the C terminus of actin, as detected by transient phosphorescence anisotropy in our previous study (27). Additionally, thioriadazine, phenothiazine, and tegaserod exhibit significantly different isoform specificity. Although the location of the bound compound on actin is not yet known, the observed diversity of compound effects is most likely explained by their diverse effects on actin structure. For example, these compounds might compete directly with actin-binding partners or induce allosteric structural changes affecting intermonomer bond formation. The key observation is that these compounds are significant modulators of actin structure and dynamics, and their functional effects show distinct specificity for cardiac and skeletal actin isoforms.
Even though a number of natural small-molecule inhibitors of actin cytoskeleton dynamics have long been recognized as valuable molecular probes for understanding the complex mechanisms of cellular function, few studies have been performed to compare their isoform-specific effects on skeletal and cardiac actin. Phalloidin, a heptapeptide toxin from the poisonous mushroom Amanita phalloides, binds tightly and specifically to polymerized actin (43), stabilizing the filaments. A comparison of phalloidin binding to three different isoforms of actin showed that a single amino acid change can alter actin's affinity for phalloidin (44). Similarly, an actin isoform-specific behavior was also observed for Clostridium botulinum C2 toxin (45). Thus, a subtle change in actin-compound interaction can change functional behavior. This kind of selectivity must reside in structural differences between the two isoforms. In the present case, the differences in how compounds bind and affect skeletal and cardiac actin must be determined by structural effects of the 4 amino acid substitutions.
Specificity of compounds for muscle types
Of all sarcomeric components, small-molecule effectors designed to target and modulate striated and smooth muscle myosin isoforms and troponin C are showing promise in preclinical and clinical trials for treatment of muscle diseases (18–20). The balance between contraction and relaxation of muscle is controlled by a shift in calcium concentration. As the components of the sarcomere are interrelated, any attempt to stabilize the contracted or relaxed state by targeting any one component, along the troponin–tropomyosin–actin–myosin continuum, tends to favor the contracted or relaxed state in other components. Thus, an ideal modulator for sarcomeric proteins should work by a delicate shift in calcium sensitivity and should have a minimal effect on downstream regulation. In addition, the compound should be muscle-specific, as desired for its safe clinical application. In our work, we focus on how modulation of actin with actin-binding compounds alters Ca2+-dependent muscle regulation as reflected in functional activity, comparing skeletal and cardiac muscle.
We have shown that several compounds in the present study differentially affect enzymatic activities of skeletal and cardiac myofibrils under activating and/or relaxing conditions. Pronounced effects were observed at pCa 4 (activating) and at pCa 8 (relaxing) with compounds honokiol, phenothiazine, tegaserod, fluphenazine, and mefloquine showing significant differences in ATPase activity for the two muscle types. Because we have shown that these compounds bind to actin (27), the changes in enzymatic activities of myofibrils can most likely be explained, by structural changes in actin and resulting changes in interaction with other sarcomeric proteins. The role of structural changes in actin is supported by our previous (27), as well as current (Table 2), observed compound-induced changes in the activity of skeletal and cardiac acto-S1, where the only proteins present are actin and myosin. Although the extent of change in acto-S1 and myofibrillar ATPase activities are not identical (Table 2), such quantitative differences are expected for several reasons: (i) myofibrillar ATPase activities are determined by interactions not only between actin and myosin, but also between actin, and other sarcomeric proteins. For example, honokiol, fluphenazine, and phenothiazine showed significant differences in ATPase activity of the two muscle systems under relaxed conditions (Fig. 5D), which could be explained by compound-specific effects on interaction between actin and Tm that changes accessibility to myosin-binding sites. (ii) Actin concentrations used for experiments in solution are much lower than in myofibrils, but observed ATPase changes in myofibrils were comparable with those in acto-S1. These compounds do not affect skeletal and cardiac myosin's Mg-ATPase in the absence of actin (27), so they probably do not directly bind to myosin. However, the possibility of interaction of these compounds with other regulatory proteins such as MyBP-C, cannot be ruled out. Skeletal and cardiac acto-S1, and the corresponding myofibrils, have significantly different levels of ATPase activity, as expected due to their different physiological functions. Several compounds induced such muscle-specific effects. For example, under activating conditions, phenothiazine and tegaserod acted as inhibitors for skeletal and cardiac acto-S1 as well as myofibrils. In relaxing conditions, honokiol, fluphenazine, and phenothiazine activated ATPase activities, but the extents of activation were significantly different between cardiac and skeletal muscle types.
Muscle type-specific effects of compounds on enzymatic activities could be due to multiple factors: (i) diversity of compound-induced changes in cardiac and skeletal actin structure, suggested by isoform-specific effects on polymerization (Fig. 3); (ii) different enzymatic properties of cardiac and skeletal myosin, due to structural differences at the actin-myosin interface, resulting in different effects of a compound on structural transitions in the actomyosin ATPase cycle; (iii) effects of amino acid substitutions in the two actin isoforms on cross-bridge dynamics (46); and (iv) different isoforms of other regulatory proteins in skeletal and cardiac isoforms and their effects on regulating the actin–myosin interaction. Thus, the effects of compounds may arise from a combination of changes in the interaction between actin and myosin as well as other sarcomeric proteins.
Therefore, compound-induced structural changes at the actin level are likely to affect either the actin–myosin interaction or Ca2+-mediated regulation of the corresponding muscle types. A key bottleneck for progressing to clinical trials is the development of compounds that bind with high affinity with the desired target. Our compounds affect actin at micromolar concentrations, indicating moderate actin-binding affinity, which can be optimized in the future through medicinal chemistry. Some of these compounds are already in use as medications for several diseases (Table 1), and some have significant side effects on muscle function (47–53). An effective drug must achieve a balance between specific therapeutic benefit and undesired side effects. Future screening of larger libraries, with the high-throughput structure-based FRET assays and secondary assays developed in this study, will help identify potential actin-binding compounds that can specifically and safely work on a particular muscle type.
Conclusion
The current study shows that actin-binding compounds, previously discovered by high-throughput FRET screening, have specific biochemical and functional effects that are distinct in cardiac and skeletal actin, and in cardiac and skeletal muscle. This sets the stage to apply the original high-throughput FRET screen to larger small-molecule libraries, leading to the discovery of novel actin-binding compounds with specific therapeutic potential for treating disorders of cardiac or skeletal muscle (27, 54).
Experimental procedures
Preparation of actin and S1
New Zealand White rabbits (purchased from Charles River, Birchwood, ME) were housed in University Research Animal Resources facility. Rabbits were euthanized by trained veterinarians, consistent with the recommendations of the American Veterinary Medical Association (AVMA) guidelines. Bovine left ventricle was isolated from fresh bovine hearts (Pel-Freez Biologicals). Actin was prepared from either rabbit skeletal or bovine cardiac acetone, using the procedure of Pardee and Spudich (55), with slight modifications, as follows. Actin was extracted from acetone powder with cold water and polymerized with 2 mm MgCl2, 30 mm KCl, and 1 mm ATP for 1.5 h. Addition of 1 mm ATP at this step ensures full polymerization. Then, 0.6 m KCl was slowly added to polymerized actin and gently stirred for 1 h. The polymerized actin was ultracentrifuged at 350,000 × g for 30 min at 4 °C and the F-actin pellet was suspended in G-Ca buffer (5 mm Tris, 0.5 mm ATP, 0.2 mm CaCl2, pH 7.5), followed by clarification at 300,000 × g for 10 min. The resultant G-actin was then extensively dialyzed in G-Ca buffer for ∼36 h with periodic changes of G-buffer. G-actin was finally clarified again at 300,000 × g for 10 min and the protein concentration was measured at 290 nm assuming the molecular mass of 42,300 Da and absorption of 0.1% protein 0.63 (56). The prepared actin was used within 2 days for polymerization and depolymerization experiments. Skeletal and cardiac S1 were prepared as described (27).
Labeling of actin with pyrene iodoacetamide
Skeletal muscle actin was prepared as described previously (57) by extracting acetone powder of rabbit skeletal muscle with cold water. Actin was polymerized with 30 mm KCl for 1 h at room temperature, and centrifuged at 350,000 × g for 30 min at 4 °C. The pellet was suspended in G buffer (10 mm Tris, 0.5 mm ATP, 0.2 mm CaCl2, pH 7.5). Pyrene-actin was prepared by labeling actin with pyrene iodoacetamide (Invitrogen) as described previously (58), with slight modifications. Actin (24 μm) was polymerized with 0.1 m KCl, 1 mm NaN3 and 20 mm Tris (pH 7.5), and the dye, freshly dissolved in N,N-dimethylformamide, was added at a concentration of 180 μm. After 18 h incubation at 23 °C, the labeling was terminated by 10 mm DTT, and actin was ultracentrifuged at 350,000 × g for 30 min at 4 °C. Labeled actin was then resuspended in G-buffer and clarified by 10 min centrifugation at 300,000 × g. Protein concentration was measured by Bradford assay using unlabeled G-actin as a standard.
Actin polymerization and depolymerization assay
Rabbit skeletal and bovine cardiac actin polymerization rates were determined by the increase in fluorescence caused by incorporation of pyrene-labeled G-actin into actin filaments (59). Pyrene-actin (5% of total actin concentration) was mixed with globular α-skeletal and α-cardiac actin in G-buffer. Polymerization was induced by the addition of either 2 mm MgCl2 or 50 mm KCl to 20 μm G-actin and the increase of pyrene fluorescence with an excitation wavelength of 350 nm was monitored at 407 nm using a Varian Cary Eclipse spectrofluorometer (total volume of 150 μl) in a microcuvette. Polymerization of actin in the presence of compound was done with a concentration of 50 μm compound with the addition of 2 mm MgCl2. For determination of the apparent half-times of polymerization, the kinetic traces were approximated by a single exponential function and the half-times were calculated from the rate constant. Relative change in polymerization was calculated from the ratio of apparent half-times of actin in the presence and absence of compound (Fig. 3). For depolymerization, an amount of 20 μm actin containing 5% pyrene actin was first polymerized with 2 mm MgCl2 for at least 2 h at 23 °C. F-actin was then diluted 1:20 into G-buffer, and the decrease in fluorescence intensity was monitored for 10 min with the same wavelength settings as in polymerization (Fig. 4). Kinetic traces of depolymerization were not approximated as single exponential as it was a linear process within the measured time frame (55, 60, 61).
Preparation of skeletal and cardiac myofibrils
Rabbit psoas muscle (fast skeletal) bundles were isolated and chemically skinned in 0.5% Triton X-100 and stored at −20 °C in 50% glycerol with 60 mm KPr, 25 mm MOPS, 2 mm MgCl2, 1 mm EGTA, 1 mm NaN3, and EDTA-free protease inhibitor mixture (pH 7.0). Muscles were used within 6 months of the initial skinning process. Skeletal myofibrils were prepared in homogenization solution containing 60 mm KPr, 25 mm MOPS, 1 mm EGTA, and 1 mm NaN3 (pH 7.0) (29). Muscle bundles were finely cut and subsequently homogenized with a PowerGen 125 Polytron (Fisher Scientific) for 10 min. Serial washing via centrifugation at 1,500 × g for 5 min and resuspension with homogenization solution was done to remove residue Triton, glycerol, and protease inhibitors. All steps in the preparation were done at 4 °C. Total myofibrillar protein concentration was measured by Bradford assay using BSA as a standard. Freshly prepared myofibrils were used in experiments within 72 h.
Fresh bovine left ventricle was cut into 1-inch pieces. These chunks were flash-frozen in liquid nitrogen and stored at −80 °C until use. Muscle pieces were thawed in base rigor buffer K60 (60 mm of KCl, 2 mm MgCl2, 20 mm MOPS, pH 7.4) containing protease (Sigma, 8340) and phosphatase inhibitors (Sigma, P0044). Thawed pieces were finely chopped with razor blade and 1 mm EGTA was added for early permeabilization and calcium removal. Muscle bundles were fragmented to myocyte-sized fragments with a PowerGen 125 Polytron (Fisher Scientific). Fragments were Dounce homogenized prior to skinning in 1.0% Triton X-100 on ice (62). The resulting myofibrils were washed multiple times as described above with K60 to remove Triton, protease, and phosphate inhibitors. Finally, myofibril solution was filtered through 70-μm nylon cell strainer and protein concentration was measured by Bradford assay with BSA as a standard. The freshly prepared myofibrils were stored at 4 °C that retained near full ATPase activity for about 24 h.
Preparation of 96-well assay plates for myofibrillar ATPase assay
The compounds of interest (27) were purchased from TargetMol (Boston, MA) at 10 mm stocks in DMSO. The compounds were then distributed into a 96-well template plate and diluted with DMSO to the appropriate concentration for the dose-response assays. Final compound concentration was varied from 0 to 100 μm across 12 wells. Final DMSO concentrations in each well were kept constant at 1%. Final assay plates were prepared by transferring 2 μl of compound from the template plates using as Mosquito liquid dispenser from TTP Labtech (Melbourne, UK). These assay plates were stored at −20 °C prior to usage.
Steady-state ATPase kinetics
An enzyme-coupled, NADH-linked ATPase assay was used to measure skeletal and cardiac myofibril ATPase activity in 96-well–microplates at 23 °C. Ionic strength was maintained below 0.2 m, so myofilaments remain insoluble and stable (63). To achieve optimal enzymatic activities, buffers for skeletal and cardiac myofibrils differed slightly (64, 65): skeletal in 50 mm MOPS, 100 mm KCl, 5 mm MgCl2, 1 mm EGTA (pH 7.0); cardiac in 20 mm MOPS, 35 mm NaCl, 5 mm MgCl2, 1 mm EGTA (pH 7.0). Each well in the microplate contained an assay mix of 0.84 mm phosphoenolpyruvate, 0.17 mm NADH, 10 units/volume of pyruvate kinase, 20 units/volume of lactate dehydrogenase. The concentration of free Ca2+ was controlled by EGTA buffering (66). Myofibrils were dispensed in microplates and were incubated with assay mix plus compound of interest for 20 min at 23 °C. The concentration of myofibrils used were 0.01 mg/ml for skeletal and 0.05 mg/ml for cardiac. The assay was started upon the addition of ATP, at a final concentration of 2.1 mm (total volume to 200 μl), and absorbance was recorded at 340 nm in a SpectraMax Plus microplate spectrophotometer from Molecular Devices (Sunnyvale, CA). The steady-state myofibrillar ATPase rate was measured from NADH oxidation, measured as the rate of decrease in absorption at 340 nm for 30 min, with absorption data collected every 15 s. All data and statistical analysis of steady-state kinetics were conducted with the OriginPro program. The results were fitted with the Hill equation,
| (Eq. 1) |
where V is the ATPase rate and n is the Hill coefficient.
ATPase activity is reported on the micromole of ATP/mg of protein/min scale. Average data are presented as mean ± S.D. Sample means are derived from eight or more experimental repeats.
The effects of drugs on ATPase activity of myofibrils were determined in the presence of 50 μm compound, as prepared above, at activating pCa of 4.0 and relaxing pCa of 8.0 (Fig. 5). The effect of 500 nm thapsigargin (a SERCA modulator) on myofibrils ATPase activity inhibition was tested across a 12-point pCa range. The effects of 50 μm tegaserod, phenothiazine, and fluphenazine on myofibril ATPase activity and pCa50 were examined across the same 12-point pCa range (Fig. 6). In all dose-response experiments, a 1% DMSO only control was included.
The steady-state acto-S1 ATPases of rabbit skeletal acto-S1 and bovine cardiac acto-S1 were determined with the same NADH-linked assay in the presence and absence of 50 μm compound, using 0.05 μm skeletal and 0.2 μm cardiac S1 at 20 μm of the corresponding actin. ATPase activity was reported as % change due to compound (Table 2).
Data availability
All data discussed are presented within the article.
Acknowledgments
Fluorescence experiments and drug plate preparations were performed at the Biophysical Technology Center, University of Minnesota. We thank Chandini Nair and Ananya Tripathi for technical assistance with earlier experiments.
Author contributions—P. G. conceptualization; P. G. and L. A. P. data curation; P. G., L. A. P., S. L., and A. W. formal analysis; P. G., L. A. P., S. L., and A.W. validation; P. G. and L. A. P. investigation; P. G. and L. A. P. visualization; P. G., L. A. P., E. P., S. L., and A. W. methodology; P. G., L. A. P., and E. P. writing-original draft; P. G., L. A. P., E. P., S. L., A. W., and D. D. T. writing-review and editing; L. A. P. and D. D. T. funding acquisition; D. D. T. resources; D. D. T. supervision.
Funding and additional information—This work was supported by National Institutes of Health Grants R01AR032961 and R37AG26160 (to D. D. T.) and T32GM008244 and F30AG057108 (to L. A. P.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of interest—D. D. T. holds equity in, and serves as President of, Photonic Pharma LLC. This relationship has been reviewed and managed by the University of Minnesota. Photonic Pharma had no role in this study. The authors declare no conflicts of interest in regard to this manuscript.
- Tn
- troponin
- Tm
- tropomyosin
- MyBP-C
- myosin-binding protein C
- SERCA
- sarcoendoplasmic reticulum calcium transport ATPase
- S1
- subfragment 1
- AM
- actomyosin
- AR
- actin-regulatory protein.
References
- 1. Batters C., Veigel C., Homsher E., and Sellers J. R. (2014) To understand muscle you must take it apart. Front. Physiol. 5, 90 10.3389/fphys.2014.00090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Galińska-Rakoczy A., Engel P., Xu C., Jung H., Craig R., Tobacman L. S., and Lehman W. (2008) Structural basis for the regulation of muscle contraction by troponin and tropomyosin. J. Mol. Biol. 379, 929–935 10.1016/j.jmb.2008.04.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Flashman E., Redwood C., Moolman-Smook J., and Watkins H. (2004) Cardiac myosin binding protein C: its role in physiology and disease. Circ. Res. 94, 1279–1289 10.1161/01.RES.0000127175.21818.C2 [DOI] [PubMed] [Google Scholar]
- 4. Freiburg A., and Gautel M. (1996) A molecular map of the interactions between titin and myosin-binding protein C: implications for sarcomeric assembly in familial hypertrophic cardiomyopathy. Eur. J. Biochem. 235, 317–323 10.1111/j.1432-1033.1996.00317.x [DOI] [PubMed] [Google Scholar]
- 5. Miyamoto C. A., Fischman D. A., and Reinach F. C. (1999) The interface between MyBP-C and myosin: site-directed mutagenesis of the CX myosin-binding domain of MyBP-C. J. Muscle Res. Cell Motil. 20, 703–715 [DOI] [PubMed] [Google Scholar]
- 6. Bhuiyan M. S., Gulick J., Osinska H., Gupta M., and Robbins J. (2012) Determination of the critical residues responsible for cardiac myosin binding protein C's interactions. J. Mol. Cell Cardiol. 53, 838–847 10.1016/j.yjmcc.2012.08.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wijnker P. J. M., Sequeira V., Kuster D. W. D., and Velden J. V. (2019) Hypertrophic cardiomyopathy: a vicious cycle triggered by sarcomere mutations and secondary disease hits. Antioxid. Redox Signal. 31, 318–358 10.1089/ars.2017.7236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Marston S. (2018) The molecular mechanisms of mutations in actin and myosin that cause inherited myopathy. Int. J. Mol. Sci. 19, 2020 10.3390/ijms19072020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Heitner S. B., Jacoby D., Lester S. J., Owens A., Wang A., Zhang D., Lambing J., Lee J., Semigran M., and Sehnert A. J. (2019) Mavacamten treatment for obstructive hypertrophic cardiomyopathy: a clinical trial. Ann. Intern. Med. 170, 741–748 10.7326/M18-3016 [DOI] [PubMed] [Google Scholar]
- 10. Hwang P. M., and Sykes B. D. (2015) Targeting the sarcomere to correct muscle function. Nat. Rev. Drug Discov. 14, 313–328 10.1038/nrd4554 [DOI] [PubMed] [Google Scholar]
- 11. Spudich J. A. (2019) Three perspectives on the molecular basis of hypercontractility caused by hypertrophic cardiomyopathy mutations. Pflugers Arch. 471, 701–717 10.1007/s00424-019-02259-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang R. R., Han T., Guo F., Liu Z. Z., Han Y. L., Chen W. C., Liu Y. Y., and Xie X. D. (2015) Lambert-Eaton myasthenic syndrome in a patient with small-cell lung cancer: a case report. Oncol. Lett. 10, 1339–1342 10.3892/ol.2015.3473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Grimm S., and Chamberlain M. (2011) Hodgkin's lymphoma: a review of neurologic complications. Adv. Hematol. 2011, 624578 10.1155/2011/624578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Turner C., and Hilton-Jones D. (2014) Myotonic dystrophy: diagnosis, management and new therapies. Curr. Opin. Neurol. 27, 599–606 10.1097/WCO.0000000000000128 [DOI] [PubMed] [Google Scholar]
- 15. Finsterer J. (2008) Primary periodic paralyzes. Acta Neurol. Scand. 117, 145–158 10.1111/j.1600-0404.2007.00963.x [DOI] [PubMed] [Google Scholar]
- 16. Wee A. S. (2004) Effects of acute and chronic denervation on human myotonia. Electromyogr. Clin. Neurophysiol. 44, 443–446 [PubMed] [Google Scholar]
- 17. Hwee D. T., Kennedy A., Ryans J., Russell A. J., Jia Z., Hinken A. C., Morgans D. J., Malik F. I., and Jasper J. R. (2014) Fast skeletal muscle troponin activator tirasemtiv increases muscle function and performance in the B6SJL-SOD1G93A ALS mouse model. PLoS ONE 9, e96921 10.1371/journal.pone.0096921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Malik F. I., Hartman J. J., Elias K. A., Morgan B. P., Rodriguez H., Brejc K., Anderson R. L., Sueoka S. H., Lee K. H., Finer J. T., Sakowicz R., Baliga R., Cox D. R., Garard M., Godinez G., et al. (2011) Cardiac myosin activation: a potential therapeutic approach for systolic heart failure. Science 331, 1439–1443 10.1126/science.1200113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Green E. M., Wakimoto H., Anderson R. L., Evanchik M. J., Gorham J. M., Harrison B. C., Henze M., Kawas R., Oslob J. D., Rodriguez H. M., Song Y., Wan W., Leinwand L. A., Spudich J. A., McDowell R. S., et al. (2016) A small-molecule inhibitor of sarcomere contractility suppresses hypertrophic cardiomyopathy in mice. Science 351, 617–621 10.1126/science.aad3456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Collibee S. E., Bergnes G., Muci A., Browne W. F. 4th., Garard M., Hinken A. C., Russell A. J., Suehiro I., Hartman J., Kawas R., Lu P. P., Lee K. H., Marquez D., Tomlinson M., Xu D., et al. (2018) Discovery of Tirasemtiv, the first direct fast skeletal muscle troponin activator. ACS Med. Chem. Lett. 9, 354–358 10.1021/acsmedchemlett.7b00546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hwee D. T., Cheng A. J., Hartman J. J., Hinken A. C., Lee K., Durham N., Russell A. J., Malik F. I., Westerblad H., and Jasper J. R. (2017) The Ca2+ sensitizer CK-2066260 increases myofibrillar Ca2+ sensitivity and submaximal force selectively in fast skeletal muscle. J. Physiol. 595, 1657–1670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vandekerckhove J., and Weber K. (1978) At least six different actins are expressed in a higher mammal: an analysis based on the amino acid sequence of the amino-terminal tryptic peptide. J. Mol. Biol. 126, 783–802 10.1016/0022-2836(78)90020-7 [DOI] [PubMed] [Google Scholar]
- 23. Mossakowska M., and Strzelecka-Gołaszewska H. (1985) Identification of amino acid substitutions differentiating actin isoforms in their interaction with myosin. Eur. J. Biochem. 153, 373–381 10.1111/j.1432-1033.1985.tb09313.x [DOI] [PubMed] [Google Scholar]
- 24. Wang Y., Ajtai K., and Burghardt T. P. (2018) Cardiac and skeletal actin substrates uniquely tune cardiac myosin strain-dependent mechanics. Open Biol. 8, 180143 10.1098/rsob.180143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Strzelecka-Gołaszewska H., Zmorzyński S., and Mossakowska M. (1985) Bovine aorta actin: development of an improved purification procedure and comparison of polymerization properties with actins from other types of muscle. Biochim. Biophys. Acta 828, 13–21 10.1016/0167-4838(85)90003-2 [DOI] [PubMed] [Google Scholar]
- 26. Ochala J., Iwamoto H., Ravenscroft G., Laing N. G., and Nowak K. J. (2013) Skeletal and cardiac alpha-actin isoforms differently modulate myosin cross-bridge formation and myofibre force production. Hum. Mol. Genet. 22, 4398–4404 10.1093/hmg/ddt289 [DOI] [PubMed] [Google Scholar]
- 27. Guhathakurta P., Prochniewicz E., Grant B. D., Peterson K. C., and Thomas D. D. (2018) High-throughput screen, using time-resolved FRET, yields actin-binding compounds that modulate actin-myosin structure and function. J. Biol. Chem. 293, 12288–12298 10.1074/jbc.RA118.002702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cooper J. A., Walker S. B., and Pollard T. D. (1983) Pyrene actin: documentation of the validity of a sensitive assay for actin polymerization. J. Muscle Res. Cell. Motil. 4, 253–262 10.1007/BF00712034 [DOI] [PubMed] [Google Scholar]
- 29. Mello R. N., and Thomas D. D. (2012) Three distinct actin-attached structural states of myosin in muscle fibers. Biophys. J. 102, 1088–1096 10.1016/j.bpj.2011.11.4027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Prochniewicz E., Lowe D. A., Spakowicz D. J., Higgins L., O'Conor K., Thompson L. V., Ferrington D. A., and Thomas D. D. (2008) Functional, structural, and chemical changes in myosin associated with hydrogen peroxide treatment of skeletal muscle fibers. Am. J. Physiol. Cell Physiol. 294, C613–626 10.1152/ajpcell.00232.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Alousi A. A., Grant A. M., Etzler J. R., Cofer B. R., Van der Bel-Kahn J., and Melvin D. (1990) Reduced cardiac myofibrillar Mg-ATPase activity without changes in myosin isozymes in patients with end-stage heart failure. Mol. Cell Biochem. 96, 79–88 10.1007/BF00228455 [DOI] [PubMed] [Google Scholar]
- 32. Krause S. M. (1990) Effect of global myocardial stunning on Ca2+-sensitive myofibrillar ATPase activity and creatine kinase kinetics. Am. J. Physiol. 259, H813–819 10.1152/ajpheart.1990.259.3.H813 [DOI] [PubMed] [Google Scholar]
- 33. Murphy A. M., and Solaro R. J. (1990) Developmental difference in the stimulation of cardiac myofibrillar Mg2+-ATPase activity by calmidazolium. Pediatr. Res. 28, 46–49 10.1203/00006450-199007000-00011 [DOI] [PubMed] [Google Scholar]
- 34. Kawas R. F., Anderson R. L., Ingle S. R. B., Song Y., Sran A. S., and Rodriguez H. M. (2017) A small-molecule modulator of cardiac myosin acts on multiple stages of the myosin chemomechanical cycle. J. Biol. Chem. 292, 16571–16577 10.1074/jbc.M117.776815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Strzelecka-Gołaszewska H., Venyaminov S., Zmorzynski S., and Mossakowska M. (1985) Effects of various amino acid replacements on the conformational stability of G-actin. Eur. J. Biochem. 147, 331–342 10.1111/j.1432-1033.1985.tb08754.x [DOI] [PubMed] [Google Scholar]
- 36. Spector I., Braet F., Shochet N. R., and Bubb M. R. (1999) New anti-actin drugs in the study of the organization and function of the actin cytoskeleton. Microsc. Res. Tech. 47, 18–37 [DOI] [PubMed] [Google Scholar]
- 37. Giganti A., and Friederich E. (2003) The actin cytoskeleton as a therapeutic target: state of the art and future directions. Prog. Cell Cycle Res. 5, 511–525 [PubMed] [Google Scholar]
- 38. Fenteany G., and Zhu S. (2003) Small-molecule inhibitors of actin dynamics and cell motility. Curr. Top. Med. Chem. 3, 593–616 10.2174/1568026033452348 [DOI] [PubMed] [Google Scholar]
- 39. Müller M., Mazur A. J., Behrmann E., Diensthuber R. P., Radke M. B., Qu Z., Littwitz C., Raunser S., Schoenenberger C. A., Manstein D. J., and Mannherz H. G. (2012) Functional characterization of the human α-cardiac actin mutations Y166C and M305L involved in hypertrophic cardiomyopathy. Cell Mol. Life Sci. 69, 3457–3479 10.1007/s00018-012-1030-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sparrow J. C., Nowak K. J., Durling H. J., Beggs A. H., Wallgren-Pettersson C., Romero N., Nonaka I., and Laing N. G. (2003) Muscle disease caused by mutations in the skeletal muscle α-actin gene (ACTA1). Neuromuscul. Disord. 13, 519–531 10.1016/S0960-8966(03)00101-9 [DOI] [PubMed] [Google Scholar]
- 41. Costa C. F., Rommelaere H., Waterschoot D., Sethi K. K., Nowak K. J., Laing N. G., Ampe C., and Machesky L. M. (2004) Myopathy mutations in α-skeletal-muscle actin cause a range of molecular defects. J. Cell Sci. 117, 3367–3377 10.1242/jcs.01172 [DOI] [PubMed] [Google Scholar]
- 42. Iwasa M., Maeda K., Narita A., Maeda Y., and Oda T. (2008) Dual roles of Gln137 of actin revealed by recombinant human cardiac muscle α-actin mutants. J. Biol. Chem. 283, 21045–21053 10.1074/jbc.M800570200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wieland T., and Faulstich H. (1978) Amatoxins, phallotoxins, phallolysin, and antamanide: the biologically active components of poisonous Amanita mushrooms. CRC Crit. Rev. Biochem. 5, 185–260 10.3109/10409237809149870 [DOI] [PubMed] [Google Scholar]
- 44. De La Cruz E. M., and Pollard T. D. (1996) Kinetics and thermodynamics of phalloidin binding to actin filaments from three divergent species. Biochemistry 35, 14054–14061 10.1021/bi961047t [DOI] [PubMed] [Google Scholar]
- 45. Aktories K., Bärmann M., Ohishi I., Tsuyama S., Jakobs K. H., and Habermann E. (1986) Botulinum C2 toxin ADP-ribosylates actin. Nature 322, 390–392 10.1038/322390a0 [DOI] [PubMed] [Google Scholar]
- 46. Bagshaw C. R. (1982) Muscle Contraction, pp. 86, Chapman and Hall, London: 10.1016/0307-4412(83)90081-X [DOI] [Google Scholar]
- 47. Blair S. D., Holcombe C., Coombes E. N., and O'Malley M. K. (1991) Leg ischaemia secondary to non-medical injection of temazepam. Lancet 338, 1393–1394 10.1016/0140-6736(91)92269-8 [DOI] [PubMed] [Google Scholar]
- 48. Clark T. J., Collins J. V., and Tong D. (1971) Respiratory depression caused by nitrazepam in patients with respiratory failure. Lancet 2, 737–738 10.1016/S0140-6736(71)92105-2 [DOI] [PubMed] [Google Scholar]
- 49. Hudson M. M., Edmonds M., and Watkins P. J. (1991) Misuse of temazepam. BMJ 303, 993 10.1136/bmj.303.6808.993-c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Koeller J., and Eble M. (1988) Mitoxantrone: a novel anthracycline derivative. Clin. Pharm. 7, 574–581 [PubMed] [Google Scholar]
- 51. Mather F. J., Simon R. M., Clark G. M., and Von Hoff D. D. (1987) Cardiotoxicity in patients treated with mitoxantrone: Southwest Oncology Group phase II studies. Cancer Treat Rep 71, 609–613 [PubMed] [Google Scholar]
- 52. Ray W. A., Chung C. P., Murray K. T., Hall K., and Stein C. M. (2009) Atypical antipsychotic drugs and the risk of sudden cardiac death. N. Engl. J. Med. 360, 225–235 10.1056/NEJMoa0806994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Stuart-Harris R., Pearson M., Smith I. E., and Olsen E. G. (1984) Cardiotoxicity associated with mitoxantrone. Lancet 2, 219–220 10.1016/S0140-6736(84)90498-7 [DOI] [PubMed] [Google Scholar]
- 54. Guhathakurta P., Prochniewicz E., and Thomas D. D. (2018) Actin-myosin interaction: structure, function and drug discovery. Int. J. Mol. Sci. 19, 2628 10.3390/ijms19092628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Pardee J. D., and Spudich J. A. (1982) Purification of muscle actin. Methods Enzymol. 85, 164–181 10.1016/0076-6879(82)85020-9 [DOI] [PubMed] [Google Scholar]
- 56. Houk T. W. Jr., and Ue K. (1974) The measurement of actin concentration in solution: a comparison of methods. Anal. Biochem. 62, 66–74 10.1016/0003-2697(74)90367-4 [DOI] [PubMed] [Google Scholar]
- 57. Prochniewicz E., Zhang Q., Howard E. C., and Thomas D. D. (1996) Microsecond rotational dynamics of actin: spectroscopic detection and theoretical simulation. J. Mol. Biol. 255, 446–457 10.1006/jmbi.1996.0037 [DOI] [PubMed] [Google Scholar]
- 58. Criddle A. H., Geeves M. A., and Jeffries T. (1985) The use of actin labelled with N-(1-pyrenyl)iodoacetamide to study the interaction of actin with myosin subfragments and troponin/tropomyosin. Biochem. J. 232, 343–349 10.1042/bj2320343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kouyama T., and Mihashi K. (2005) Fluorimetry study of N-(1-pyrenyl)iodoacetamide-labelled F-actin: local structural change of actin protomer both on polymerization and on binding of heavy meromyosin. Eur. J. Biochem. 114, 33–38 10.1111/j.1432-1033.1981.tb06167.x [DOI] [PubMed] [Google Scholar]
- 60. Fujiwara I., Vavylonis D., and Pollard T. D. (2007) Polymerization kinetics of ADP- and ADP-Pi-actin determined by fluorescence microscopy. Proc. Natl. Acad. Sci. U.S.A. 104, 8827–8832 10.1073/pnas.0702510104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Fujiwara I., Zweifel M. E., Courtemanche N., and Pollard T. D. (2018) Latrunculin A accelerates actin filament depolymerization in addition to sequestering actin monomers. Curr. Biol. 28, 3183–3192.e2 10.1016/j.cub.2018.07.082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Solaro R. J., Pang D. C., and Briggs F. N. (1971) The purification of cardiac myofibrils with Triton X-100. Biochim. Biophys. Acta 245, 259–262 10.1016/0005-2728(71)90033-8 [DOI] [PubMed] [Google Scholar]
- 63. Chen X., Tume R. K., Xu X., and Zhou G. (2017) Solubilization of myofibrillar proteins in water or low ionic strength media: classical techniques, basic principles, and novel functionalities. Crit. Rev. Food Sci. Nutr. 57, 3260–3280 10.1080/10408398.2015.1110111 [DOI] [PubMed] [Google Scholar]
- 64. Ma Y. Z., and Taylor E. W. (1994) Kinetic mechanism of myofibril ATPase. Biophys. J. 66, 1542–1553 10.1016/S0006-3495(94)80945-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Smith S. H., and Fuchs F. (1999) Effect of ionic strength on length-dependent Ca2+ activation in skinned cardiac muscle. J. Mol. Cell Cardiol. 31, 2115–2125 10.1006/jmcc.1999.1043 [DOI] [PubMed] [Google Scholar]
- 66. Bers D. M., Patton C. W., and Nuccitelli R. (2010) A practical guide to the preparation of Ca2+ buffers. Methods Cell Biol. 99, 1–26 10.1016/B978-0-12-374841-6.00001-3 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data discussed are presented within the article.