Abstract
The genetic relationships among 24 Indian jujube cultivars (Ziziphus mauritiana Lam.) were evaluated by genotyping the microsatellite loci using simple sequence repeat (SSR) markers. The SSR loci were scored by fluorescent labelling and automated detection systems for the high-throughput capillary electrophoresis and high-resolution gel electrophoresis. Out of the 29 newly characterized SSR loci, 26 were considered as polymorphic with a total of 181 alleles obtained. The number of alleles ranged from 2–12, while the polymorphism information content ranged from 0.08–0.83, and the expected and observed heterozygosity were 0.04–0.83 and 0.04–0.82, respectively. The allele pattern of Indian jujube for all SSR loci confirmed its karyotype as tetraploid. Similarity coefficients and UPGMA dendrogram revealed that the Taiwanese cultivars consisted of a large ‘A’ clade, which is further divided into ‘A1’ and ‘A2’ groups, and the ‘B’ clade where both are rooted by the wild accession, ‘Chad native’. These four genetic clusters were supported by the results of PCoA and the assignment test. The excess of heterozygotes based on F-statistics was attributed to its mating system as outcrossing and self-incompatible, and the introgression of the presumed mutation-derived cultivars with genetic admixture. Based on this study, SSR markers offer valuable information on the genetic relationship of this tropical fruit tree which is basically in agreement with the genealogy of its breeding history.
Keywords: Genetic diversity, Genetic structure, Microsatellite DNA, Ziziphus mauritiana, Crop breeding, Horticulture, Plant biology, Plant genetics, Biodiversity, DNA barcoding
Genetic diversity; Genetic structure; Microsatellite DNA; Ziziphus mauritiana; Crop breeding; Horticulture; Plant biology; Plant genetics; Biodiversity; DNA barcoding.
1. Introduction
Lesser known fruits are genetically very diverse groups grown in temperate, subtropical and tropical regions and have been recognized for their human health benefits. Most of these fruits have high content of non-nutritive, nutritive, and bioactive compounds such as flavonoids, phenolics, anthocyanins, phenolic acids, as well as nutritive compounds such as sugars, essential oils, carotenoids, vitamins, and minerals [1, 2, 3].
Indian jujube (Ziziphus mauritiana Lam.) is among those less recognized fruits globally. It belongs to the genus Ziziphus of the family Rhamnaceae that includes approximately 86 species found in the tropical and subtropical regions of the northern hemisphere. Chinese jujube (Ziziphus jujube Mill.) and Indian jujube are the most important commercialized species. Basic chromosome numbers of x = 10, 12 and 13 have been shown for Ziziphus species [4] of which 2n = 4x = 48 for Indian jujube [4, 5]. Most of the Indian jujube varieties are tetraploid, but the cultivar ‘Illaichi’ is an octaploid (2n = 8x = 96) [6]. Indian jujube had been used around 1,000 BC, and it is believed to originate from Central Asia and spread to North Africa, India, South China, Myanmar and Australia [7].
Recently, DNA markers have been widely used to assess genetic relationships and cultivar identification. These molecular markers were used for several Chinese jujube studies [8, 9, 10, 11, 12, 13, 14, 15], but few for Indian jujubes. Among those reported to evaluate genetic relationships, genetic diversity and cultivar identification of several Indian jujubes were inter-simple sequence repeat (ISSR) and random amplified polymorphic DNA (RAPD) [16, 17, 18]. However, the recent popular molecular markers for plant systems are the simple sequence repeats (SSR) or microsatellites. The SSRs are 1–6 bp tandem repeats [19]. They are characterized with high mutation rates, high number of alleles and abundant in genomes. These sequences are considered useful and excellent molecular markers in population genetics, linkage mapping, genetic fingerprinting and taxonomic studies [20] even among closely related genotypes [21]. High levels of genetic polymorphism derived from SSRs have been observed because of their allelic diversity due to replication slippage [22, 23]. The codominant and high polymorphic characteristics of SSR are useful for genetic relationship and cultivar identification of tropical fruit trees such as guavas [24], pineapples [25], wax apples [26] and mangoes [27]. Lately, 38 SSR loci from Indian jujube have been isolated and characterized [28].
Indian jujube is an economically important crop that is also extensively cultivated in Taiwan [29]. There are several cultivars developed by breeders and farmers which show that the propagation of Indian jujube is incredibly active [29, 30, 31]. These cultivars are also propagated asexually, thus it is difficult to protect the right of the breeder. This study aims to address the needs of understanding the genetic background of Indian jujube cultivars by using molecular markers to effectively establish breeding strategies and legally protect the new cultivars. Specifically, SSR markers were used to investigate the genetic relationship among 24 Indian jujube cultivars in Taiwan.
2. Materials and methods
2.1. Plant materials
Twenty-four Indian jujube cultivars were collected and cultivated at the Kaohsiung District Agricultural Research and Extension Station (KDARES), Taiwan (Table 1). The J1 to J23 cultivars were obtained from the local tribes based on the differences of morphological characteristics from the inventory of tropical fruit trees, and hence, asexually propagated at the KDARES for breeding purposes. The J24 was a direct germplasm exchange from India by scientist cooperation through the Agricultural Technical Cooperation.
Table 1.
Indian jujube cultivars used in this study.
| No. | Cultivars | Voucher | Origin | Genetic background |
|---|---|---|---|---|
| J1 | Kaolang 1 | C. C. Tsai 3101 | Taiwan | unknown |
| J2 | Kaolang 2 | C. C. Tsai 3102 | Taiwan | mutant from Kaolang 1 |
| J3 | Kaolang 3 | C. C. Tsai 3103 | Taiwan | mutant from Kaolang 1 |
| J4 | Tsueimi | C. C. Tsai 3104 | Taiwan | mutant from Kaolang 1 |
| J5 | Mejao | C. C. Tsai 3105 | Taiwan | unknown |
| J6 | Cento | C. C. Tsai 3106 | Taiwan | mutant from Mejao |
| J7 | Gingtao | C. C. Tsai 3107 | Taiwan | mutant from Mejao |
| J8 | Dayeh | C. C. Tsai 3108 | Taiwan | mutant from Mejao |
| J9 | Chungyeh | C. C. Tsai 3109 | Taiwan | mutant from Mejao |
| J10 | Tsueishiang | C. C. Tsai 3110 | Taiwan | mutant from Mejao |
| J11 | Tianmi | C. C. Tsai 3111 | Taiwan | unknown |
| J12 | Kaohsiung 2 | C. C. Tsai 3112 | Taiwan | unknown |
| J13 | Kaohsiung 3 | C. C. Tsai 3113 | Taiwan | unknown |
| J14 | Kaohsiung 5 | C. C. Tsai 3114 | Taiwan | unknown |
| J15 | Yuguan | C. C. Tsai 3115 | Taiwan | unknown |
| J16 | Biyuan | C. C. Tsai 3116 | Taiwan | unknown |
| J17 | Hongyung | C. C. Tsai 3117 | Taiwan | unknown |
| J18 | Hsinsuchi | C. C. Tsai 3118 | Taiwan | unknown |
| J19 | Huangguan | C. C. Tsai 3119 | Taiwan | unknown |
| J20 | Telong | C. C. Tsai 3120 | Taiwan | unknown |
| J21 | Roulong | C. C. Tsai 3121 | Taiwan | unknown |
| J22 | Kaohsiung 6 | C. C. Tsai 3122 | Taiwan | unknown |
| J23 | Shuemi | C. C. Tsai 3123 | Taiwan | unknown |
| J24 | Chad native | C. C. Tsai 3124 | India | native species |
Voucher specimens were deposited at the herbarium of the National Museum of Natural Science, Taiwan (TNM).
2.2. DNA extraction, SSR loci characterization and PCR amplification
Genomic DNA was extracted from the mature leaf samples using the protocol of the Plant Genomic DNA Extraction Kit (RBC Bioscience, Taipei, Taiwan). The prior work of Chiou et al. (2012) utilized 131 out of the 241 isolated SSR loci to design the primer pairs for the flanking regions of SSR loci. Subsequently, 74 SSR loci were characterized to obtain the 38 polymorphic SSR loci for further studies of Indian jujubes. In this study, only 57 of the aforementioned primer pairs were characterized anew to obtain polymorphic SSR loci based on the criteria described by Chiou et al. (2012). The polymorphic SSR loci with highest PIC values were selected to assess the genetic relationship of the 24 Indian jujube cultivars enumerated by Chiou et al. (2012).
To detect the microsatellite genotypes, we used two systems: ABI PRISM 3700 DNA Sequencer (Applied Biosystems) and LI-COR 4300 DNA analyzer (LI-COR, Lincoln, Nebraska USA). Both systems employ fluorescent labelling and automated detection, which when compared, obtained the same results. The gel and fluorescence-based fragment analysis system of the LI-COR 4300 DNA analyzer is particularly useful and suitable for crop breeding research due to the sensitive detection of mutations and data visualization for genotyping [32]. Breeders could easily identify those cultivars and potential mutations based on the high-resolution genotype images from LI-COR 4300 DNA analyzer. However, the designed forward primers for the selected SSR loci were elongated at M13 (-21) 18 bp sequence (5′-TGTAAAACGACGGCCAGT-3′) based on fluorescent labelling in order to reduce the cost of the PCR product detection [33]. The designed primer pairs were first tested for PCR amplification and then used to amplify the samples from 24 Indian jujube cultivars after optimization. The PCR conditions were as follows: total volume 25 ul with 20 ng of template DNA, 1x PCR buffer, 0.2 mM of each dNTP, 0.2 mM of each SSR xu/4specific primer and 0.25 U Taq DNA polymerase (Promega, Madison, Wisconsin, USA). A two-step PCR amplification was conducted. The first thermocycling profiles included: initial denaturation at 94 °C for 3 min, followed by 20 cycles of 30 s denaturation at 94 °C, 30 s annealing at 58 °C, 40 s extension at 72 °C and a final extension for 7 min at 72 °C. Subsequently, 0.075 mM M13 primer 5′-labelled with IRDye for LI-COR 4300 DNA analyzer was added in this PCR reaction mixture. The second thermo cycling profiles were as follows: initial denaturation at 94 °C for 3 min, followed by 10 cycles of 30 s denaturation at 94 °C, 30 s annealing at 58 °C, 40 s extension at 72 °C and a final extension for 7 min at 72 °C. Samples were denatured in the loading dye (10 mg/ml blue dextran in formamide) and separated using 6.5% polyacrylamide gel (19:1,7 M urea) electrophoresis in a LI-COR 4300 DNA analyzer. Fragment lengths were determined with the aid of an external standard (50–500 bp, GE Healthcare, USA) and an in-house amplified internal standard (Allele Locator 1.03 software; Amersham Biosciences, India). Also, the forward primer of these SSR loci added 18 bp tail sequence (5′-TGTAAAACGACGGCCAGT-3′) for the detection by ABI PRISM 3700 DNA Sequencer. These additional sequences are complementary to universal primer M13, which could be labelled with three fluorescent primers: 6-FAM (blue), HEX (green) and NED (yellow). The PCR conditions and thermocycling profiles were the same as enumerated above. The amplification products were analyzed further with the ABI PRISM 3700 DNA Sequencer and coded by using GeneMapper v.3.7 (Applied Biosystems) to confirm the accuracy of the data.
2.3. Data analysis
In this study, the degree of polymorphism for the 29 newly characterized SSR loci, including the number of alleles (NA), expected heterozygosity (gene diversity) corrected for sample size (HE), observed heterozygosity (H), the three fixation indices (FIT, FIS, and FST) of F-statistics [34], the coefficient of gene differentiation (GST) [35] and fixation indices (RST) of R-statistics [36] were calculated using SPAGeDi [37]. To evaluate the selective neutrality of microsatellite markers, the Ewens-Watterson neutrality test was performed by Popgene 1.31 (Yeh et al. 1997). The polymorphism information content (PIC) [38] was calculated using PowerMarker version 3.25 [39]. There were three types of test conducted for bottleneck analysis: sign test, standardized difference test and Wilcoxon signed rank test under different mutation models such as Infinite Allele Model (IAM), Stepwise Mutation Model (SMM) and Two Phase Model (TPM) using Bottleneck 1.2.02 (Cornuet and Luikart, 1996).
The dissimilar genetic distance between Indian jujube accessions was estimated according to Bowcock et al. (1994) and Ciampolini et al. (1995) [40,41]. This distance was calculated based on the pairwise comparison between individuals causing a multilocus genetic similarity value complementary to the multilocus genetic distance (Dm). It is then converted as the dissimilar genetic distance with 1 – Dm. The cluster dendrogram reconstruction was derived from the pairwise dissimilar distance matrix using the unweighted pair-group method (UPGMA) (MEGA version 5.05) [42].
The Principal Coordinates Analysis (PCoA) was carried out to evaluate the relationship among tetraploid Indian jujube cultivars and the genotypic group structures using the Lynch genetic distance matrix [43] with the POLYSAT software [44]. The Bayesian-clustering assignment test in STRUCTURE ver. 2.3.4 [45, 46, 47] was used to estimate the genetic compositions and genotypic group structures that evaluate best for 24 Indian jujube cultivars/lines of their genotypic grouping and degree of genetic admixture. The posterior probability of K from 1 to 16 was estimated by the Markov chain Monte Carlo (MCMC) method using the admixture model [48] in 10,000,000 steps with a 1,000,000-step burn-in for each run. The posterior probability of each grouping number was replicated 20 independent runs to evaluate the consistency of the results. The Delta K (ΔK) method [49] (STRUCTURE HARVESTER v. 0.6.8) [50] was performed to generate the dynamic plot of ΔK and the mean of LnP (K) to evaluate the best fit on the number of grouping. The number of K with the largest ΔK was considered the best fit.
3. Results
3.1. Characterization of SSR loci, SSR-PCR products
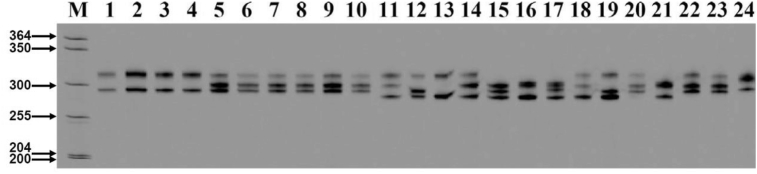
Twenty-nine newly characterized SSR loci were tested for 24 Indian jujube cultivars after optimization, of which 26 SSR loci were observed as polymorphic and the other 3 were monomorphic (Table 2). Some SSR loci showed either one or two PCR products in each sample. This indicates homogeneity or heterogeneity of the sample, respectively (Figure 1). Other SSR loci had more than two alleles (Figure 2). PCR amplification was used for 29 SSR loci to carry out the genetic diversity analysis of 24 Indian jujube cultivars.
Table 2.
Characteristics of the 29 polymorphic microsatellite loci isolated from Indian jujube (Ta, optimized annealing temperature).
| SSR Locus | Primers (5’→3′) | Fluorophore for Li-Cor system | Fluorophore for ABI system | Allelic size (bp) | Repeat Motif | Ta (°C) | GenBank Accession No. |
|---|---|---|---|---|---|---|---|
| Zma30 | F: ATATTTTCGGCTCCTCACCG R: ATGTGAAGATGACCCGACCG |
IRDye | 6-FAM | 325–374 | (GA)16 | 58 | MG385144 |
| Zma32 | F: TGTTTTCTTCTATGGCACAG R: ATGACGAAGAACCCTGAAGC |
IRDye | HEX | 222–350 | (AC)4 (AG)25N (CT)14 (AT)14 | 58 | MG385145 |
| Zma41 | F: GTGGTGACCGGGAATCGTG R: GGAGTGAATAGTGACCCGAGG |
IRDye | NED | 180 | (GA)23 | 58 | MG385146 |
| Zma51 | F: GTGGGAAGTTTTTGACGCCGCT R: GTGAGTGCACAGTGGCACCT |
IRDye | 6-FAM | 290–314 | (AG)19 | 58 | MG385147 |
| Zma53 | F: TGAATAGTGACCGACGGGT R: TATGGTGGTCCGGTGCATG |
IRDye | HEX | 188–204 | (AG)18 | 58 | MG385148 |
| Zma57 | F: CCTAAGCTTTCATGTTCCCTCC R: GCAATGATCCCCAAGCTGTC |
IRDye | HEX | 196–226 | (AG)17 | 58 | MG385149 |
| Zma58 | F: TACACCATGTGCCAGCTCT R: GAGAGTAGGCCACGTCAAG |
IRDye | 6-FAM | 288–310 | (TC)29 (AC)4 | 58 | MG385150 |
| Zma59 | F: CAACAATTTGCGGCTAACTC R: GAGAAGAAGAAGCCATGTGT |
IRDye | NED | 165–181 | (AG)18 | 58 | MG385151 |
| Zma73 | F: AATTTGGGTGGCCGGATGGA R: TTCGACCAATGGGGAAAGGAG |
IRDye | NED | 176–200 | (AG)27 | 58 | MG385152 |
| Zma93 | F: GCTACATCCTACACAACGCCT R: TGCAATTCACCAACTGGCACA |
IRDye | HEX | 233–275 | (AG)18 | 58 | MG385153 |
| Zma122 | F: ACCATATAGCCAACCCCTGGT R: CCCTGATCCATGCAAGCGT |
IRDye | 6-FAM | 254–296 | (AG)28 | 58 | MG385154 |
| Zma131 | F: TCCTGTCATGGGCTAGCTG R: CCTCGAAAAACAACCCCTCC |
IRDye | NED | 143–174 | (AG)22 | 58 | MG385155 |
| Zma147 | F: TCCTCCTGGACCGACACGT R: AACCGACCTCGTGGAGTGCA |
IRDye | HEX | 249–265 | (AG)25 | 58 | MG385156 |
| Zma148 | F: GGATGGAGAAAGACTACAAATTC R: CTGTTCGCATGAACGTGGCGAG |
IRDye | HEX | 214–250 | (AG)29 | 58 | MG385157 |
| Zma158 | F: GCCGGAAGTTTCTACGGCT R: TTGCCTCTCGTTGCCTTGC |
IRDye | NED | 125–135 | (AG)18 | 58 | MG385158 |
| Zma166 | F: AGAGGCATTGCTTCGCTGGA R: TCCAAACGTACAAGGCTTCGT |
IRDye | HEX | 231–255 | (AG)11N (GT)5 (AT)21 | 58 | MG385159 |
| Zma167 | F: CGAAGCTTTAGCCAGCTTACGT R: TTCACTCCCTCCCCCAACG |
IRDye | 6-FAM | 290–322 | (AG)17N (AG)19 | 58 | MG385160 |
| Zma173 | F: AGACGAAAAACGGGGGTGG R: CCATTTGGACCAGCCACGT |
IRDye | NED | 182–190 | (AG)25 | 58 | MG385161 |
| Zma180 | F: CCAGCGTGTGCATGGTTGG R: GGCAAACAGTGTCACCCACA |
IRDye | NED | 155 | (AG)24 | 58 | MG385162 |
| Zma185 | F: TCCTTCTCTGCGAATGGCA R: TCAAATCCTCCAAACCCCA |
IRDye | HEX | 188–200 | (AG)20 | 58 | MG385163 |
| Zma187 | F: AAGAGAAACTCCAGCTGCT R: TGTAACAGAGGCCATACACA |
IRDye | HEX | 194–241 | (AG)50 | 58 | MG385164 |
| Zma202 | F: CATGTGGCTCGGTGGTGGT R: TAAAAAGGGGCCCGCAAGT |
IRDye | NED | 172–182 | (AG)19 | 58 | MG385165 |
| Zma206 | F: AGCATCCAAACAGAGCGGGA R: CGAGAGACTCGAGCACTGC |
IRDye | NED | 156–180 | (AG)20 | 58 | MG385166 |
| Zma258 | F: CGTTAATCCAAGCAAACCCCAC R: AGTGCTCTGTCCTTGATCCCCA |
IRDye | 6-FAM | 270–310 | (AG)19 | 58 | MG385167 |
| Zma262 | F: TCCAATAGGAGCCTCACCA R: TCGACAAAAGGTCCCGAGG |
IRDye | NED | 166 | (AG)31 | 58 | MG385168 |
| Zma264 | F: AAGAGGATGCGGAGTTGGT R: CAACCTGTCTCGTGAGTGC |
IRDye | HEX | 220–255 | (AG)22 | 58 | MG385169 |
| Zma265 | F: AGAAGGTTGGGAAGGAGGT R: GCTTTGCTCGACCCCAACA |
IRDye | HEX | 190–212 | (AG)17 | 58 | MG385170 |
| Zma282 | F: ACGGTGTTTCTGGTGGACC R: GATCGACCCCTGGGAGTGA |
IRDye | HEX | 204–236 | (AG)21 | 58 | MG385171 |
| Zma283 | F: CCAATTGTTCAAGGTGTGAGAG R: TCACTTGCCACATTACAGAC |
IRDye | NED | 166–190 | (CT)10N [32]10 (TTG)7 | 58 | MG385172 |
Figure 1.
J181 SSR locus analysis of polymorphism in 24 Indian jujube cultivars. M: DNA marker. Lanes 1–24 represent different cultivars (see Table 1).
Figure 2.
J58 SSR locus analysis of polymorphism in 24 Indian jujube cultivars. M: DNA marker. Lanes 1–24 represent different cultivars (see Table 1).
3.2. Genetic diversity of Indian jujube cultivars in Taiwan
For the 26 polymorphic SSR loci, a total of 181 alleles were obtained ranging from 2 to 12 per locus (Table 3) with a mean of 5.24 (Table 3). The H and HE ranged from 0.04 (Zma173) to 0.82 (Zma282) and 0.04 (Zma173) to 0.83 (Zma282) with a mean of 0.48 and 0.41, respectively. Moreover, the PIC value of the locus ranged from 0.08 (Zma30, Zma57, Zma173, and Zma187) to 0.83 (Zma282) with a mean of 0.46 (Table 3). Among the loci, the Zma282 had the most variations at 12 alleles, 0.83 for HE, 0.82 for H, and 0.83 for PIC indicating a locus with the highest polymorphism. The three fixation indices, FIT, FIS, and FST had estimates ranging from -0.78 to 0.47, -1 to -0.2 and 0 to 0.59, respectively. The corresponding means of the three indices are as follows: -0.17, -0.57 and 0.26, all of which were detected statistically significant. Nevertheless, the Ewens-Watterson test indicates that the polymorphic SSR loci were selectively neutral except for Zma73 and Zma282 (Table 4). The results of the bottleneck tests were shown to be significant suggesting that recent bottleneck was shaping these Indian jujube cultivars (Table 5). This was confirmed based on the results of Sign test and Wilcoxon test involving the 24 cultivars as shown by the excess of heterozygosity based on the IAM, TPM, and SMM mutation models (Table 5).
Table 3.
The summary of genetic variation and fixation index based on 29 SSR loci for all strains of Ziziphus mauritiana Lam. calculated by SPAGeDi which include mean of NA (number of alleles), HE (expected heterozygosity, corrected for sample size), H (observed heterozygosity), FIT, FIS, FST, GST, RST, and PIC (polymorphism information content) by PowerMarker.
| Locus | NA | HE | H | FIT | FIS | FST | GST | RST | PIC |
|---|---|---|---|---|---|---|---|---|---|
| Zma30 | 2 | 0.05 | 0.06 | 0.43∗ | -0.20∗ | 0.52∗ | 0.25 | 0.69 | 0.08 |
| Zma32 | 10 | 0.79 | 0.78 | -0.27∗ | -0.33∗ | 0.05∗ | -0.21 | 0.22 | 0.78 |
| Zma41 | 1 | 0.00 | 0.00 | - | - | - | - | - | 0.00 |
| Zma51 | 5 | 0.37 | 0.38 | 0.41∗ | -0.20∗ | 0.51∗ | -0.25 | 0.50 | 0.63 |
| Zma53 | 3 | 0.52 | 0.52 | 0.47∗ | -0.26∗ | 0.58∗ | 0.18 | 0.58 | 0.43 |
| Zma57 | 2 | 0.54 | 0.53 | -0.78∗ | -1.00∗ | 0.11∗ | -0.73 | 0.56 | 0.08 |
| Zma58 | 9 | 0.74 | 0.74 | -0.15∗ | -0.62∗ | 0.29∗ | -0.10 | 0.23 | 0.80 |
| Zma59 | 4 | 0.68 | 0.67 | -0.44∗ | -0.50∗ | 0.04∗ | -0.34 | 0.02 | 0.22 |
| Zma73 | 3 | 0.62 | 0.62 | -0.20∗ | -0.20∗ | 0.00∗ | -0.19 | 0.01 | 0.15 |
| Zma93 | 6 | 0.42 | 0.42 | 0.10∗ | -0.32∗ | 0.32∗ | -0.02 | 0.15 | 0.73 |
| Zma122 | 7 | 0.68 | 0.67 | -0.26∗ | -1.00∗ | 0.37∗ | -0.22 | 0.45 | 0.64 |
| Zma131 | 6 | 0.64 | 0.63 | -0.30∗ | -1.00∗ | 0.35∗ | -0.29 | 0.44 | 0.70 |
| Zma147 | 4 | 0.71 | 0.71 | -0.24∗ | -0.25∗ | 0.01∗ | -0.17 | 0.00 | 0.28 |
| Zma148 | 8 | 0.73 | 0.73 | -0.14∗ | -0.62∗ | 0.29∗ | -0.25 | 0.51 | 0.76 |
| Zma158 | 8 | 0.57 | 0.56 | -0.17∗ | -1.00∗ | 0.41∗ | -0.40 | 0.58 | 0.79 |
| Zma166 | 9 | 0.75 | 0.74 | -0.12∗ | -0.33∗ | 0.16∗ | -0.17 | 0.20 | 0.74 |
| Zma167 | 10 | 0.80 | 0.80 | 0.00∗ | -1.00∗ | 0.50∗ | -0.09 | 0.39 | 0.82 |
| Zma173 | 2 | 0.04 | 0.04 | 0.43∗ | -0.20∗ | 0.52∗ | 0.00 | 0.52 | 0.08 |
| Zma180 | 1 | 0.00 | 0.00 | - | - | - | - | - | 0.00 |
| Zma185 | 7 | 0.73 | 0.73 | 0.08∗ | -1.00∗ | 0.54∗ | -0.08 | 0.32 | 0.70 |
| Zma187 | 2 | 0.69 | 0.68 | -0.44∗ | -0.50∗ | 0.04∗ | -0.39 | 0.07 | 0.08 |
| Zma202 | 4 | 0.62 | 0.62 | -0.34∗ | -0.33∗ | 0.00∗ | -0.33 | -0.02 | 0.34 |
| Zma206 | 3 | 0.57 | 0.56 | -0.71∗ | -1.00∗ | 0.14∗ | -0.60 | 0.58 | 0.15 |
| Zma258 | 6 | 0.73 | 0.73 | -0.21∗ | -0.38∗ | 0.12∗ | -0.27 | 0.12 | 0.67 |
| Zma262 | 1 | 0.00 | 0.00 | - | - | - | - | - | 0.00 |
| Zma264 | 5 | 0.61 | 0.61 | 0.17∗ | -1.00∗ | 0.59∗ | -0.09 | 0.41 | 0.49 |
| Zma265 | 8 | 0.64 | 0.64 | 0.21∗ | -0.40∗ | 0.43∗ | -0.20 | 0.45 | 0.79 |
| Zma282 | 12 | 0.83 | 0.82 | -0.21∗ | -0.33∗ | 0.09∗ | -0.19 | 0.02 | 0.83 |
| Zma283 | 4 | 0.61 | 0.61 | 0.02∗ | -1.00∗ | 0.51∗ | -0.24 | 0.37 | 0.48 |
| All loci | 5.24 | 0.41 | 0.48 | -0.17∗ | -0.57∗ | 0.26∗ | -0.23 | 0.24 | 0.46 |
Note: Asterisks indicate statistical significance (P < 0.05).
NA number of alleles, Ne number of effective alleles, HE expected heterozygosity, PIC polymorphism information content.
Table 4.
The Ewens-Watterson test for the selective neutrality of 29 SSR loci by using Popgene.
| Obs. F | SE | L95 | U95 | |
|---|---|---|---|---|
| Zma30 | 0.93 | 0.04 | 0.38 | 0.98 |
| Zma32 | 0.22 | 0.03 | 0.22 | 0.81 |
| Zma41 | 1.00 | ----- | ----- | ----- |
| Zma51 | 0.34 | 0.03 | 0.24 | 0.87 |
| Zma53 | 0.62 | 0.03 | 0.29 | 0.92 |
| Zma57 | 0.66 | 0.03 | 0.25 | 0.86 |
| Zma58 | 0.37 | 0.03 | 0.23 | 0.84 |
| Zma59 | 0.43 | 0.03 | 0.28 | 0.93 |
| Zma73 | 0.17∗ | 0.03 | 0.23 | 0.82 |
| Zma93 | 0.29 | 0.02 | 0.20 | 0.75 |
| Zma122 | 0.40 | 0.02 | 0.22 | 0.81 |
| Zma131 | 0.53 | 0.03 | 0.26 | 0.85 |
| Zma147 | 0.22 | 0.04 | 0.28 | 0.93 |
| Zma148 | 0.33 | 0.02 | 0.24 | 0.81 |
| Zma158 | 0.34 | 0.03 | 0.26 | 0.87 |
| Zma166 | 0.40 | 0.03 | 0.29 | 0.92 |
| Zma167 | 0.26 | 0.03 | 0.25 | 0.88 |
| Zma173 | 0.99 | 0.03 | 0.50 | 0.99 |
| Zma180 | 1.00 | ----- | ----- | ----- |
| Zma185 | 0.34 | 0.03 | 0.30 | 0.93 |
| Zma187 | 0.46 | 0.02 | 0.24 | 0.81 |
| Zma202 | 0.32 | 0.04 | 0.32 | 0.95 |
| Zma206 | 0.66 | 0.03 | 0.24 | 0.86 |
| Zma258 | 0.45 | 0.02 | 0.20 | 0.77 |
| Zma262 | 1.00 | ----- | ----- | ----- |
| Zma264 | 0.63 | 0.03 | 0.25 | 0.87 |
| Zma265 | 0.27 | 0.02 | 0.22 | 0.81 |
| Zma282 | 0.20∗ | 0.03 | 0.25 | 0.88 |
| Zma283 | 0.68 | 0.04 | 0.38 | 0.99 |
These statistics were calculated using 1000 simulated samples.
Obs. F - Observed sum of the square of allelic frequency.
SE- Standard error of the mean.
L95- Lower 95% confidence limit.
U95- Upper 95% confidence limit.
p < 0.05.
Table 5.
Tests for bottleneck under three microsatellite mutation models.
| IAM | TPM | SMM | |
|---|---|---|---|
| Sign Test | |||
| He/Ho | 14.5/26∗ | 15.06/26∗ | 15.29/26∗ |
| P | 0.22 | 0.41 | 0.00 |
| Standardized | |||
| T2 | 2.48 | -0.67 | -7.43 |
| P | 0.01 | 0.25 | 0.00 |
| Wilcoxon test | |||
| P (one tail for H deficiency) | 0.99 | 0.38 | 0.00 |
| P (one tail for H excess) | 0.01∗ | 0.63∗ | 0.99∗ |
IAM = infinite allele model; TPM = two-phase model; SMM = stepwise mutation model; Sign Test = number of loci with heterozygosity excess; Wilcoxon test = Wilcoxon rank test with probability of heterozygosity excess; Ho/He = observed and expected number of loci with heterozygosity excess under the infinite allele model (IAM), the two-phase model (TPM), and the stepwise mutation model (SMM); P = probability. H = heterozygosity.
p < 0.05.
3.3. Cultivar identification and similarity test among the cultivars
For all the 29 SSR markers tested, estimates of all possible pair-wise genetic similarity ranged from 1 to 0.30 with an average of 0.74 (Table 6). Accessions ‘Kaohsiung 6’ and ‘Chad native’ were found to be least similar genetically with a value of 0.30 (Table 6). In contrast, two groups exhibited the highest genetic similarity of 1. First, ‘Kaolang1’, ‘Kaolang2’, ‘Kaolang3’ and ‘Tsueimi’ belonged to one group, and the other group consisted of ‘Mejao’, ‘Cento’, ‘Gingtao’, ‘Daych’, ‘Chungyeh’ and ‘Tsueishiang’. The construction of dendrogram was based on UPGMA analysis to evaluate relationship patterns (Figure 3). All cultivars were divided into two major clades and rooted by ‘Chad native’ due to long genetic distance. The ‘A’ clade included 14 cultivars while the ‘B’ clade had 9 cultivars. The ‘A’ clade was further subdivided into ‘A1’ and ‘A2’ groups. However, some cultivars cannot be separated from these groups as a presumed mutation-derived cultivar. For instance, ‘Kaolang 2’, ‘Kaolang 3’ and ‘Tsueimi’ were likely derived from ‘Kaolang1’, while ‘Cento’, ‘Gingtao’, ‘Daych’, ‘Chungyeh’, and ‘Tsueishiang’ from ‘Mejao’.
Table 6.
Genetic distance matrix of 24 Indian jujube cultivars using 29 SSR loci (J1–J24 for Indian jujube cultivars refer to Table1).
| J1 | J2 | J3 | J4 | J5 | J6 | J7 | J8 | J9 | J10 | J11 | J12 | J13 | J14 | J15 | J16 | J17 | J18 | J19 | J20 | J21 | J22 | J23 | J24 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| J1 | 1.00 | |||||||||||||||||||||||
| J2 | 1.00 | 1.00 | ||||||||||||||||||||||
| J3 | 1.00 | 1.00 | 1.00 | |||||||||||||||||||||
| J4 | 1.00 | 1.00 | 1.00 | 1.00 | ||||||||||||||||||||
| J5 | 0.76 | 0.76 | 0.76 | 0.76 | 1.00 | |||||||||||||||||||
| J6 | 0.76 | 0.76 | 0.76 | 0.76 | 1.00 | 1.00 | ||||||||||||||||||
| J7 | 0.76 | 0.76 | 0.76 | 0.76 | 1.00 | 1.00 | 1.00 | |||||||||||||||||
| J8 | 0.76 | 0.76 | 0.76 | 0.76 | 1.00 | 1.00 | 1.00 | 1.00 | ||||||||||||||||
| J9 | 0.76 | 0.76 | 0.76 | 0.76 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | |||||||||||||||
| J10 | 0.76 | 0.76 | 0.76 | 0.76 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | ||||||||||||||
| J11 | 0.83 | 0.83 | 0.83 | 0.83 | 0.79 | 0.79 | 0.79 | 0.79 | 0.79 | 0.79 | 1.00 | |||||||||||||
| J12 | 0.86 | 0.86 | 0.86 | 0.86 | 0.78 | 0.78 | 0.78 | 0.78 | 0.78 | 0.78 | 0.86 | 1.00 | ||||||||||||
| J13 | 0.80 | 0.80 | 0.80 | 0.80 | 0.73 | 0.73 | 0.73 | 0.73 | 0.73 | 0.73 | 0.82 | 0.8 | 1.00 | |||||||||||
| J14 | 0.73 | 0.73 | 0.73 | 0.73 | 0.72 | 0.72 | 0.72 | 0.72 | 0.72 | 0.72 | 0.83 | 0.77 | 0.78 | 1.00 | ||||||||||
| J15 | 0.72 | 0.72 | 0.72 | 0.72 | 0.73 | 0.73 | 0.73 | 0.73 | 0.73 | 0.73 | 0.77 | 0.8 | 0.76 | 0.89 | 1.00 | |||||||||
| J16 | 0.72 | 0.72 | 0.72 | 0.72 | 0.68 | 0.68 | 0.68 | 0.68 | 0.68 | 0.68 | 0.75 | 0.78 | 0.78 | 0.84 | 0.86 | 1.00 | ||||||||
| J17 | 0.72 | 0.72 | 0.72 | 0.72 | 0.69 | 0.69 | 0.69 | 0.69 | 0.69 | 0.69 | 0.73 | 0.73 | 0.73 | 0.76 | 0.79 | 0.86 | 1.00 | |||||||
| J18 | 0.73 | 0.73 | 0.73 | 0.73 | 0.76 | 0.76 | 0.76 | 0.76 | 0.76 | 0.76 | 0.83 | 0.77 | 0.81 | 0.87 | 0.8 | 0.76 | 0.71 | 1.00 | ||||||
| J19 | 0.78 | 0.78 | 0.78 | 0.78 | 0.74 | 0.74 | 0.74 | 0.74 | 0.74 | 0.74 | 0.78 | 0.85 | 0.78 | 0.84 | 0.88 | 0.83 | 0.77 | 0.78 | 1.00 | |||||
| J20 | 0.72 | 0.72 | 0.72 | 0.72 | 0.76 | 0.76 | 0.76 | 0.76 | 0.76 | 0.76 | 0.75 | 0.74 | 0.76 | 0.84 | 0.82 | 0.77 | 0.74 | 0.82 | 0.8 | 1.00 | ||||
| J21 | 0.66 | 0.66 | 0.66 | 0.66 | 0.71 | 0.71 | 0.71 | 0.71 | 0.71 | 0.71 | 0.77 | 0.71 | 0.72 | 0.71 | 0.72 | 0.7 | 0.69 | 0.76 | 0.72 | 0.68 | 1.00 | |||
| J22 | 0.76 | 0.76 | 0.76 | 0.76 | 0.77 | 0.77 | 0.77 | 0.74 | 0.77 | 0.77 | 0.77 | 0.77 | 0.73 | 0.84 | 0.85 | 0.74 | 0.72 | 0.81 | 0.85 | 0.85 | 0.72 | 1.00 | ||
| J23 | 0.74 | 0.74 | 0.74 | 0.74 | 0.95 | 0.95 | 0.95 | 0.95 | 0.95 | 0.95 | 0.77 | 0.74 | 0.73 | 0.74 | 0.74 | 0.68 | 0.69 | 0.78 | 0.77 | 0.77 | 0.72 | 0.79 | 1.00 | |
| J24 | 0.33 | 0.33 | 0.33 | 0.33 | 0.33 | 0.33 | 0.33 | 0.33 | 0.33 | 0.33 | 0.35 | 0.33 | 0.40 | 0.33 | 0.32 | 0.34 | 0.31 | 0.33 | 0.31 | 0.35 | 0.32 | 0.30 | 0.34 | 1.00 |
Figure 3.
Dendrogram showing the genetic relationships among 24 Indian jujube cultivars using SSR markers. Scale bar represents the genetic distance.
The PCoA and assignment test were used to reassign the clustering of Indian jujube cultivars without any assumptions from prior classification. PCoA results revealed the separation between the wild ‘Chad native’ and the other 23 cultivars (Figure 4A). Furthermore, the admixture patterns of all domesticated and commercial cultivars in three axes can explain 79.90% of the variation (31.61%, 21.78%, and 21.76% of the first, second and third axis, respectively) (Figure 4A). Excluding the wild accession, three other major groups were separated in the PCoA plot (Figure 4B) which is consistent with the dendogram (Figure 3). The ‘A1’ group in UPGMA dendrogram was distributed between second and third quadrants, and ‘A2’ group in the first quadrant, while the ‘B’ group in the fourth quadrant (Figure 4B). Specifically, ‘A1’ group was further divided into two subgroups that distinctly distributed ‘Kaolang1’, ‘Kaolang 2’, ‘Kaolang 3’ and ‘Tsueimi’ in the second quadrant, and ‘Tianmi’, ‘Kaohsiung2’, and ‘Kaohsiung3’ in the third quadrant. Apparently, the best fit for the number of grouping was implied as four with ΔK = 162.292 (STRUCTURE HARVESTER v. 0.6.8) [50] based on the 29 microsatellite loci (Figure 5A). When K = 4, four genetic compositions were calculated using the Bayesian-clustering method. Hence, the 24 Indian jujube cultivars were separated into four groups (Figure 5B) based on four different compositions (Table 7). This is consistent with the results of UPGMA dendrogram and PCoA plot except for ‘Tianmi’, ‘Kaohsiung2’, ‘Kaohsiung3’ and ‘Roulong’. Along with ‘Shuemi’, these five cultivars were detected to have a genetic admixture of two compositions (Figure 5B) suggesting a possible hybrid origin [24, 48].
Figure 4.
The PCoA analysis performed by POLYSAT based on 29 microsatellite loci from 24 strains of Ziziphus mauritiana Lam. (A) The PCoA plot with the Chad-native strain. (B) The PCoA plot without the Chad-native strain.
Figure 5.
Plots of (A) the log likelihood and ΔK and (B) the best clustering at K = 4 estimated by the program Structure analysis of the full data set of 24 strains of Ziziphus mauritiana Lam. The vertical lines represent the 24 strains.
Table 7.
Genetic compositions of Indian jujube cultivars based on the Bayesian-clustering method.
| Cultivars | Composition 1 | Composition 2 | Composition 3 | Composition 4 |
|---|---|---|---|---|
| Kaolang 1 | 0.997 | 0.001 | 0.001 | 0.001 |
| Kaolang 2 | 0.997 | 0.001 | 0.001 | 0.001 |
| Kaolang 3 | 0.997 | 0.001 | 0.001 | 0.001 |
| Tsueimi | 0.997 | 0.001 | 0.001 | 0.001 |
| Mejao | 0.001 | 0.996 | 0.001 | 0.001 |
| Cento | 0.001 | 0.996 | 0.001 | 0.001 |
| Gingtao | 0.001 | 0.996 | 0.001 | 0.001 |
| Dayeh | 0.001 | 0.996 | 0.001 | 0.001 |
| Chungyeh | 0.001 | 0.996 | 0.001 | 0.001 |
| Tsueishiang | 0.001 | 0.996 | 0.001 | 0.001 |
| Tianmi | 0.287 | 0.010 | 0.688 | 0.016 |
| Kaohsiung 2 | 0.252 | 0.004 | 0.670 | 0.074 |
| Kaohsiung 3 | 0.379 | 0.003 | 0.610 | 0.008 |
| Kaohsiung 5 | 0.005 | 0.013 | 0.976 | 0.005 |
| Kaohsiung 6 | 0.064 | 0.004 | 0.849 | 0.082 |
| Yuguan | 0.010 | 0.066 | 0.918 | 0.006 |
| Biyuan | 0.002 | 0.002 | 0.992 | 0.004 |
| Hongyung | 0.002 | 0.002 | 0.992 | 0.003 |
| Hsinsuchi | 0.002 | 0.005 | 0.949 | 0.043 |
| Huangguan | 0.012 | 0.017 | 0.963 | 0.009 |
| Telong | 0.003 | 0.002 | 0.990 | 0.005 |
| Roulong | 0.006 | 0.034 | 0.461 | 0.499 |
| Shuemi | 0.003 | 0.633 | 0.023 | 0.341 |
| Chad native | 0.001 | 0.001 | 0.001 | 0.998 |
4. Discussion
Chiou et al. (2012) selected 14 higher polymorphic SSR loci [Zma-25, Zma-29, Zma-107, Zma-161, Zma-168, Zma-181 (Figure 1), Zma-182, Zma-189, Zma-192, Zma-210, Zma-230, Zma-236, Zma-257, and Zma-279] as the standard polymorphic molecular markers to detect genetic variation and reconstruct the genetic relationship of the 24 Indian jujube cultivars [28]. In this study, however, 26 out of the 29 newly characterized SSR loci were found to be polymorphic and consequently selected to evaluate the genetic relationship of these cultivars. Although the stutter bands of PCR products were resolved [52, 53], most SSR loci (89.66%) (26/29) were observed to have more than two alleles. This result confirmed the karyotype of Indian jujube as tetraploid (2n = 4x = 48) [4,5]. Besides, SSR loci duplication also shows more than two bands in PCR products which can be a source of inaccuracy. Other possible causes of stutter bands in SSR-PCR products are commonly found among dinucleotide repeat units particularly those with larger repeat numbers [52, 54]. The stutter products are results from the slippage of DNA polymerase which is a natural process for SSR mutations [51]. It is common for SSR markers interfered with a high ratio of stutter products, especially in polyploid plants [55]. However, most stutter bands were shown to be minor and shorter bands compared to the major bands [56]. Therefore, bands considered as minor and stutter upon careful inspection were not included in the analysis of this study.
The obtained HE values of Indian jujube were closer to other tropical fruit trees such as mango cultivars [27], wax apple [26] and camu-camu [57], but much lower compared to Japanese plum cultivars [58], apple cultivars [59], Tunisian orange [60], Asian pear [61] and Chinese jujube [62]. These fruit cultivars have strong self-incompatibility [62, 63, 64] including Indian jujube [7]. Self-incompatibility is a mechanism for outcrossing that tends to maintain a high degree of heterogeneity in crops [65]. However, these Indian jujube cultivars were shown otherwise relative to other self-incompatible fruit trees likely due to narrow genetic base when first introduced to Taiwan [29]. Several other variants eventually might have risen as cultivated mutants (Table 1) made by artificial selection. Moreover, the results in this study revealed that microsatellite primers were consistent with that of genomic microsatellite studies in mango (0–0.756 with a mean of 0.525) [66] and Chinese jujube (0.25–0.88 with a mean of 0.56) [62]. This indicates that SSR as a molecular marker is useful in investigating genetic relationships of Indian jujube cultivars in Taiwan.
The negative values of FIS and FIT would mean an excess of heterozygotes as a result of mating between more distant relatives than the average within subpopulations [67, 68]. These values among domesticated cultivars also suggest outcrossing due to self-incompatibility based on the genetic evidence. The results are likewise in agreement with that of spontaneous mutations where alleles always have identical genetic composition using SSR markers [69, 70, 71]. Nevertheless, several studies demonstrated that retrotransposon markers effectively detect spontaneous mutations [72]. These reports imply that SSR markers are less suitable for these mutations, but are favorable for cultivar identification [24, 25, 27]. Because of this limitation, it is suggested that the commercial cultivars like ‘Kaolang2’, ‘Kaolang3’, ‘Tsueimi’, ‘Cento’, ‘Gingtao’, ‘Daych’, ‘Chungyeh’ and ‘Tsueishiang’ are considered descendants of either ‘Kaolang1’ or ‘Mejao’ by spontaneous mutations. Indian jujube cultivars were domesticated from wild jujube (Z. rotundifolia) [73, 74] between 1500 BC to 300 AD based on Indian archaeological and literary records [51]. The ability of different taxa such as Z. nummularia, Z. oenoplia, Z. rugosa, Z. sativa, Z. vulgaris and Z. xylopyrus to cross freely had formed Z. rotundifolia that enriched the gene pool of Z. mauritiana by increasing the genetic variations of their adaptability to different environments [51]. Breeders cultivated different varieties and selected descendants based on disease resistance, fruit size and sugar contents [51]. It was introduced from India to Indochina several times and cultivated in Southern Taiwan around 1944 [75]. This shows that the genetic composition of SSR loci might be contributed by various pollen flow resulting to admixture in some cultivated varieties such as ‘Tianmi’, ‘Kaohsiung2’, ‘Kaohsiung3’, ‘Roulong’ and ‘Shuemi’ in Taiwan. Other cultivars that might have been generated by spontaneous mutations include ‘Kaolang1’, ‘Mejao’ and their mutation-derived cultivars that had exactly identical compositions based on SSR markers [69, 70, 71].
The Indian jujube is one of the commercially important fruit crops in Taiwan. Lately, several new cultivated varieties were developed by breeders using both spontaneous mutation technology and the traditional artificial hybridization. The standard identification system of SSR marker is an important method to aid breeders in the improvement of commercial cultivars. In this study, we developed 29 primer sets consisting of 26 polymorphic and 3 monomorphic microsatellite loci from Indian jujube. These SSR markers detected that spontaneous mutations might have risen recently exhibiting identical genetic compositions with their potential progenitor. The cultivated varieties with genetic admixture were results of the past hybridization or introgression caused by artificial or natural occurrence. Therefore, the 29 new primer sets for Indian jujube SSR loci reported here are useful for evaluating genetic diversity, developing a standard system for cultivar identification and analyzing lineage of this tropical fruit tree.
Declarations
Author contribution statement
Chu-Ying Chiou: Conceived and designed the experiments; Performed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Huei-Chuan Shih: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Chi-Chu Tsai, Yu-Chung Chiang: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Xiao-Lei Jin: Performed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Ya-Zhu Ko: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Junaldo A. Mantiquilla: Analyzed and interpreted the data; Wrote the paper.
I-Szu Weng: Performed the experiments.
Funding statement
This research was supported by the Ministry of Science and Technology, Taiwan (MOST 105-2621-B-110-003-MY3 and MOST 105-2621-B-110-001) to Y.-C. Chiang and partially supported by the Higher Education Sprout Project of NSYSU.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
Achievement of this study is dedicated to the memory of the scientific contributions of Dr. C.C.T. who is the third author. Dr. C.C.T. died from an unexpected stroke on November 5th 2015.
References
- 1.Ersoy N., Kupe M., Sagbas H.I., Ercisli S. Phytochemical diversity among barberry (Berberis vulgaris L.) Not. Bot. Horti Agrobot. Cluj-Napoca. 2018;46(2):198–204. [Google Scholar]
- 2.Guney M., Kafkas S., Koc A., Aras S., Keles H., Karci H. Characterization of quince (Cydonia oblonga Mill.) accessions by simple sequence repeat markers. Turk. J. Agric. For. 2019;43:69–79. [Google Scholar]
- 3.Senica M., Stampar F., Mikulic-Petkovsek M. Different extraction processes affect the metabolites in blue honeysuckle (Lonicera caerulea L. subsp. edulis) food products. Turk. J. Agric. For. 2019;43:576–585. [Google Scholar]
- 4.Srivastava H.C. Floral biology and genetics of ber (Zizyphus mauritiana L.)--a review. Agric. Rev. 1980;1:27–133. [Google Scholar]
- 5.Daulta B.S., Sareen P.K. Studies on chromosome number and in vitro pollen viability and germination in ber (Zizyphus mauritiana Lamk) Haryana Agric. Univ. J. Res. 1980;10:60–64. [Google Scholar]
- 6.Nehra N.S., Sareen P.K., Chitkara S.D. Cytological studies in genus Zizyphus. Cytologia. 1983;48:103–107. [Google Scholar]
- 7.Morton J.F., Dowling C.F. Indian Jujube. In Fruits of warm climates. In: Mortan J.F., Miami F.L., editors. Center for New Crops & Plant Products. Purdue University: Lafayette, Ind; USA: 1987. pp. 272–275. [Google Scholar]
- 8.Li J.D., Bi H.T., Li H.T., Li Z.S., Feng J.C. I International Jujube Symposium, China. 2008. Genetic analysis of Ziziphus jujuba 'Huizao' using ISSR markers; pp. 135–142. [Google Scholar]
- 9.Lu J.I., Mao Y., Shen L., Peng S.H., Liu M. Application of AFLP markers for identification of hybrids from open pollinated Dongzao (Zizyphus jujuba Mill.) progenies. Acta Hortic. Sin. 2005;32:680. [Google Scholar]
- 10.Peng J.Y., Shu H.R., Peng S.Q. To address the problem of infraspecific classification of Ziziphus jujuba Mill. using RAPD data. Acta Pharmacol. Sin. 2002;40:89–94. [Google Scholar]
- 11.Peng J.Y., Shu H.R., Sun Z.X., Peng S.Q. RAPD analysis of germplasm resources on Chinese date. Acta Hortic. Sin. 2000;27:171–176. [Google Scholar]
- 12.Bai R.X. Hebei Agricultural University; Hebei: 2008. Studies on Genetic Diversity and Core Collection Construction of Ziziphus jujuba Germsplasm Resources Using AFLP and SRAP Markers. Ph. D. Dissertation. [Google Scholar]
- 13.Wang S., Liu Y., Ma L., Liu H., Tang Y., Wu L., Pang X. Isolation and characterization of microsatellite markers and analysis of genetic diversity in Chinese jujube (Ziziphus jujuba Mill.) PloS One. 2014;9 doi: 10.1371/journal.pone.0099842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Y.K., Tian J.B., Wang Y.Q., Sui C.L., Li D.K., Huang C.L. AFLP analysis of jujube cultivars and strains. J. Fruit Sci. 2007;2:146–150. [Google Scholar]
- 15.Wen Y.F., Gang H.E. Genetic relationships among jujube cultivars suitable for cultivation in South China. J. Fruit Sci. 2007;24:640–643. [Google Scholar]
- 16.Saha D., Srivastava S.C., Ramani R. Genetic relationships among fruit cultivars and host plants of Indian lac insect in ber (Ziziphus mauritiana Lam.) revealed by RAPD and ISSR markers. Indian J. Biotechnol. 2013;12:170–177. [Google Scholar]
- 17.Zhi F.J., Jia Y.L., Liang H.Y., Lu R.J., Chu F.J. Identification of RAPD-cultivar of jujube. Acta Agric. Bor. Sin. 2009;1:110–114. [Google Scholar]
- 18.Su H.S., Chen C.C. Development of Horticulture, National Chung Hsing University; Taichung, Taiwan: 2005. Assessment of Genetic Diversity Among Indian Jujube (Zizyphus mauritiana Lam.) Cultivars by RAPD and ISSR Markers. Master Thesis. [Google Scholar]
- 19.Litt M., Luty J.A. A hypervariable microsatellite revealed by in vitro amplification of a dinucleotide repeat within the cardiac muscle actin gene. Am. J. Hum. Genet. 1989;44:397–401. [PMC free article] [PubMed] [Google Scholar]
- 20.Hite J.M., Eckert K.A., Cheng K.C. Factors affecting fidelity of DNA synthesis during PCR amplification of d(C-A) n-d(G-T) n microsatellite repeats. Nucleic Acids Res. 1996;24:2429–2434. doi: 10.1093/nar/24.12.2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown S.M., Hopkins M.S., Mitchell S.E., Senior M.L., Wang T.Y., Duncan R.R., Gonzalez-Candelas F., Kresovich S. Multiple methods for the identification of polymorphic simple sequence repeats (SSRs) in sorghum [Sorghum bicolor (L.) Moench] Theor. Appl. Genet. 1996;93:190–198. doi: 10.1007/BF00225745. [DOI] [PubMed] [Google Scholar]
- 22.Tautz D., Renz M. Simple sequences are ubiquitous repetitive components of eukaryotic genomes. Nucleic Acids Res. 1984;12:4127–4138. doi: 10.1093/nar/12.10.4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tautz D., Schlötterer C. Simple sequences. Curr. Opin. Genet. Dev. 1994;4:832–837. doi: 10.1016/0959-437x(94)90067-1. [DOI] [PubMed] [Google Scholar]
- 24.Kanupriya, Latha P.M., Aswath C., Reddy L., Padmakar B., Vasugi C., Dinesh M.R. Cultivar identification and genetic fingerprinting of guava (Psidium guajava) using microsatellite markers. Int. J. Fruit Sci. 2011;11:184–196. [Google Scholar]
- 25.Shoda M., Urasaki N., Sakiyama S., Terakami S., Hosaka F., Shigeta N., Nishitani C., Yamamoto T. DNA profiling of pineapple cultivars in Japan discriminated by SSR markers. Breed Sci. 2012;62:352–359. doi: 10.1270/jsbbs.62.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lai J.M., Tsai C.C., Yen C.R., Ko Y.Z., Chen S.R., Weng I.S., Lin Y.S., Chiang Y.C. Molecular characterization of twenty polymorphic microsatellite markers in the polyploid fruit tree species Syzygium samarangense (Myrtaceae) Genet. Mol. Res. 2015;14:13013–13021. doi: 10.4238/2015.October.21.22. [DOI] [PubMed] [Google Scholar]
- 27.Tsai C.C., Chen Y.K., Chen C.H., Weng I.S., Tsai C.M., Lee S.R., Chiang Y.C. Cultivar identification and genetic relationship of mango (Mangifera indica) in Taiwan using 37 SSR markers. Sci. Hortic. 2013;164:196–201. [Google Scholar]
- 28.Chiou C.Y., Chiang Y.C., Chen C.H., Yen C.R., Lee S.R., Lin Y.S., Tsai C.C. Development and characterization of 38 polymorphic microsatellite markers from an economically important fruit tree, the Indian jujube. Am. J. Bot. 2012;99:e199–e202. doi: 10.3732/ajb.1100500. [DOI] [PubMed] [Google Scholar]
- 29.Chiou C.Y., Yen C.R. Variation of flowering characteristics and pollen viability among Indian jujube (Ziziphus mauritiana Lam.) cultivars. J. Int. Coop. 2011;6:1–16. [Google Scholar]
- 30.Chio C.Y., Yen C.R. Forcing culture development and industry adjustment of ber (Ziziphus mauritiana Lam.) in Taiwan. In: Wu T.C., Yeh W.P., Chen M.S., Hsu C.M., editors. The Symposium of Forcing Culture Development and Industry Adjustment of Fruit in Taiwan. Taichung District Agricultural Research and Extension Station, Council of Agriculture: Taichung; Taiwan: 2017. pp. 153–170. [Google Scholar]
- 31.Wang T.N., Liu B.C. The breeding achievement of fruit crops in Taiwan. In: Wang T.N., Liu B.C., editors. Proceedings of the Symposium on the Fruit Crop Industry in Taiwan. Fengshan Tropical Horticultural Experiment Branch, Agricultural Research Institute, Council of Agriculture: Kaohsiung, Taiwan; 2005. pp. 44–73. [Google Scholar]
- 32.Szurman-Zubrzycka M., Chmielewska B., Gajewska P., Szarejko I. Mutation detection by analysis of DNA heteroduplexes in TILLING populations of diploid species. In: Jankowicz-Cieslak T., Tai H.T., Kumlehn J., Till B.J., editors. Biotechnologies for Plant Mutation Breeding: Protocols. Springer; Cham, Switzerland: 2017. pp. 281–303. [Google Scholar]
- 33.Schuelke M. An economic method for the fluorescent labeling of PCR fragments. Nat. Biotechnol. 2000;18:233–234. doi: 10.1038/72708. [DOI] [PubMed] [Google Scholar]
- 34.Wright S. The genetical structure of populations. Ann. Eugen. 1949;15:323–354. doi: 10.1111/j.1469-1809.1949.tb02451.x. [DOI] [PubMed] [Google Scholar]
- 35.Nei M. Analysis of gene diversity in subdivided populations. Proc. Natl. Acad. Sci. Unit. States Am. 1973;70:3321–3323. doi: 10.1073/pnas.70.12.3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Slatkin M. A measure of population subdivision based on microsatellite allele frequencies. Genetics. 1995;139:457–462. doi: 10.1093/genetics/139.1.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hardy O.J., Vekemans X. SPAGeDi: a versatile computer program to analyse spatial genetic structure at the individual or population levels. Mol. Ecol. Notes. 2002;2:618–620. [Google Scholar]
- 38.Shete S., Tiwari H., Elston R.C. On estimating the heterozygosity and polymorphism information content value. Theor. Popul. Biol. 2000;57:265–271. doi: 10.1006/tpbi.2000.1452. [DOI] [PubMed] [Google Scholar]
- 39.Liu K., Muse S.V. PowerMarker: an integrated analysis environment for genetic marker analysis. Bioinformatics. 2005;21:2128–2129. doi: 10.1093/bioinformatics/bti282. [DOI] [PubMed] [Google Scholar]
- 40.Bowcock A.M., Ruiz-Linares A., Tomfohrde J., Minch E., Kidd J.R., Cavalli-Sforza L.L. High resolution of human evolutionary trees with polymorphic microsatellites. Nature. 1994;368:455–457. doi: 10.1038/368455a0. [DOI] [PubMed] [Google Scholar]
- 41.Ciampolini R., Moazami-Goudarzi K., Vaiman D., Dillmann C., Mazzanti E., Foulley J.L., Leveziel H., Cianci D. Individual multilocus genotypes using microsatellite polymorphisms to permit the analysis of the genetic variability within and between Italian beef cattle breeds. J. Anim. Sci. 1995;73:3259–3268. doi: 10.2527/1995.73113259x. [DOI] [PubMed] [Google Scholar]
- 42.Tamura K., Peterson D., Peterson N., Stecher G., Nei M., Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lynch M. The similarity index and DNA fingerprinting. Mol. Biol. Evol. 1990;7:478–484. doi: 10.1093/oxfordjournals.molbev.a040620. [DOI] [PubMed] [Google Scholar]
- 44.Clark L.V., Jasieniuk M. POLYSAT: an R package for polyploid microsatellite analysis. Mol. Ecol. Resour. 2011;11:562–566. doi: 10.1111/j.1755-0998.2011.02985.x. [DOI] [PubMed] [Google Scholar]
- 45.Falush D., Stephens M., Pritchard J.K. Inference of population structure using multilocus genotype data: linked loci and correlated allele frequencies. Genetics. 2003;164:1567–1587. doi: 10.1093/genetics/164.4.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Falush D., Stephens M., Pritchard J.K. Inference of population structure using multilocus genotype data: dominant markers and null alleles. Mol. Ecol. Notes. 2007;7:574–578. doi: 10.1111/j.1471-8286.2007.01758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pritchard J.K., Stephens M., Donnelly P. Inference of population structure using multilocus genotype data. Genetics. 2000;155:945–959. doi: 10.1093/genetics/155.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hubisz M.J., Falush D., Stephens M., Pritchard J.K. Inferring weak population structure with the assistance of sample group information. Mol. Ecol. Resour. 2009;9:1322–1332. doi: 10.1111/j.1755-0998.2009.02591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Evanno G., Regnaut S., Goude t J. Detecting the number of clusters of individuals using the software STRUCTURE: a simulation study. Mol. Ecol. 2005;14:2611–2620. doi: 10.1111/j.1365-294X.2005.02553.x. [DOI] [PubMed] [Google Scholar]
- 50.Earl D.A., vonHoldt B.M. Structure HARVESTER: a website and program for visualizing STRUCTURE output and implementing the Evanno method. Conserv. Genet. Resour. 2012;4:359–361. [Google Scholar]
- 51.Awasthi O.P., More T.A. Genetic diversity and status of Ziziphus in India. ISHS Acta. Hortic. 2009;840:33–40. [Google Scholar]
- 52.Kunkel T.A., Patel S.S., Johnson K.A. Error-prone replication of repeated DNA sequences by T7 DNA polymerase in the absence of its processivity subunit. Proc. Natl. Acad. Sci. Unit. States Am. 1994;91:6830–6834. doi: 10.1073/pnas.91.15.6830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shinde D., Lai Y., Sun F., Arnheim N. Taq DNA polymerase slippage mutation rates measured by PCR and quasi-likelihood analysis:(CA/GT) n and (A/T) n microsatellites. Nucleic Acids Res. 2003;31:974–980. doi: 10.1093/nar/gkg178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Clarke L.A., Rebelo C.S., Gonçalves J., Boavida M.G., Jordan P. PCR amplification introduces errors into mononucleotide and dinucleotide repeat sequences. Mol. Pathol. 2001;54:351. doi: 10.1136/mp.54.5.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pfeiffer T., Roschanski A.M., Pannell J.R., Korbecka G., Schnittler M. Characterization of microsatellite loci and reliable genotyping in a polyploid plant, Mercurialis perennis (Euphorbiaceae) J. Hered. 2011;102:479–488. doi: 10.1093/jhered/esr024. [DOI] [PubMed] [Google Scholar]
- 56.Walsh P.S., Fildes N.J., Reynolds R. Sequence analysis and characterization of stutter products at the tetranucleotide repeat locus vWA. Nucleic Acids Res. 1996;24:2807–2812. doi: 10.1093/nar/24.14.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Šmíd J., Kalousová M., Mandák B., Houška J., Chládová A., Pinedo M., Lojka B. Morphological and genetic diversity of camu-camu [Myrciaria dubia (Kunth) McVaugh] in the Peruvian Amazon. PloS One. 2017;12 doi: 10.1371/journal.pone.0179886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Carrasco B., Díaz C., Moya M., Gebauer M., García-González R. Genetic characterization of Japanese plum cultivars (Prunus salicina) using SSR and ISSR molecular markers. Cienc. Investig. Agrar. 2012;39:533–543. [Google Scholar]
- 59.Sikorskaite S., Gelvonauskiene D., Stanys V., Baniulis D. Characterization of microsatellite loci in apple (Malus × domestica Borkh.) cultivars. Zemdirbyste. 2012;99:131–138. [Google Scholar]
- 60.Mahjbi A., Oueslati A., Baraket G., Salhi-Hannachi A., Azouzi S.Z. Assessment of genetic diversity of Tunisian orange, Citrus sinensis (L.) Osbeck using microsatellite (SSR) markers. Genet. Mol. Res. 2016;15 doi: 10.4238/gmr.15026564. [DOI] [PubMed] [Google Scholar]
- 61.Nishio S., Takada N., Saito T., Yamamoto T., Iketani H. Estimation of loss of genetic diversity in modern Japanese cultivars by comparison of diverse genetic resources in Asian pear (Pyrus spp.) BMC Genet. 2016;17:81. doi: 10.1186/s12863-016-0380-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xu C., Gao J., Du Z., Li D., Wang Z., Li Y., Pang X. Identifying the genetic diversity, genetic structure and a core collection of Ziziphus jujuba Mill. var. jujuba accessions using microsatellite markers. Sci. Rep. 2016;6:31503. doi: 10.1038/srep31503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hegedűs A. Review of the self-incompatibility in apple (Malus x domestica Borkh., syn.: Malus pumila Mill.) Int. J. Hortic. Sci. 2006;12:31–36. [Google Scholar]
- 64.Okie W.R., Weinberger J.H. Plums. In: Janick J., Moore J.N., editors. I. John Wiley and Sons, Inc.; New York: 1996. (Fruit Breeding). Tree and tropical fruits. [Google Scholar]
- 65.Wang M.L., Chen Z.B., Barkley N.A., Newman M.L., Kim W., Raymer P., Pederson G.A. Characterization of seashore paspalum (Paspalum vaginatum Swartz) germplasm by transferred SSRs from wheat, maize and sorghum. Genet. Resour. Crop Evol. 2006;53:779–791. [Google Scholar]
- 66.Chiang Y.C., Tsai C.M., Chen Y.K.H., Lee S.R., Chen C.H., Lin Y.S., Tsai C.C. Development and characterization of 20 new polymorphic microsatellite markers from Mangifera indica (Anacardiaceae) Am. J. Bot. 2012;99:e117–e119. doi: 10.3732/ajb.1100443. [DOI] [PubMed] [Google Scholar]
- 67.Wright S. The interpretation of population structure by F-statistics with special regard to systems of mating. Evolution. 1965;19:395–420. [Google Scholar]
- 68.Nagylaki T. Fixation indices in subdivided populations. Genetics. 1998;148:1325–1332. doi: 10.1093/genetics/148.3.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Galli Z., Halász G., Kiss E., Heszky L., Dobránszki J. Molecular identification of commercial apple cultivars with microsatellite markers. Hortscience. 2005;40:1974–1977. [Google Scholar]
- 70.Garkava-Gustavsson L., Kolodinska Brantestam A., Sehic J., Nybom H. Molecular characterisation of indigenous Swedish apple cultivars based on SSR and S-allele analysis. Hereditas. 2008;145:99–112. doi: 10.1111/j.0018-0661.2008.02042.x. [DOI] [PubMed] [Google Scholar]
- 71.Hokanson S., Lamboy W., Szewc-McFadden A., McFerson J. Microsatellite (SSR) variation in a collection of Malus (apple) species and hybrids. Euphytica. 2001;118:281–294. [Google Scholar]
- 72.Venturi S., Dondini L., Donini P., Sansavini S. Retrotransposon characterisation and fingerprinting of apple clones by S-SAP markers. Theor. Appl. Genet. 2006;112:440–444. doi: 10.1007/s00122-005-0143-8. [DOI] [PubMed] [Google Scholar]
- 73.Awasthi O.P., Pathak R.K., Pandey S.D. Effect of sodicity and salinity levels on four scion cultivars budded on Indian jujube (Ziziphus mauritiana) Indian J. Agric. Sci. 1994;65:363–367. [Google Scholar]
- 74.Awasthi O.P., Pathak R.K., Pandey S.D. Sodicity and salinity on survival and nutrient status of four scion cultivars budded on Indian jujube (Zizyphus mauritiana Lamk.) Trop. Agric. 1997;74:238–242. [Google Scholar]
- 75.Cho C.Z. Indian jujube. In: Huang S.N., Huang H.Y., editors. In Reasonable Fertilization of Crops (Fruit Trees), Tainan District Agriculture Improvement Center Special Monograph. 2005. pp. 51–55. No. 3, Tainan, Taiwan. [Google Scholar]