Abstract
Purpose:
The purpose of this study was to investigate the clinical efficacy of salvage percutaneous radiofrequency ablation in patients with unresectable colorectal cancer liver metastases.
Methods:
The cohort consisted of 81 patients with 126 colorectal cancer liver metastases who underwent radiofrequency ablation between January 2012 and September 2016. The clinical data and ablation data were retrospectively analyzed. The local tumor progression-free survival, overall survival, and prognostic factors were analyzed using the log-rank test and Cox regression model.
Results:
The technique success rate was 99.21%. The primary efficacy rate was 100% at the 1-month follow-up. Minor complications were observed in 2 patients, which recovered within 1 week. The median local tumor progression-free survival time of all patients was 29.8 months. The absence of subsequent chemotherapy was an independent predictor of a shorter local tumor progression-free survival time (P < 0.001, hazard ratio: 2.823, 95% confidence interval: 1.603, 4.972). The median overall survival time was 26.8 months. A lesion size greater than 3 cm (P = 0.011, hazard ratio: 2.112, 95% confidence interval: 1.188, 3.754) and the presence of early local tumor progression (P = 0.011, hazard ratio: 2.352, 95% confidence interval: 1.217, 4.545) were related to a shorter survival time.
Conclusions:
Percutaneous radiofrequency ablation is safe in patients with colorectal cancer liver metastases refractory from chemotherapy. Subsequent chemotherapy is important to enhance local control. Small lesions and favorable early responses are related to prolonged overall survival.
Keywords: colorectal cancer liver metastases, unresectable, radiofrequency ablation, local tumor progression, overall survival, predictor
Introduction
Colorectal cancer is the most common tumor among gastrointestinal malignancies and the third most frequent cause of cancer-related death in Western countries.1 The liver is the main site of metastasis, which accounts for more than 50% of cases of colorectal cancer. Only 20–30% of patients with colorectal cancer liver metastases (CRCLMs) are suitable for radical resection,2 which is regarded as the main cause of cancer-related death.3 Most patients are not candidates for surgery when they are diagnosed because of extensive tumor burden or tumor location adjacent to important vascular or biliary ducts. For these patients, systemic therapy or molecular-targeted treatment is recommended as part of the treatment strategy, and the survival benefits are significant; in addition, some patients are converted to resection candidates.4,5 Furthermore, through combining molecular-targeted treatment and chemotherapy, a median overall survival (OS) of up to 30 months can be achieved in patients with advanced CRCLMs.6,7 However, few patients with CRCLMs achieve complete response, and 30–50% of patients show progression of disease quickly and do not respond to 2 lines of systemic chemotherapy, with a median OS of 7.1–8.8 months.8 Therefore, more aggressive local therapy based on palliative systemic therapy or molecular-targeted treatment is applied for unresectable CRCLMs.
Radiofrequency ablation (RFA) is a treatment modality that is increasingly being used.9,10 Previous studies have demonstrated 5-year OS rates after ablation of CRCLMs ranging from 21% to 47.8%.11 Although RFA has been established as a treatment option for selected patients with unresectable liver malignancies in the last 2 decades, the efficacy of this approach remains controversial.12 In a recent multidisciplinary international consensus study concerning treatment strategies for liver metastases, RFA was not even mentioned as a treatment option for CRCLMs.13 One reason may be that the associated local recurrence rate of up to 40% is concerning14; on the other hand, data regarding the effects on OS compared with the standard of care, i.e. systemic treatment, are lacking.10,12,15 Encouragingly, a randomized phase II trial demonstrated that aggressive local treatment (RFA ± resection) can prolong OS in patients with unresectable CRCLMs.16
The aim of this retrospective study was to analyze the efficacy of salvage RFA for patients with unresectable CRCLMs, focusing on the influencing factors related to the OS, local recurrence rate, and rate of complications.
Materials and Methods
Participants
This study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki, and the Institutional Review Board of Fudan University Shanghai Cancer Center approved the study (approval no. 1706173-7-1707). Signed informed consent was obtained from all patients. From January 2012 to September 2016, 81 patients with CRCLMs underwent computed tomography (CT)-guided percutaneous liver tumor RFA. One patient terminated the ablation procedure because of intolerable pain and was excluded from the survival analysis.
Of 80 patients, 52 (65.00%) received chemotherapy prior to liver ablation. A total of 18 of them (18/52, 34.62%) responded to size enlargement, and 34 patients (34/52, 65.38%) showed new liver lesions following at least 2 cycles of chemotherapy. The chemotherapy regimens mainly consisted of FOLFOX or FOLFIRI. There were 9 (11.25%) patients considered unresectable cases by an institutional multidisciplinary team, due to the tumor location or presence of comorbidities. Other 12 (15.00%) patients experienced liver recurrence after surgical resection, and 7 (8.75%) patients refused to undergo hepatectomy. All patients were recommended treatment combined with systemic chemotherapy if there were no chemotherapy contraindications. The patient characteristics are summarized in Table 1.
Table 1.
Baseline Clinical Characteristics of the Patients Before Ablation (n = 80).
| Factors | N (%) |
|---|---|
| Age (year) | 59 ± 10.98 (24-82) |
| ≤60 | 39(48.75) |
| > 60 | 41(51.25) |
| Gender | |
| Male | 52(65.00) |
| Female | 28(35.00) |
| Primary tumor site | |
| Rectum | 34(42.50) |
| Colon | 46(57.50) |
| Primary tumor differentiation | |
| Medium | 58(72.50) |
| Poor | 22(27.50) |
| Lymph node metastases | |
| No | 35(43.75) |
| Yes | 45(56.25) |
| Primary tumor Ki67 status | |
| ≤ 50% | 7(21.21) |
| > 50% | 26(78.79) |
| Liver metastases | |
| Synchronous | 36(45.00) |
| Metachronous early (≤ 12 mo) | 19(23.75) |
| Metachronous late (> 12 mo) | 25(31.25) |
| Preablation treatment | |
| Chemotherapy | 33(41.25) |
| No | 47(58.75) |
| Extrahepatic metastases | |
| No | 52(65.00) |
| Yes | 28(35.00) |
| CEA level before ablation ng/mL | |
| ≤ 30 | 52(65.00) |
| > 30 | 28(35.00) |
| Preablation lesion size | 2.5 ± 1.2 |
| ≤30 | 54(67.50%, n = 93) |
| >30 | 26(32.50%, n = 32) |
| Metastases number | |
| ≤2 | 58(72.50) |
| >2 | 22(27.50) |
| Subsequent chemotherapy | |
| No | 23(28.75) |
| Yes | 57(71.25) |
RFA Procedures
All patients signed an informed consent form prior to undergoing RFA and were administered diazepam (10 mg) via muscle injection pre-ablation. A total of 3 interventional physicians with at least 5 years of experience implemented the ablations. The ablations were performed by CT guidance with local anesthesia, and electrocardiogram monitoring was used for all procedures. The MedSphere (MedSphere International, Inc., Shanghai, China) and RITA (RITA Medical Systems, Mountain View, CA) electrodes were used for the ablations. All ablations were performed according to the manufacturer’s protocol with the aim of creating an ablation defect at least 5 mm larger than the largest tumor diameter. An immediate post-procedure CT scan was performed to evaluate the ablation zones and complications. The minimal ablative margin was measured and documented as previous.17 Patients were transported to the ward after ablation and received tranexamic acid and sodium chloride injections (0.5 g) via intravenous drip q.d. for 2 days.
The complications were classified according to the Society of Interventional Radiology clinical practice guidelines as major (requirement of additional therapy and lengthened hospital stay) and minor (with no consequences and requiring no therapy or nominal therapy).10
Follow-Up
Contrast-enhanced CT or magnetic resonance (MR) imaging, as well as serum carcinoembryonic antigen (CEA) level tests, were performed at 1, 3, 6, and 12 months and then at 6-month intervals following the ablation procedure. The 1-month imaging results were used as a baseline for subsequent response evaluation. The technique success was evaluated based on the immediate CT findings after ablation, which was defined as the target tumor being treated according to the pre-protocol and covered completely.10 The technique efficacy was assessed based on the 1-month imaging follow-up results. No obvious signs of nodular or irregular enhanced foci were considered total ablation. Local tumor progression (LTP) was determined based on the appearance of tumor foci within 1 cm of the edge of the ablation zone on contrast-enhanced CT or MR imaging after the initial imaging study had confirmed adequate ablation.10 Patients with LTP were recommended to performed either re-ablation or chemotherapy. LTP-free survival was defined as the time interval between ablation and the first imaging sign of LTP. OS was defined as the time interval between ablation and death due to any cause.
Statistical Analysis
Survival data were analyzed using the Kaplan–Meier method. The median follow-up time was calculated using the reverse Kaplan–Meier method. The log-rank test was employed for univariate analysis of clinical features. Factors with P values less than 0.05 were considered candidates for multivariate analysis, which was performed using Cox regression. A P value less than 0.05 was considered statistically significant. All analyses were conducted using SPSS version 22.0 (IBM, Armonk, NY, USA).
Results
Technique Effectiveness
The ablation Procedure was not completed in 1 patient with 1 lesion because the patient could not endure the pain during ablation. Therefore, the technique success rate was 99.21% (125/126). In total, 80 patients (52 men and 28 women, median age: 59 years, range: 24-82) with 125 CRCLM lesions underwent ablation. The diameter of lesions was 2.5 ± 1.2 cm (range: 1.0-6.4). The 1-month imaging follow-up showed complete ablation of all lesions (primary efficacy rate of 100%).
Complications
Minor complications occurred in 2 patients, with both involving subcapsular hemorrhage. These 2 patients were administered intramuscular hemostatic agent injections without blood transfusion. Follow-up imaging indicated that the hematoma resolved within 2 weeks. No severe complications were observed.
LTP and Prognostic Factors
During a median follow-up period of 51.2 months [95% confidence interval (CI): 44.1, 58.2 months], LTP developed in 59 of 125 lesions (47.2%). The 1-, 2-, and 3-year cumulative LTP rates were 40.7%, 48.6%, and 53.4%, respectively. The estimated median LTP-free survival time of all patients was 29.8 months (95% CI: 11.8, 34.1 months). Of the 59 LTP lesions, a secondary RFA session was performed for 10 lesions (16.95%). Three of them (3/10, 30%) were completely ablated without local progression until the study was completed; and 7 (7/10, 70%) experienced LTP within 6 months following re-ablation. Other 49 of the 59 patients (83.05%) with LTP tumors received systemic chemotherapy. The estimated median LTP-free survival time of patients with subsequent chemotherapy was not reached, and that of patient without subsequent chemotherapy was 8.4 months (95% CI: 6.2, 14.4 months).
In univariate analysis, a lesion diameter larger than 3 cm (P = 0.031), ablative margin less than 5 mm (P = 0.029), CEA level greater than 30 ng/mL (P = 0.032), and absence of subsequent chemotherapy (P < 0.001) were related to shorter LTP-free survival (Table 2). Multivariate analysis revealed that only the absence of subsequent chemotherapy was an independent predictor of shorter LTP-free survival [P < 0.001, hazard ratio (HR): 2.823, 95% CI: 1.603, 4.972] (Table 2, Figure 1).
Table 2.
Univariate Analysis for Predictors of LTPFS and Overall Survival.
| Factors | Median LTPFS (mo) | P value | Factors | Median OS (mo) | P value |
|---|---|---|---|---|---|
| Age, yo | 0.857 | 0.178 | |||
| ≤ 60 | 14.7 | 30.9 | |||
| > 60 | 17.1 | 25.7 | |||
| Gender | 0.654 | .643 | |||
| Male | 17.1 | 28.3 | |||
| Female | 14.7 | 25.7 | |||
| Primary tumor site | 0.997 | 0.046 | |||
| Rectum | 17.1 | 25.1 | |||
| Colon | 15.9 | 30.9 | |||
| Primary tumor differentiation | 0.657 | 0.054 | |||
| Medium | 16.0 | 30.9 | |||
| Poor | 11.8 | 16.5 | |||
| Lymph node metastases | 0.232 | 0.392 | |||
| No | 11.2 | 30.9 | |||
| Yes | 34.1 | 21.5 | |||
| Primary tumor Ki67 status | 0.842 | 0.277 | |||
| ≤ 50% | NR | ||||
| > 50% | 16.0 | 11.9 | |||
| Liver metastases | 0.319 | 0.503 | |||
| Synchronous | 11.0 | 26.6 | |||
| Metachronous early (≤ 12 mo) | 29.8 | 20.7 | |||
| Metachronous late (> 12 mo) | 34.1 | 32.7 | |||
| Preablation treatment | 0.120 | .231 | |||
| Chemotherapy | 9.4 | 22.7 | |||
| No | 29.8 | 28.3 | |||
| Extrahepatic metastases | 0.484 | 0.375 | |||
| No | 17.1 | 28.3 | |||
| Yes | 11.0 | 22.7 | |||
| CEA level before ablation ng/mL | 0.003 | 0.008 | |||
| ≤ 30 | 35.5 | 35.5 | |||
| > 30 | 18.7 | 20.2 | |||
| Preablation lesion size mm | 0.049 | 0.002 | |||
| ≤ 30 | 29.8 | 32.7 | |||
| > 30 | 9.4 | 21.7 | |||
| In vicinity of liver vessel | 0.466 | 0.67 | |||
| No | 16.0 | 27.3 | |||
| Yes | 7.5 | 26.5 | |||
| Subcapsule lesion | 0.357 | 0.699 | |||
| No | 14.7 | 26.5 | |||
| Yes | 29.8 | 27.3 | |||
| Metastases site | 0.455 | 0.255 | |||
| Left lobe | 16.0 | 26.5 | |||
| Right lobe | 9.4 | 27.4 | |||
| Metastases number | 0.861 | 0.236 | |||
| ≤ 2 | 15.9 | 30.9 | |||
| > 2 | 11.2 | 26.5 | |||
| Ablative margin mm | 0.029 | 0.71 | |||
| ≤ 5 | 14.7 | 22.7 | |||
| > 5 | NR | 27.3 | |||
| Subsequent chemotherapy | 0.002 | 0.016 | |||
| No | 8.4 | 19.5 | |||
| Yes | NR | NR | |||
| Evaluation on 6-month | 0.001 | ||||
| No-LTP | 32.5 | ||||
| LTP | 15.5 | ||||
| Extrahepatic metastases site | 0.000 | ||||
| 0 | 28.3 | ||||
| 1 | 35.9 | ||||
| 2 | 11.1 |
LTPFS = local tumor progression free survival, OS = overall survival, CEA = carcinoembryonic antigen, LTP = local tumor progression, NR = not reached.
Figure 1.
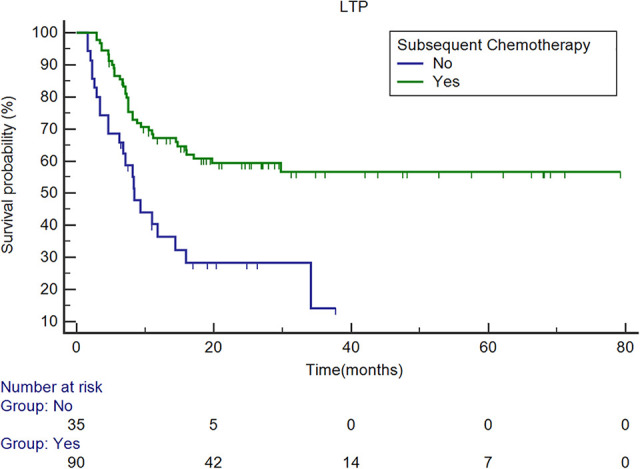
Survival curves of LTPFS with/without subsequent chemotherapy. The overall estimated median LTPFS was 29.8 months (95% CI: 14.4, 34.1 months). The estimated median LTPFS in patients who received subsequent chemotherapy was not reach, and that of patients who did not receive subsequent chemotherapy was 8.4 months (95% CI: 6.2, 14.4 months). Multivariate analysis revealed that the absence of subsequent chemotherapy was an independent predictor (P < 0.001, HR: 2.823, 95% CI: 1.603, 4.972). LTPFS = local tumor progression-free survival; CI = confidence interval; HR = hazard ratio.
Survival Results
During the period of follow-up, 54 (67.5%) patients died, 3 (3.75%) were lost, and 23 (28.75) survived. The estimated median OS was 26.8 months, with a 95% CI of 21.4 and 32.1 months. The cumulative 1-, 3-, and 5-year survival rates were 81.2%, 32.1% and 23.9%, respectively.
In univariate analysis, a CEA level greater than 30 ng/mL (P = 0.008) before ablation, lesion diameter larger than 3 cm (P = 0.002), presence of rectal cancer (P = 0.046), not receiving subsequent chemotherapy (P < 0.001), more than 2 sites of extra-hepatic metastases (P < 0.001), and the presence of LTP at the 6-month follow-up (P = 0.001) were predictors of shorter survival (Table 2). Multivariate analysis revealed that a lesion diameter larger than 3 cm (P = 0.011, HR: 2.112, 95% CI: 1.188, 3.754) and presence of early LTP (LTP at the 6-month follow-up) (P = 0.011, HR: 2.352, 95% CI: 1.217, 4.545) were independent predictors of shorter survival (Table 3, Figures 2 and 3).
Table 3.
Multiparametric Analysis for LTPFS and OS.
| LTPFS | OS | ||||||
|---|---|---|---|---|---|---|---|
| Predictors | HR | 95% CI | P value | Predictors | HR | 95% CI | P value |
| Absent of subsequent chemotherapy | 2.823 | 1.603, 4.972 | <0.001 | lesion size > 30 mm | 2.112 | 1.188, 3.754 | 0.011 |
| early LTP (6 months) | 2.352 | 1.217, 4.545 | 0.011 | ||||
LTPFS = local tumor progression free survival, OS = overall survival, HR = hazard ratio, CI = confident interval, LTP = local tumor progression.
Figure 2.
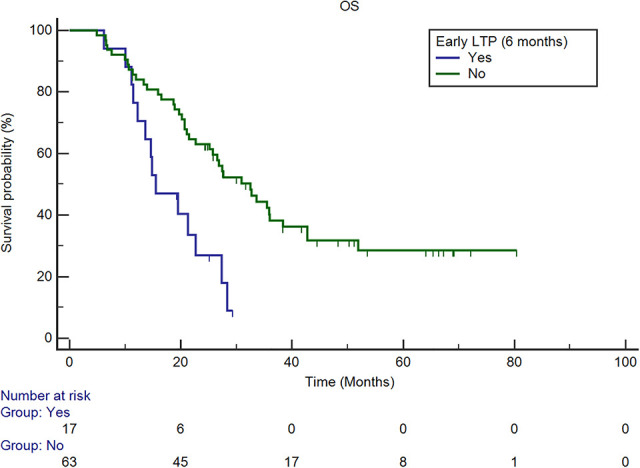
Survival curves of OS for patients with and without early LTP. The estimated median OS of patients with LTP at 6 months was 15.5 months (95% CI: 11.4, 22.7 months), and that of patients without LTP at 6-month was 32.5 months (95% CI: 22.7, 38.4 months). Multivariate analysis revealed that the presence of early LTP (LTP at the 6-month follow-up) was an independent predictor after adjusting for potential confounders (P = 0.011, HR: 2.352, 95% CI: 1.217, 4.545). OS = overall survival; LTP = local tumor progression; CI = confidence interval; HR = hazard ratio.
Figure 3.
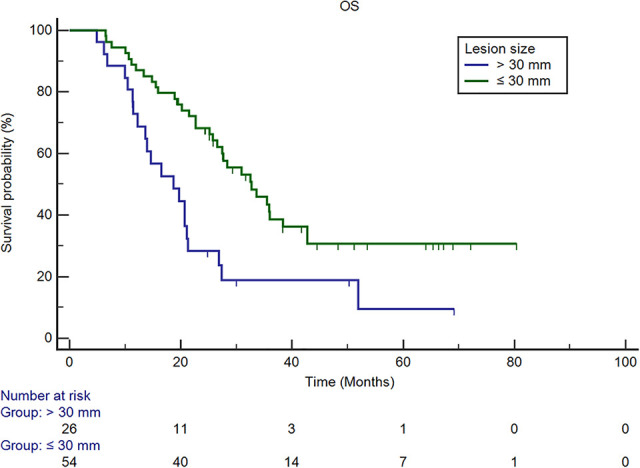
Survival curves of OS based on lesion size. The estimated median OS of patients with lesion diameters >3 cm was 18.7 months (95% CI: 12.2, 21.3 months), and that of patients ≤3 cm was 32.7 months (95% CI: 25.7, 42.7 months). Multivariate analysis revealed that lesion diameters >3 cm were independent predictors after adjusting for potential confounders (P = 0.011, HR: 2.112, 95% CI: 1.188, 3.754). OS = overall survival; CI = confidence interval; HR = hazard ratio.
Discussion
The relatively high local tumor recurrence or local progression rate is the main concern related to thermal ablation of CRCLMs.18 This study showed an overall LTP rate of 47.2% (59/125), which was comparable to that found in previous studies, with an LTP rate of approximately 40%.19,20 A lesion size larger than 3 cm, locating in the vicinity of large vessels, and inadequate ablation margins are well-known predictors of local progression.15,19 However, in the current cohort, lesion diameter and ablative margins were not independent prognostic factors. The influence of lesion size on LTP may have been weakened because most (78.4%, 98/125) lesion sizes were smaller than 3 cm in this study. We sought to achieve ablative margins as close to 10 mm as possible and evaluated ablation zone coverage immediately after ablation. Therefore, we attained at least a 5-mm ablative margin in most treatment sessions (76%, 95/125). This may be the reason why ablative margins were not an independent predictor of LTP. On the other hand, as found in other studies,16,21 we revealed that systemic chemotherapy following ablation was independently associated with improved local control. In addition, we noted that most LTP cases (83%, 49/59) developed within 12 months after ablation. This finding reminded us that close and intense follow-up is necessary in the first year after ablation.
In the treatment of CRCLMs, although RFA has been proposed as an alternative to hepatic resection for small liver metastases, microwave ablation (MWA) can achieve better ablation effects for tumors larger than 5 cm and is superior to RFA or partial hepatectomy.22 However, only a few studies have evaluated the role of MWA in CRCLMs.15 Shady et al. compared MWA and RFA for CRCLMs and found that there was no difference in the LTP rate between RFA and MWA (P = 0.84), but ablation margins of 5 mm or smaller were a significant predictor of shorter LTP-free survival for RFA (P < 0.001).20 Stereotactic body radiotherapy (SBRT) is another option for local tumor ablation for CRCLMs. Due to its high-accuracy target localization, SBRT is able to be used to treat relatively large lesions with minimal normal liver tissue impairment, compared to that with traditional radiotherapy. For lesions locating in the vicinity of large blood vessels, SBRT can deliver high doses of radiation without concern regarding the remnant liver volume. The clinical efficiency of SBRT is comparable to that of RFA or MWA according to a series of retrospective studies and a systematic review that reported 1- and 2-year local control rates of 67% and 59.3%, respectively.23 Furthermore, as biologically equivalent doses are an independent prognostic factor for local control,24 confirmatory trials exploring optimal schedules are lacking. On the other hand, strategies combining SBRT with other anti-tumor treatments may improve outcomes. A recent study revealed a 2-years local control rate of 89.5% using SBRT combined with resection for initially unresectable CRCLMs.25 The safety and clinical efficacy of liver radiotherapy combined with immunotherapy are under investigation in several trials. The initial results have shown that combination therapy is quite tolerable.26 However, large randomized control trials comparing SBRT with other thermal ablation strategies are needed.
In previous studies, the median survival times following ablation for unresectable CRCLMs ranged from 13.9 to 45.6 months.16,27 The discrepancy is mainly due to variations in participant selection and treatment modalities. In a study by Wang et al., the patients with unresectable CRCLM treated by RFA had a median OS of 13.9 months and obtained survival benefits27; nevertheless, in patients with untreated CRCLMs, the median OS was approximately 7.5 months.28 In a randomized phase II trial for unresectable CRCLMs, the median OS was 45.6 months in the combined-modality arm (systemic treatment plus radiofrequency ablation ± resection) vs. 40.5 months in the systemic-treatment arm. The participant selection criteria in this study including: <10 lesions and no extrahepatic disease.16 In this study, almost all patients were refractory after at least 2 lines of chemotherapy, with an improved median OS (28.6 months) than that previously reported for patients who failed 2 lines of systemic chemotherapy (who had a median OS of 7.1–8.8 months).8
As reported previously, we revealed that patients with tumor sizes larger than 3 cm had a poor prognosis.29-31 However, another prognostic factor, the number of liver tumors, was not an independent predictor of survival in this study. This discrepancy may have been due to the relatively lower lesion-to-patient ratio (1.56, 125/80) in this study. In addition, we found that the local response at 6 months was significantly related to the survival benefit. This result indicated that close follow-up and more intense systemic therapy are important for these patients. Moreover, it indicated that patient selection is important for the clinical outcomes of CRCLM ablation. Even with total ablation, some patients experience early local progression. Furthermore, for patients without local progression at the 6-month evaluation, longer follow-up intervals (such as 6-month intervals) and a delay in systemic therapy may provide them with an improved quality of life. However, this hypothesis needs further investigation. Our study had several limitations. First, the retrospective design introduced bias to the patient selection and data acquisition. Furthermore, heterogeneous pre-procedure modalities might have influenced the survival outcomes. Finally, most patients in our study were refractory to chemotherapy prior to ablation. These patients with dismal prognosis were not the best candidates for ablation therapy.
Conclusion
Percutaneous RFA is a safe treatment modality for CRCLMs, even in patients who are refractory to chemotherapy. Receiving subsequent chemotherapy after ablation may enhance local tumor control. Patients with improved early local control and small tumor sizes have improved OS.
Abbreviations
- CEA
carcinoembryonic antigen
- CRCLMs
colorectal cancer liver metastases
- CT
computed tomography
- LTP
local tumor progression
- MR
magnetic resonance
- OS
overall survival
- RFA
radiofrequency ablation
- SBRT
stereotactic body radiotherapy
Footnotes
Authors’ Note: Ying Wang and Guang-Yuan Zhang contributed equally to this work. All authors specify that this manuscript has not been published in whole or in part nor is it being considered for publication elsewhere. This study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki, and the Institutional Review Board of Fudan University Shanghai Cancer Center approved the study (approval no. 1706173-7-1707). Signed informed consent was obtained from all patients.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article This study was supported in part by grants from the National Natural Science Foundation of China (No. 81501562), the National Key Research and Development Program of China (No. 2016YFC0106203), and the Program of Shanghai Hospital Development Center (No. SHDC22017102).
ORCID iD: Ying Wang  https://orcid.org/0000-0002-0838-4366
https://orcid.org/0000-0002-0838-4366
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. doi:10.3322/caac.21442 [DOI] [PubMed] [Google Scholar]
- 2. Hackl C, Neumann P, Gerken M, Loss M, Klinkhammer-Schalke M, Schlitt HJ. Treatment of colorectal liver metastases in Germany: a ten-year population-based analysis of 5772 cases of primary colorectal adenocarcinoma. BMC Cancer. 2014;14:810 doi:10.1186/1471-2407-14-810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Leonard GD, Brenner B, Kemeny NE. Neoadjuvant chemotherapy before liver resection for patients with unresectable liver metastases from colorectal carcinoma. J Clin Oncol. 2005;23(9):2038–2048. doi:10.1200/JCO.2005.00.349 [DOI] [PubMed] [Google Scholar]
- 4. Adam R, Delvart V, Pascal G, et al. Rescue surgery for unresectable colorectal liver metastases downstaged by chemotherapy: a model to predict long-term survival. Ann Surg. 2004;240(4):644–657; discussion 657-8. doi:10.1097/01.sla.0000141198.92114.f6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Folprecht G, Gruenberger T, Bechstein WO, et al. Tumour response and secondary resectability of colorectal liver metastases following neoadjuvant chemotherapy with cetuximab: the CELIM randomised phase 2 trial. Lancet Oncol. 2010;11(1):38–47. doi:10.1016/S1470-2045(09)70330-4 [DOI] [PubMed] [Google Scholar]
- 6. Heinemann V, von Weikersthal LF, Decker T, et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15(10):1065–1075. doi:10.1016/S1470-2045(14)70330-4 [DOI] [PubMed] [Google Scholar]
- 7. Simkens LH, van Tinteren H, May A, et al. Maintenance treatment with capecitabine and bevacizumab in metastatic colorectal cancer (CAIRO3): a phase 3 randomised controlled trial of the Dutch Colorectal Cancer Group. Lancet. 2015;385(9980):1843–1852. doi:10.1016/S0140-6736(14)62004-3 [DOI] [PubMed] [Google Scholar]
- 8. Grothey A, Marshall JL, Seery TE. Current options for third-line treatment of metastatic colorectal cancer. Clin Adv Hematol Oncol. 2016;14(3 Suppl 3):1–15. [PubMed] [Google Scholar]
- 9. van Amerongen MJ, Jenniskens SFM, van den Boezem PB, Futterer JJ, de Wilt JHW. Radiofrequency ablation compared to surgical resection for curative treatment of patients with colorectal liver metastases—a meta-analysis. HPB (Oxford). 2017;19(9):749–756. doi:10.1016/j.hpb.2017.05.011 [DOI] [PubMed] [Google Scholar]
- 10. Ahmed M, Solbiati L, Brace CL, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria—a 10-year update. J Vasc Interv Radiol. November 2014;25(11):1691–1705 e4. doi:10.1016/j.jvir.2014.08.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Odisio BC, Yamashita S, Huang SY, et al. Local tumour progression after percutaneous ablation of colorectal liver metastases according to RAS mutation status. Br J Surg. 2017;104(6):760–768. doi:10.1002/bjs.10490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sartori S, Tombesi P, Di Vece F. Thermal ablation in colorectal liver metastases: lack of evidence or lack of capability to prove the evidence? World J Gastroenterol. 2016;22(13):3511–3515. doi:10.3748/wjg.v22.i13.3511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Adam R, de Gramont A, Figueras J, et al. Managing synchronous liver metastases from colorectal cancer: a multidisciplinary international consensus. Cancer Treat Rev. 2015;41(9):729–741. doi:10.1016/j.ctrv.2015.06.006 [DOI] [PubMed] [Google Scholar]
- 14. Takahashi H, Akyuz M, Aksoy E, Karabulut K, Berber E. Local recurrence after laparoscopic radiofrequency ablation of malignant liver tumors: results of a contemporary series. J Surg Oncol. 2017;115(7):830–834. doi:10.1002/jso.24599 [DOI] [PubMed] [Google Scholar]
- 15. Meijerink MR, Puijk RS, van Tilborg A, et al. Radiofrequency and microwave ablation compared to systemic chemotherapy and to partial hepatectomy in the treatment of colorectal liver metastases: a systematic review and meta-analysis. Cardiovasc Intervent Radiol. 2018;41(8):1189–1204. doi:10.1007/s00270-018-1959-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ruers T, Van Coevorden F, Punt CJ, et al. Local treatment of unresectable colorectal liver metastases: results of a randomized phase ii trial. J Natl Cancer Inst. 2017;109(9):djx015 doi:10.1093/jnci/djx015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang X, Sofocleous CT, Erinjeri JP, et al. Margin size is an independent predictor of local tumor progression after ablation of colon cancer liver metastases. Cardiovasc Intervent Radiol. 2013;36(1):166–175. doi:10.1007/s00270-012-0377-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Petre EN, Sofocleous C. Thermal ablation in the management of colorectal cancer patients with oligometastatic liver disease. Visc Med. 2017;33(1):62–68. doi:10.1159/000454697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Berber E, Pelley R, Siperstein AE. Predictors of survival after radiofrequency thermal ablation of colorectal cancer metastases to the liver: a prospective study. J Clin Oncol. 2005;23(7):1358–1364. doi:10.1200/JCO.2005.12.039 [DOI] [PubMed] [Google Scholar]
- 20. Shady W, Petre EN, Do KG, et al. Percutaneous microwave versus radiofrequency ablation of colorectal liver metastases: ablation with clear margins (A0) provides the best local tumor control. J Vasc Interv Radiol. 2018;29(2):268–275 e1 doi:10.1016/j.jvir.2017.08.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Solbiati L, Ahmed M, Cova L, Ierace T, Brioschi M, Goldberg SN. Small liver colorectal metastases treated with percutaneous radiofrequency ablation: local response rate and long-term survival with up to 10-year follow-up. Radiology. 2012;265(3):958–968. doi:10.1148/radiol.12111851 [DOI] [PubMed] [Google Scholar]
- 22. Sparchez Z, Mocan T, Hajjar NA, et al. Percutaneous ultrasound guided radiofrequency and microwave ablation in the treatment of hepatic metastases. A monocentric initial experience. Med Ultrason. 2019;21(3):217–224. doi:10.11152/mu-1957 [DOI] [PubMed] [Google Scholar]
- 23. Petrelli F, Comito T, Barni S, et al. Stereotactic body radiotherapy for colorectal cancer liver metastases: a systematic review. Radiother Oncol. 2018;129(3):427–434. doi:10.1016/j.radonc.2018.06.035 [DOI] [PubMed] [Google Scholar]
- 24. Chang DT, Swaminath A, Kozak M, et al. Stereotactic body radiotherapy for colorectal liver metastases: a pooled analysis. Cancer. 2011;117(17):4060–4069. doi:10.1002/cncr.25997 [DOI] [PubMed] [Google Scholar]
- 25. Ihnat P, Skacelikova E, Vavra P, Jonszta T, Ihnat Rudinska L, Tomaskova H. Novel strategy in the treatment of liver metastases—hepatic resection combined with stereotactic body radiotherapy. Asian J Surg. 2020;43(9):902–906. doi:10.1016/j.asjsur.2019.11.016 [DOI] [PubMed] [Google Scholar]
- 26. Bang A, Wilhite TJ, Pike LRG, et al. Multicenter evaluation of the tolerability of combined treatment with PD-1 and CTLA-4 immune checkpoint inhibitors and palliative radiation therapy. Int J Radiat Oncol Biol Phys. 2017;98(2):344–351. doi:10.1016/j.ijrobp.2017.02.003 [DOI] [PubMed] [Google Scholar]
- 27. Wang Y, Zheng J, Chen H, et al. A prognostic nomogram for colorectal cancer liver metastases after percutaneous thermal ablation. Int J Hyperthermia. 2018;34(6):853–862. doi:10.1080/02656736.2017.1368095 [DOI] [PubMed] [Google Scholar]
- 28. Stangl R, Hofmann AA, Charnley RM, Scheele J. Factors influencing the natural history of colorectal liver metastases. Lancet. 1994;343(8910):1405–1410. doi:10.1016/s0140-6736(94)92529-1 [DOI] [PubMed] [Google Scholar]
- 29. Hamada A, Yamakado K, Nakatsuka A, et al. Radiofrequency ablation for colorectal liver metastases: prognostic factors in non-surgical candidates. Jpn J Radiol. 2012;30(7):567–574. doi:10.1007/s11604-012-0089-0 [DOI] [PubMed] [Google Scholar]
- 30. Cirimbei C, Rotaru V, Chitoran E, Pavaleanu O, Cirimbei SE. Immediate and long-term results of radiofrequency ablation for colorectal liver metastases. Anticancer Res. 2017;37(11):6489–6494. doi:10.21873/anticanres.12105 [DOI] [PubMed] [Google Scholar]
- 31. Shady W, Petre EN, Gonen M, et al. Percutaneous radiofrequency ablation of colorectal cancer liver metastases: factors affecting outcomes—a 10-year experience at a single center. Radiology. 2016;278(2):601–611. doi:10.1148/radiol.2015142489 [DOI] [PMC free article] [PubMed] [Google Scholar]


