Abstract
Many promising drug candidates and pharmaceutical compounds fail due to idiosyncratic adverse drug reactions (IADRs), often arising from the formation of reactive metabolites. Among the “structural alerts” responsible, anilines are well-known to undergo deleterious metabolic processing, yet isosteric replacement strategies remain limited. Herein we discuss current art and potential new avenues of saturated isosteres to mitigate aniline-related toxicities.
Keywords: Bioisosteres, structural alerts, reactive metabolites, anilines, aminonorbornanes
In the last two decades, advancements in automation, technology, and reaction science have shifted the landscape of pharmaceutical development. Among these shifts, the ability to rapidly evaluate chemical entities in high-throughput formats has become standard practice due to enabling technologies in both the synthesis and analysis of new compounds. C–N bond forming protocols have been particularly transformative for discovery efforts; however, the operational simplicity and popularity of these reactions (e.g., Buchwald–Hartwig and Chan–Lam cross couplings, SNAr) have increasingly shifted chemical space toward Csp2-rich scaffolds.
As a result, aryl amines have become a prevalent substructure in high-throughput screening libraries but have proven to be particularly disruptive in late-stage pharmaceutical development due to their propensity for reactive metabolite (RM) formation. Cytochrome P450 (CYP450)-mediated oxidation of the aniline motif affords highly electrophilic species (i.e., quinone-imines) that form covalent linkages to bystander proteins or CYP450s themselves in an indiscriminate fashion, leading to increased risk for drug–drug interactions or idiosyncratic adverse drug interactions.1 Nearly one-third of drugs with idiosyncratic adverse drug reactions (IADRs) that have been labeled with black-box-warnings or completely withdrawn from the market contain the aniline functionality, and many of these examples have evidence for aniline bioactivation and RM formation.2−4
This association of anilines as precursors to RMs prompted our own group to consider effective strategies for mitigating aniline-derived toxicity such that failed drug candidates could be re-engineered with preserved efficacy but enhanced safety. As such, the use of isosteres represents an attractive approach to address this issue, as isosteric replacement aims to closely mimic the efficacy of a lead compound while improving physicochemical properties. With respect to anilines, available isosteres are limited in application, scope, and diversity. We aim to highlight in this Viewpoint current approaches to aniline isosterism and offer an outlook on new structures to serve as metabolically innocuous aniline isosteres.
Current Approaches to Aniline Isosterism
As benzene itself is a structural alert susceptible to RM formation, benzene isosterism has been targeted in a number of different applications. The unifying feature of these approaches has been the use of small, saturated carbocycles to supplant the arene, and the appeal of this strategy is multifaceted. Saturated carbocycles increase Fsp3 and three-dimensionality,5,6 are more resistant to RM formation and CYP-inhibition,7 and often represent novel intellectual property. The most common examples of benzene isosteres are bicyclo[1.1.1]pentane (BCP),8−10 bicyclo[2.2.2]octane (BCO),11−13 and cubane (CUB),14 although several alternative structures have been proposed recently but have yet to be realized synthetically or applied in a biological setting.15 Conversely, despite the well-known metabolic liabilities of anilines, a subclass of substituted benzenes, examples of aniline isosterism are sparsely reported in the literature. Among compounds containing these motifs, it is difficult to quantify the number of pharmaceutical entities that have been intentionally modified with a saturated structure to improve ADME properties and those that simply incorporate these motifs as de novo building blocks.
Broad comparison of physical parameters of aminoBCP, aminoBCO, aminoCUB, and aminonorbornane (aminoNB) against the analogous aniline reveals that the isosteres provide general spatial approximations of the parent structure (Figure 1A).16 Out of the 198 patents disclosing 1-aminoBCPs, all structures were exclusively mono- or disubstituted at the bridgehead position, resulting in linear structures. This substitution pattern is prevalent in the other scaffolds as well. Out of 310 patents containing either 1-aminoNBs or 1-aminoBCOs, only 6 patents contain structures with a substitution pattern other than “para” despite having 11 and 13 unique sites for substitution, respectively.17 This analysis of the patent literature highlights an important limitation of these carbocycles: despite having multiple sites available for substitution, the vast majority of reported structures are only functionalized at the bridgehead position. The reason for this void of chemical diversity within the field of aniline (and more generally, benzene) isosterism lies in the limited number of synthetic methods available to prepare and functionalize these saturated hydrocarbon scaffolds.
Figure 1.
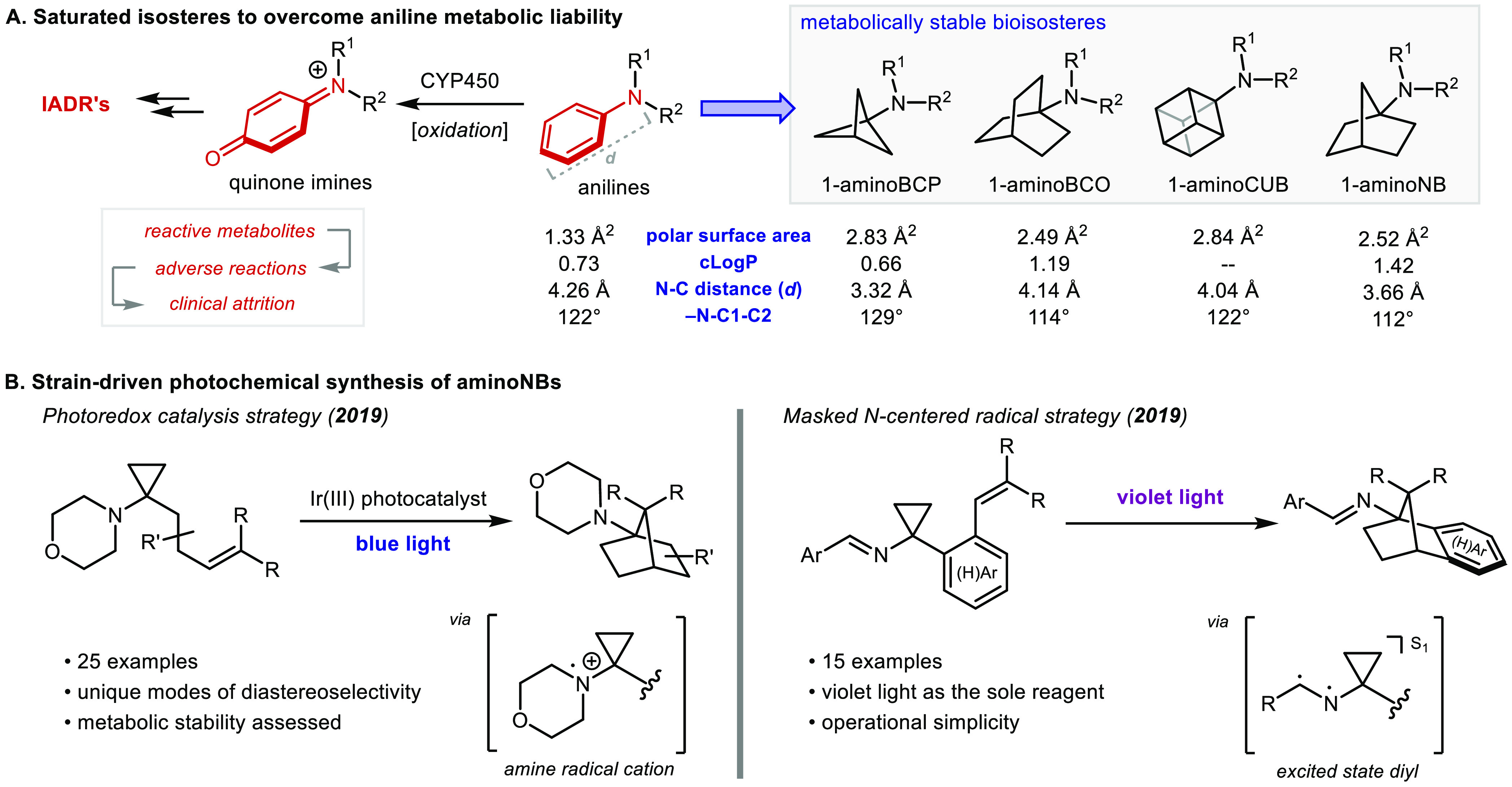
(A) Saturated isosteres of aniline to overcome metabolic liability. (B) Strain-driven photochemical synthesis of aminoNBs.
Current synthetic methods only allow access to para-substituted mimics of anilines, but this only represents a fraction of drugs which contain considerably more diversity. Of the 200 top selling drugs of 2018, 48 contain an aniline motif, but among these only 12 are solely para-substituted. In order to address this limitation, our laboratory became engaged in developing flexible and scalable synthetic protocols to access diversely substituted 1-aminoNBs to leverage in aniline isosteric applications.
Efforts within our group have resulted in facile synthetic access to the aminoNB scaffold via a robust photochemical cyclization strategy. Two distinct methods have been developed, providing entry into uniquely decorated aminoNB cores. The first approach employs photoredox catalysis, an emerging technology within drug discovery programs,18 and the second exploits direct photochemical excitation of imines using visible light as the sole reagent necessary to enact the transformation (Figure 1B).19 The mild nature of these reactions tolerates a wide array of functional groups and has proven to be scalable in continuous flow processing, and importantly, all sites of substitution are diversifiable with these approaches. Initial stability assays in rat and human liver microsomes, in addition to metabolite identification, have demonstrated that the aminoNBs generally have improved metabolic profiles compared to their aniline counterparts. These new methods can be easily translated to medicinal chemistry applications, and our hope is that these innovative building blocks will be incorporated into existing therapeutics to yield improved safety profiles and reinvigorate promising leads.
Future Outlook
Despite the recent advances in accessing diversely substituted carbocyclic structures, a number of challenges remain for the isosteric replacement of anilines to be broadly applicable. The ability to selectively synthesize and strategically manipulate small carbocyclic frameworks remains limited. Lack of imaginative new methods and commercial availability of precursors (e.g., BCP and CUB bridgehead dicarboxylates) has considerably narrowed the region of chemical space being interrogated for pharmaceutical applications. Due to the increased complexity of saturated carbocycles compared to planar anilines, these isosteres have the potential to be more versatile than their aromatic precursors. Only advancements in synthetic technology will be able to provide the structural diversity and scrupulous control over physicochemical properties required to mimic densely functionalized anilines.
Even meeting these criteria, careful considerations need to be made to select an appropriate isostere for any given application; exchanging an aryl amine to an alkyl amine has the potential to significantly perturb protonation state, and removal of arenes may disrupt π–π stacking interactions within the binding pocket. However, conscientious design can prevent these issues. Inductive withdrawing groups can modulate the pKa of alkyl amines, and access to a large library of isosteres of varying sizes, shapes, and bond angles will increase the likelihood of achieving an optimal fit within a binding pocket, recouping or even improving upon the energetic contributions of π-stacking.
Anilines have persisted as staple components of drug development libraries due to their accessibility and ease of handling despite being stereotyped as the most “notorious” of all structural alerts.20 It is unsurprising then that a significant fraction of drugs labeled with black box warnings or completely withdrawn from the market contain the aniline motif, and some of the consequences of bioactivation have been as severe as patient death.2 However, pharmaceutical compounds containing this predictable liability continue to be overlooked as these toxicities are tolerated in patients with traditionally poor outcomes and for areas of unmet medical need. Instead, can advancements in reaction science and investment in aniline isosterism overcome this bias to continue to incorporate anilines into new therapies? We foresee a great benefit from developing rationally designed, inert structures with multiple sites for diversification that complement the current armamentarium of aniline isosteres and hope to see further advancements in this area in the near future.
Acknowledgments
T.M.S. and L.A.C. are grateful for funding provided by the NIH NIGMS (R01-GM127774).
Author Contributions
The manuscript was written through contributions of all authors.
Views expressed in this editorial are those of the authors and not necessarily the views of the ACS.
The authors declare no competing financial interest.
This paper was published ASAP on February 21, 2020, with an error in Figure 1. The corrected version was reposted on February 25, 2020.
References
- Walsh J. S.; Miwa G. T. Bioactivation of Drugs: Risk and Drug Design. Annu. Rev. Pharmacol. Toxicol. 2011, 51, 145–167. 10.1146/annurev-pharmtox-010510-100514. [DOI] [PubMed] [Google Scholar]
- Stepan A. F.; Walker D. P.; Bauman J.; Price D. A.; Baillie T. A.; Kalgutkar A. S.; Aleo M. D. Structural Alert/Reactive Metabolite Concept as Applied in Medicinal Chemistry to Mitigate the Risk of Idiosyncratic Drug Toxicity: A Perspective Based on the Critical Examination of Trends in the Top 200 Drugs Marketed in the United States. Chem. Res. Toxicol. 2011, 24, 1345–1410. 10.1021/tx200168d. [DOI] [PubMed] [Google Scholar]
- Kalgutkar A. S. Should the Incorporation of Structural Alerts be Restricted in Drug Design? An Analysis of Structure-Toxicity Trends with Aniline-Based Drugs. Curr. Med. Chem. 2014, 22, 438–464. 10.2174/0929867321666141112122118. [DOI] [PubMed] [Google Scholar]
- Orr S. T. M.; Ripp S. L.; Ballard T. E.; Henderson J. L.; Scott D. O.; Obach R. S.; Sun H.; Kalgutkar A. S. Mechanism-Based Inactivation (MBI) of Cytochrome P450 Enzymes: Structure-Activity Relationships and Discovery Strategies to Mitigate Drug-Drug Interaction Risks.. J. Med. Chem. 2012, 55, 4896–4933. 10.1021/jm300065h. [DOI] [PubMed] [Google Scholar]
- Ritchie T. J.; Macdonald S. J. F. The impact of aromatic ring count on compound developability – are too many aromatic rings a liability in drug design?. Drug Discovery Today 2009, 14, 1011. 10.1016/j.drudis.2009.07.014. [DOI] [PubMed] [Google Scholar]
- Lovering F.; Bikker J.; Humblet C. Escape from Flatland: Increasing Saturation as an Approach to Improving Clinical Success. J. Med. Chem. 2009, 52, 6752–6756. 10.1021/jm901241e. [DOI] [PubMed] [Google Scholar]
- Lovering F. Escape from Flatland 2: complexity and promiscuity. MedChemComm 2013, 4, 515–519. 10.1039/c2md20347b. [DOI] [Google Scholar]
- Stepan A. F.; Subramanyam C.; Efremov I. V.; Dutra J. K.; O’Sullivan T. J.; DiRico K. J.; McDonald W. S.; Won A.; Dorff P. H.; Nolan C. E.; Becker S. L.; Pustilnik L. R.; Riddel D. R.; Kauffman G. W.; Kormos B. L.; Zhang L.; Lu Y.; Capetta S. H.; Green M. E.; Karki K.; Sibley E.; Atchison K. P.; Hallgren A. J.; Oborski C. E.; Robshaw A. E.; Sneed B.; O’Donnell C. J. Application of the Bicyclo[1.1.1]pentane Motif as a Nonclassical Phenyl Ring Bioisostere in the Design of a Potent and Orally Active γ-Secretase Inhibitor. J. Med. Chem. 2012, 55, 3414–3424. 10.1021/jm300094u. [DOI] [PubMed] [Google Scholar]
- Nicolaou K. C.; Vourloumis D.; Totokotsopoulos S.; Papakyriakou A.; Karsunky H.; Fernando H.; Gavrilyuk J.; Webb D.; Stepan A. F. Synthesis and Biopharmaceutical Evaluation of Imatinib Analogues Featuring unusual Structural Motifs. ChemMedChem 2016, 11, 31–37. 10.1002/cmdc.201500510. [DOI] [PubMed] [Google Scholar]
- Measom N. D.; Down K. D.; Hirst D. J.; Jamieson C.; Manas E. S.; Patel V. K.; Somers D. O. Investigation of a Bicyclo[1.1.1]pentane as a Phenyl Replacement within a LpPLA2 Inhibitor. ACS Med. Chem. Lett. 2017, 8, 43–48. 10.1021/acsmedchemlett.6b00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auberson Y. P.; Brocklehurst C.; Furegati M.; Fessard T. C.; Koch G.; Decker A.; La Vecchia L.; Briard E. Improving Nonspecific Binding and Solubility: Bicycloalkyl Groups and Cubanes as para-Phenyl Bioisosteres. ChemMedChem 2017, 12, 590–598. 10.1002/cmdc.201700082. [DOI] [PubMed] [Google Scholar]
- Zhong M.; Peng E.; Huang N.; Huang Q.; Huq A.; Lau M.; Colonno R.; Li L. Discovery of functionalized bisimidazoles bearing cyclic aliphatic-phenyl motifs as HCV NS5A inhibitors. Bioorg. Med. Chem. Lett. 2014, 24, 5731–3737. 10.1016/j.bmcl.2014.10.057. [DOI] [PubMed] [Google Scholar]
- Aguilar A.; Lu J.; Liu L.; Du D.; Bernard D.; McEachern D.; Przybranowski S.; Li X.; Luo R.; Wen B.; Sun D.; Wang H.; Wen J.; Wang G.; Zhai Y.; Guo M.; Yang D.; Wang S. Discovery of 4-((3′R,4′S,5′R)-6″-Chloro-4′-(3-chloro-2-fluorophenyl)-1′-ethyl-2″-oxodispiro[cyclohexane-1,2′-pyrrolidine-3′,3″-indoline]-5′-carboxamido)bicyclo[2.2.2]octane-1-carboxylic Acid (AA-115/APG-115): A Potent and Orally Active Murine Double Minute 2 (MDM2) Inhibitor in Clinical Development. J. Med. Chem. 2017, 60, 2819–2839. 10.1021/acs.jmedchem.6b01665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalmers B. A.; Xing H.; Houston S.; Clark C.; Ghassabian S.; Kuo A.; Cao B.; Reitsma A.; Murray C-E. P.; Stok J. E.; Boyle G. M.; Pierce C. J.; Littler S. W.; Winkler D. A.; Bernhardt P. V.; Pasay C.; De Voss J. J.; McCarthy J.; Parson P. G.; Walter G. H.; Smith M. T.; Cooper H. M.; Nilsson S. K.; Tsanktsidis J.; Savage G. P.; Williams C. M. Validating Eaton’s Hypothesis: Cubane as a Benzene Bioisostere. Angew. Chem., Int. Ed. 2016, 55, 3580–3585. 10.1002/anie.201510675. [DOI] [PubMed] [Google Scholar]
- Mykhailiuk P. K. Saturated bioisosteres of benzene: where to go next?. Org. Biomol. Chem. 2019, 17, 2839–2849. 10.1039/C8OB02812E. [DOI] [PubMed] [Google Scholar]
- Values were calculated from ground state equilibrium geometries using the ωB97x-D G-31 G* level of theory in Spartan.
- 198 patents containing aminoBCPs, 207 patents containing aminoBCO, 103 patents containing aminoNBs, and 20 patents containing CUBs have been registered since 2000. The search was performed in December 2019 in Reaxys DB.
- Staveness D.; Sodano T. M.; Li K.; Burnham E. A.; Jackson K. D.; Stephenson C. R. J. Providing a New Aniline Bioisostere through the Photochemical Production of 1-Aminonorbornanes. Chem. 2019, 5, 215–226. 10.1016/j.chempr.2018.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staveness D.; Collins J. L. III; McAtee R. C.; Stephenson C. R. J. Exploiting Imine Photochemistry for Masked N-Centered Radical Reactivity. Angew. Chem. 2019, 131, 19176–19182. 10.1002/ange.201909492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalgutkar A. S.; Dalvie D. Predicting Toxicities of Reactive Metabolite-Positive Drug Candidates. Annu. Rev. Pharmacol. Toxicol. 2015, 55, 35–54. 10.1146/annurev-pharmtox-010814-124720. [DOI] [PubMed] [Google Scholar]



