Abstract
Recent years have seen an unprecedented level of innovation in allosteric drug discovery and development, with multiple drug candidates advancing into clinical studies. From early examples of allosteric drugs like GABAA receptor modulators (benzodiazepines) in the 1960s to more recent GPCR negative allosteric modulators of CCR5 (maraviroc) approved in 2007, the opportunities for interrogating allosteric sites in drug discovery have expanded to other target classes such as protein–protein interactions, kinases, and nuclear hormone receptors. In this Innovation Letter, the authors highlight the latest advances of allosteric drug discovery from different target classes and novel emerging chemical modalities beyond small molecules.
Keywords: Allosteric modulator, PPI, kinases, covalent inhibitor, nuclear hormone receptor, new chemical modalities
A contemporary definition of allostery refers to the process by which biological macromolecules (mainly proteins) transfer the effect of binding at one site to another, often distal, functional site, enabling the regulation of activity.1 Each receptor owns a precise binding site for its respective endogenous molecules, describing this site as the orthosteric binding site. Orthosteric binding sites tend to be highly conserved across different receptor subtypes. Allosteric sites are structurally, conformationally, and usually functionally different than the corresponding receptor orthosteric pockets. In numerous examples, binding of direct-acting (or orthosteric) agonists has shown undesired side-effects or led to receptor desensitization, internalization, or downregulation for long duration studies.2 In comparison to orthosteric synthetic ligands, allosteric modulators have been reputed to offer several distinct potential advantages.3 Allosteric ligands may grant high levels of selectivity by binding to sites that are less preserved across receptor subtype families. In addition, an allosteric modulator that lacks functional agonist activity has an effect solely in the presence of the endogenous ligand, protecting the spatial and temporal action of the natural ligand. Furthermore, many efforts have established improved chemical tractability and physicochemical properties of allosteric versus orthosteric compounds.4
Allosteric ligands can exhibit diverse modes of pharmacology, comprising, in general, positive allosteric modulators (abbreviated as PAMs), which potentiate agonist mediated receptor response, and negative allosteric modulators (NAMs), which noncompetitively reduce activity. Silent or neutral allosteric modulators (SAMs or NALs) bind at allosteric sites and can block the effect of PAMs or NAMs; however, they have no impact on orthosteric ligand responses. For more detailed explanation of complex binding cooperativity allosteric modalities and examples, see the recent review by Coughlin et al.5 and references included.
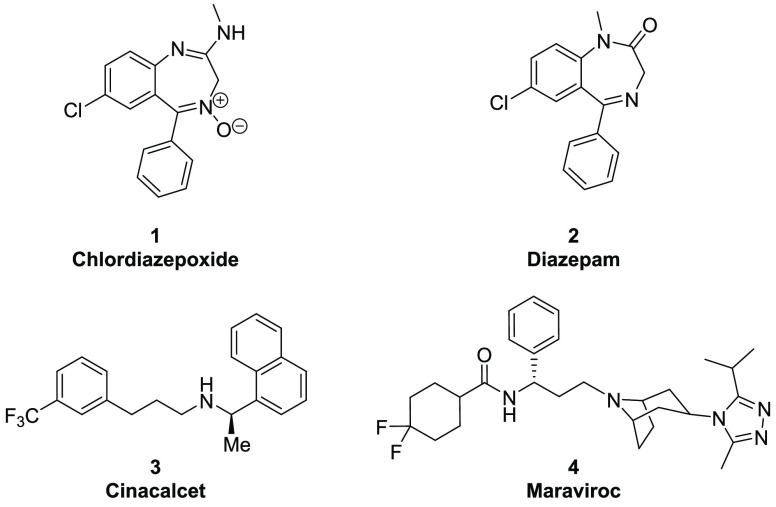
Although the allostery concept was originally applied in bacterial regulatory enzymes and hemoglobin, other protein classes, such as ion channels and GPCRs, quickly followed with successful examples in the clinic. In particular, GABAA receptor positive receptor modulators benzodiazepines, like chlordiazepoxide (1, Librium) and diazepam (2, Valium), were approved in the 1960s (Figure 1). In 2004, cinacalcet (3, Sensipar, an allosteric activator of calcium-sensing receptor) was one of the initial allosteric GPCR modulators to obtain regulatory approval for commercialization. Maraviroc (4, Selzentry) is a chemokine CCR5 receptor NAM designed as an anti-HIV agent and approved in 2007.
Figure 1.
Historical allosteric modulator drugs.
From the chemistry perspective, allosteric modulators tend to have favorable physicochemical properties as investigated by Westen and co-workers.6 Their analysis of a large compound collection of allosteric and non-allosteric ligands from the ChEMBL database concluded that allosteric modulators have a propensity to be smaller, more lipophilic, and rigid compounds, than their corresponding orthosteric ligands. However, one of the main limitations for targeting allosteric compounds is the knowledge of the corresponding binding sites for specific receptors and how to identify those allosteric sites (which screening assay format to use) in early Lead Generation efforts.
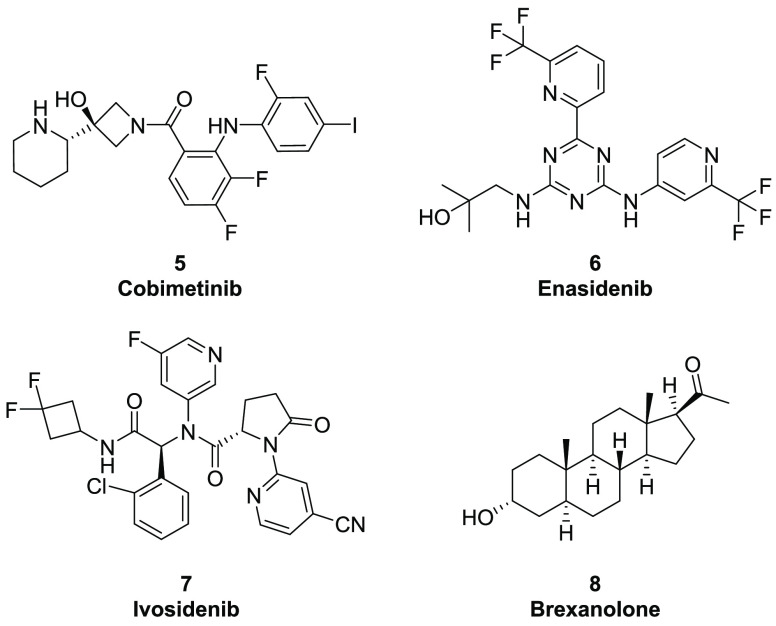
In recent years, the exponential advancement in structure biology, X-ray crystallography, and cryo-EM has offered unprecedented opportunities7,8 to leverage the insights into allostery and enable the discovery and development of novel allosteric chemical modalities9 for treatment of various human diseases. From the perspective of reaching clinical status and beyond, several new allosteric modulators have recently been approved (Figure 2). Cobimetinib (5, Cotellic), an allosteric MEK1/2 inhibitor codeveloped by Exelixis/Genentech, was approved in 2015 for unresectable or metastatic melanoma with BRAF mutation. Isocitrate dehydrogenase 2 (IDH2) allosteric inhibitor, enasidenib (6, Idhifa), was approved for acute myeloid leukemia (AML) in 2017.10 Ivosidenib (7, Tibsovo), an isocitrate dehydrogenase 1 (IDH1) allosteric inhibitor,11 received FDA approval in 2018 for treating patients with an IDH1 mutation and suffering from refractory AML. In 2019, brexanolone (8, Zulresso), a positive allosteric modulator of GABAA receptor developed by Sage Therapeutics, was approved for postpartum depression (PPD).
Figure 2.
Recently approved allosteric modulators.
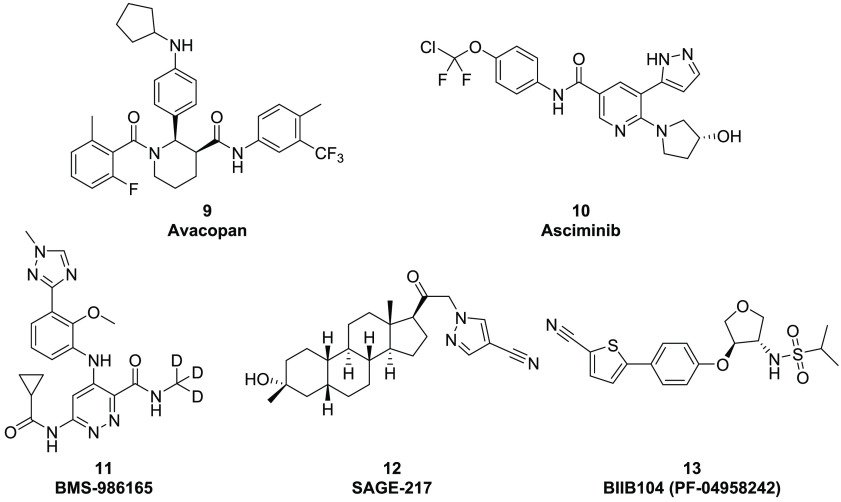
In addition to several recently approved drugs, there are many allosteric modulators currently in late stage clinical studies (Figure 3). Avacopan (9), a C5a allosteric inhibitor,12 is in Phase 3 clinical trials for the treatment of anti-neutrophil cytoplasmic autoantibody (ANCA) vasculitis. Asciminib (10, ABL001) is an allosteric inhibitor of the tyrosine kinase ABL-00113 developed by Novartis and in Phase 3 trial for chronic myelogenous leukemia. BMS-986165 (11), an allosteric Tyk2 inhibitor14 developed by Bristol-Myers Squibb (BMS), is in Phase 3 clinical studies for plaque psoriasis. SAGE-217 (12), a GABAA receptor positive allosteric modulator15 developed by Sage Therapeutics, is in Phase 3 clinical trials for major depression disorder and PPD. BIIB104 (13, also known as PF-04958242)16 is an AMPA receptor PAM in Phase 2 human studies by Biogen for improvement of cognitive impairment related to schizophrenia. SAGE-718, a novel NMDA receptor PAM, is advancing to Phase 2 clinical studies.17
Figure 3.
Allosteric modulators in clinical studies.
In the next section of this manuscript, we are going to highlight innovations in allosteric modulation across different target classes.
Allosteric Modulators of Protein–Protein Interactions
Targeting protein–protein interactions (PPI) has great potential for drug discovery, but modulation of protein–protein interaction by small molecules has long been considered challenging.18 Generally speaking, protein–protein (PP) interfaces do not exhibit deep hydrophobic pockets commonly found at enzyme active sites. As a result, small molecule orthosteric PPI modulators have to garner energy from many shallow binding pockets on the surface to compete with the much larger interface areas the endogenous ligands use. Nevertheless, over the past decade, vast progress has been made and a number of novel PPI modulators seem promising in preclinical studies and clinical trials. Unlike orthosteric PPI ligands, allosteric PPI modulators bind, by definition, at a site other than the protein–protein interfaces, which allows fine-tuning function in terms of partial inhibition, agonism, and dose dependency.19 Similarly to other allosteric family targets, PPI allosteric sites may provide better subtype selectivity, since allosteric sites are largely more diverse between related proteins than the active sites.
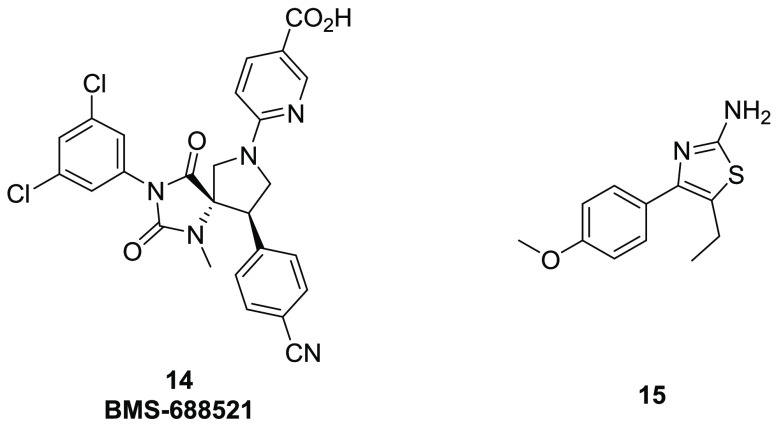
One example of a small molecule allosteric PPI modulator is BMS-68852120 (14, Figure 4), which was discovered as an antagonist of leukocyte function associated antigen-1 (LFA-1, or CD11a/CD18 or αLβ2 using integrin nomenclature) and disruptor of the interactions between integrin LFA-1 and the protein known as intercellular adhesion molecule 1 (ICAM-1). The binding between LFA-1 and ICAMs is considered crucial for cell adhesion. Preclinical and clinical studies with efalizumab (a humanized anti-LFA-1 antibody) confirmed LFA-1 as a viable immunological target. From an early lead, the BMS team was able to identify 14, which showed excellent in vitro potency and ex vivo efficacy. It was shown to be efficacious at a 1 mg/kg BID dose in a mouse allergic eosinophilic lung inflammation model. X-ray cocrystal studies indicated that 14 sticks to the I-Domain allosteric site and prevents conformational changes required for converting the I-Domain to the high affinity, open conformation, thus disrupting the interaction of LFA-1 to ICAM-1.
Figure 4.
Examples of allosteric PPI modulators.
Another example of allosteric PPIs is Bushweller’s21 reported studies on Runx1/CBFβ PPI disruptors (Figure 4). Runt-related transcription factor 1 (Runx1) and core-binding factor subunit β (CBFβ) are two subunits of core binding factors. They perform vital functions in hematopoiesis and are targets of chromosomal translocations in leukemia. The interaction of the CBFβ-smooth muscle myosin heavy chain fusion protein to Runx1 is crucial for leukemogenesis, which makes targeting the protein–protein (Runx1 and CBFβ) interaction an appealing approach for leukemia. Virtual screening of a 70,000 member, commercially available, compound library resulted in 35 confirmed hits. These compounds were then screened by NMR studies to detect chemical shift changes in 2D-NMR studies (15N–1H or 13C–1H HSQC) of proteins. The activity of the compounds was later confirmed with FRET and ELISA assays, and chemical optimization was carried out to improve potency and physicochemical properties. Compound 15 (Figure 4) was identified as a lead compound with low micromolar affinity, which also successfully blocked the protein–protein interaction of Runx1 to CBFβ. Compound 15 showed dose-dependent antiproliferative effects in ME-1 cells, and similar growth inhibition was also observed in cell lines such as KH-2, HL-60, and U937. They also applied the NMR chemical shift perturbations to dock the compounds to CBFβ and showed the compounds bind at a site offset from the interfacial region of CBFβ and behave as allosteric inhibitors of this protein–protein interaction.
Allosteric Modulators of Ion Channels
As mentioned earlier, allosteric modulation of ion channels has been an active area of R&D since the discovery of allostery. It continues to attract researchers to find safe and effective ion channel regulators to treat disease ranging from CNS to cardiovascular diseases.
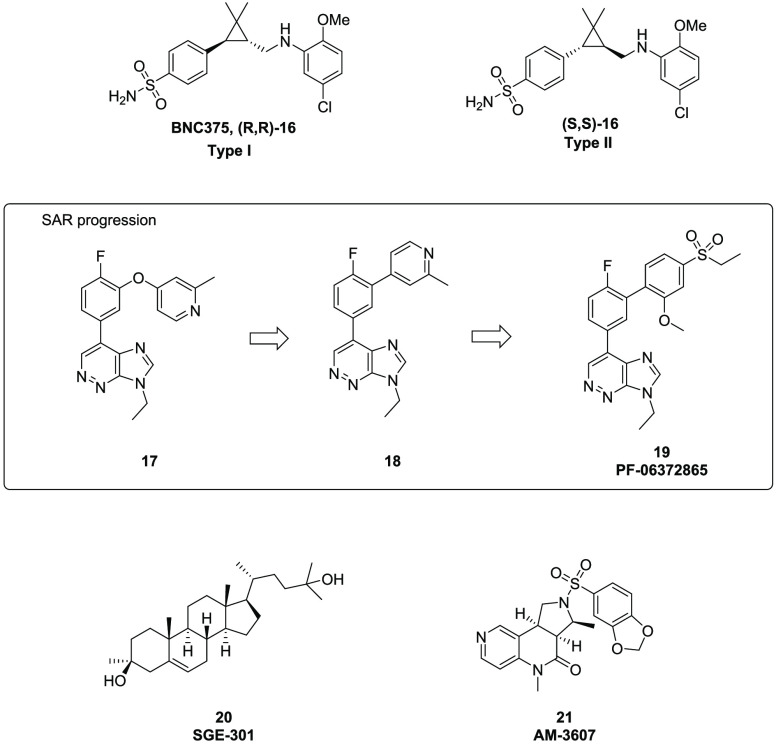
A number of studies have shown that potentiation of alpha 7 nicotinic acetylcholine receptors (α7 nAChRs) can improve cognition, which presents PAMs of α7 nAChRs as a promising target to treat cognitive impairment related to Alzheimer’s disease or schizophrenia.22 Based on their function, α7 nAChRs PAMs can be divided into two classifications. Type I PAMs amplify peak channel response to acetylcholine and do not influence channel desensitization kinetics, whereas Type II PAMs increase channel response with a delay on receptor desensitization. Harvey and his team at Bionomics recently reported the discovery of a novel class of compounds with either Type I or Type II PAM activity that can be tuned by the relative stereochemistry around the central cyclopropyl ring. For instance, (R,R)-16 (BNC375,23Figure 5) and its analogues are Type I PAMs, whereas (S,S)-16 and its analogues are Type II PAMs. Additional profiling suggested that good CNS-drug like properties make it an interesting candidate for further preclinical studies.
Figure 5.
Ion channel allosteric modulators.
GABA is the major inhibitory neurotransmitter in the CNS, and drugs targeting the GABAA receptor have shown diverse pharmacology, including anxiolytic and anticonvulsant effects. In an effort to discover α1-sparing, α2/3 PAMs for the treatment of anxiety and other disorders, a Pfizer group24 designed and synthesized a number of novel scaffolds based on similarity searches and molecular overlays of known GABAA receptor ligands. Although the initial hit, compound 17 (Figure 5), was only moderately active (α2 Ki = 33 nM), it exhibited the desired functional selectivity (α1 PAM = −22%, α2 PAM = 54%; percentage values refer to the level of enhancement measured after activation of the receptor with GABA as described in the reference) and in vitro profile. Further optimization led to compound 18, which showed both improved activity and GABA subtype selectivity (α2 Ki = 13 nM), α1 PAM: −5%, α2 PAM = 71%). Fine tuning the potency and physicochemical properties resulted in discovery of PF-06372865 (19, Figure 5), which was advanced to preclinical safety studies and subsequently identified as a clinical candidate.
Other recent reports in this area include Sage Therapeutics’ NMDA (N-methyl- d-aspartate receptor) PAM SGE-30125 (20) and Amgen’s AM-3607 (21), an allosteric glycine receptor potentiator26 (Figure 5).
Allosteric Modulators of G-Protein Coupled Receptors (GPCRs)
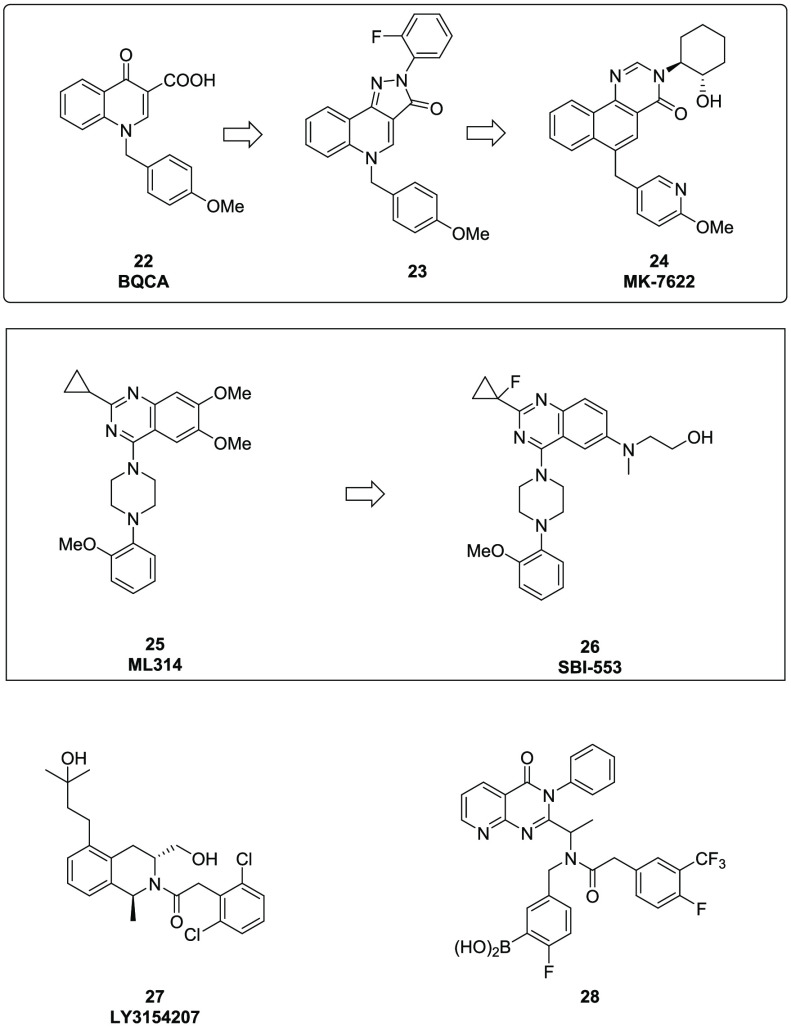
GPCRs are another area of extensive allosteric research. For this Innovations paper, we are highlighting a few of the most recent examples in the field. A Merck group recently published their work on MK-7622,27 a first-in-class M1 PAM for the treatment of Alzheimer’s disease (AD). AD comprises the gradual and irreversible degeneration of cholinergic neurons. Cholinergic signaling takes place through acetylcholine (ACh), which is an endogenous ligand of nicotinic and muscarinic receptors. Muscarinic receptors are class A GPCRs covering five subtypes (M1 to M5). The M1 subtype is predominantly expressed in the cortical areas, hippocampus, and striatum, and it is believed to be crucial in memory and higher brain function. High throughput screening of their internal compound collection identified BQCA (22, Figure 6), which displays moderate M1 activity (0.66 μM). The carboxylic acid moiety on 22 is believed to limit its CNS exposure; however, medicinal chemistry efforts using bioisostere replacements led to compound 23 (0.41 μM). Fine tuning modifications of the central core structure afforded compound MK-7622 (24), which is an M1-selective modulator with a promising preclinical pharmacokinetic and pharmacodynamic profile. This compound was in Phase 2 proof-of-concept clinical studies for the treatment of cognitive dysfunction in AD patients. However, the clinical trial was recently halted after a futility analysis showed that MK-7622 did not demonstrate cognition improvement at 12 weeks.28
Figure 6.
GPCR allosteric modulators.
Dopamine receptors are members of the class A GPCRs that play key roles mediating cellular responses to dopamine, a neurotransmitter with multiple functions systemically and in the brain. In recent years, dopamine receptors have drawn extensive interest as targets for both peripheral and CNS disorders. Among five known dopamine receptor subtypes, D1 has been mostly investigated and studies have shown that activation of D1 receptor is potentially beneficial for Parkinson’s disease, AD, schizophrenia, depression, and sleep disorders.
Until recently, orthosteric activation of D1 receptors has been the primary focus, with an emphasis on catechol derivatives, structurally mimicking dopamine. However, development of these compounds has been proven quite difficult due to the suboptimal pharmacokinetic profile and low receptor selectivity over the D5 subtype.29 In 2019, a group from Lilly reported the discovery of LY3154207 (27, Figure 6), a novel potent and subtype selective human D1 PAM.30 Starting from a single micromolar hit from an HTS screen of more than half million compounds, the team optimized the initial thieno-piperidine hit by improving potency, solubility, and intrinsic clearance simultaneously. Extensive SAR exploration led to three additional potent, structurally similar analogs; however, LY3154207 was ultimately identified as a development candidate. Through extensive NMR and X-ray studies, the authors confirmed that 27, and its analogs, bind to a novel binding site in the intracellular loop 2 (ICL2), which is unrelated to the known D1 orthosteric agonist site. In a locomotor activity test utilizing humanized-D1 mice, the activity of 27 exhibited effects distinct from orthosteric agonists, displaying neither a bell-shaped dose–response relationship over a wide dose range nor induced tachyphylaxis. Moreover, the compound displayed efficacy in a mouse model of early Parkinson’s disease and is in Phase 2 clinical studies for the treatment of Lewy body dementia.
As indicated through nomenclature, GPCRs are coupled to G proteins that bind and hydrolyze guanosine 5-triphosphate (GTP) to mediate downstream signaling. Recent research in GPCR signaling has revealed that GPCR can also signal trough G protein independent pathways; β-arrestin dependent signaling is recognized as one of the most important G protein independent pathways. One example of a β-arrestin biased GPCR modulator is the NTR1 inhibitor SBI-553.31
Neurotensin receptor 1 (NTR1) is a GPCR widely expressed in the CNS and has been linked to a broad variety of CNS disorders. Using a HTS approach of NTR1/ β-arrestin-GTP complexes, a combined Sanford-Burnham/Duke team was able to identify a lead compound ML314 (25, Figure 6), which showed full agonist activity against the NTR1 receptor but selectivity over the NTR2 subtype. Compound 25 was subsequently tested in a Ca2+ mobilization assay and displayed no significant response, suggesting it functions as a β-arrestin biased agonist. Compound 25 was moderately potent (EC50 = 2.87 μM) and showed efficacy in several in vivo animal models; however, it demonstrated very low oral bioavailability (< 5 %F in rat) preventing its path forward. Further optimization led to the discovery of SBI-553 (26, Figure 6), which offered approximately a 10-fold potency improvement, and significantly improved bioavailability (45 %F in rat) and CNS penetration.
The chemokine receptor CXCR3, which is highly expressed on activated T cells, performs a critical role in the regulation of the human immune system response. Dysregulation of CXCR3 is linked to several pathologies like autoimmune diseases, cancer, vascular disease, and transplant rejection. To design a biased β-arrestin-2 negative allosteric modulator of CXCR3, a team from Friedrich Alexander University built a homology model based on reported small molecule CXCR3 allosteric ligands. They utilized a boronic acid moiety as a probe to identify key anchor amino acid residues. This effort led to the discovery of two new allosteric binding sites on CXCR3 and compound 28,32 the first reported biased β-arrestin-2 NAM of CXCR3 (Figure 6). Compound 28 is an approximately 24-fold more potent NAM of CXCL11-mediated β-arrestin-2 recruitment compared to the corresponding G-protein activation pathway. In addition, this molecule exhibited negative cooperativity comparable to the competitive antagonism in the β-arrestin-2 recruitment assay and decreased negative cooperativity in the [35S]-GTPγS incorporation assay.
Allosteric Modulators of Kinases
Small-molecule kinase inhibitors have proven to be effective therapeutics for human diseases, especially in oncology and immunology disorders. While most kinase inhibitors bind to the ATP-binding pocket, targeting allosteric pockets of kinases outside of the highly conserved ATP pocket has been considered an attractive alternative to overcome issues of selectivity and drug resistance.
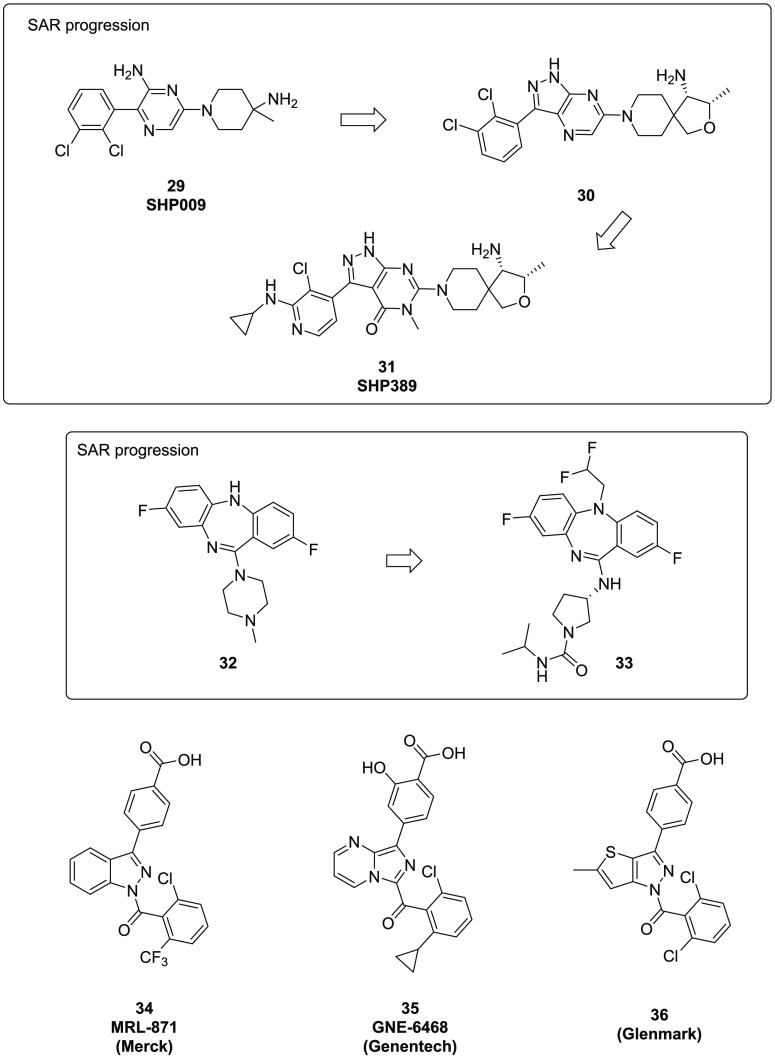
SHP2 is a non-receptor protein tyrosine phosphatase within the mitogen-activated protein kinase (MAPK) pathway, mediating cell growth and differentiation, which has been a very active area for oncology research. In 2016, a Novartis team disclosed the discovery of SHP009,33 a selective albeit moderately potent and orally bioavailable SHP2 inhibitor (29, Figure 7). A cocrystal structure of the molecule bound to SHP2 demonstrated that the compound stabilizes an inactive conformation through simultaneous binding to the interface of the N-terminal SHP2, C-terminal SH2P, and the protein tyrosine phosphatase domains, resembling the published inactive apo SHP2 crystal structure. To improve the potency of SHP-009, a series of 5,6-membered-ring fused analogs were designed utilizing a homology model.34 One analog, compound 30, was 10-fold more potent than 29 in a biochemical assay and further exhibited remarkable antiproliferative effects in a KYSE520 cell assay (IC50 = 0.46 μM). Unfortunately, compound 30 was also a potent hERG inhibitor (IC50 = 0.004 μM). Reducing hERG channel activity by decreasing the lipophilicity of the scaffold resulted in the identification of SHP38935 (31, Figure 7) which had significantly attenuated hERG activity (IC50 = 17 μM). In the KYSE520 cell assay, 31 displayed excellent antiproliferative effects (IC50 = 0.72 μM). However, mouse pharmacokinetic studies showed it has low oral bioavailability and poor permeability, which prevented further evaluation of 31 in mouse tumor xenograft models.
Figure 7.
Kinase and nuclear receptor allosteric modulators.
PAKs (p21-activated kinases) are a family of serine/threonine protein kinases that are effectors of Rac/Cdc42 GTPases and play a critical role in cell proliferation, survival, and angiogenesis. In recent years, they have attracted significant interest as novel targets for oncology and neurodegenerative diseases. Several PAK inhibitors have been extensively studied, such as PF-3758309,36 FRAX486,37 and FRAX597.38 Approaches toward allosteric modulation have been pursued aiming to achieve better selectivity across the kinome.39 In 2015, a Novartis team40 screened a small library using full-length PAK1 protein, in search of allosteric and selective PAK1 inhibitors. The initial hit (32, Figure 7) was weakly active against PAK1 (IC50 = 12 uM). X-ray cocrystallography of 32 indicated the compound bound to a novel allosteric site of PAK1, and subsequent optimization provided 33, which displayed nanomolar inhibition (PAK1 IC50 = 5.2 nM) and high selectivity against other PAKs. In Su86.86 cells, this compound displayed dose-dependent inhibition of PAK1 autophosphorylation and inhibited proliferation at a concentration of approximately 2 μM.
Allosteric Modulators of Nuclear Receptors
Nuclear receptors (NR) are a class of multidomain proteins which regulate genes that affect varied processes such as reproduction, development, inflammation, and metabolism. In recent years, allosteric modulation of NR function has emerged as an interesting area of drug discovery. Several studies targeting allosteric NR like retinoic acid related orphan receptor γ (RORγ), retinoid X receptor (RXRα), androgen receptor (AR), farnesoid X receptor (FXR), thyroid hormone receptor (TR), or peroxisome proliferator-activated receptor gamma (PPARγ) have been reported.41 One interesting example is a recent Merck discovery of allosteric RORγ inverse agonist MRL-87142 (34, Figure 7). RORγ is an NR subclass which regulates the differentiation and functionality of immune Th17 cells. Due to its therapeutic potential in autoimmune disease, numerous compounds have advanced toward clinical studies; however, those efforts focused on orthosteric approaches up to 2015. In that year, Merck disclosed the breakthrough discovery of a novel allosteric RORγ modulator while investigating a series of benzoic acid derivatives (Figure 7, 34, MRL-871). Applying structure-based drug discovery, the team identified a novel allosteric site on RORγ receptor separated from the orthosteric binding pocket. They hypothesized that allosteric binding of these benzoic acid derivatives induced a conformational change of the receptor, probably through an additional interaction of the carboxylic acid moiety with the receptor. Compound 34 was tested in a time-resolved fluorescence resonance energy transfer (TR-FRET) coactivator recruitment assay yielding an IC50 = 6 nM, and in a cellular hPBMC (human peripheral blood mononuclear cell) IL17A assay, showing inhibition (IC50 = 40 nM). Following on this innovation, other companies such as Genentech43 and Glenmark44 have developed additional acid containing ligands (35 and 36 respectively, Figure 7) presumed to bind to the same allosteric site.
Covalent Allosteric Modulators
Covalent allosteric modulators potentially combine the pharmacological advantages of allosteric modulators and the attributes of high potency, long duration of action, and low drug resistance of covalent ligands. This approach has been gaining increasing recognition as a novel modality in drug discovery.45
One example is the recent research reported on Akt1 kinase allosteric modulators.46 Ser/Thr kinase Akt (also referred to as Protein Kinase B) is a key player in the P13K/Akt/mTOR pathway and P13K/Akt signaling, influencing cell cycle and apoptosis.
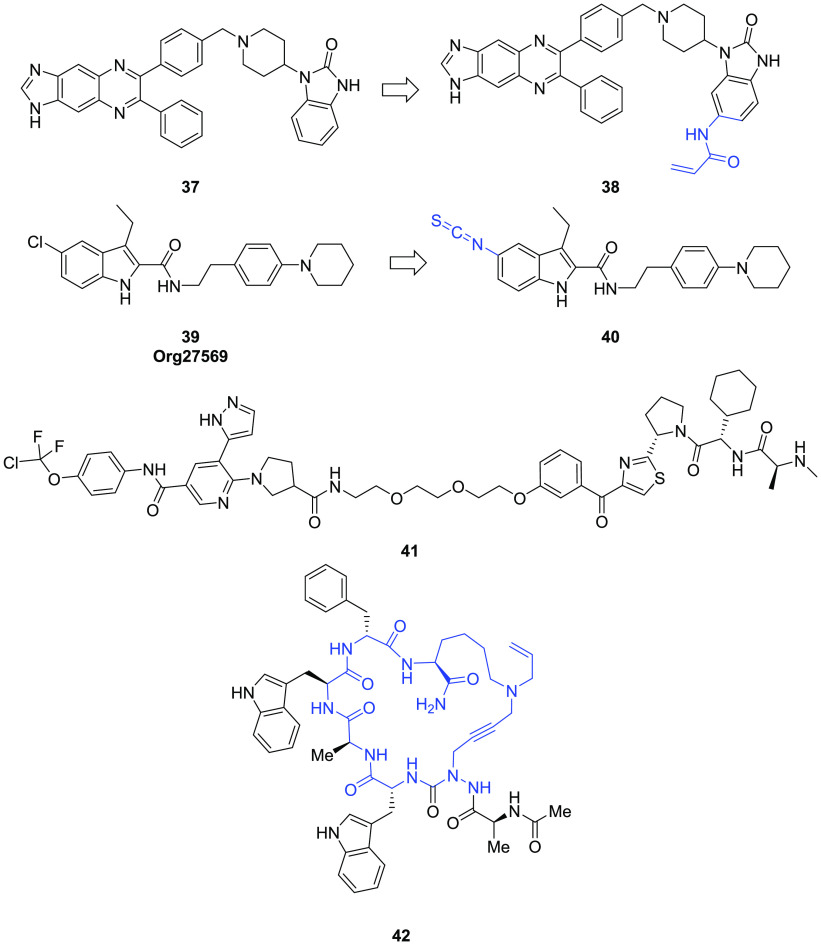
Merck reported the first allosteric Akt inhibitor,47 compound 37 (Figure 8), which showed dual activity in Akt1 (IC50 = 58 nM) and Akt2 (IC50 = 210 nM). A cocrystal structure of Akt1 complexed with allosteric inhibitor 37 revealed that compound 37 binds to an allosteric binding site created at the interface of the pleckstrin homology (PH) domain-dependent covalent domain and the N-, C-lobes of the kinase domain. Building on this knowledge, Weisner et al.48 recently designed a novel covalent allosteric modulator (38, Figure 8), by attaching a Michael acceptor as the covalent binding warhead. In the Akt binding assay, 38 showed increased binding affinity to Akt1 (in comparison to the noncovalent allosteric modulator 37). The crystal structure of compound 38 showed that the electrophilic warhead indeed forms a covalent bond with Cys310 stabilizing the inactive kinase conformation.
Figure 8.
Covalent allosteric modulators and allosteric modulators beyond small molecules.
An additional example of covalent allosteric modulation comes from targeting the cannabinoid 1 receptor (CB1R). CB1R is a GPCR class A, mainly expressed in the brain and involved in several physiological processes, such as mood, cardiovascular regulation, learning, and memory. Small molecule modulators of CB1 had long been an active area for drug research.49 Org2756950 (39, Figure 8), the first allosteric modulator targeting CB1R signaling, has emerged as the most potent CB1R PAM of the orthosteric ligand CP55940 in functional assays (IC50 = 0.29 nM). It is believed that it induces a conformational change of the CB1R and results in downstream activation of ERK signaling. Recently, Kulkarni et al.51 replaced the chlorine atom at the C5 position of 39 with an isothiocyanate group, providing compound 40 (Figure 8), which has the potential to participate in covalent interaction with CB1R. Functional assays revealed that 40 was more potent and efficacious than the corresponding analog 39 in CB1R-dependent β-arrestin recruitment and cAMP accumulation, displaying the highest functional selectivity (83-fold) for β-arrestin against cAMP. Compared with the parent 39, compound 40 did not demonstrate inverse agonism, which can potentially alleviate psychotropic side-effects produced by CB1R orthosteric antagonists or inverse agonists.
In the next section of this manuscript, we are highlighting some examples of novel chemical modalities beyond small molecules that are able to display an allosteric mechanism of action.
Allosteric Protein Degraders
Protein degradation or PROTAC has gained substantial attention in drug discovery in recent years.52 Currently, several drug target proteins, e.g. androgen receptor, estrogen receptor, and bromodomain containing 4, have been degraded effectively via this approach which utilizes the ubiquitin–proteasome system. The first few protein degraders have recently initiated phase 1 clinical trials in 2019.53
While protein degradation of BCR-ABL has been reported by several research groups, most attempts have focused on introducing an orthosteric ligand of the protein of interest (POI) into hybrid molecules. A collaboration effort between Takeda and Naito’s group, however, took a different approach,54 linking an allosteric ligand moiety of BCR-ABL with an inhibitor of apoptosis (IAP) binding moiety. The hybrid molecule, compound 41 (Figure 8), displayed effective degradation of BCR-ABL protein with submicromolar potency. In cell-based studies, 41 inhibited growth in K562 and KCL-22 chronic myeloid leukemia cell lines. Although the pharmacokinetic profile of compound 41 is far from ideal, this study had demonstrated the possibility to expand allosteric regulation to protein degradation technology. Further studies on allosteric protein degradation may offer additional advantages over current PROTAC technologies.
Novel Peptides and Peptidomimetics as Allosteric Modulators
Peptides as a drug modality can offer certain beneficial attributes such as potent pharmacological activity, high specificity, and minimal toxicity. However, the lack of oral bioavailability aggravated with low stability under physiological conditions limit their therapeutic use. In the past several years, novel research has been reported identifying peptides and peptidomimetics as allosteric modulators. Lubell and co-workers55 have synthesized several aza-cyclopeptides to target the CD36 receptor system. The CD36 receptor is a multifunctional glycoprotein, expressed in a variety of cell types, and regulates the innate immune response associated with pneumonia, tuberculosis, malaria, leishmaniasis, and HIV infection. Apart from infectious diseases, it is also involved in metabolic disorders such as atherosclerosis, age-related macular degeneration, AD, cancer, and diabetes. Using a multiple component “A3 -macrocyclization” strategy, Lubell’s group prepared a diverse set of cyclic azapeptides. Some cyclic aza-GHRP-6 analogs (42, Figure 8) exhibited allosteric modulation56 of CD36 receptor with potent affinity. The aza-cyclopeptides also modulated the toll-like receptor agonist-induced overproduction of nitric oxide and reduced pro-inflammatory cytokine and chemokine release in macrophages.
Monoclonal Antibodies as Allosteric Modulators
Monoclonal antibodies acting as antagonists to receptors by blocking the natural ligand binding site (orthosteric site) have been extensively studied. In contrast, the use of antibodies to target receptors at allosteric sites has not been thoroughly investigated. Interestingly, a group from XOMA Corporation reported studies of three categories of human monoclonal antibodies which bind allosterically to the insulin receptor (INSR).57 They also demonstrated that antibodies can modulate the INSR in vitro and in animal models. XMetA, a positive allosteric activator of the INSR, confirmed activation of the INSR and effectively ameliorated diabetes in mouse models. XMetS, a PAM of the INSR, does not directly potentiate the INSR; however, it increases the receptor’s ability to bind insulin and potentiate insulin signaling. XMetD, an inhibitor of INSR signaling, reverses insulin-induced hypoglycemia in an animal model of hyperinsulinemia. XOMA 358 (X358)58 is a human monoclonal antibody to the insulin receptor acting as a NAM of insulin signaling. It is under development as a novel treatment of hyperinsulinemic hypoglycemia.
In summary, significant progress in expanding novel modalities of allosteric modulation has been achieved in the past few years. Multiple allosteric modulators are advancing to clinical trials, and many have achieved regulatory approval. Moreover, we have observed how the concept of allosteric modulation has been applied to other targets beyond the traditional ion channels and GPCRs. Recent history has revealed a significant wave of innovation in allosteric modulators of novel drug target classes such as protein–protein interactions, kinases, nuclear hormones, and others. The driver for this broader research is the potential favorable profile of allosteric modulators when confronting typical challenges in drug development, such as subtype selectivity. Furthermore, the concept of allosteric modulation has been widened to emerging chemical modalities with examples toward covalent inhibitors, protein degraders, large peptidomimetics, and even allosteric monoclonal antibodies. We envision an unprecedented era of innovation for novel allosteric modalities that hopefully will continue to translate to meaningful therapies for patients and their families.
Glossary
Abbreviations:
- HTS
high through-put screening
- GPCR
G protein-coupled receptor
- NAL
neutral allosteric modulator
- NAM
negative allosteric modulator
- PAM
positive allosteric modulator
- PPI
protein–protein interactions
- R&D
research and development
The authors declare no competing financial interest.
References
- Motlagh H. N.; Wrabl J. O.; Li J.; Hilser V. J. The ensemble nature of allostery. Nature 2014, 508, 331–9. 10.1038/nature13001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenthur C. J.; Gentry P. R.; Mathews T. P.; Lindsley C. W. Drugs for allosteric sites on receptors. Annu. Rev. Pharmacol. Toxicol. 2014, 54, 165–184. 10.1146/annurev-pharmtox-010611-134525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussinov R.; Tsai C. J. Allostery in disease and in drug discovery. Cell 2013, 153, 293–305. 10.1016/j.cell.2013.03.034. [DOI] [PubMed] [Google Scholar]
- Melancon B. J.; Hopkins C. R.; Wood M. R.; Emmitte K. A.; Niswender C. M.; Christopoulos A.; Conn P. J.; Lindsley C. W. Allosteric modulation of seven transmembrane spanning receptors: theory, practice, and opportunities for central nervous system drug discovery. J. Med. Chem. 2012, 55, 1445–1464. 10.1021/jm201139r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coughlin Q.; Hopper A. T.; Blanco M. J.; Tirunagaru V.; Robichaud A. J.; Doller D. Allosteric Modalities for Membrane-Bound Receptors: Insights from Drug Hunting for Brain Diseases. J. Med. Chem. 2019, 62, 5979–6002. 10.1021/acs.jmedchem.8b01651. [DOI] [PubMed] [Google Scholar]
- van Westen G. J.; Gaulton A.; Overington J. P. Chemical, target, and bioactive properties of allosteric modulation. PLoS Comput. Biol. 2014, 10, e1003559. 10.1371/journal.pcbi.1003559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu S.; He X.; Ni D.; Zhang J. Allosteric modulator discovery: from serendipity to structure-based design. J. Med. Chem. 2019, 62, 6405–6421. 10.1021/acs.jmedchem.8b01749. [DOI] [PubMed] [Google Scholar]
- García-Nafría J.; Tate C. G. Cryo-electron microscopy: Moving beyond x-ray crystal structures for drug receptors and drug development. Annu. Rev. Pharmacol. Toxicol. 2020, 60, 51–71. 10.1146/annurev-pharmtox-010919-023545. [DOI] [PubMed] [Google Scholar]
- Blanco M. J.; Gardinier K.. New Chemical Modalities and Strategic Thinking in Early Drug Discovery. ACS Med. Chem. Lett. 2020, 11, 228−231 10.1021/acsmedchemlett.9b00582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E. S. Enasidenib: first global approval. Drugs 2017, 77, 1705–1711. 10.1007/s40265-017-0813-2. [DOI] [PubMed] [Google Scholar]
- Popovici-Muller J.; Lemieux R. M.; Artin E.; Saunders J. O.; Salituro F. G.; Travins J.; Cianchetta G.; Cai Z.; Zhou D.; Cui D.; Chen P. Discovery of AG-120 (Ivosidenib): a first-in-class mutant IDH1 inhibitor for the treatment of IDH1 mutant cancers. ACS Med. Chem. Lett. 2018, 9, 300–305. 10.1021/acsmedchemlett.7b00421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H.; Kim H. R.; Deepak R. K.; Wang L.; Chung K. Y.; Fan H.; Wei Z.; Zhang C. Orthosteric and allosteric action of the C5a receptor antagonists. Nat. Struct. Mol. Biol. 2018, 25, 472–481. 10.1038/s41594-018-0067-z. [DOI] [PubMed] [Google Scholar]
- Schoepfer J.; Jahnke W.; Berellini G.; Buonamici S.; Cotesta S.; Cowan-Jacob S. W.; Dodd S.; Drueckes P.; Fabbro D.; Gabriel T.; Groell J. M. Discovery of asciminib (ABL001), an allosteric inhibitor of the tyrosine kinase activity of BCR-ABL1. J. Med. Chem. 2018, 61, 8120–8135. 10.1021/acs.jmedchem.8b01040. [DOI] [PubMed] [Google Scholar]
- Wrobleski S. T.; Moslin R.; Lin S.; Zhang Y.; Spergel S.; Kempson J.; Tokarski J. S.; Strnad J.; Zupa-Fernandez A.; Cheng L.; Shuster D. Highly selective inhibition of tyrosine kinase 2 (TYK2) for the treatment of autoimmune diseases: discovery of the allosteric inhibitor BMS-986165. J. Med. Chem. 2019, 62, 8973–8995. 10.1021/acs.jmedchem.9b00444. [DOI] [PubMed] [Google Scholar]
- Martinez Botella G.; Salituro F. G.; Harrison B. L.; Beresis R. T.; Bai Z.; Blanco M. J.; Belfort G. M.; Dai J.; Loya C. M.; Ackley M. A.; Althaus A. L. Neuroactive steroids. 2. 3α-Hydroxy-3β-methyl-21-(4-cyano-1 H-pyrazol-1′-yl)-19-nor-5β-pregnan-20-one (SAGE-217): a clinical next generation neuroactive steroid positive allosteric modulator of the (γ-aminobutyric acid) A receptor. J. Med. Chem. 2017, 60, 7810–7819. 10.1021/acs.jmedchem.7b00846. [DOI] [PubMed] [Google Scholar]
- Shaffer C. L.; Patel N. C.; Schwarz J.; Scialis R. J.; Wei Y.; Hou X. J.; Xie L.; Karki K.; Bryce D. K.; Osgood S. M.; Hoffmann W. E. The Discovery and Characterization of the α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic Acid (AMPA) Receptor Potentiator N-{(3 S, 4 S)-4-[4-(5-Cyano-2-thienyl) phenoxy] tetrahydrofuran-3-yl} propane-2-sulfonamide (PF-04958242). J. Med. Chem. 2015, 58, 4291–308. 10.1021/acs.jmedchem.5b00300. [DOI] [PubMed] [Google Scholar]
- Sage Therapeutics’ Website . Sage Therapeutics Announces Planned Progression of SAGE-718 to Phase 2 in Huntington’s Disease and Presentations at the 2019 Annual Meeting of the American College of Neuropsychopharmacology (ACNP). https://investor.sagerx.com/news-releases/news-release-details/sage-therapeutics-announces-planned-progression-sage-718-phase-2. (accessed December 16, 2019).
- Naylor M. R.; Bockus A. T.; Blanco M. J.; Lokey R. S. Cyclic peptide natural products chart the frontier of oral bioavailability in the pursuit of undruggable targets. Curr. Opin. Chem. Biol. 2017, 38, 141–147. 10.1016/j.cbpa.2017.04.012. [DOI] [PubMed] [Google Scholar]
- Ni D.; Lu S.; Zhang J. Emerging roles of allosteric modulators in the regulation of protein-protein interactions (PPIs): A new paradigm for PPI drug discovery. Med. Res. Rev. 2019, 39, 2314–2342. 10.1002/med.21585. [DOI] [PubMed] [Google Scholar]
- Watterson S. H.; Xiao Z.; Dodd D. S.; Tortolani D. R.; Vaccaro W.; Potin D.; Launay M.; Stetsko D. K.; Skala S.; Davis P. M.; Lee D.; et al. Small Molecule Antagonist of Leukocyte Function Associated Antigen-1 (LFA-1): Structure– Activity Relationships Leading to the Identification of 6-((5 S, 9 R)-9-(4-Cyanophenyl)-3-(3, 5-dichlorophenyl)-1-methyl-2, 4-dioxo-1, 3, 7-triazaspiro [4.4] nonan-7-yl) nicotinic Acid (BMS-688521). J. Med. Chem. 2010, 53, 3814–3830. 10.1021/jm100348u. [DOI] [PubMed] [Google Scholar]
- Gorczynski M. J.; Grembecka J.; Zhou Y.; Kong Y.; Roudaia L.; Douvas M. G.; Newman M.; Bielnicka I.; Baber G.; Corpora T.; Shi J.; et al. Allosteric inhibition of the protein-protein interaction between the leukemia-associated proteins Runx1 and CBFβ. Chem. Biol. 2007, 14, 1186–1197. 10.1016/j.chembiol.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Timmermann D. B.; Grønlien J. H.; Kohlhaas K. L.; Nielsen EØ; Dam E.; Jørgensen T. D.; Ahring P. K.; Peters D.; Holst D.; Chrsitensen J. K.; Malysz J. An allosteric modulator of the α7 nicotinic acetylcholine receptor possessing cognition-enhancing properties in vivo. J. Pharmacol. Exp. Ther. 2007, 323, 294–307. 10.1124/jpet.107.120436. [DOI] [PubMed] [Google Scholar]
- Harvey A. J.; Avery T. D.; Schaeffer L.; Joseph C.; Huff B. C.; Singh R.; Morice C.; Giethlen B.; Grishin A. A.; Coles C. J.; Kolesik P. Discovery of BNC375, a Potent, Selective, and Orally Available Type I Positive Allosteric Modulator of α7 nAChRs. ACS Med. Chem. Lett. 2019, 10, 754–760. 10.1021/acsmedchemlett.9b00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen R. M.; Blakemore D.; Cao L.; Flanagan N.; Fish R.; Gibson K. R.; Gurrell R.; Huh C. W.; Kammonen J.; Mortimer-Cassen E.; Nickolls S. A. Design and Identification of a Novel, Functionally Subtype Selective GABAA Positive Allosteric Modulator (PF-06372865). J. Med. Chem. 2019, 62, 5773–5796. 10.1021/acs.jmedchem.9b00322. [DOI] [PubMed] [Google Scholar]
- La D. S.; Salituro F. G.; Martinez Botella G.; Griffin A. M.; Bai Z.; Ackley M. A.; Dai J.; Doherty J. J.; Harrison B. L.; Hoffmann E. C.; Kazdoba T. M. Neuroactive Steroid N-Methyl-d-aspartate Receptor Positive Allosteric Modulators: Synthesis, SAR, and Pharmacological Activity. J. Med. Chem. 2019, 62, 7526–7542. 10.1021/acs.jmedchem.9b00591. [DOI] [PubMed] [Google Scholar]
- Bregman H.; Simard J. R.; Andrews K. L.; Ayube S.; Chen H.; Gunaydin H.; Guzman-Perez A.; Hu J.; Huang L.; Huang X.; Krolikowski P. H. The discovery and hit-to-lead optimization of tricyclic sulfonamides as potent and efficacious potentiators of glycine receptors. J. Med. Chem. 2017, 60, 1105–1125. 10.1021/acs.jmedchem.6b01496. [DOI] [PubMed] [Google Scholar]
- Beshore D. C.; N. Di Marco C.; Chang R. K.; Greshock T. J.; Ma L.; Wittmann M.; Seager M. A.; Koeplinger K. A.; Thompson C. D.; Fuerst J.; Hartman G. D. MK-7622: A first-in-class M1 positive allosteric modulator development candidate. ACS Med. Chem. Lett. 2018, 9, 652–656. 10.1021/acsmedchemlett.8b00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss T.; Li J.; Cummings J.; Farlow M.; Assaid C.; Froman S.; Leibensperger H.; Snow-Adami L.; McMahon K. B.; Egan M.; Michelson D. Randomized, controlled, proof-of-concept trial of MK-7622 in Alzheimer’s disease. Alzheimers Dement (N Y). 2018, 4, 173–181. 10.1016/j.trci.2018.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall A.; Provins L.; Valade A. Novel strategies to activate the dopamine D1 receptor: recent advances in orthosteric agonism and positive allosteric modulation. J. Med. Chem. 2019, 62, 128–140. 10.1021/acs.jmedchem.8b01767. [DOI] [PubMed] [Google Scholar]
- Hao J.; Beck J. P.; Schaus J. M.; Krushinski J. H.; Chen Q.; Beadle C. D.; Vidal P.; Reinhard M. R.; Dressman B. A.; Massey S. M.; Boulet S. L.; et al. Synthesis and Pharmacological Characterization of 2-(2, 6-Dichlorophenyl)-1-((1 S, 3 R)-5-(3-hydroxy-3-methylbutyl)-3-(hydroxymethyl)-1-methyl-3, 4-dihydroisoquinolin-2 (1 H)-yl) ethan-1-one (LY3154207), a Potent, Subtype Selective, and Orally Available Positive Allosteric Modulator of the Human Dopamine D1 Receptor. J. Med. Chem. 2019, 62, 8711–8732. 10.1021/acs.jmedchem.9b01234. [DOI] [PubMed] [Google Scholar]
- Uslaner J. M.; Kuduk S. D.; Wittmann M.; Lange H. S.; Fox S. V.; Min C.; Pajkovic N.; Harris D.; Cilissen C.; Mahon C.; Mostoller K. Preclinical to human translational pharmacology of the novel M1 positive allosteric modulator MK-7622. J. Pharmacol. Exp. Ther. 2018, 365, 556–566. 10.1124/jpet.117.245894. [DOI] [PubMed] [Google Scholar]
- Bernat V.; Admas T. H.; Brox R.; Heinemann F. W.; Tschammer N. Boronic acids as probes for investigation of allosteric modulation of the chemokine receptor CXCR3. ACS Chem. Biol. 2014, 9, 2664–2677. 10.1021/cb500678c. [DOI] [PubMed] [Google Scholar]
- Garcia Fortanet J.; Chen C. H.; Chen Y. N.; Chen Z.; Deng Z.; Firestone B.; Fekkes P.; Fodor M.; Fortin P. D.; Fridrich C.; Grunenfelder D. Allosteric inhibition of SHP2: identification of a potent, selective, and orally efficacious phosphatase inhibitor. J. Med. Chem. 2016, 59, 7773–7782. 10.1021/acs.jmedchem.6b00680. [DOI] [PubMed] [Google Scholar]
- Bagdanoff J. T.; Chen Z.; Acker M.; Chen Y. N.; Chan H.; Dore M.; Firestone B.; Fodor M.; Fortanet J.; Hentemann M.; Kato M. Optimization of Fused Bicyclic Allosteric SHP2 Inhibitors. J. Med. Chem. 2019, 62, 1781–1792. 10.1021/acs.jmedchem.8b01725. [DOI] [PubMed] [Google Scholar]
- Sarver P.; Acker M.; Bagdanoff J. T.; Chen Z.; Chen Y. N.; Chan H.; Firestone B.; Fodor M.; Fortanet J.; Hao H.; Hentemann M. 6-Amino-3-methylpyrimidinones as Potent, Selective, and Orally Efficacious SHP2 Inhibitors. J. Med. Chem. 2019, 62, 1793–1802. 10.1021/acs.jmedchem.8b01726. [DOI] [PubMed] [Google Scholar]
- Murray B. W.; Guo C.; Piraino J.; Westwick J. K.; Zhang C.; Lamerdin J.; Dagostino E.; Knighton D.; Loi C. M.; Zager M.; Kraynov E. Small-molecule p21-activated kinase inhibitor PF-3758309 is a potent inhibitor of oncogenic signaling and tumor growth. Proc. Natl. Acad. Sci. U. S. A. 2010, 107, 9446–9451. 10.1073/pnas.0911863107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan B. M.; Duron S. G.; Campbell D. A.; Vollrath B.; Rao B. S.; Ko H. Y.; Lin G. G.; Govindarajan A.; Choi S. Y.; Tonegawa S. Rescue of fragile X syndrome phenotypes in Fmr1 KO mice by the small-molecule PAK inhibitor FRAX486. Proc. Natl. Acad. Sci. U. S. A. 2013, 110, 5671–5676. 10.1073/pnas.1219383110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licciulli S.; Maksimoska J.; Zhou C.; Troutman S.; Kota S.; Liu Q.; Duron S.; Campbell D.; Chernoff J.; Field J.; Marmorstein R. FRAX597, a small molecule inhibitor of the p21-activated kinases, inhibits tumorigenesis of neurofibromatosis type 2 (NF2)-associated Schwannomas. J. Biol. Chem. 2013, 288, 29105–29114. 10.1074/jbc.M113.510933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobitz H.; Jahnke W.; Cowan-Jacob S. Expanding the opportunities for modulating kinase targets with allosteric approaches. Curr. Top. Med. Chem. 2016, 17, 59–70. 10.2174/1568026616666160719165314. [DOI] [PubMed] [Google Scholar]
- Karpov A. S.; Amiri P.; Bellamacina C.; Bellance M. H.; Breitenstein W.; Daniel D.; Denay R.; Fabbro D.; Fernandez C.; Galuba I.; Guerro-Lagasse S. Optimization of a dibenzodiazepine hit to a potent and selective allosteric PAK1 inhibitor. ACS Med. Chem. Lett. 2015, 6, 776–81. 10.1021/acsmedchemlett.5b00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer F. A.; Leijten-van de Gevel I. A.; de Vries R. M.; Brunsveld L. Allosteric small molecule modulators of nuclear receptors. Mol. Cell. Endocrinol. 2019, 485, 20–34. 10.1016/j.mce.2019.01.022. [DOI] [PubMed] [Google Scholar]
- Scheepstra M.; Leysen S.; Van Almen G. C.; Miller J. R.; Piesvaux J.; Kutilek V.; Van Eenennaam H.; Zhang H.; Barr K.; Nagpal S.; Soisson S. M. Identification of an allosteric binding site for RORγt inhibition. Nat. Commun. 2015, 6, 8833. 10.1038/ncomms9833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauber B. P.; Gobbi A.; Robarge K.; Zhou A.; Deng Y.; Eidenschenk C.; Everett C.; Johnson A. R.; La H.; Ouyang W.; Wong H.; Barnard A.; Ganguli A.; Hawkins J.; Norman M.; Salmon G.; Summerhill S.; Cao J.; Tang W. Discovery of imidazo[1,5-a]pyridines and -pyrimidines as potent and selective RORc inverse agonists. Bioorg. Med. Chem. Lett. 2015, 25, 2907–2912. 10.1016/j.bmcl.2015.05.055. [DOI] [PubMed] [Google Scholar]
- Chaudhari S. S.; Thomas A.; Dhone S. V.; Khairatkar-Josh N.; Bajpai M.. Bicyclic heterocyclic compounds as ROR gamma modulators. PCT Int. Appl. WO2015/008234.
- Lu S.; Zhang J. Designed covalent allosteric modulators: an emerging paradigm in drug discovery. Drug Discovery Today 2017, 22, 447–453. 10.1016/j.drudis.2016.11.013. [DOI] [PubMed] [Google Scholar]
- Uhlenbrock N.; Smith S.; Weisner J.; Landel I.; Lindemann M.; Le T. A.; Hardick J.; Gontla R.; Scheinpflug R.; Czodrowski P.; Janning P. Structural and chemical insights into the covalent-allosteric inhibition of the protein kinase Akt. Chem. Sci. 2019, 10, 3573–3585. 10.1039/C8SC05212C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z.; Robinson R. G.; Fu S.; Barnett S. F.; Defeo-Jones D.; Jones R. E.; Kral A. M.; Huber H. E.; Kohl N. E.; Hartman G. D.; Bilodeau M. T. Rapid assembly of diverse and potent allosteric Akt inhibitors. Bioorg. Med. Chem. Lett. 2008, 18, 2211–2214. 10.1016/j.bmcl.2007.10.023. [DOI] [PubMed] [Google Scholar]
- Weisner J.; Gontla R.; van der Westhuizen L.; Oeck S.; Ketzer J.; Janning P.; Richters A.; Mühlenberg T.; Fang Z.; Taher A.; Jendrossek V. Covalent-allosteric kinase inhibitors. Angew. Chem., Int. Ed. 2015, 54, 10313–10316. 10.1002/anie.201502142. [DOI] [PubMed] [Google Scholar]
- Smith T. H.; Sim-Selley L. J.; Selley D. E. Cannabinoid CB1 receptor-interacting proteins: novel targets for central nervous system drug discovery?. Br. J. Pharmacol. 2010, 160, 454–466. 10.1111/j.1476-5381.2010.00777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn K. H.; Mahmoud M. M.; Kendall D. A. Allosteric modulator ORG27569 induces CB1 cannabinoid receptor high affinity agonist binding state, receptor internalization, and Gi protein-independent ERK1/2 kinase activation. J. Biol. Chem. 2012, 287, 12070–12082. 10.1074/jbc.M111.316463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni P. M.; Kulkarni A. R.; Korde A.; Tichkule R. B.; Laprairie R. B.; Denovan-Wright E. M.; Zhou H.; Janero D. R.; Zvonok N.; Makriyannis A.; Cascio M. G. Novel electrophilic and photoaffinity covalent probes for mapping the cannabinoid 1 receptor allosteric site (s). J. Med. Chem. 2016, 59, 44–60. 10.1021/acs.jmedchem.5b01303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai A. C.; Crews C. M. Induced protein degradation: an emerging drug discovery paradigm. Nat. Rev. Drug Discovery 2017, 16, 101–114. 10.1038/nrd.2016.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benchekroun M. The advent of directed protein degraders in drug discovery. Future Drug. Discovery 2019, 1, FDD16. 10.4155/fdd-2019-0019. [DOI] [Google Scholar]
- Shimokawa K.; Shibata N.; Sameshima T.; Miyamoto N.; Ujikawa O.; Nara H.; Ohoka N.; Hattori T.; Cho N.; Naito M. Targeting the allosteric site of oncoprotein BCR-ABL as an alternative strategy for effective target protein degradation. ACS Med. Chem. Lett. 2017, 8, 1042–1047. 10.1021/acsmedchemlett.7b00247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J.; Mulumba M.; Ong H.; Lubell W. D. Diversity-Oriented Synthesis of Cyclic Azapeptides by A3-Macrocyclization Provides High-Affinity CD36-Modulating Peptidomimetics. Angew. Chem., Int. Ed. 2017, 56, 6284–6288. 10.1002/anie.201611685. [DOI] [PubMed] [Google Scholar]
- Danelius E.; Ohm R. G.; Ahsanullah A.; Mulumba M.; Ong H.; Chemtob S.; Erdélyi M.; Lubell W. D. Dynamic Chirality in the Mechanism of Action of Allosteric CD36 Modulators of Macrophage-Driven Inflammation. J. Med. Chem. 2019, 62, 11071–11079. 10.1021/acs.jmedchem.9b00918. [DOI] [PubMed] [Google Scholar]; Dynamic Chirality in the Mechanism of Action of Allosteric CD36 Modulators of Macrophage-Driven Inflammation.
- Corbin J. A.; Bhaskar V.; Goldfine I. D.; Bedinger D. H.; Lau A.; Michelson K.; Gross L. M.; Maddux B. A.; Kuan H. F.; Tran C.; Lao L. Improved glucose metabolism in vitro and in vivo by an allosteric monoclonal antibody that increases insulin receptor binding affinity. PLoS One 2014, 9, e88684. 10.1371/journal.pone.0088684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K. W.; Neale A.; Gordon A.; Roessig J.; Bezwada P.; Vukelich S.; Goldfine I.; Rubin P. Attenuation of insulin action by an allosteric insulin receptor antibody in healthy volunteers. J. Clin. Endocrinol. Metab. 2017, 102, 3021–3028. 10.1210/jc.2017-00822. [DOI] [PubMed] [Google Scholar]