Important Compound Classes
Title
Small Molecule Inhibitors of DYRK1/CLK and Uses Thereof
Patent Publication Number
WO 2020/069418 A1
Publication Date
April 02, 2020
Priority Application
US 62/738,540
Priority Date
September 28, 2018
Inventors
Hulme, C.; Foley, C.
Assignee Company
Arizona Board of Regents on Behalf of the University of Arizona; University Services Annex, 4th Floor, P.O. Box 210300A, Tucson, Arizona 85721, USA.
Disease Area
Cancer
Biological Target
Dual specificity tyrosine phosphorylation regulated kinase-1 A (DYRK1A).
Summary
Alzheimer’s disease (AD) is the primary cause of dementia in the elderly, and the incidence of AD reaches nearly 40% in patients 85 years of age and older. According to the National Institute of Health, in most people with AD, symptoms first appear in their mid-60s. Estimates vary, but experts suggest that more than 5.5 million Americans, most of them age 65 or older, may have dementia caused by AD (https://www.nia.nih.gov/health/alzheimers-disease-fact-sheet, accessed 2020-04-06).
This neurodegenerative disorder is characterized by neuronal death, loss of gray matter in the frontal cortex and hippocampus, accumulation of amyloid plaques, and neurofibrillary tangles (NFTs), which may lead to memory loss and dementia. NFTs are thought to be mediated by excessive phosphorylation of tau proteins that are inactive and would form multiple aggregates. Under normal circumstances, tau proteins are cytoplasmic proteins involved in the stabilization of microtubules. Another hypothesis called the β-amyloid cascade hypothesis indicates that the deposition of insoluble β-amyloid plaques is the cause for the neuronal death seen in patients with AD. However, the buildup of NFTs and the insoluble hyperphosphorylated tau proteins is said to be the cause for neuronal death. Nonetheless, the etiology and neuronal pathology are highly dependent on the dual specificity tyrosine phosphorylation regulated kinase-1 A (DYRK1A), which regulates neuronal differentiation, cell cycle, and synaptic transmission. DYRKs are members of another set of families of the CMGC (CDK, MAPK, GSK3 and CLK) group of kinases, which are characterized by their dual specificity in autophosphorylation activity and serine/threonine kinase activity. The DYRK subfamily comprises the YAKs and class I (DYRK1A and DYRK1B) and class II (DYRK2, DYRK3 and DYRK4) DYRKs, and the DYRKs are multifaceted kinases, regulating a great variety of cellular processes including proteasomal degradation, gene expression, and chromatin remodelling.
The early onset of AD in Down syndrome (DS) individuals has been linked to DYRK1A overexpression and histopathological features found in the brains of AD patients, including extracellular β-amyloid plaques and hyperphosphorylated tau protein. Increased DYRK1A levels are present in the brains of patients not only with AD but as well as other neurodegenerative diseases such as Huntington, Parkinson, and Pick syndromes. In addition, DYRK1A increases the secretase-mediated cleavage of amyloid precursor protein (APP) into Aβ peptides and also phosphorylates APP directly. DYRK1A is important in neuronal development and plays a variety of functional roles within the adult central nervous system, and its overexpression may be a significant factor leading to cognitive deficits in patients with AD and DS. Given the central role of DYRK1A in the development and progression of AD, DYRK1A has emerged as a high priority target for inhibition or degradation, offering a novel approach for the treatment of AD. There are reports which show evidence that DYRK1A plays a role in diabetes and β-cell proliferation.
Limited treatment options exist for cognitive deficiencies associated with DS and AD. Consequently, small molecule inhibition or the degradation of DYRK1A activity in the brain may provide pharmaceutical intervention in mental impairment associated with AD and many other neurodegenerative diseases. Currently, inhibition of excess DYRK1A activity with natural products epigallocatechin-3-gallate (EGCg) and harmine has been shown to improve cognitive deficits. However, these drugs are not significantly selective and have numerous off-target activities that compromise their utility.
This Patent Highlight shows knowledge-based design efforts that led to novel small molecule series of structurally unique 6,5-heterocyclic DYRK1A inhibitors, which may be beneficial as cognitive enhancers and to treat a variety of disease states including Parkinson’s disease, glioblastomas, Pick’s disease, Huntington’s disease, and additional tauopathies.
Definitions
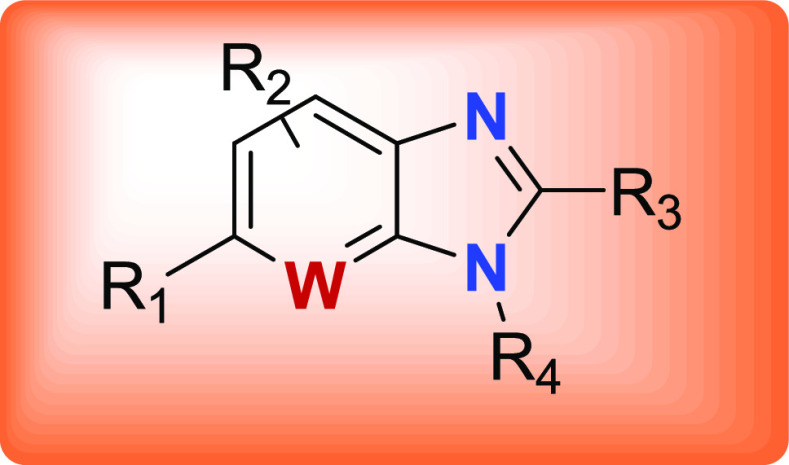
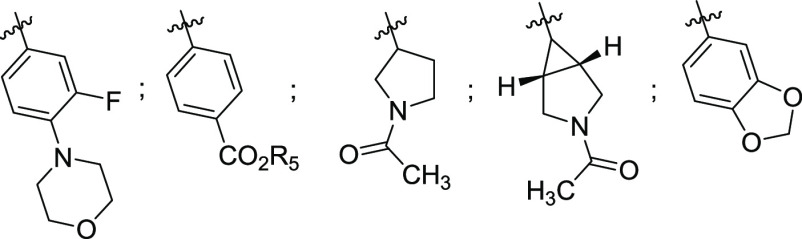
R1 = H, F, heterocycle, alkyl, and so forth,
R2 = H, F,
R3 = H, CH3, N, isopropyl, etc.,
W = N, CH, CF,
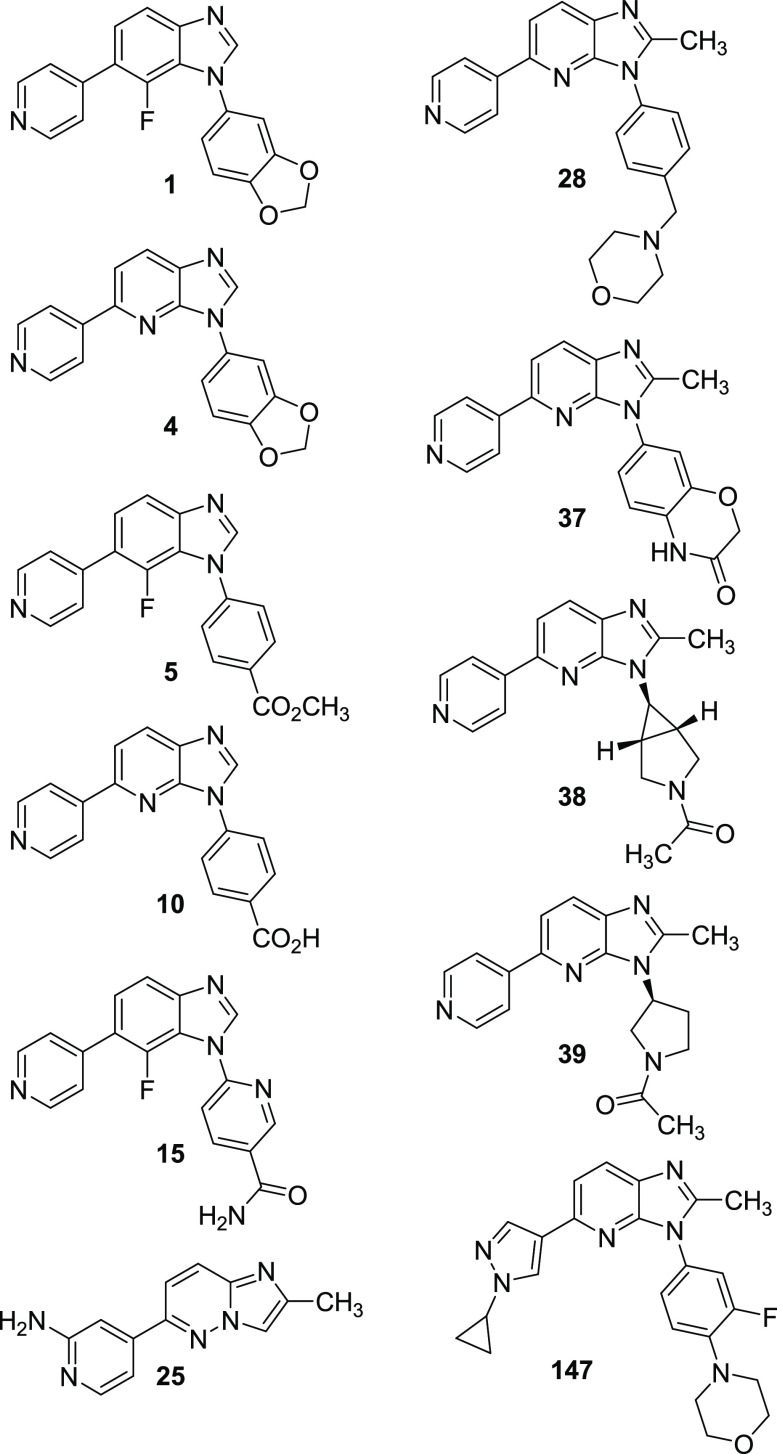
Key Structures
Biological Data
The table below
shows the results of
affinity testing of the compounds of representative compounds with
DYRK1A [DYRK1A affinity Key (KD)], where + + + = 0.5–100
nM, + + = 101–500 nM.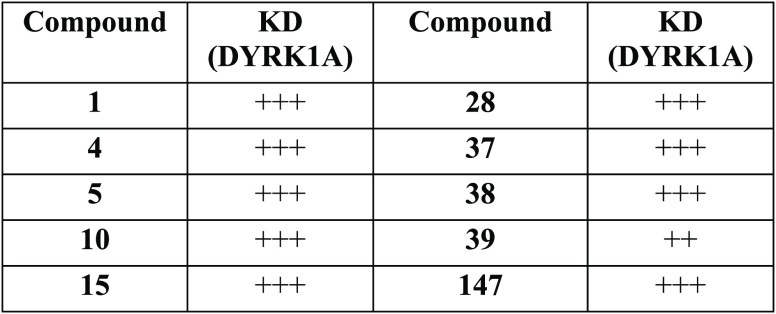
Recent Review Articles
-
1.
Scavuzzo M. A.; Borowiak M.. Sci. Transl. Med. 2020, 12, 7359..
-
2.
Arbones M. L.; Thomazeau A.; Nakano-Kobayashi A.; Hagiwara M.; Delabar J. M.. Pharmacol. Ther. 2019, 194, 199.
-
3.
Jarhad D. B.; Mashelkar K. K.; Kim H.; Noh M.; Jeong L. S.. J. Med. Chem. 2018, 61, 9791.
-
4.
Pathak A.; Rohilla A.; Gupta T.; Akhtar M. J.; Haider M. R.; Sharma K.; Haider K.; Yar M. S.. Eur. J. Med. Chem. 2018, 158, 559.
-
5.
Feki A.; Hibaoui Y.. Brain Sci. 2018, 8, 187.
-
6.
Rad S. K.; Arya A.; Karimian H.; Arya A.; Arya A.; Madhavan P.; Rizwan F.; Koshy S.; Prabhu G.. Drug Des. Dev. Ther. 2018, 12, 3999..
The author declares no competing financial interest.