Abstract
Introduction
This review aims at describing different types of hydrogels in context to their composition, fabrication techniques and other specific features along with an insight into the latest advancements including smart hydrogels, 3D printed, programmable, shape memory and self-healing hydrogels for their applicability as scaffold in maxillofacial bone and cartilage tissue regeneration.
Methods
Electronic database searches were undertaken on PubMed, Ovid, Medline, Embase, ProQuest and science direct for English language literature, published for application of hydrogels in maxillofacial bone and cartilage tissue engineering. The search items used in this article were hydrogel, bone and cartilage tissue engineering, maxillofacial, clinical trials. Reviews and in vitro studies were excluded.
Results
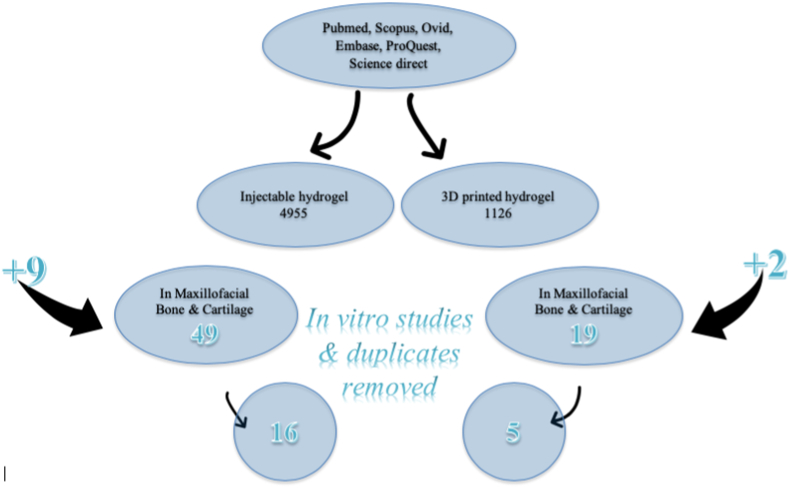
Search for injectable hydrogel showed 4955 articles, when restricted to bone tissue engineering results were reduced to 463 and for cartilage engineering to 335; when we limited it to maxillofacial bone and cartilage tissue engineering, search results showed 49 articles to which 9 additional articles were included from references, after exclusion of in-vitro studies and duplicates 16 articles were obtained for our study. Similarly, for 3D printed hydrogels, result showed 1126 articles, which got restricted to 19 when searched for maxillofacial bone and cartilage engineering, then 2 additional articles were included directly from references, and finally after exclusion of the invitro studies and duplicates, a total of 5 articles were obtained.
Conclusion
Modifications in hydrogel can improve the mechanical properties, biocompatibility and unique chemistries for its use in bone and cartilage tissue engineering for future research
Keywords: Injectable hydrogels, Maxillofacial tissue engineering, Smart hydrogels
1. Introduction
Hydrogels are hydrophilic polymer networks formed through crosslinking of monomer or polymer chains via covalent and/or noncovalent interactions.1 They are smart biomaterials which have gathered significant appeal to the biomedical researchers due to the advancements in terms of their fabrication and applications. Hydrogels have existed for a long time as conjoined hydroxyethyl methacrylate hydrogels and in current times they serve as biomaterial of choice for fabrication of scaffold in bone and cartilage tissue engineering. Hydrogel based scaffolds are self-sufficient, three dimensional structures exhibiting viscous as well as elastic properties to allow for dissemination and attachment of molecules and cells.
The various biomedical applications where hydrogels have been successfully used includes cell therapeutics, wound healing, scaffolds for cartilage or bone regeneration and for sustained release of drugs. Hydrogel based tissue engineering have traversed through a series of advancements with latest being injectable hydrogel and 3D printed hydrogels.
This study aims to provide an overview of recent designs of hydrogels particularly injectable hydrogels and 3D printed hydrogels along with their pros and cons in context to their applicability in maxillofacial bone and cartilage tissue engineering so as to identify the knowledge gap in order to direct future research.
2. Method of data collection
Electronic database searches were undertaken on PubMed, Ovid, Medline, Embase, ProQuest and science direct for English language literature, published for application of hydrogels in maxillofacial bone and cartilage tissue engineering. The search items used in this article were hydrogel, bone and cartilage tissue engineering, maxillofacial, clinical trials. Reviews and in vitro studies were excluded.
Search for (hydrogel* OR “hydrogel scaffold”) showed 37,091 results, when it was limited to injectable, it was reduced to 4955 articles. Fig. 1. Although the search results for bone tissue engineering were reduced to 463 and for cartilage engineering to 335; when we limited it to maxillofacial bone and cartilage tissue engineering, search results showed 49 articles to which 9 additional articles were included from references, but when in-vitro studies and duplicates were removed, 16 articles were obtained for our study (Table 1).
Fig. 1.
Search criteria.
Table 1.
Details of studies employing different injectable hydrogels and their outcomes.
| S No | Author | Composition | Tissue | Study Model | Outcomes |
|---|---|---|---|---|---|
| 1 | Sanz26 2012 | Hyaluronic acid‐based hydrogels containing nanohydroxyapatite and different concentrations of BMP‐2 (0, 5 and 150 μg/ml) | Bone | 28 rats mandibular bone | -Significant increase in mandibular bone volume correlated with the amount of BMP‐2 loaded in hydrogel |
| 2 | Cao84 2012 | NGF-carrying collagen/nano-hydroxyapatite/alginate hydrogel | Bone | 35 New Zealand white rabbits | -Col/nHA/Alg hydrogel as an NGF delivery during the consolidation phase of distraction osteogenesis increased regeneration and new bone formation |
| 3 | Kim85 2013 | Collagen sponge with hBMSCs and hydrogel in a polycaprolactone outer box | Bone | rabbit mandibular defect | -Collagen sponge, hydrogel, and rhBMP-2 effective for bone large mandibular defects |
| 4 | Jo86 2015 | rhBMP2 with hydrogel | Bone | 4 beagle dogs mandibular alveolar ridges | |
| 5 | Song20 2017 | Calcium phosphate cement with cell-encapsulating hydrogel microfibers of partially-oxidized alginate with various concentrations (0–0.8%) of fibrinogen (Alg-Fb MF). | Bone | Rat mandibular defect | −0.4% fibrinogen most enhanced cell migration, release and proliferation -new bone area fraction of (42.1 ± 7.8) % in the defects > 3-fold |
| 6 | Seo87 2018 | In situ gelling polymer solutions with or without BMP-2 | Bone | 3 young male beagle dogs mandible | -significantly increased bone to implant contact vertical bone, higher osseointegration |
| 7 | Fahmy-Garcia71 2018 | Collagen-I based Recombinant Peptide microspheres dispersed in three hydrogels: high mannuronate (SLM) alginate, high guluronate (SLG) alginate, hyaluronan derivative (HApN) | Bone | subcutaneously in rats | -SLM, SLG loaded with BMP-2 induce ectopic bone formation -SLG alginate best for bone formation de novo |
| 8 | Jung68 2018 | Multivalent ion-based in situ gelling polysaccharide hydrogel | Bone | mandibular defect model of miniature pigs | -Sustained release of BMP-2 -Improved osteogenic differentiation -Enhanced Bone regeneration |
| 9 | Jung70 2019 | In situ gelling alginate (ALG)/hyaluronic acid (HA) hydrogel containing vancomycin (antibiotic) and bone morphogenetic protein-2 (BMP-2; growth factor) | Bone | osteomyelitis rat model | -Effective anti-bacterial activity without significant cytotoxicity -Enhanced bone regeneration |
| 10 | Lei88 2019 | Silica nanoparticle-embedded core–shell structured poly (ethyleneglycol)-b-poly (lactic-co-glycolicacid)-b-poly (N-isopropylacrylamide hydrogel | Bone | rat mandibular bone defect | -neurogenesis and enhanced bone formation. |
| 11 | Imada89 2019 | bFGF-containing gelatin hydrogel | Bone | 43 rats | -promoted socket healing |
| 12 | Pan90 2020 | Composite scaffold of hydrogel/hydroxyapatite | Bone | mandibular incisors of rats following tooth extraction | -new bone area enhanced >50%, alveolar ridge >60% after 4 weeks -promoted bone and soft tissue |
| 13. | Wang91 2020 | CS scaffolds containing adipose-derived stem cell, vascular endothelial growth factor, and bone morphogenetic protein-2-loaded nanoHA/poly lactic-co-glycolic acid microspheres. | Bone | critical-sized mandibular bone defects in rabbits | -BMP-2/VEGF demonstrated greater new bone formation, faster healing, and better callus remodelling |
| 14 | Chen8 2016 | Carboxymethylated pullulan/chondroitin sulfate hydrogel | Cartilage | mouse subcutaneous | -Good cyto compatibility -Facilitates chondrogenesis -Acceptable bio-compatibility |
| 15. | Chen60 2017 | Hyaluronic acid/RGD-functionalized pectin hydrogel | Cartilage | mouse subcutaneous | -Supports, facilitates chondrogenesis -Optimal biocompatibility |
| 16 | Zhong92 2020 | Decellularized meniscus extracellular matrix (mECM) for in situ delivery of rat BMSCs | Cartilage | meniscal defect in a SD rat model. | - Promotes chondrogenic/fibrochondrogenic differentiation -Reduction of osteophyte formation prevention of joint space narrowing |
Similarly, for 3D printed hydrogels, result showed 1126 articles, which got restricted to 19 when searched for maxillofacial bone and cartilage engineering, then 2 additional articles were included directly from references, and finally after exclusion of the in-vitro studies and duplicates, a total of 5 articles were obtained and are discussed in Table 2.
Table 2.
Details of studies employing different 3D printed hydrogels and their outcomes.
| S No | Author | Composition | Tissue | Study Model | Outcomes |
|---|---|---|---|---|---|
| 1 | Cui93 2019 | Tough polyion complex hydrogel was synthesized with multiwalled carbon nanotubes with rat bone marrow-derived mesenchymal stem cells | Bone | calvarial defect of Sprague-Dawley rats | -exhibited good biocompatibility and facilitated osteogenic differentiation of rBMSCs. |
| 2 | Visscher78 2019 | Hybrid alginate hydrogel/poly (ε-caprolactone) | Auricular cartilage | -High cell survival -Improved mechanical strength -Enhanced chondrogenesis |
|
| 3 | Zhou94 2019 | Alpha-beta titanium alloy (Ti6Al4V) with Cell matrix hydrogel and adipose derived stem cells | Bone | full-thickness mandibular defect rat | -enhanced osteogenic differentiation |
| 4 | Zhang95 2020 | PCL/hydrogel composite scaffolds loaded with dual bioactive small molecules (resveratrol and strontium ranelate) | Bone | rat model with a critical-sized mandibular bone defect | -Enhanced angiogenesis and inhibited osteoclast activities, -Synergistically promoted MSC osteogenic differentiation |
| 5 | Kuss96 2020 |
Polycaprolactone/hydroxyapatite scaffolds coated with Human adipose derived mesenchymal stem cells and human umbilical vein endothelial cells encapsulated within bioactive hydrogels | subcutaneously implanted into nude mice | -Facilitated microvessel and lumen formation and promoted anastomosis of vascular networks of human origin with host murine vasculature |
2.1. Injectable hydrogels
Modern day biomedical researchers are directing their research to develop injectable hydrogels for application and role in phototherapy, angiogenesis, osteogenic processes and bone remodelling. Injectable hydrogels have proven a great potential as scaffolds for use in three-dimensional cell culture in tissue engineering, as they can retain water, are similar to extracellular matrix (ECM), have a porous framework to allow for cell transplantation and proliferation, minimal invasive properties, can deliver nutrients in a controlled manner and can match any irregular defect.2
The progress in the fabrication of hydrogels has led to an innovative class of materials called “smart injectable hydrogels” that have tunable properties controlled by specific stimuli.3
2.2. Types of injectable hydrogel
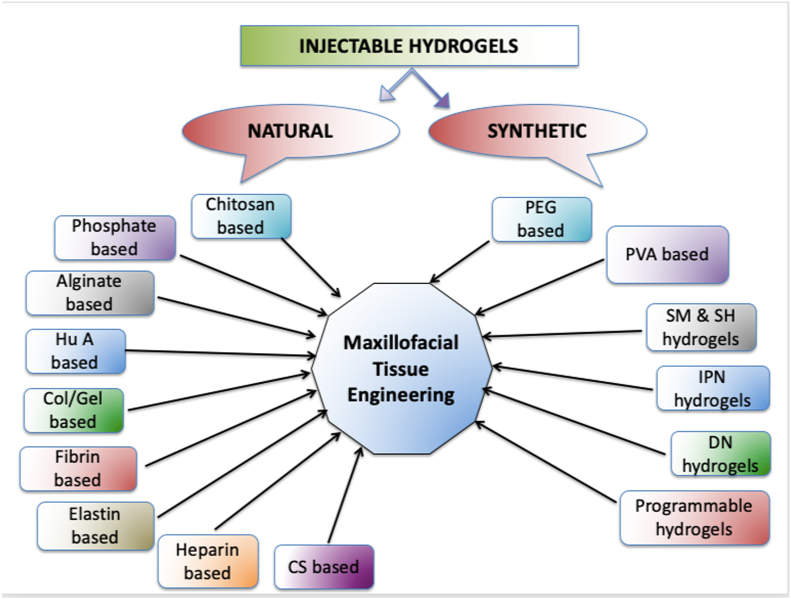
Injectable hydrogels are being prepared using both natural and synthetic biomaterials including chitosan, collagen or gelatin, alginate, hyaluronic acid, heparin, chondroitin sulfate, poly ethylene glycol (PEG), and poly vinyl alcohol (PVA).
Injectable hydrogels can be fabricated through physical methods involving weak secondary forces, chemical methods involving covalent cross-linking enzymatically, photo-cross-linking, Schiff base cross-linking, Michael addition or click chemistry-mediated to design and produce ion-sensitive, pH-sensitive, or temperature-sensitive hydrogels.3 Fig. 2.
Fig. 2.
Types of injectable hydrogels.
2.3. Natural hydrogels
Natural hydrogels embodies bioactive components within itself to improve cell recognition, adhesion, proliferation, and biological repair. Such hydrogels are usually based on polysaccharides such as alginate, agarose, chitosan, and hyaluronic acid (HA), or proteins such as collagen, gelatin, and fibrin. Although they closely simulate the biological properties of the ECM, their main drawbacks include poor mechanical properties, fast and unpredictable degradation under biological conditions depending upon individual characteristics, enzyme levels, and site where they have been injected.3 However if they can be modified chemically with addition of specific macromolecules, these limitations could be overcome by improved mechanical properties and tuned biodegradability. In a nutshell, the basic challenge with injectable hydrogels is to achieve fast gelation, good mechanical strength, and cytocompatibility, therefore all the modifications in composition and fabrication methodology revolves around achieving these goals.
2.4. Chitosan based hydrogels
Chitosan is one of the most abundant polymers and derived by alkaline deacetylation of chitin in the exoskeleton of crustaceans.2 It was observed that the electrostatic interactions between chitosan and gelatin, can improve the mechanical properties of the hydrogel. Lavanya et al. (2020) demonstrated fabrication of thermo/pH-responsive injectable hydrogel from this natural biopolymer chitosan.4 Another injectable chitosan-based-hydrogel has been prepared by conjugating chitosan with carboxymethyl chloride and α-cyclodextrin, to show improved water solubility and crosslinking with polyethylene glycol (PEG).5
Cui et al., 2019 fabricated a nanoengineered photo cross-linkable in situ-forming hydrogel comprising chitosan and 2D nano-silicates. These reinforced hydrogels promoted proliferation, attachment and differentiation of stem cells in vitro and calvarial healing in vivo. They exhibited increase in Young's modulus and decrease in its degradation rate.6 Zhang et al. also fabricated supramolecular hydrogels based on nano clay and guanidine-rich chitosan to be used as injectable and moldable osteo-inductive carriers.7
Chen et al. investigated thermo-sensitive chitosan/β-glycerophosphate/hydroxyapatite (Ch/GP/HA) hydrogels prepared using sol-gel method, for osteogenic differentiation of stem cells.8 These stem cells had a higher alkaline phosphatase (ALP) activity and showed better up-regulation of gene expression of Runx-2, Col I, ALP and osteocalcin (OCN). Saravanan also observed that the injectable chitosan/beta glycerophosphate hydrogels had a good potential for use in bone tissue regeneration.9
In order to fulfil the basic requisites of fast gelation, good mechanical strength, and cytocompatibility, Wasupalli et al., 2020 developed polyelectrolyte complex fiber-reinforced HA chitosan composite hydrogels that exhibited enhanced mechanical strength, reduced gel time, and excellent cytocompatibility.10
Further studies including Chitosan with GP in the ratio (9:1, v/v) with nano-HA, exhibited formation of a thermosensitive hydrogel in-situ which possessed the most sustained drug release profile.11 Addition of nHA in the hydrogel enhanced swelling, protein adsorption, osteoblast differentiation in vitro and accelerated bone formation in vivo with deposition of apatite and collagen.12
A chitin-CaSO4-nano-fibrin based injectable shear thin hydrogel showed an improved angiogenic potential with elastic modulus increased 1.67 times more than the control.13 Similarly, chitin-polylactic glycolic acid (PLGA) hydrogel with CaSO4/FGF-18 showed enhanced bone regeneration.14
2.5. Phosphate based hydrogels
Hydrogels act as binders for the inorganic minerals, modulate cell colonization, and influence the tissue healing through their ability to transport drugs and cells.15
When hydrogels with calcium phosphate (CP) granules were studied in femoral epiphysis defects in rabbits, they demonstrated their role as a carrier, aiding in cell colonization, exhibiting slow degradation, and faster permeability for tissue ingrowth between the granules.16 Safwat et al. developed an injectable nano-fibrillated cellulose/biphasic CP hydrogel for use in bone regeneration.17
Production of injectable, biodegradable, dual-gelling macromer with stem cells within hydrogels by incorporating phosphate groups improved bio-integration and facilitated its degradation.18 Chen et al. also incorporated biphasic CP microparticles in injectable thermo-responsive hydrogel to modulate bone cell proliferation and differentiation.19 Another injectable CP hydrogel containing stem cells and microfibers with varying concentrations (0–0.8%) of fibrinogen, was developed to achieve desirable degradability, deliver cells timely and maintain cell viability.20 It was observed that when the porosity of injectable hydrogel was 62%, its injection did not harm cell viability, and all the types of cells could proliferate and differentiate to their osteogenic lineage.21 It was demonstrated that an injectable hydrogel can be tuned as required, through crystallization of the sodium-magnesium-phosphate nanosheets, to accelerate bone healing and osseointegration.22
2.6. Alginate based (Alg) hydrogels
Alginate polysaccharide is obtained from brown algae and is one of the most used materials for cartilage tissue engineering as it is non-immunogenic as well as non-toxic. However, it is not strong enough to maintain the structure and shape and also lacks cell adhesion ability, therefore needs to be modified so as to improve its mechanical properties. A mechanically strong injectable hydrogel prepared from calcium phosphate–alginate cement was found to support adhesion, proliferation and differentiation of angiogenic and osteogenic cells.23, 24
Another injectable, biodegradable, oxidized Alg/hyaluronic acid hydrogel with primary chondrocytes, 6 weeks after implantation in mice, yielded effective cartilage regeneration. In another study, biocompatible and biodegradable hydrogel prepared by blending Alg and chitosan with fibrin nanoparticles, showed improved swelling ratio, degradation profile, compressive strength, and elastic module.3 An injectable alginate-based adhesive, photocross linked hydrogel with tunable mechanical properties, prepared for stem cell delivery, was subcutaneously implanted in mice peri-implantitis to confirm the biodegradability, biocompatibility, and osteo conductivity with complete bone regeneration around ailing dental implants with peri-implant bone loss.25
2.7. Hyaluronic acid (HuA) based hydrogels
HuA is a component of the ECM of an adult cartilage that interacts with chondrocytes via surface receptors CD44, and promotes chondrogenic differentiation. HuA hydrogels form the natural hydrated media for cells to grow and differentiate. However, the biomechanical stability of HuA hydrogels is low; so have to be used with strong polymers to improve their mechanical properties.2 Research has shown them to transport bone morphogenetic protein-2 (BMP-2) and their subperiosteal injection in rats demonstrated good bone augmentation when it incorporated nano-HA and BMP-2 while use.26
HuA/type I collagen hydrogel stimulated chondrocyte proliferation and enhanced proteoglycan synthesis.27 When fibrin was mixed with high molecular weight (MW) HuA, it promoted chondrocytic differentiation in vitro, while the presence of collagen changed the viscoelasticity and improved the biomechanical properties of the hydrogel (25% higher Young's modulus). As the risks related to the use of collagen are high, a fibrin-based hydrogel was developed in combination with high-MW HuA, with properties appropriate for implantation, promoted ECM production and cell delivery capabilities.2
HuA/PEG hydrogel with better chondrogenic capabilities was synthesized with stem cells exhibiting higher viability and proliferation. Chitosan has excellent features such as biocompatibility, structural resemblance to glycosaminoglycan, and easily forms ionic complexes, so Park and colleagues30 designed a HuA hydrogel with methacrylated glycol chitosan and loaded it with chondrocytes, to allow their proliferation with increased deposition of cartilaginous ECM.
HuA is mechanical unstable and undergoes rapid hydrolytic degradation, hence a chemically modified in situ photo-cross-linkable HuA scaffold was used for articular cartilage repair with chondrocytes and evaluated in vitro for deposition of cartilaginous matrix.31 3D printed methacrylated HuA, porous and custom made scaffolds have also been used for chondrogenic differentiation. In hydrogels containing higher concentration of methacrylated HuA polymer, stem cells could differentiate without any osteogenic stimulus. Maleated HuA, a photo-cross-linkable system, was also assessed for in situ biomineralization. Nano-composite hydrogels, with improved properties for soft/hard tissue regeneration, embedded with bovine chondrocytes, have demonstrated viability and proliferation of cells.32 HuA hydrogel with cellulose nanocrystals and stem cells also has shown good cell proliferation and spreading within the gel.33
2.8. Collagen (Col) or gelatin (gel) based hydrogels
Gelatin, a natural protein obtained by degradation of collagen, has a high biodegradation rate, poor mechanical stability and high solubility. However, when chemical strategies like amino gelatin, oxidized dextran, and four-arm PEG-acrylate are employed, the resulting hydrogel exhibits better mechanical properties, biodegradability, and biocompatibility.
Type I and type II collagens were embedded with chondrocytes and used in controlled concentration to increase the compressive modulus and cell features, cartilage-specific ECM was secreted.34 Collagen hydrogel conjugating with carbon dot nanoparticles enhanced hydrogel stability and stiffness owing to conjugation with carbon dot nanoparticles and ‘genipin’ crosslinking. This hydrogel presented a 21-fold higher compression modulus and a 39.3% lower degradation rate than the pure collagen hydrogel and induced chondrogenic differentiation of stem cells.35 When enzymatic cross-linkable gelatin and functionalized gold nanoparticles were used to promote osteo-differentiation, phenol crosslinking was found suitable for injectable hydrogels for bone regeneration.36
Gel-methacryloyl (MA) hydrogels cross-linking showed comparable properties as photo-polymerizing Gel-MA. However, the crosslinked Gel-MA system showed lesser adverse effects on viability and metabolic activity of chondrocytes for up to 35 days, and allowed thick (10 mm) homogenous cross-linking density throughout the construct.37 Similarly, when a collagen type II hydrogel laden with chondrocytes was injected into damaged rabbit cartilage, the gel bonded to the adjacent bone and cartilage within minutes, with good cartilage regeneration.38 Type II collagen, HuA, chondrocytes and chondrogenic growth factors, formed an injectable hydrogel capable of forming a stable structure directly in situ for cartilage repair.39 Injectable macroporous gelatin microribbon based hydrogel scaffolds showed enhanced stem cells survival and accelerated bone regeneration after injection in mice.40,41
2.9. Fibrin based hydrogels
Fibrin, a natural protein involved in coagulation mechanism, is commonly employed either alone or in combination to improve cell attachment, proliferation, and differentiation for cartilage regeneration. Injectable fibrin based hydrogel can induce angiogenesis and in situ neovascularization when used with PEG and stem cells. Fibrin hydrogel with cartilage ECM microparticles and transforming growth factor-β 3 (TGF-β 3) has been successfully used for regeneration of auricular cartilage. When used with alginate particles it has shown improved cartilage regeneration in vitro and in vivo.2
A dense fibrin in the hydrogel, limits the cell spreading, proliferation, and ECM production, while a porous and fibrillar structure of alginate/fibrin hydrogel better supports cellular interactions. Its compression modulus is significantly lower than pure fibrin and alginate hydrogels.3
2.10. Elastin based hydrogels
Elastin, present in skin, blood vessels, and lungs, is an insoluble protein that confers elastic properties to natural tissues, and has been widely involved in cartilage tissue engineering.3
When an injectable elastin-based hydrogel with tunable gelation, improved structural stability, and high biocompatibility, is blend with soluble elastin aggregates, chondroitin sulfate, HA, and collagen, the resultant hydrogel is capable of restoring its original dimensions and water content even after following multicycle mechanical compression.42 Heparin based Hydrogels.
Injectable biodegradable hydrogels, allow sustained delivery of therapeutic drugs. A heparin based temperature-sensitive polyε-caprolactone (PCL) was used as a carrier, as they can undergo temperature-induced sol-to-gel transitions in an aqueous solution. Its gelation rate, mechanical strength, and viscosity was tunable by varying the density of PCL copolymers.43
2.11. Chondroitin sulfate (CS) based hydrogels
A highly crosslinked network hydrogel created by chemical crosslinking of CS, casein, and silica nanospheres allowed the hydrogel to be adaptable (faster or slower, as needed) by altering the polymer proportion. These hydrogels were cyto-compatible, and can be highly considered for controlled and sustained drug release purposes, as well as scaffolds.44
An adhesive and self-healing polysaccharide hydrogel was developed with CS and a polymer by UV light irradiation to improve the mechanical strength and tune its compliance and cohesive energy for better adhesion ability, rapid self-healing and good cytocompatibility.45 An injectable enzymatically crosslinked carboxymethylated pullulan/CS hydrogel was successfully used for cartilage regeneration.46
2.12. Synthetic hydrogels
Synthetic hydrogels are programmable and reproducible but lack biodegradability and biological activity. The common synthetic hydrogels include PEG, PVA and polyhydroxyethyl methacrylate (PHEMA) because of their high water holding ability, nontoxicity, and chemical interactions. Synthetic biodegradable hydrogels include copolymers of PEG, PCL and polylactic-co-glycolic acid (PLGA) and have drawn much attention because their properties can be tuned.
Injectable thermosensitive hydrogel of PLGA-PEG containing HA provides enhanced mechanical properties and bioactivity.47 The modulus of the hydrogels enhances in HA-content dependent manner, and the acidic pH of the environment gets neutralized by HA. This composite released a small molecule dye for up to two weeks, and increased release with addition of HA.48 Another injectable cyto-compatible, biodegradable thermo-responsive hydrogel was developed using PCL-PEG-polypropylene glycol PPG copolymer with various PEG:PPG ratios for better cell differentiation.49
A chitin-PLGA composite incorporated CaSO4 and FGF-18 to fabricate a shear-thinning injectable hydrogel for enhanced defect margin adaptability, better handling, and injection into deeper tissues.50 It demonstrated a 7-fold increase in the elastic modulus, in vitro osteogenic differentiation, and enhanced expression of ALP, RUNX2, BMP-2, OCN, OPN.
2.13. Polyethylene glycol (PEG) based hydrogels
Posritong developed a hydrogel containing PEG and 10% gelatin, curable by visible-light and characterized its viscosity, gelation time, swelling, degradation, and drug release behaviour; observed it to resist free flowing before in situ polymerization, making it suitable for use as an injectable carrier.51
2.14. Polyvinyl alcohol (PVA) based hydrogels
PVA hydrogels possess several chemical properties like inertness, stability, biocompatibility, and pH-responsiveness, and hence have been used for biomedical applications in areas of targeted drug delivery and tissue engineering.50 PVA based hydrogel printed by an extrusion 3D machine have shown success in customized repair of artificial cartilage. Introduction of Graphene Oxide–HA caused weakening of inter-molecular hydrogen bonds, reduced entanglement density, and improved dynamic viscosity. The solution exhibited enhanced shear-thinning behaviour, improving the printability and printing accuracy.52
2.15. Other hydrogels
Achieving stem cell differentiation in a three-dimensional microenvironment still remains a challenge.6 An injectable whitlockite nanoparticle hydrogel developed with incorporation of angiogenic drug dimethyloxalylglycine, showed better protein adsorption and expression of RUNX2, COL and OPN.53 Carbonate microparticles containing Mg induced gellan gum hydrogel, demonstrated highest cell growth in hydrogels containing an equimolar Ca:Mg ratio.54
Literature reports novel injectable hydrogels for cartilage and bone tissue engineering.3,55, 56, 57, 58, 59 A soluble, thermally induced, partially cross-linked hydrogel, demonstrated that cross-linking was responsible for gelation, while the other abated viscosity. Heating the solution above the phase transition temperature led to self-assembly of particles into a physical gel.55 These biomaterials if contain cell-targeting signals such as RGD (Arg-Gly-Asp), show better biocompatibility.60 A modified alginate hydrogel was developed using mesoporous silica nanoparticles incorporated with adhesion peptide containing RGD, to promote osteo-differentiation of stem cells. The results suggested that a stimulation in proliferation and osteo-differentiation stages could enhance the survivability, expansion, and osteogenesis.61
In order to overcome the limitations of available natural and synthetic hydrogels, certain chemical modifications were done to develop interpenetrating polymer networks (IPN) hydrogels, double network hydrogels, shape memory and self-healing hydrogels which have improved mechanical properties, better biocompatibility, and unique chemistries for bone and cartilage tissue engineering.
2.16. Shape memory (SM) and self-healing (SH) hydrogels
The drawbacks of injectable hydrogels were related to gelation time, biomechanical compatibility, and tissue-level function. Mechanical damage and cracks in the surrounding tissue didn't allow the injectable hydrogels to perform their function for long time. SM and SH hydrogels represent an innovative approach to prevent such damage and restore the original morphology by maintaining cell viability and tissue function.
SM hydrogels showed improved tensile strength (2.3 MPa). Similarly, self-healing hydrogels showed high tensile strength (0.7–1.7 MPa) and elongation at break (800–900%).3 However, thermally induced SM hydrogels have limited applications in the biomedical field as they use heat to trigger their self-healing properties. Also most of the SM hydrogels are based on co-valent cross-linked polymers that provide only shape memory. Meng and colleagues developed a self-healable SM supramolecular hydrogel using alginate phenylboronic acid (Alg-PBA) and PVA to address these issues.2
2.17. Interpenetrating polymer network (IPN) hydrogels
Semi-IPNs and IPNs have emerged as innovative tissue engineering hydrogels as they combine the favourable properties of each polymeric component of the IPNs or semi-IPNs to form a new material with totally properties. When two layers of the IPN are created, the resultant hydrogel should exhibit improved mechanical properties, allow cell adhesion and proliferation.
A dual-response hydrogel with ceramic additives fabricated in situ through UV-initiated polymerization, exhibited an IPN with ceramics particles dispersed in between the polymer network, and demonstrated high strength, sensitivity to pH and temperature; while adding ceramics did not decrease their stability.63 Others studied these dual-response IPN hydrogels with ceramics by photopolymerization for consolidation of traumatic bone fragments after fracture.64 Other teams evaluated self-healable and pH-sensitive high-strength water-soluble chitosan/PVA chemically cross-linked semi-IPN hydrogel,65 a gelatin and silk fibroin IPN hydrogel,66 and ultrasonic assisted chitosan-gelatin/polyvinyl pyrrolidone IPN hydrogel and found them with better bone engineering properties.67
2.18. Double-network (DN) hydrogels
Double network injectable IPN hydrogels were developed to protect the stem cells during injection, as they are capable of being cross-linked in situ. These hydrogels are developed by using a brittle polyelectrolyte as the first network and a neutral ductile polymer as the second, to enhance fracture energy against mechanical stress.
Jung and colleagues prepared in situ gelling Alg/HA hydrogels to tune the gelling rate using CaSO4 as a crosslinking agent and Na2HPO4 as crosslinking retardation agent for sustained release of BMP-2 in mandibular defect of miniature pigs. They observed that the osteogenic differentiation improved with increased HA composition in the hydrogel.68 Visible light-cured glycol chitosan hydrogels containing BMP-2 and TGF-β1 were found to demonstrate controlled sustained release of growth factors for 30 days, no cytotoxicity against the osteoblast cell line, improved mRNA expressions of ALP, type I collagen, OCN, increased bone volume and bone mineral density in tibia defect sites.69 An in situ gelling Alg/HA hydrogel containing vancomycin and BMP-2 growth factor was found as a therapeutic tool for osteomyelitis management.70
Some researchers used collagen-I based recombinant peptide microspheres as a carrier for BMP-2 in three hydrogels injected subcutaneously in rats, namely high mannuronate alginate, high guluronate alginate, and thermo-responsive hyaluronan derivative, where the latter showed the least release of BMP-2. Vascularization was significantly higher in guluronate alginate and hyaluronan than in mannuronate alginate hydrogel.71
These in situ forming injectable hydrogel allow a better spatial and temporal control, enhance patient compliance and comfort. Carbohydrate polymers are mostly used for manufacturing injectable in situ-forming hydrogels as they are easily available, are biocompatible, have other required physiochemical properties and are present as a modifiable functional moiety.72
2.19. Programmable hydrogels
Programmable hydrogels can change their properties and functions, reversibly and/or sequentially as needed. They are different from the stimulus responsive hydrogels where changes are passive and can't be stopped or reversed once started. For these programmable hydrogels, internal factors are covalent and/or noncovalent interactions and external factors are environmental stimuli required to trigger internal factors. The change of these programmable hydrogels depends on how the polymers chain, their structures, and their functional groups. There are three categories of stimuli including biological (DNA, proteins, enzymes, small metabolites like glucose and ATP), chemical (Reduction-oxidation reactions, ions and pH) and physical (temperature, light, mechanical force, electric or magnetic field) that can induce changes in the programmable hydrogels. With the stimulation, programmable hydrogels can undergo functional changes in dimension, mechanical support, cell attachment and molecular sequestration.1
CRISPR technology, a gene editing tool, developed a group of stimuli-responsive hydrogels by exploiting the program to activate hydrogels to convert the biological information into property changes to allow for a range of in vitro uses in tissue engineering, bioelectronics, and diagnostics.73
2.20. 3D printed hydrogels
3D bioprinting is an emerging modality of tissue engineering where the computer-aided biocompatible structure is built layer-by-layer using bio ink and stem cells cultured in the 3D printed scaffold. Of all the techniques, micro-extrusion technique is popularly employed to fabricate constructs comprising hydrogels encapsulating the chondrocytes or the stem cells. Cell-laden hydrogels can be natural polymers like alginate and collagen or synthetic polymers like Gel MA and polyethylene glycol dimethacrylate.74,75
A composite of chitosan, nano-HA with pre-osteoblast cells was printed successfully with an extruder-based bioprinter, and compared with alginate and alginate-HA hydrogel, the most widely used solution in bioprinting. Chitosan was found superior to alginate in terms of cell proliferation and differentiation.76
3D-printed molds having cryogel/hydrogel with porosities ranging from 79.7 to 87.2% and high interconnectivity, have been used to create patient-specific tissue engineered scaffolds in three cleft craniofacial defects. The cryogels could swell to almost 1500% of their original dry weight while hydrogel swelling could reach 500%, demonstrating the ability to fill a defect site.77
Visscher and colleagues designed and manufactured a hybrid cartilage for ear reconstruction using 3D-printed PCL scaffold with varying porosities, and using alginate as cell carrier. In alginate, 83% chondrocytes survived after 21 days and produced cartilage-like matrix in vitro.78 PCL scaffolds with 600 μm distances between strands exhibited the best mechanical properties but at 300 μm distances were the most optimal for printing.
2.21. Challenges associated with use of injectable hydrogel as scaffold
Despite the several successful clinical trials, there are significant challenges with the hydrogels for their use as scaffold for bone or cartilage tissue engineering, that include lack of appropriate spatial and temporal control leading to poor cell penetration and irregular cell seeding; difficulty in engineering complex tissues with multiple cell types and ECM composition; poor mechanical properties of hydrogels at both macroscopic and microscopic levels, applications limited to only soft and non-load-bearing tissues; and absence of microvasculature that restricts transportation of nutrients and signalling molecules leading to loss of viability and function of the seeded cells.
Appropriate polymer concentration in the hydrogel is very important as its higher concentration improves the mechanical strength and viscosity while a lower concentration improves cell proliferation and differentiation. Addition of stiff thermoplastic polymer such as PCL to cell-laden hydrogels acts as a frame to reinforce the construct by modulating the polymer percentage, as well as attain the equilibrium of the compressive modulus in the desired range for cartilage engineering.79 However, slower rate of degradation of PCL is a potential limitation since the residual PCL may act as a barrier to tissue formation. To overcome this, Visser et al. suggested reduction of the residual PCL by increasing its porosity using melt-electro writing technique with a voltage gated nozzle tip to enable fabrication of very thin (diameter approx. 0.8 μm) PCL with 93–98% porosity, while stiffness and yielding strains remain comparable to native cartilage.80 Another way to minimise residual polymer is to use a polymer with higher degradation rate like poly hydroxymethylglycolide-co-caprolactone or PLGA. But the acidic by-products of PLGA may cause adverse inflammatory responses, and make it a concern for its future applications.81
The strategy of modifying the microenvironment can be a promising approach for craniofacial bone tissue engineering.82 An alginate and matrigel when embedded with bioactive glass microparticles, enhanced the osteogenic differentiation regardless of the decrease in elasticity of the hydrogel.83
3. Conclusion
The research on development, evaluation and characterization of hydrogel and its prospective use as scaffold for cartilage or bone regeneration has gained momentum over the last decade. The main reason to focus on hydrogel as scaffold is mainly because of its superior biocompatibility, innate resemblance to extra cellular matrix, and its potential use in 3D architecture.
In this review, effort has been made to furnish details about the different types of hydrogel, along with various additive components and their advantages, to be used as scaffold for specific application in maxillofacial tissue engineering. The limitations and challenges while using injectable or 3D printed hydrogels as scaffold are also highlighted to direct future research.
Declaration of competing interest
Authors have no conflict of interest to declare.
Acknowledgement
Indian Council of Medical Research (Bioengineering): Biomaterial for designing of mandibular bony scaffold: Evaluation of polymer-ceramic nano composite scaffold with BMP for critical size mandibular defect. (5/3/8/290/2015).
References
- 1.Wang Y. Programmable hydrogels. Biomaterials. 2018;178:663–680. doi: 10.1016/j.biomaterials.2018.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raucci M.G., D'Amora U., Ronca A., Ambrosio L. Injectable functional biomaterials for minimally invasive surgery. Adv Healthc Mater. 2020 Jun 2 doi: 10.1002/adhm.202000349. [DOI] [PubMed] [Google Scholar]
- 3.Liu M., Zeng X., Ma C., Yi H., Ali Z., Mou X., Li S., Deng Y., He N. Injectable hydrogels for cartilage and bone tissue engineering. Bone Res. 2017;5:17014. doi: 10.1038/boneres.2017.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lavanya K., Chandran S.V., Balagangadharan K., Selvamurugan N. Temperature- and pH-responsive chitosan-based injectable hydrogels for bone tissue engineering. Mater Sci Eng C Mater Biol Appl. 2020;111 doi: 10.1016/j.msec.2020.110862. [DOI] [PubMed] [Google Scholar]
- 5.Saekhor K., Udomsinprasert W., Honsawek S., Tachaboonyakiat W. Preparation of an injectable modified chitosan-based hydrogel approaching for bone tissue engineering. Int J Biol Macromol. 2019;123:167–173. doi: 10.1016/j.ijbiomac.2018.11.041. [DOI] [PubMed] [Google Scholar]
- 6.Cui Z.K., Kim S., Baljon J.J., Wu B.M., Aghaloo T., Lee M. Microporous methacrylated glycol chitosan-montmorillonite nanocomposite hydrogel for bone tissue engineering. Nat Commun. 2019;10(1):3523. doi: 10.1038/s41467-019-11511-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang X., Fan J., Lee C.S., Kim S., Chen C., Lee M. Supramolecular hydrogels based on nanoclay and guanidine-rich chitosan. injectable and moldable osteoinductive carriers. ACS Appl Mater Interfaces. 2020;12(14):16088–16096. doi: 10.1021/acsami.0c01241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen Y., Zhang F., Fu Q., Liu Y., Wang Z., Qi N. In vitro proliferation and osteogenic differentiation of human dental pulp stem cells in injectable thermo-sensitive chitosan/β-glycerophosphate/hydroxyapatite hydrogel. J Biomater Appl. 2016;31(3):317–327. doi: 10.1177/0885328216661566. [DOI] [PubMed] [Google Scholar]
- 9.Saravanan S., Vimalraj S., Thanikaivelan P., Banudevi S., Manivasagam G. A review on injectable chitosan/beta glycerophosphate hydrogels for bone tissue regeneration. Int J Biol Macromol. 2019;121:38–54. doi: 10.1016/j.ijbiomac.2018.10.014. [DOI] [PubMed] [Google Scholar]
- 10.Wasupalli G.K., Verma D. Injectable and thermosensitive nanofibrous hydrogel for bone tissue engineering. Mater Sci Eng C Mater Biol Appl. 2020;107 doi: 10.1016/j.msec.2019.110343. [DOI] [PubMed] [Google Scholar]
- 11.Morsi N.M., Nabil Shamma R., Osama Eladawy N., Abdelkhalek A.A. Bioactive injectable triple acting thermosensitive hydrogel enriched with nano-hydroxyapatite for bone regeneration: in-vitro characterization, Saos-2 cell line cell viability and osteogenic markers evaluation. Drug Dev Ind Pharm. 2019;45(5):787–804. doi: 10.1080/03639045.2019.1572184. [DOI] [PubMed] [Google Scholar]
- 12.Dhivya S., Saravanan S., Sastry T.P., Selvamurugan N. Nanohydroxyapatite-reinforced chitosan composite hydrogel for bone tissue repair in vitro and in vivo. J Nanobiotechnol. 2015;13:40. doi: 10.1186/s12951-015-0099-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arun Kumar R., Sivashanmugam A., Deepthi S., Bumgardner J.D., Nair S.V., Jayakumar R. Nano-fibrin stabilized CaSO4 crystals incorporated injectable chitin composite hydrogel for enhanced angiogenesis & osteogenesis. Carbohydr Polym. 2016;140:144–153. doi: 10.1016/j.carbpol.2015.11.074. [DOI] [PubMed] [Google Scholar]
- 14.Sivashanmugam A., Charoenlarp P., Deepthi S., Rajendran A., Nair S.V., Iseki S., Jayakumar R. Injectable shear-thinning CaSO4/FGF-18-incorporated chitin-PLGA hydrogel enhances bone regeneration in mice cranial bone defect model. ACS Appl Mater Interfaces. 2017;9(49):42639–42652. doi: 10.1021/acsami.7b15845. [DOI] [PubMed] [Google Scholar]
- 15.D'Este M., Eglin D. Hydrogels in calcium phosphate moldable and injectable bone substitutes: sticky excipients or advanced 3-D carriers? Acta Biomater. 2013;9(3):5421–5430. doi: 10.1016/j.actbio.2012.11.022. [DOI] [PubMed] [Google Scholar]
- 16.Daculsi G., Uzel A.P., Weiss P., Goyenvalle E., Aguado E. Developments in injectable multiphasic biomaterials. The performance of microporous biphasic calcium phosphate granules and hydrogels. J Mater Sci Mater Med. 2010;21(3):855–861. doi: 10.1007/s10856-009-3914-y. [DOI] [PubMed] [Google Scholar]
- 17.Safwat E., Hassan M.L., Saniour S., Zaki D.Y., Eldeftar M., Saba D., Zazou M. Injectable TEMPO-oxidized nanofibrillated cellulose/biphasic calcium phosphate hydrogel for bone regeneration. J Biomater Appl. 2018;32(10):1371–1381. doi: 10.1177/0885328218763866. [DOI] [PubMed] [Google Scholar]
- 18.Watson B.M., Vo T.N., Tatara A.M., Shah S.R., Scott D.W., Engel P.S., Mikos A.G. Biodegradable, phosphate-containing, dual-gelling macromers for cellular delivery in bone tissue engineering. Biomaterials. 2015;67:286–296. doi: 10.1016/j.biomaterials.2015.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen J.P., Tsai M.J., Liao H.T. Incorporation of biphasic calcium phosphate microparticles in injectable thermoresponsive hydrogel modulates bone cell proliferation and differentiation. Colloids Surf B Biointerfaces. 2013;110:120–129. doi: 10.1016/j.colsurfb.2013.04.028. [DOI] [PubMed] [Google Scholar]
- 20.Song Y., Zhang C., Wang P., Bao C., Weir M.D., Reynolds M.A. Engineering bone regeneration with novel cell-laden hydrogel microfiber-injectable calcium phosphate scaffold. Mater Sci Eng C. 2017;75:895–905. doi: 10.1016/j.msec.2017.02.158. [DOI] [PubMed] [Google Scholar]
- 21.Wang L., Zhang C., Li C., Zhao L., Xu H.H.K. Injectable calcium phosphate with hydrogel fibers encapsulating induced pluripotent, dental pulp and bone marrow stem cells for bone repair. Mater Sci Eng C. 2016;69(69):1125–1136. doi: 10.1016/j.msec.2016.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laurenti M., Al Subaie A., Abdallah M.N., Cortes A.R.G., Ackerman J.L., Vali H. Two-dimensional magnesium phosphate nanosheets form highly thixotropic gels that up-regulate bone formation. Nano Lett. 2016;16(8):4779–4787. doi: 10.1021/acs.nanolett.6b00636. [DOI] [PubMed] [Google Scholar]
- 23.Han W.M., Anderson S.E., Mohiuddin M., Barros D., Nakhai S.A., Shin E. Synthetic matrix enhances transplanted satellite cell engraftment in dystrophic and aged skeletal muscle with comorbid trauma. Sci Adv. 2018;4(8) doi: 10.1126/sciadv.aar4008. eaar4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zheng Y., Huang K., You X., Huang B., Wu J., Gu Z. Hybrid hydrogels with high strength and biocompatibility for bone regeneration. Int J Biol Macromol. 2017;104:1143–1149. doi: 10.1016/j.ijbiomac.2017.07.017. [DOI] [PubMed] [Google Scholar]
- 25.Hasani-Sadrabadi M.M., Sarrion P., Pouraghaei S., Chau Y., Ansari S., Li S., Aghaloo T., Moshaverinia A. An engineered cell-laden adhesive hydrogel promotes craniofacial bone tissue regeneration in rats. Sci Transl Med. 2020;12(534):eaay6853. doi: 10.1126/scitranslmed.aay6853. [DOI] [PubMed] [Google Scholar]
- 26.Martínez-Sanz E., Varghese O.P., Kisiel M., Engstrand T., Reich K.M., Bohner M. Minimally invasive mandibular bone augmentation using injectable hydrogels. J Tiss Eng Reg Med. 2012;6(3):s15–s23. doi: 10.1002/term.1593. [DOI] [PubMed] [Google Scholar]
- 27.Liao H.T., Tsai M.J., Brahmayya M., Chen J.P. Bone regeneration using adipose-derived stem cells in injectable thermo-gelling hydrogel scaffold containing platelet-rich plasma and biphasic calcium phosphate. Int J Mol Sci. 2018;19(9):2537. doi: 10.3390/ijms19092537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park S.H., Yun B.G., Won J.Y., Yun W.S., Shim J.H., Lim M.H. New application of three-dimensional printing biomaterial in nasal reconstruction. Laryngoscope. 2017;127:1036–1043. doi: 10.1002/lary.26400. [DOI] [PubMed] [Google Scholar]
- 31.Nettles D.L., Vail T.P., Morgan M.T., Grinstaff M.W., Setton L.A. Photocrosslinkable hyaluronan as a scaffold for articular cartilage repair. Ann Biomed Eng. 2004;32:391. doi: 10.1023/b:abme.0000017552.65260.94. [DOI] [PubMed] [Google Scholar]
- 32.Poldervvart M.T., Goversen B., De Ruijter M., Abbadessa A., Melchels F.P., Öner F.C. 3d bioprinting of methacrylated hyaluronic acid (meha) hydrogel with intrinsic osteogenicity. PloS One. 2017;12 doi: 10.1371/journal.pone.0177628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Domingues R.M., Silva M., Gershovich P., Betta S., Babo P., Caridade S.G. Development of injectable hyaluronic acid/cellulose nanocrystals bionanocomposite hydrogels for tissue engineering applications. Bioconjugate Chem. 2015;26:1571. doi: 10.1021/acs.bioconjchem.5b00209. [DOI] [PubMed] [Google Scholar]
- 34.Yuan B., Raucci M.G., Fan Y., Zhu X., Yang X., Zhang X., Santin M., Ambrosio L. Injectable strontium-doped hydroxyapatite integrated with phosphoserine-tethered poly(epsilon-lysine) dendrons for osteoporotic bone defect repair. J Mater Chem B. 2018;6:7974. doi: 10.1039/c8tb02526f. [DOI] [PubMed] [Google Scholar]
- 35.Lu Z., Liu S., Le Y., Qin Z., He M., Xu F. An injectable collagen-genipin-carbon dot hydrogel combined with photodynamic therapy to enhance chondrogenesis. Biomat. 2019;218 doi: 10.1016/j.biomaterials.2019.05.001. [DOI] [PubMed] [Google Scholar]
- 36.Lee D., Heo D.N., Nah H.R., Lee S.J., Ko W.K., Lee J.S. Injectable hydrogel composite containing modified gold nanoparticles: implication in bone tissue regeneration. Int J Nanomed. 2018;13:7019–7031. doi: 10.2147/IJN.S185715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lim K.S., Klotz B.J., Lindberg G.C.J., Melchels F.P.W., Hooper G.J., Malda J. Visible light cross-linking of gelatin hydrogels offers an enhanced cell microenvironment with improved light penetration depth. Macromol Biosci. 2019;19(6) doi: 10.1002/mabi.201900098. [DOI] [PubMed] [Google Scholar]
- 38.Funayama A., Niki Y., Matsumoto H., Maeno S., Yatabe T., Morioka H. Repair of full-thickness articular cartilage defects using injectable type II collagen gel embedded with cultured chondrocytes in a rabbit model. J Orthop Sci. 2008;13:225. doi: 10.1007/s00776-008-1220-z. [DOI] [PubMed] [Google Scholar]
- 39.Kontturi L.S., Järvinen E., Muhonen V., Collin E.C., Pandit A.S., Kiviranta I. An injectable, in situ forming type ii collagen/hyaluronic acid hydrogel vehicle for chondrocyte delivery in cartilage tissue engineering. Drug Del Transl Res. 2014;4:149. doi: 10.1007/s13346-013-0188-1. [DOI] [PubMed] [Google Scholar]
- 40.Ueno M., Lo C.W., Barati D., Conrad B., Lin T., Kohno Y. Interleukin-4 overexpressing mesenchymal stem cells within gelatin-based microribbon hydrogels enhance bone healing in a murine long bone critical-size defect model. J Biomed Mater Res. 2020 May 3 doi: 10.1002/jbm.a.36982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tang Y., Tong X., Conrad B., Yang F. Injectable and in situ crosslinkable gelatin microribbon hydrogels for stem cell delivery and bone regeneration in vivo. Theranostics. 2020;10(13):6035–6047. doi: 10.7150/thno.41096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fathi A., Mithieux S.M., Wei H., Chrzanowski W., Valtchev P., Weiss A.S., Dehghani F. Elastin based cell-laden injectable hydrogels with tunable gelation, mechanical and biodegradation properties. Biomaterials. 2014;35:5425. doi: 10.1016/j.biomaterials.2014.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sim H.J., Thambi T., Lee D.S. Heparin-based temperature-sensitive injectable hydrogels for protein delivery. J Mater Chem B. 2015;3:8892–8901. doi: 10.1039/c5tb01399b. [DOI] [PubMed] [Google Scholar]
- 44.Simão A.R., Fragal V.H., Lima A.M.O., Pellá M.C.G., Garcia F.P., Nakamura C.V. pH-responsive hybrid hydrogels: chondroitin sulfate/casein trapped silica nanospheres for controlled drug release. Int J Biol Macromol. 2020;148:302–315. doi: 10.1016/j.ijbiomac.2020.01.093. [DOI] [PubMed] [Google Scholar]
- 45.Deng Z., He Y., Wang Y.J., Zhao Y., Chen L. Chondroitin sulfate hydrogels based on electrostatic interactions with enhanced adhesive properties: exploring the bulk and interfacial contributions. Soft Matter. 2020 Jun 18 doi: 10.1039/d0sm00547a. [DOI] [PubMed] [Google Scholar]
- 46.Chen F., Yu S., Liu B., Ni Y., Yu C., Su Y. An injectable enzymatically crosslinked carboxymethylated pullulan/chondroitin sulfate hydrogel for cartilage tissue engineering. Sci Rep. 2016;6:20014. doi: 10.1038/srep20014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lin G., Cosimbescu L., Karin N.J., Tarasevich B.J. Injectable and thermosensitive PLGA-g-PEG hydrogels containing hydroxyapatite: preparation, characterization and in vitro release behavior. Biomed Mater. 2012;7(2) doi: 10.1088/1748-6041/7/2/024107. [DOI] [PubMed] [Google Scholar]
- 48.Alexander A., Khan J., Saraf S., Saraf S. Polyethylene glycol (PEG)-Poly(N-isopropylacrylamide) (PNIPAAm) based thermosensitive injectable hydrogels for biomedical applications. Eur J Pharm Biopharm. 2014;88(3):575–585. doi: 10.1016/j.ejpb.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 49.Brewer K., Gundsambuu B., Facal Marina P., Barry S.C., Blencowe A. Thermoresponsive poly(ε-caprolactone)-poly(ethylene/propylene glycol) copolymers as injectable hydrogels for cell therapies. Polymers. 2020;12(2):367. doi: 10.3390/polym12020367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lu H.D., Charati M.B., Kim I.L., Burdick J.A. Injectable shear-thinning hydrogels engineered with a self-assembling Dock-and-Lock mechanism. Biomat. 2012;33:2145–2153. doi: 10.1016/j.biomaterials.2011.11.076. [DOI] [PubMed] [Google Scholar]
- 51.Posritong S., Flores Chavez R., Chu T.G., Bruzzaniti A. A Pyk2 inhibitor incorporated into a PEGDA-gelatin hydrogel promotes osteoblast activity and mineral deposition. Biomed Mater. 2019;14(2) doi: 10.1088/1748-605X/aafffa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Meng Y., Cao J., Chen Y., Yu Y., Ye L. 3D printing of a poly(vinyl alcohol)-based nano-composite hydrogel as an artificial cartilage replacement and the improvement mechanism of printing accuracy. J Mater Chem B. 2020;4:677–690. doi: 10.1039/c9tb02278c. [DOI] [PubMed] [Google Scholar]
- 53.Yegappan R., Selvaprithiviraj V., Amirthalingam S., Mohandas A., Hwang N.S., Jayakumar R. Injectable angiogenic and osteogenic carrageenan nanocomposite hydrogel for bone tissue engineering. Int J Biol Macromol. 2019;122:320–328. doi: 10.1016/j.ijbiomac.2018.10.182. [DOI] [PubMed] [Google Scholar]
- 54.Douglas T.E., Łapa A., Reczyńska K., Krok-Borkowicz M., Pietryga K., Samal S.K. Novel injectable, self-gelling hydrogel-microparticle composites for bone regeneration consisting of gellan gum and calcium and magnesium carbonate microparticles. Biomed Mater. 2016;(6):11. doi: 10.1088/1748-6041/11/6/065011. 065011. [DOI] [PubMed] [Google Scholar]
- 55.Amirova A., Rodchenko S., Kurlykin M., Tenkovtsev A., Krasnou I., Krumme A., Filippov A. Synthesis and investigation of thermo-induced gelation of partially cross-linked poly-2-isopropyl-2-oxazoline in aqueous media. Polymers. 2020;12(3):698. doi: 10.3390/polym12030698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bachmann B., Spitz S., Schädl B., Teuschl A.H., Redl H., Nürnberger S., Ertl P. Stiffness matters: fine-tuned hydrogel elasticity alters chondrogenic redifferentiation. Front Bioeng Biotechnol. 2020;8:373. doi: 10.3389/fbioe.2020.00373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cai H., Wang P., Xu Y., Yao Y., Liu J., Li T. BMSCs-assisted injectable Col I hydrogel-regenerated cartilage defect by reconstructing superficial and calcified cartilage. Regen Biomater. 2020;7(1):35–45. doi: 10.1093/rb/rbz028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xu X., Liu Y., Fu W., Yao M., Ding Z., Xuan J. Poly(N-isopropylacrylamide)-Based thermoresponsive composite hydrogels for biomedical applications. Polymers. 2020;12(3):580. doi: 10.3390/polym12030580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hoang Thi T.T., Sinh L.H., Huynh D.P., Nguyen D.H., Huynh C. Self-assemblable polymer smart-blocks for temperature-induced injectable hydrogel in biomedical applications. Front Chem. 2020;8:19. doi: 10.3389/fchem.2020.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen F., Ni Y., Liu B., Zhou T., Yu C., Su Y., Zhu X., Yu X., Zhou Y. Self-crosslinking and injectable hyaluronic acid/RGD-functionalized pectin hydrogel for cartilage tissue engineering. Carbohydr Polym. 2017;166:31–44. doi: 10.1016/j.carbpol.2017.02.059. [DOI] [PubMed] [Google Scholar]
- 61.Luo Z., Zhang S., Pan J., Shi R., Liu H., Lyu Y. Time-responsive osteogenic niche of stem cells: a sequentially triggered, dual-peptide loaded, alginate hybrid system for promoting cell activity and osteo-differentiation. Biomaterials. 2018;163:25–42. doi: 10.1016/j.biomaterials.2018.02.025. [DOI] [PubMed] [Google Scholar]
- 63.Gulyuz U., Okay O. Self-healing poly(acrylic acid) hydrogels with shape memory behavior of high mechanical strength. Macromolecules. 2014;47:6889. [Google Scholar]
- 64.de Lima G.G., Elter J.K., Chee B.S., Magalhães W.L.E., Devine D.M., Nugent M.J.D., de Sá M.J.C. A tough and novel dual-response PAA/P(NiPAAM-co-PEGDMA) IPN hydrogels with ceramics by photopolymerization for consolidation of bone fragments following fracture. Biomed Mater. 2019;14(5) doi: 10.1088/1748-605X/ab2fa3. [DOI] [PubMed] [Google Scholar]
- 65.Liu X., Yang W., Xiao C. Self-healable and pH-sensitive high-strength water-soluble chitosan/chemically cross-linked polyvinyl alcohol semi-IPN hydrogel. Int J Biol Macromol. 2019;138:667–672. doi: 10.1016/j.ijbiomac.2019.07.169. [DOI] [PubMed] [Google Scholar]
- 66.Park S., Edwards S., Hou S., Boudreau R., Yee R., Jeong K.J. A multi-interpenetrating network (IPN) hydrogel with gelatin and silk fibroin. Biomater Sci. 2019;7(4):1276–1280. doi: 10.1039/c8bm01532e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang Y., Zhang X., Qiu D., Li Y., Yao L., Duan J. Ultrasonic assisted microwave synthesis of poly (Chitosan-co-gelatin)/polyvinyl pyrrolidone IPN hydrogel. Ultrason Sonochem. 2018;40:714–719. doi: 10.1016/j.ultsonch.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 68.Jung S.W., Byun J.H., Oh S.H., Kim T.H., Park J.S., Rho G.J. Multivalent ion-based in situ gelling polysaccharide hydrogel as an injectable bone graft. Carbohydr Polym. 2018;180:216–225. doi: 10.1016/j.carbpol.2017.10.029. [DOI] [PubMed] [Google Scholar]
- 69.Yoon S.J., Yoo Y., Nam S.E., Hyun H., Lee D.W., Um S. The cocktail effect of BMP-2 and TGF-β1 loaded in visible light-cured glycol chitosan hydrogels for the enhancement of bone formation in a rat tibial defect model. Mar Drugs. 2018;16(10):351. doi: 10.3390/md16100351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jung S.W., Oh S.H., Lee I.S., Byun J.H., Lee J.H. In situ gelling hydrogel with anti-bacterial activity and bone healing property for treatment of osteomyelitis. Tissue Eng Regen Med. 2019;16(5):479–490. doi: 10.1007/s13770-019-00206-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fahmy-Garcia S., Mumcuoglu D., de Miguel L., Dieleman V., Witte-Bouma J., van der Eerden B.C.J. Novel in situ gelling hydrogels loaded with recombinant collagen peptide microspheres as a slow-release system induce ectopic bone formation. Adv Healthc Mater. 2018;7(21):e1800507. doi: 10.1002/adhm.201800507. [DOI] [PubMed] [Google Scholar]
- 72.Mathew A.P., Uthaman S., Cho K.H., Cho C.S., Park I.K. Injectable hydrogels for delivering biotherapeutic molecules. Int J Biol Macromol. 2018;110:17–29. doi: 10.1016/j.ijbiomac.2017.11.113. [DOI] [PubMed] [Google Scholar]
- 73.English M.A., Soenksen L.R., Gayet R.V., de Puig H., Angenent-Mari N.M., Mao A.S. Programmable CRISPR-responsive smart materials. Science. 2019;365:780–785. doi: 10.1126/science.aaw5122. [DOI] [PubMed] [Google Scholar]
- 74.Daly A.C., Cunniffe G.M., Sathy B.N., Jeon O., Alsberg E., Kelly D.J. 3D bioprinting of developmentally inspired templates for whole bone organ engineering. Adv Healthc Mater. 2016;5:2353–2362. doi: 10.1002/adhm.201600182. [DOI] [PubMed] [Google Scholar]
- 75.Zopf D.A., Mitsak A.G., Flanagan C.L., Wheeler M., Green G.E., Hollister S.J. Computer aided-designed, 3-dimensionally printed porous tissue bioscaffolds for craniofacial soft tissue reconstruction. Otolaryngol Head Neck Surg. 2015;152:57–62. doi: 10.1177/0194599814552065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Demirtaş T.T., Irmak G., Gümüşderelioğlu M. A bioprintable form of chitosan hydrogel for bone tissue engineering. Biofabrication. 2017;9(3) doi: 10.1088/1758-5090/aa7b1d. [DOI] [PubMed] [Google Scholar]
- 77.de la Lastra A.A., Hixon K.R., Aryan L., Banks A.N., Lin A.Y., Hall A.F., Sell S.A. Tissue engineering scaffolds fabricated in dissolvable 3D-printed molds for patient-specific craniofacial bone regeneration. J Funct Biomater. 2018;9(3):46. doi: 10.3390/jfb9030046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Visscher D.O., Gleadall A., Buskermolen J.K., Burla F., Segal J., Koenderink G.H. Design and fabrication of a hybrid alginate hydrogel/poly(ε-caprolactone) mold for auricular cartilage reconstruction. J Biomed Mater Res B Appl Biomater. 2019;107(5):1711–1721. doi: 10.1002/jbm.b.34264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lee J.S., Hong J.M., Jung J.W., Shim J.H., Oh J.H., Cho D.W. 3D printing of composite tissue with complex shape applied to ear regeneration. Biofabrication. 2014;6 doi: 10.1088/1758-5082/6/2/024103. [DOI] [PubMed] [Google Scholar]
- 80.Visser J., Melchels F.P., Jeon J.E., van Bussel E.M., Kimpton L.S., Byrne H.M. Reinforcement of hydrogels using three-dimensionally printed microfibres. Nat Commun. 2015;6:6933. doi: 10.1038/ncomms7933. [DOI] [PubMed] [Google Scholar]
- 81.Seyednejad H., Gawlitta D., Kuiper R.V., de Bruin A., van Nostrum C.F., Vermonden T., Dhert W.J., Hennink W.E. In vivo biocompatibility and biodegradation of 3D-printed porous scaffolds based on a hydroxyl-functionalized poly(epsilon-caprolactone) Biomat. 2012;33:4309–4318. doi: 10.1016/j.biomaterials.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 82.Olate-Moya F., Arens L., Wilhelm M., Mateos-Timoneda M.A., Engel E., Palza H. Chondroinductive alginate-based hydrogels having Graphene Oxide for 3D printed scaffold fabrication. ACS Appl Mater Interfaces. 2020;12(4):4343–4357. doi: 10.1021/acsami.9b22062. [DOI] [PubMed] [Google Scholar]
- 83.Sevari S.P., Shahnazi F., Chen C., Mitchell J.C., Ansari S., Moshaverinia A. Bioactive glass-containing hydrogel delivery system for osteogenic differentiation of human dental pulp stem cells. J Biomed Mater Res. 2020;108(3):557–564. doi: 10.1002/jbm.a.36836. [DOI] [PubMed] [Google Scholar]
- 84.Cao J., Wang L., Lei D.L., Liu Y.P., Du Z.J. Local injection of nerve growth factor via a hydrogel enhances bone formation during mandibular distraction osteogenesis. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;113(1):48–53. doi: 10.1016/j.tripleo.2011.01.021. [DOI] [PubMed] [Google Scholar]
- 85.Kim J., Yang H.J., Cho T.H., Lee S.E., Park Y.D., Kim H.M. Enhanced regeneration of rabbit mandibular defects through a combined treatment of electrical stimulation and rhBMP-2 application. Med Biol Eng Comput. 2013;51(12):1339–1348. doi: 10.1007/s11517-013-1106-x. [DOI] [PubMed] [Google Scholar]
- 86.Jo J.H., Choi S.W., Choi J.W., Paik D.H., Kang S.S., Kim S.E., Jeon Y.C., Huh J.B. Effects of different rhBMP-2 release profiles in defect areas around dental implants on bone regeneration. Biomed Mater. 2015;(4):10. doi: 10.1088/1748-6041/10/4/045007. 045007. [DOI] [PubMed] [Google Scholar]
- 87.Seo B.B., Chang H.I., Choi H., Koh J.T., Yun K.D., Lee J.Y. New approach for vertical bone regeneration using in situ gelling and sustained BMP-2 releasing poly(phosphazene) hydrogel system on peri-implant site with critical defect in a canine model. J Biomed Mater Res B Appl Biomater. 2018;106(2):751–759. doi: 10.1002/jbm.b.33885. [DOI] [PubMed] [Google Scholar]
- 88.Lei L., Liu Z., Yuan P., Jin R., Wang X. Injectable colloidal hydrogel with mesoporous silica nanoparticles for sustained co-release of microRNA-222 and aspirin to achieve innervated bone regeneration in rat mandibular defects. J Mater Chem B. 2019;7(16):2722–2735. doi: 10.1039/c9tb00025a. [DOI] [PubMed] [Google Scholar]
- 89.Imada M., Yagyuu T., Ueyama Y., Maeda M., Yamamoto K., Kurokawa S., Jo J.I., Tabata Y., Tanaka Y., Kirita T. Prevention of tooth extraction-triggered bisphosphonate-related osteonecrosis of the jaws with basic fibroblast growth factor: an experimental study in rats. PloS One. 2019;14(2) doi: 10.1371/journal.pone.0211928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pan Y., Zhao Y., Kuang R., Liu H., Sun D., Mao T. Injectable hydrogel-loaded nano-hydroxyapatite that improves bone regeneration and alveolar ridge promotion. Mater Sci Eng C. 2020;116 doi: 10.1016/j.msec.2020.111158. [DOI] [PubMed] [Google Scholar]
- 91.Wang T., Guo S., Zhang H., Chen Y., Cai Y. Injectable hydrogel delivering bone morphogenetic protein-2, vascular endothelial growth factor, and adipose-derived stem cells for vascularized bone tissue engineering. J Drug Deliv Sci Technol. 2020;57 [Google Scholar]
- 92.Zhong G., Yao J., Huang X., Luo Y., Wang M., Han J. Injectable ECM hydrogel for delivery of BMSCs enabled full-thickness meniscus repair in an orthotopic rat model. Bioactive Materials. 2020;5(4):871–879. doi: 10.1016/j.bioactmat.2020.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cui H., Yu Y., Li X., Sun Z., Ruan J., Wu Z. Direct 3D printing of a tough hydrogel incorporated with carbon nanotubes for bone regeneration. J Mater Chem. 2019;45(7):7207–7217. doi: 10.1039/c9tb01494b. [DOI] [PubMed] [Google Scholar]
- 94.Zhou X., Zhang D., Wang M., Zhang D., Xu Y. Three-dimensional printed titanium scaffolds enhance osteogenic differentiation and new bone formation by cultured adipose tissue-derived stem cells through the IGF-1R/AKT/mammalian target of rapamycin complex 1 (mTORC1) Pathway. Med Sci Monit. 2019;25:8043–8054. doi: 10.12659/MSM.918517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang W., Shi W., Wu S., Kuss M., Jiang X., Untrauer J.B., Reid S.P., Duan B. 3D printed composite scaffolds with dual small molecule delivery for mandibular bone regeneration. Biofabrication. 2020;12(3) doi: 10.1088/1758-5090/ab906e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kuss M.A., Wu s Wang Y., Untrauer J.B. Prevascularization of 3D printed bone scaffolds by bioactive hydrogels and cell co-culture. J Biomed Mater Res B Appl Biomater. 2018;106(5):1788–1798. doi: 10.1002/jbm.b.33994. [DOI] [PMC free article] [PubMed] [Google Scholar]