Abstract
The Medtronic MiniMed 670G system delivers insulin to patients with type 1 diabetes mellitus (T1DM) using either its hybrid closed-loop (HCL) “Auto Mode” feature or an open-loop mode. In this retrospective, cross-sectional analysis, we quantified the association between time in Auto Mode and both haemoglobin A1c (HbA1c) and time in range (TIR, sensor glucose 70–180 mg/dL) among 96 paediatric and young adult patients with T1DM. The median percentage time in Auto Mode was 38.5% (interquartile range 0%–64%). The percentage time in Auto Mode significantly correlated with HbA1c after adjustment for covariables (β = −0.008, P = 0.014). Each daily 3.4-h increase in Auto Mode time was associated with a 0.1% decrease in HbA1c. Auto Mode time was also correlated with TIR after adjustment for covariables (β = 0.14, P = 0.02): for each daily 8.6-h increase in Auto Mode time, TIR increased by 5%. While Auto Mode use was low, increased time in Auto Mode was associated with a significantly lower HbA1c and increased TIR. These findings emphasize the importance of identifying strategies to improve the ease of use of HCL systems.
1 |. INTRODUCTION
The Medtronic MiniMed 670G (670G) system is the first commercially available hybrid closed-loop (HCL) insulin pump system for use in type 1 diabetes mellitus (T1DM).1 This automated insulin delivery system provides insulin using either its HCL “Auto Mode” feature or an open-loop “Manual Mode”. The 670G pivotal trial and larger real-world studies show that glycaemia improves after initiation of Auto Mode in both paediatric and adolescent populations.1,2 These improvements include increased time in range (TIR, defined as sensor glucose, SG, 70–180 mg/dL),1–4 reduced glycaemic variability3,4 and lower haemoglobin A1c (HbA1c).1,3,4 However, in our paediatric practice, the proportion of time that patients spend in Auto Mode varies considerably. Patients report the 670G system frequently exits from Auto Mode to Manual Mode for several reasons including sustained hyper-glycaemia, not providing sensor calibrations at the appropriate time and inconsistent sensor readings. Given the variability in use of the Auto Mode feature, we aimed to quantify the relationship between percentage time spent in Auto Mode with HbA1c and TIR among adolescent and young adult patients using the 670G in real-world paediatric endocrine practice.
2 |. METHODS
In this retrospective, cross-sectional study, we identified patients from 10 to 21 years of age who were treated at the Eskind Pediatric Diabetes Center at the Vanderbilt University Medical Center and utilized the 670G system for diabetes management between February 1 and December 13, 2018. Data were extracted from any 670G user who had an HbA1c value and 670G pump download obtained on the same day. We additionally collected the HbA1c value that immediately preceded initiation of the 670G system. We extracted all data in Table S1 (see Supporting Information) for the preceding 14 or 30 days from the 670G insulin pump download report. Additional clinical information obtained via chart review included age, sex, race, insurance, anthropometric data, duration of T1DM and treatment modality used before the 670G. Our protocol excluded individuals with 0% sensor wear time or <14 days of insulin pump data available.
The clinical decision to utilize the 670G system was based upon individualized patient and provider discussions without any specific indications. Patients underwent two 1–2-h training sessions with a certified diabetes educator: general 670G training at the initiation of Manual Mode then Auto Mode-focused training before initiation of the HCL feature.
We conducted multivariate linear regression analyses with the dependent variables HbA1c (primary outcome) and TIR (secondary outcome). We prespecified 13 independent variables that we hypothesized could be related to glycaemic outcomes. We used these variables to construct a parsimonious model with at most six degrees of freedom. The models presented here are those with the highest coefficient of determination. Data are summarized as medians (interquartile range, IQR). Statistical analysis was conducted using IBM SPSS Statistics for Windows, version 25 (IBM Corp., Armonk, New York).
3 |. RESULTS
The analysis included 96 adolescents and young adults (61 females) with T1DM. Their median age was 16.6 years (IQR 13.9–18.0 years) and the median body mass index was 23.85 kg/m2 (IQR 20.6–27.9 kg/m2). Median change in HbA1c from just before 670G initiation to the time of data collection was a reduction of 0.4% (IQR −1.1% to 0.2%). Figure 1 summarizes key therapeutic and glycaemic parameters from this cohort, while Table S1 (see Supporting Information) contains additional demographic and insulin pump-based metrics.
FIGURE 1.
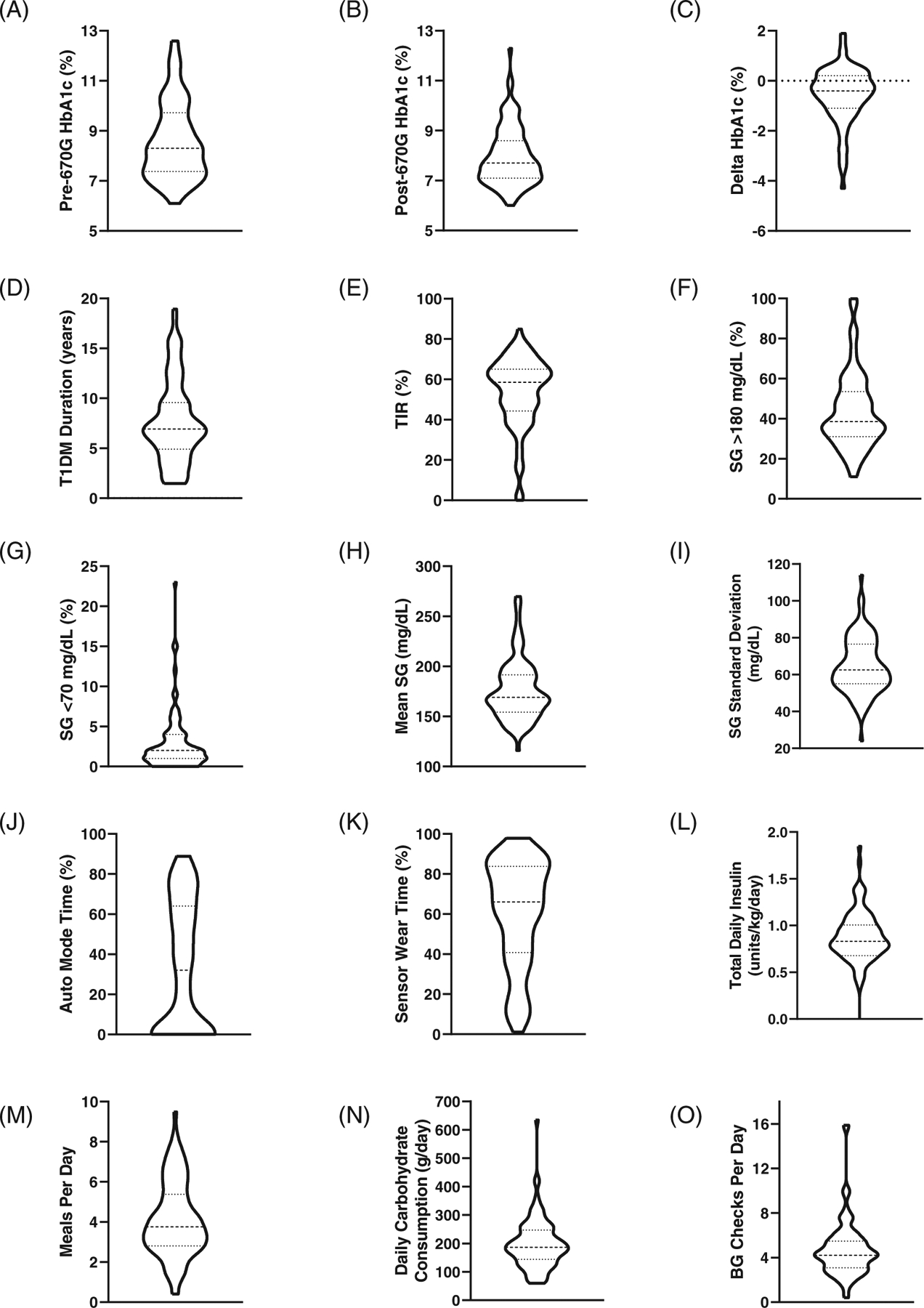
Participant characteristics. Violin plots depict the frequency distribution of the data, with wider sections representing a higher density of participants and narrower sections representing a lower density of participants. Solid vertical line represents zero participants. Additional data depicted in the violin plot include median (thick dotted line) and interquartile range (thin dotted lines), see Table S1 (Supporting Information) for numeric median and interquartile range values. Delta HbA1c depicts change in HbA1c from just before initiation of MiniMed 670G use and time of data collection. Abbreviations: BG, blood glucose; HbA1c, haemoglobin A1c; SG, sensor glucose; TIR, time in range
3.1 |. Time in Auto Mode significantly correlated with haemoglobin A1c
Bivariate analysis of the effect of Auto Mode time on HbA1c revealed a significant inverse correlation (R2 = 0.163, P <0.001; Figure 2A). In the multivariate linear regression analysis with HbA1c as the dependent variable, a significant regression equation was found with a coefficient of determination (R2) of 0.618 (P <0.001). The mean daily percentage time in Auto Mode significantly correlated with HbA1c after adjustment for covariables (β = −0.008, P = 0.014; Figure 2C). Additional statistically significant covariables in the model included total daily dose of insulin, grams of carbohydrates consumed per day, number of meals per day, standard deviation of SG and number of blood glucose checks per day (Figure 2C). In the population analysed, each 3.4-h increase in Auto Mode time per day was associated with a 0.1% decrease in HbA1c when adjusted for covariables.
FIGURE 2.
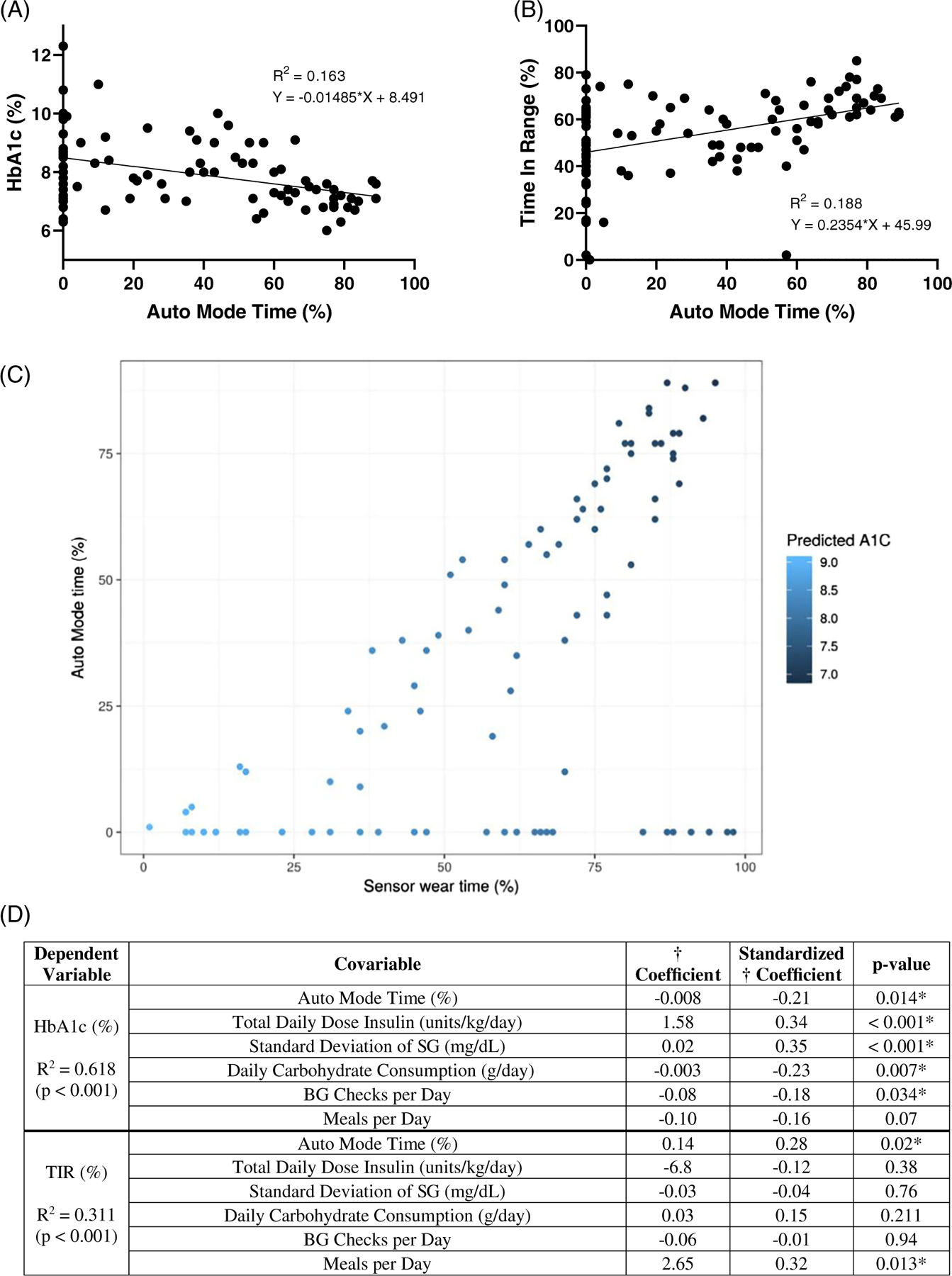
Bivariate and multivariate linear regression analysis. A, Scatterplot depicting bivariate analysis of the effect of Auto Mode time (%) on HbA1c (%). B, Scatterplot depicting bivariate analysis of effect of Auto Mode time (%) on TIR (%). C, Auto Mode time and sensor wear time prediction of HbA1c. Scatterplot depicting predicted HbA1c from a linear model using sensor wear time and Auto Mode time as predictors. Light blue shaded points represent higher predicted HbA1c, dark blue shaded points represent lower predicted HbA1c. D, Correlates with HbA1c and TIR in multilinear regression analysis. Abbreviations: BG, blood glucose; HbA1c, haemoglobin A1c; SG, sensor glucose; TIR, time in range. *Significant P <0.05
3.2 |. Time in Auto Mode significantly correlated with time in range
Bivariate analysis of the effect of Auto Mode time on TIR revealed a significant direct correlation (R2 = 0.188, P <0.001; Figure 2B). In the multivariate linear regression analysis with TIR as the dependent variable, a significant regression equation was found with R2 = 0.311 (P <0.001). The mean daily percentage time in Auto Mode significantly correlated with TIR after adjustment for covariables (β = 0.14, P = 0.02; Figure 2C). The other statistically significant covariable in the model was number of meals per day (Figure 2C). Each 8.6-h increase in Auto Mode time per day was associated with a 5% increase in TIR per day after adjustment for covariables. Median TIR in the full cohort versus those with >50% Auto Mode time was 59.0% (IQR 44.3%–65.0%) versus 64.0% (IQR 59.0%–69.0%), respectively. Additional bivariate analyses of variables of interest are depicted in Figure S1A–D (see Supporting Information).
4 |. DISCUSSION
Our analysis indicates increased Auto Mode time is associated with lower HbA1c and increased TIR among adolescents and young adults with T1DM. To our knowledge, this study is the first to quantify the therapeutic benefit of spending more time in Auto Mode in real-world clinical practice. In the cohort analysed, each 3.4-h increase in Auto Mode time per day was associated with a 0.1% lower HbA1c. Likewise, each 8.6-h increase in Auto Mode time per day was associated with 5% more TIR. These findings are consistent with previous 670G trials that demonstrated improvements in glycaemia, including increased TIR1–4 and reduction in HbA1c.1,3,4 The ability of automated insulin delivery to improve glycaemia may particularly benefit adolescents and young adults, who have mostly struggled with meeting therapeutic targets. In the T1DM Exchange Registry cohort, only 17% of teenagers aged 13–17 years reached the recommended target HbA1c of <7.5%, whereas 14% of patients aged 17–25 years reached the target HbA1c of <7.0%.5 In our cohort, the median HbA1c was 7.7%, but the median Auto Mode time was only 32.0%. Thus, it is likely that a substantially higher number of our patients could meet glycaemic targets if the proportion of Auto Mode time was increased.
Additionally, the potential long-term benefit of each 0.1% reduction in HbA1c with an increase of 3.4 h of Auto Mode time is underscored by findings from the Diabetes Control and Complications Trial (DCCT). If the findings from the DCCT were extrapolated to the present cohort, each 0.1% reduction in HbA1c would be associated with an average microvascular risk reduction of 5.7% for retinopathy progression, 3.2% for microalbuminuria, 5.7% for macroalbuminuria and 3.9% for neuropathy.6 These data suggest increasing Auto Mode time has the potential to aid in reducing the risk of long-term diabetes complications.
This analysis provides additional evidence that patients struggle to remain in Auto Mode and would benefit from increased time in HCL mode. An observational study of 83 paediatric and young adult patients showed 19% of patients completely discontinued using the 670G after an average follow-up time of 8 months.7 Cited reasons for discontinuation included calibration requirements, problems with sensor durability or adhesion, skin irritation and forced exits from Auto Mode.7 The percentage time spent in Auto Mode was also highly variable, ranging from 10% to 90%.7 Although the percentage of time in Auto Mode was comparatively higher in the pivotal 670G trial, Auto Mode time declined from a peak of 87% after the first 7 days of use to 72% at the 3-month endpoint in the adolescent and young adult subanalysis.1 However, there are some indications that adaptations to remain in HCL may improve glycaemic control. In an investigation of an alternative Medtronic HCL system algorithm, making correction boluses for hyperglycaemia more aggressive, bolusing prandial insulin from SG values, and loosening parameters to remain in Auto Mode reduced the number of alarms eightfold and Auto Mode exits fourfold compared with the standard Medtronic HCL algorithm.8 These modifications were accompanied by greater improvements in TIR.8 Collectively, these data indicate Auto Mode time varies considerably among 670G users, and measures to increase Auto Mode time could improve glycaemia substantially.
The opportunity to improve HbA1c and TIR by increasing HCL time is particularly relevant as newer automated insulin delivery systems approach clinical implementation. These new control systems, including the Tandem Control-IQ, Diabeloop DBLG1, Medtronic 780G and Insulet Omnipod Horizon, will need to address the issue of frequent exits from the HCL system if they are to enhance glycaemic control significantly in the paediatric and adolescent T1DM population.
Measures of dietary intake were also significant covariates in the multivariable regression models. In the HbA1c analysis, greater carbohydrate consumption was associated with lower HbA1c. In the TIR analysis, a larger number of meals recorded per day were associated with more TIR when adjusted for covariables. It is possible that an increased number of meals and carbohydrates recorded in the insulin pump are surrogates of adherence (e.g. bolusing insulin before eating).
Given the known benefits of sensor-augmented pump therapy on glycaemia,9 we additionally analysed the relationship of the sensor wear time with HbA1c and TIR. Auto Mode time absolutely depends on sensor wear time (i.e. users cannot remain in Auto Mode without wearing the sensor); however, our multivariable linear regression analysis was insufficiently powered to differentiate between the impacts of Auto Mode time versus sensor wear time on the key glycaemic outcomes. Using a likelihood ratio test, we determined that either Auto Mode time or sensor wear time (or both) is significantly associated with HbA1c when controlling for other covariates in the model (P = 0.016). As Figure 2C suggests, patients who had both higher sensor wear times and increased Auto Mode time had incrementally lower HbA1c than patients with higher sensor wear time but less Auto Mode time.
Limitations of this study include the retrospective, cross-sectional design, which restricted available insulin pump data to 14–30 days for each participant. Additionally, extrapolation of findings to the general T1DM population may be limited given that they are specific to HCL users, a population of patients who possibly have greater resources to manage their diabetes than their peers do. However, with broadening commercial availability of additional HCL systems in the near future, we anticipate increasing the closed-loop system use will widen the relevance of our study findings.
In conclusion, whereas the MiniMed 670G system has demonstrated the potential to improve glycaemic outcomes, our findings suggest that these improvements are diminished when time spent in HCL is limited by frequent Auto Mode exits. In our clinical practice and other recently published cohorts,7 time spent in Auto Mode is substantially lower than in previously published trials.1,3,4 Future automated insulin delivery systems that reduce the burden of staying in the closed-loop mode have the potential to enhance glycaemic control substantially in the paediatric and adolescent T1DM population. Reducing barriers to remaining in the closed-loop mode of any future HCL insulin pump systems will be imperative for achieving optimum improvements in glycaemic control.
Supplementary Material
Funding information
Eunice Kennedy Shriver National Institute of Child Health & Human Development, Grant/Award Number: K12HD087023; Ruth L. Kirschstein National Research Service Award (NRSA) for Research Training in Diabetes and Endocrinology, Grant/Award Number: T32DK007061; Vanderbilt Diabetes Research and Training Center, Grant/Award Number: DK020593
Footnotes
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of this article.
REFERENCES
- 1.Messer LH, Forlenza GP, Sherr JL, et al. Optimizing hybrid closed-loop therapy in adolescents and emerging adults using the MiniMed 670G system. Diabetes Care. 2018;41:789–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stone MP, Agrawal P, Chen X, et al. Retrospective analysis of 3-month real-world glucose data after the MiniMed 670G system commercial launch. Diabetes Technol Ther. 2018;20:689–692. [DOI] [PubMed] [Google Scholar]
- 3.Garg SK, Weinzimer SA, Tamborlane WV, et al. Glucose outcomes with the in-home use of a hybrid closed-loop insulin delivery system in adolescents and adults with type 1 diabetes. Diabetes Technol Ther. 2017; 19:155–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Forlenza GP, Pinhas-Hamiel O, Liljenquist DR, et al. Safety evaluation of the MiniMed 670G system in children 7–13 years of age with type 1 diabetes. Diabetes Technol Ther. 2019;21:11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miller KM, Foster NC, Beck RW, et al. Current state of type 1 diabetes treatment in the U.S.: updated data from the T1D Exchange Clinic Registry. Diabetes Care. 2015;38:971–978. [DOI] [PubMed] [Google Scholar]
- 6.Nathan DM, Bayless M, Cleary P, et al. Diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: advances and contributions. Diabetes. 2013;62:3976–3986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goodwin D, Waldman G, Lyons J, Oladunjoye A, Steil G. Challenges in implementing hybrid closed loop insulin pump therapy (Medtronic 670G) in a ‘real world’ clinical setting. J Endocrinol Soc. 2019;3(Suppl. 1):OR14–OR15. [Google Scholar]
- 8.de Bock M, Dart J, Hancock M, Smith G, Davis EA, Jones TW. Performance of Medtronic hybrid closed-loop iterations: results from a randomized trial in adolescents with type 1 diabetes. Diabetes Technol Ther. 2018;20:693–697. [DOI] [PubMed] [Google Scholar]
- 9.Poolsup N, Suksomboon N, Kyaw AM. Systematic review and meta-analysis of the effectiveness of continuous glucose monitoring (CGM) on glucose control in diabetes. Diabetol Metab Syndr. 2013;5:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


