Abstract
Traditionally thought of as a pediatric diagnostic and therapeutic dilemma, the diagnostic rate and spectrum of inborn errors of metabolism (IEM) in the adult population is largely unknown. A retrospective chart review of patients seen by the Michigan Medicine Adult Medical Genetics Clinic for clinical evaluation from 2014 to 2018 was conducted. Patients referred for a primary indication possibly consistent with an IEM were considered. Variables included age at genetic evaluation, symptom onset age, sex, clinical course, organ systems involved, developmental history, family history and prior genetic testing. Of patients evaluated during the study period, 112 were referred for an indication possibly consistent with an IEM and underwent a complete biochemical workup with an IEM diagnostic rate of 9.8% achieved. An additional 9.8% were diagnosed with a non-IEM genetic diagnosis. Management changes were implemented in all IEM diagnoses. Metabolic disorders in the adult population are under-recognized and under-diagnosed. This report demonstrates the need for clinicians to consider these diagnoses in adults and either refer to a genetics clinic or initiate a biochemical workup. As advances in diagnosis, treatment, and life expectancy of patients with IEMs increases, recognizing and diagnosing these conditions can significantly impact care.
Keywords: Medical genetics, Inborn errors of metabolism, Adult genetics, Diagnostic rate
Highlights
-
•
Inborn errors of metabolism are underdiagnosed in adult populations.
-
•
Biochemical testing yielded a 10% diagnostic rate in our adult genetics clinic.
-
•
Testing was used to diagnose common and very uncommon diagnoses in our clinic.
-
•
Patients with both biochemical and non-biochemical disorders present similarly.
1. Introduction
Inborn errors of metabolism (IEMs) represent a group of approximately 500 monogenic disorders caused by defects in a biochemical pathway leading to an accumulation of a substrate or toxic metabolite, or deficiency of a product, and subsequent end organ dysfunction. IEMs are individually rare, but collectively numerous, with an estimated cumulative worldwide incidence of 1/2500 live births [1,2]. Inheritance patterns vary and include autosomal recessive (most common), autosomal dominant, X-linked, and mitochondrial. Importantly, the diagnosis of an IEM often has lifesaving therapeutic implications for patients through the use of dietary modifications, medications, organ transplantation and more recently ongoing clinical trials for gene therapy [3]. This treatment potential has resulted in the incorporation of many IEMs on newborn screening (NBS) panels and recommendations a biochemical workup be initiated early in the evaluation for lack of attainment or regression in milestones, intellectual disability, lethargy, gastrointestinal symptoms, seizures and other significant medical issues [4].
Previously believed to primarily affect children, adults with both known diagnoses and undiagnosed IEMs represent a growing population [5,6]. A population-based United Kingdom study from 1999 to 2003 identified that 23% of patients diagnosed with an IEM were diagnosed in adulthood, with a separate cohort revealing 40% of IEM diagnoses were made in adulthood [5,7]. NBS has also resulted in the diagnosis of patients that may not present until late adolescence or adulthood as has been seen in late-onset glutaric acidemia type 2, carnitine palmitoyl transferase deficiency type 2, medium-chain acyl-CoA dehydrogenase deficiency, primary carnitine deficiency, X-linked adrenoleukodystrophy, and late onset Pompe Disease [8]. Clinical practice guidelines and management algorithms are still being developed for these individuals detected “pre-symptomatically”, and the degree to which these individuals follow up and receive care later in life for these adult-onset conditions is largely unknown, and beyond the scope of this paper [8]. The diagnosis of IEMs in adults outside of newborn screening is often difficult due to attenuated phenotypes, variable expressivity, missed diagnoses in childhood, considerable overlapping phenotypes with other disorders, and under recognition by providers [5,9]. Additionally, most IEM references focus on pediatric presentations of disease where “classical” signs and symptoms are more typical [10]. There are also very few centers with dedicated medical genetics clinics for adults, despite increased demand for their services [[11], [12], [13], [14], [15], [16]]. As a result, IEMs affecting adults are likely underdiagnosed and, for those provided a diagnosis, there is often a significant delay in diagnosis beyond what is already seen in pediatric IEM patients [6,7].
Here we present the retrospective study of the diagnostic evaluation of patients seen for indications possibly consistent with an IEM in the adult Medical Genetics Clinic at Michigan Medicine. We report the diagnostic rate and spectrum of IEMs diagnosed in our clinic, the indications for biochemical testing, and presenting features of patients most likely to result in a biochemical diagnosis. A significant percentage of all diagnoses made in clinic were for IEMs with each diagnosis resulting in changes in patient management recommendations. The information presented here has important practice implications, not only for medical geneticists but also any clinician caring for these patients.
2. Patients and methods
We performed a retrospective chart review of patients seen in the Adult Medical Genetics Clinic at Michigan Medicine from July 2014 through December 2018. The study period start date coincided with the start date of a clinical biochemical geneticist in this clinic. Study approval was obtained by the University of Michigan Institutional Review Board. Inclusion criteria included all patients seen in the Michigan Medicine Adult Medical Genetics Clinic for a primary indication possibly consistent with an IEM (Table 1) and any patient seen in clinic who underwent biochemical screening testing as part of their diagnostic workup. Only patients who underwent all recommended laboratory (biochemical and molecular) tests were included for study. Patients referred for an indication possibly consistent with an IEM but found to have a phenotype strongly suggestive of a non-IEM diagnosis such as 22q11.2 deletion syndrome were excluded from study. Additionally, patients referred to the clinic for genetic counseling of a previously made diagnosis were excluded, as were patients referred to the clinic for non-IEM genetic conditions (e.g. Huntington's disease, thoracic aortic aneurysms) 90 underwent biochemical testing through our clinic. Routine biochemical testing included a combination of blood and urine tests (Supplementary Table 1). There was no standard battery of testing for all patients, rather testing was individualized and guided by phenotype and clinical suspicion, though many had plasma amino and urine organic acids assessed. Cerebrospinal fluid (CSF) analysis is occasionally utilized in biochemical testing, however was not implemented for evaluation of any patient in our cohort due to the invasive nature of the procedure. The remaining 38 patients had previously received biochemical testing prior to referral and did not undergo additional biochemical testing through our clinic. 16 were excluded from analysis as recommended testing was not completed due to insurance issues or patient being lost to follow-up, leaving a total of 112 patients analyzed.
Table 1.
Reason for referral included in study.
| Reasons for Referral Included in Study |
|---|
| Developmental delay |
| Cognitive impairment/Intellectual disability |
| Autism |
| Behavioral difficulties |
| Peripheral neuropathy |
| Hypotonia |
| Seizures/epilepsy |
| Encephalopathy |
| Recurrent stroke/stroke-like episodes |
| Chronic fatigue/Weakness |
| Myopathy/Muscle Atrophy |
| Rhabdomyolysis |
| Dystonia/Movement disorders |
| Pyramidal signs/Spastic paraparesis |
| Ataxia |
| Ocular concerns (Ectopia lentis, retinitis pigmentosa, ophthalmoplegia) |
| Musculoskeletal complaints |
| Cardiomyopathy |
| Suggestive labs/imaging |
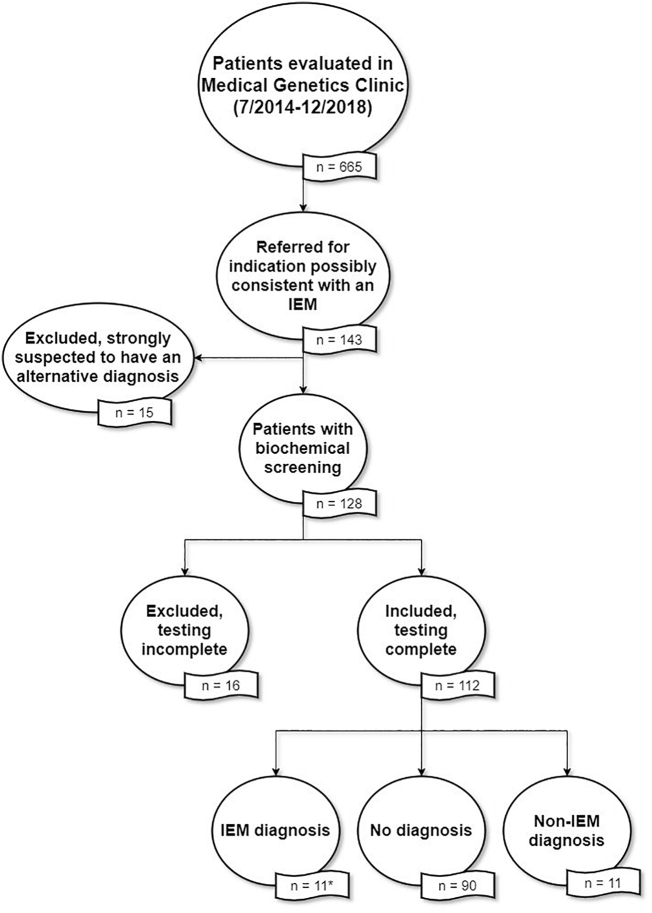
During the study period, 2042 patients were seen in the Medical Genetics Clinic. Referrals are triaged by Medical Genetics Clinic staff (by senior genetic counselors, with clinical geneticists providing input if requested). Patients are triaged for each clinician based on areas of expertise and/or interest. During this time period, 665 patients were seen by our clinical biochemical geneticist. Of those, 143 were referred for an indication possibly consistent with an IEM, which was inclusive of all referrals for the indications in Table 1. Fifteen were excluded based on initial strong clinical suspicion of a non-IEM diagnosis. The remaining 128 patients underwent some degree of biochemical screening (Fig. 1).
Fig. 1.
Flowchart of eligibility of patients considered for inclusion. Eligible indications included: developmental delay, cognitive impairment, autism spectrum disorder, myopathy, rhabdomyolysis, encephalopathy, “rule out metabolic disorder”, or “rule out mitochondrial disorder”. *For analysis purposes, only unique diagnoses (1 per family) were considered. Therefore, n = 8 for analysis.
Based on physician clinical evaluation and reviews of prior workup and diagnostic testing, a binary yes/no phenotype dataset was created for all eligible patients across all body systems and other developmental/ neurodevelopmental categories. The clinical system phenotypes assessed for abnormalities were: neurologic, ophthalmologic, ear/nose/throat (Oto), cardiac, pulmonary, gastrointestinal, genitourinary, integument, endocrine, hematologic, immunologic, musculoskeletal and psychiatric. A number of other non-body system categories were also assessed in a binary way: presence of dysmorphic features, global developmental delay (DD, categorized by presence of impairments in two or more developmental domains), autism spectrum disorder or autism-like features, cognitive impairment (CI), developmental regression, presence of seizures and ability to live independently. Positive or negative family history of similar condition was also ascertained. In addition to these binary-curated features, all patients had demographic information (age, sex, race/ethnicity), reason for referral, age of onset of symptoms, ICD-10 codes, and collation of any prior genetic/molecular testing including screening metabolic labs, karyotype, chromosomal microarray, or other single gene or panel based molecular testing. Outcome of biochemical testing (if performed) was also noted.
Statistical analysis was completed using R version 3.4.4. For phenotypic statistical analysis siblings were randomly withdrawn from the data set so only one individual from each group of siblings was included given significant overlap with presentation. Fisher's exact tests and Kruskal-Wallis tests were used to test the associations of diagnosis types with clinical and demographic variables. P values were adjusted for multiple comparisons using the false discovery rate correction [17].
3. Results
3.1. Demographics
In total, 112 patients underwent a biochemical workup in this study, including 2 sets of siblings (Table 2, Table 3).
Table 2.
Demographics.
| Category | Number (%) | |
|---|---|---|
| Patient number (Total 665) | 112 (16.8%) | |
| Average age at time of evaluation (years) | 36.9 | |
| Sex | Female | 60 (53.6%) |
| Male | 52 (46.4%) | |
| Race/ Ethnicity | Caucasian | 97 (86.6%) |
| African | 7 (6.3%) | |
| Native American | 1 (0.9%) | |
| Asian | 2 (1.8%) | |
| Middle Eastern | 3 (2.7%) | |
| Multiracial | 2 (1.8%) | |
| Non-Hispanic or Latino | 110 (98.2%) | |
| Hispanic or Latino | 2 (1.8%) | |
Table 3.
Clinical characteristics of patients with complete biochemical screening. *Several patients presented with features of multiple categories, so were counted in each.
| Category | Number (%) | |
|---|---|---|
| Symptom Onset | Infancy (0-3y) | 47 (42.0%) |
| Childhood (4-11y) | 7 (6.3%) | |
| Adolescence (12-18y) | 4 (3.6%) | |
| Adulthood (19y+) | 51 (45.5%) | |
| Unknown | 3 (2.7%) | |
| Clinical Course * | Acute | 6 (5.4%) |
| Acute-relapsing | 22 (19.6%) | |
| Diurnal variation | 1 (0.9%) | |
| Progressive | 43 (38.4%) | |
| Static/Chronic | 50 (44.6%) | |
| Asymptomatic (Family History Only) | 2 (1.8%) | |
| Triggers | Fasting | 1 (0.9%) |
| Eating | 3 (2.7%) | |
| Exercise | 20 (17.9%) | |
| Fever | 4 (3.6%) | |
| Catabolism | 12 (10.7%) | |
| Post-Partum | 0 | |
| None Identified | 72 (64.3%) | |
| Organ Systems Involved | Neurologic | 86 (76.8%) |
| Dysmorphism | 7 (6.3%) | |
| Ophthalmologic | 22 (19.6%) | |
| Oto | 13 (11.6%) | |
| Integument | 8 (7.1%) | |
| Cardiac | 14 (12.5%) | |
| Pulmonary | 8 (7.1%) | |
| Gastrointestinal | 25 (22.3%) | |
| Genitourinary | 8 (7.1%) | |
| Musculoskeletal | 71 (63.4%) | |
| Endocrinologic | 18 (16.1%) | |
| Immunologic | 4 (3.6%) | |
| Hematologic | 8 (7.1%) | |
| Psychiatric | 14 (12.5%) | |
| Asymptomatic (Family History Only) | 2 (1.8%) | |
| # Organ Systems Involved | 0 | 2 (1.8%) |
| 1 | 21 (18.8%) | |
| 2 | 44 (39.3%) | |
| 3 | 16 (14.3%) | |
| 4 | 11 (9.8%) | |
| 5 | 10 (8.9%) | |
| 6+ | 8 (7.1%) | |
| Development * | Normal | 69 (61.6%) |
| Cognitive Impairment | 31 (27.7%) | |
| Developmental Delay | 38 (33.9%) | |
| Autism | 9 (8.0%) | |
| Regression | 12 (10.7%) | |
| Family History | Yes | 49 (43.8%) |
| No | 63 (56.3%) | |
There was an approximately equal number of males and females, and patients had an average age at the time of evaluation of 36.9 years (age range 17–87 years). The majority of patients evaluated identified as Caucasian and Non-Hispanic (86.6%).
3.2. Diagnostic rate
Of those who underwent a biochemical workup, a diagnosis was made in 19.6% of patients (n = 22) with 9.8% diagnosed with an IEM (n = 11) and 9.8% diagnosed with a non-IEM diagnosis (n = 11) confirmed with molecular testing (Table 4). Of the 11 biochemical diagnoses, 64% (n = 7) had biochemical testing that was either strongly suggestive or diagnostic prior to referral (n = 3) or through our clinic (n = 4). These diagnoses included alkaptonuria, mitochondrial encephalomyopathy, lactic acidosis, and stroke-like episodes (MELAS), S-adenosylhomocysteine hydrolase deficiency (SAHH), mevalonic aciduria (two siblings), McArdle disease, and Fabry disease. The remaining 4 patients were 3 siblings diagnosed with Segawa syndrome who never underwent the diagnostic CSF testing and one patient with PANK2-associated neurodegeneration with brain iron accumulation (NBIA).
Table 4.
Diagnoses in this cohort.
| Diagnosis | OMIM Number | Number Diagnosed | Suggestive Biochemical Test | Molecular Confirmatory Test | Change in Management? | |
|---|---|---|---|---|---|---|
| Inborn Error of Metabolism (IEM) | Alkaptonuria | 203500 | 1 | UOA | Single gene sequencing | Yes |
| Mitochondrial encephalopathy, lactic acidosis, and stroke-like episodes (MELAS) | 540000 | 1 | Lactate | WES | Yes | |
| S-adenosylhomocysteine hydrolase (SAHH) deficiency | 613752 | 1 | PAA | WES | Yes | |
| Mevalonic aciduria (MVA) | 610377 | 2 | UOA | Single gene sequencing | Yes | |
| McArdle disease | 232600 | 1 | CK | Single gene sequencing | Yes | |
| Segawa disease | 605407 | 3 | WES | Yes | ||
| PANK2-associated neurodegeneration with brain iron accumulation (NBIA) | 234200 | 1 | NGS panel | Yes | ||
| Fabry disease | 301500 | 1 | α-galactosidase enzyme activity | Single gene sequencing | Yes | |
| Non-IEM Diagnosis | Fragile X | 300624 | 1 | Fragile X testing | No | |
| 16p11.2 deletion syndrome | 611913 | 1 | CMA | No | ||
| Marfan syndrome | 154700 | 1 | Single gene sequencing | Yes | ||
| 18q deletion syndrome | 601808 | 1 | CMA | No | ||
| 48,XXYY | 1 | Karyotype | No | |||
| 175 kb gain 7q36.1, 742 kb loss 8p23.1 | 1 | CMA | No | |||
| GRIN2B-related disorder | 613970 | 1 | WES | No | ||
| Myhre syndrome | 139210 | 1 | WES | No | ||
| Cerebellar ataxia, mental retardation, and disequilibrium syndrome 2 (CAMRQ2) | 610185 | 1 | WES | No | ||
| Mohr-Tranebjaerg syndrome | 304700 | 1 | NGS panel | No | ||
| Laing distal myopathy | 160500 | 1 | WES | No | ||
The overall diagnostic yield of a biochemical workup in our cohort was 6.3% (7/112) though when combined with a full molecular workup was 9.8% (11/112). Importantly, a change in management was made or is planned in all of the patients diagnosed with an IEM in our cohort.
A diagnosis was not reached for 80.4% of patients (n = 90) who underwent biochemical screening. Of these 90 patients, 16 of the 90 (17.8%) had negative whole exome sequencing (including mitochondrial DNA), with many having previous NGS panel testing performed that was normal/nondiagnostic. An additional four (4.4%) had negative dual mitochondrial genome testing. 13 of the 90 (14.4%) had negative single gene or NGS panel testing but did not undergo whole exome testing. 16 (17.8%) had negative chromosomal microarray testing, and there were 5 (5.6%) who had negative Fragile X testing with some of the above patients receiving multiple tests. In total 47 patients (52%) did not receive molecular testing for various reasons including lack of insurance coverage or low clinical suspicion as the most common reasons.
3.3. Phenotypic comparison
In our phenotype analysis comparing patients diagnosed with an IEM to patients diagnosed with a non-IEM, those with an IEM diagnosis were more likely to have an acute-relapsing or progressive course to their symptomatology, in comparison to those who received a non-IEM diagnosis who tended to have a more static/chronic course (Supplemental Table 2). No significant difference in age of onset was observed with 72.5% and 81.8% of patients with an IEM diagnosis and non-IEM diagnosis, respectively, showing symptoms in either infancy or childhood (Supplemental Table 2). The average time to diagnosis was 26 years from symptom onset, regardless of diagnosis class (IEM vs non-IEM) (Supplemental Table 2). There additionally was no difference seen between diagnostic groups when considering the developmental course with only the minority of patients with normal cognitive development (78% with developmental delay, cognitive impairment, or developmental regression; p-value = 0.319; adjusted p-value = 0.585). A brief description of the IEM diagnoses in this cohort can be found in the Supplementary Data.
Though not statistically significant, those patients who received diagnoses (IEM and non-IEM) were more likely to have concrete/finite or objective signs and symptoms, rather than non-specific symptoms (such as chronic/generalized fatigue/malaise, non-specific pain or weakness, gastroparesis, chronic nausea, frequent headache (p-value = 0.177; adjusted p-value 0.539), when compared to undiagnosed patients. The majority of individuals receiving a diagnosis (IEMs and non-IEMs) had symptoms beginning in infancy (68.4%) compared to those not receiving a diagnosis who had only had findings presenting in infancy in 34.4% of cases. Additionally, most individuals not receiving a diagnosis initially presented with symptoms in adulthood (51.1%) with those provided with a diagnosis (IEM and non-IEM) less likely to have symptoms starting in adulthood (21.1%). Individuals in this cohort receiving a diagnosis (IEM and non-IEM) were more likely to have delayed development compared to those who did not receive a diagnosis with 70% of undiagnosed individuals having normal development compared to only 6% of those given a diagnosis (p-value = 0.003; adjusted p-value = 0.096). In comparison to non-IEM diagnoses, those receiving an IEM diagnosis trended toward having a more acute-relapsing or progressive course, versus a static/chronic course (p-value = 0.004; adjusted p-value 0.104). A comparison of IEM-diagnosed patients to undiagnosed patients was also performed with no statistical difference seen between most phenotypic presentations with the exception of clinical course where IEM patients were more likely to have an acute-relapsing or progressive course (p-value = 0.004; adjusted p-value = 0.086).
4. Discussion
Here we present the diagnostic rate and spectrum of inborn errors of metabolism diagnosed in the Adult Medical Genetics Clinic at Michigan Medicine. In a method consistent with previously published reports in the pediatric population, our evaluation strategy involved a combination of personal and family history, physical examination, previous record review, and diagnostic evaluation with both biochemical testing and molecular analysis, where indicated [18,19]. In our cohort, the overall diagnostic rate for indications possibly consistent with an IEM was 19.6%, with 9.8% diagnosed with an IEM, and 9.8% diagnosed with a non-IEM diagnosis. The estimated yield of biochemical testing in patients with DD and/or CI have ranged from less than 1%, to nearly 5% of cases, and in highly selected groups, up to 14% [18,[20], [21], [22], [23], [24]]. Our diagnostic yield of 6.3% with biochemical testing (up to 9.8% with added molecular testing) is at the higher end of these estimates, but is important to note given the novel study population, with previous studies focused largely on children. Additionally, when comparing patients provided with a diagnosis through our clinic (IEMs and non-IEMs) to patients not receiving a diagnosis we found that patients receiving any diagnosis were more likely to have signs and symptoms presenting before adulthood and were more likely to have a history of developmental delay.
In our patients, a variety of IEM diagnoses were made including relatively common and well-known conditions such as Fabry disease and MELAS as well as very rare conditions such as alkaptonuria and SAHH deficiency. All patients diagnosed with an IEM had a recommended change in their management as a result of their diagnosis. The recognition and early diagnosis of IEMs is especially important in early childhood when developmental outcomes can be most significantly altered. However, the potential for improved outcomes after diagnosis and treatment can still be quite high, regardless of age at diagnosis. This is exhibited by the patients diagnosed with PANK2-associated NBIA and Fabry disease in our cohort. While cocktail initiation was not beneficial for our patient diagnosed with MELAS, counseling on the natural history of the condition resulted in a referral to our palliative care clinic for complex care management.
The prevalence of IEMs in the adult population is currently unknown, as are the types and numbers of patients being evaluated for suspected IEMs, but it is likely IEMs in this population are significantly underdiagnosed. As medical and therapeutic advances are made and with increased awareness, more children with IEMs are surviving into adulthood. However, there are likely many adults with IEMs who presented in childhood that were missed, in addition to those with true symptom onset during adulthood. Previous work has shown adults with IEMs are often seen and cared for by various subspecialists with limited medical genetics training resulting in missed, delayed, or incorrect diagnoses [6,7,15,25]. Further work is necessary to identify best practices to assist non-genetics healthcare providers with the evaluation and/or appropriate referral of patients possibly affected with IEMs [15]. This is especially important given the increased demand for genetic services, and resultant decreased time allotted for clinic visits, in addition to the limited number of practicing geneticists caring for adult populations [11,13,14].
Identifying a unique set of presenting signs and symptoms of IEMs could help in differentiation from non-IEM patients, however, we unfortunately found few differences between the phenotypes of all patients seen in our clinic. The only phenotype more commonly present in IEM-diagnosed patients was a fluctuation of clinical signs or symptoms especially when triggered by fasting, fever, catabolism, or exercise. Importantly, we chose to present adjusted p-values for reference purposes given the large number of comparisons performed. Further work with larger populations of patients will be important to evaluate the true significance of this difference. No other differences were seen between diagnosis and phenotypic presentation, further stressing the importance of a biochemical workup in all patients with a presentation possibly consistent with an IEM. Similar to the recommended biochemical screening of all patients affected with intellectual disability, this could potentially decrease the delay in diagnosis experienced by many patients with IEMs [19]. This importance is highlighted by the patient diagnosed with SAHH deficiency. This patient was initially referred to our clinic for genetic counseling of a previously given diagnosis of autosomal-recessive LGMD based on a heterozygous VUS in POMT found on NGS panel testing. Following genetic counseling it was identified he was affected with a more complex phenotype with subsequent biochemical testing suggestive of the eventual correct diagnosis. This case also demonstrates the importance and benefit of a comprehensive evaluation of all patients seen in clinic [11].
The use of biochemical testing also has the potential to provide a diagnosis faster and in a more cost-effective manner when evaluating adults with phenotypes possibly consistent with an IEM. Many biochemical screening tests are readily available to most clinicians, require no additional paperwork, and have a relatively quick turnaround time and if suggestive allows for subsequent targeted single gene testing. However, as can be noted even amongst our patient cohort, in many cases whole exome sequencing (WES) may be identified as the cheaper option when compared to panel testing depending on insurance coverage, as was the case for our IEM patients who underwent WES. For context, the Centers for Medicare and Medicaid Services (CMS) uses Current Procedural Terminology (CPT) codes to determine payment for specific testing. While the fee rate for most biochemical analysis falls under $50.00 per test (with many below $25.00 per test), the CMS fee for trio-based clinical exome sequencing is $30,067.00 (though notably self-pay options for trio-based exomes may be as low as $2500), making biochemical screening a cost- and time-effective adjunct to molecular testing [26].
An important aspect of this work also lies in the composition of the field of adult medical genetics. Currently, of all certified American Board of Medical Genetics and Genomics physicians, only 10% are certified in Internal Medicine with 67% certified in Pediatrics.[27] Early medical geneticists in the 1950s were primarily Internal Medicine trained but following the introduction of cytogenetic diagnostic techniques and the subsequent ability to provide diagnoses in childhood, the field transitioned to what it is today [28]. Recent work assessing the medical genetics workforce found adult patients with genetics conditions were a population significantly at risk of not receiving genetic services with approximately half of all adults with genetic conditions waiting more than 5 years for a diagnosis following symptom onset [11]. With the increased use of precision medicine in oncology and the increasing number of combined Internal Medicine-Medical Genetics programs in the US, the number of Internal Medicine trained medical geneticists will hopefully increase. This increase in Internal Medicine-trained medical geneticists will increase awareness and the presence of adult geneticists in subspecialty clinics ideally resulting in increased diagnoses for adults with genetic conditions.
The data presented here has some limitations important to note. The sample was limited to a single provider at a single clinic from a single institution with a relatively small numbers of patients with 3 of the diagnoses part of a sibship. Though we report on only patients seen by a single clinical biochemical geneticist, all referrals with indications possibly consistent with an IEM were triaged to be seen by this geneticist. Additionally, as this is a referral-based population, there is clearly selection bias in that these patients were the ones whom other clinicians felt should be referred to a medical genetics clinic. Unlike many pediatric departments where the majority of genetic testing is performed in a pediatric genetics clinic, at our institution a large number of non-genetic clinics perform genetic testing on adults. As a result, the data presented here likely represents only a fraction of patients seen at our institution during the study time who would qualify for study inclusion. This selection bias is further supported by the limited number of patients seen in our cohort with relatively common IEMs such as MELAS and Fabry disease. Not all patients seen in our clinic underwent a full evaluation as a result of poor insurance coverage, patient/guardian decision to not pursue testing, or were lost to follow-up so were not included in our study.
Despite these limitations, our patient cohort further demonstrates that patients with IEMs can present at any time and with varied presentations. Though IEMs often have symptom-onset during childhood and may be missed, meaningful medical management changes can still occur even if the diagnosis is delayed. Therefore, consideration of this class of disorders during adulthood is imperative. Future studies more inclusive of multi-site clinics with additional providers would be valuable in expanding the understanding of phenotypes and predictive value of testing for IEMs in the adult populations, as well as to expand the visibility of this class of disorders. Though much of the research in this field is geared toward a pediatric population when the majority of patients are diagnosed, this cohort, as well as others, demonstrates that these diagnoses are indeed being made in adulthood. If these patients are to benefit from the treatment modalities being discovered, a diagnosis must first be considered, before it is made.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ymgmr.2020.100653.
Contributor Information
Kristen N. Lee, Email: lekriste@med.umich.edu.
Wendy Uhlmann, Email: WUhlmann@med.umich.edu.
Lauren Hipp, Email: hipplau@med.umich.edu.
Shane C. Quinonez, Email: squinon@med.umich.edu.
Appendix A. Supplementary data
Supplementary material
References
- 1.Waters D., Adeloye D., Woolham D., Wastnedge E., Patel S., Rudan I. Global birth prevalence and mortality from inborn errors of metabolism: a systematic analysis of the evidence. J. Glob. Health. 2018;8(2):1–12. doi: 10.7189/jogh.08.021102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tebani A., Abily-Donval L., Afonso C., Marret S., Bekri S. Clinical metabolomics: the new metabolic window for inborn errors of metabolism investigations in the post-genomic era. Int. J. Mol. Sci. 2016;17(7):1–25. doi: 10.3390/ijms17071167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fukao T., Nakamura K. Advances in inborn errors of metabolism. J. Hum. Genet. 2019;64(65) doi: 10.1038/s10038-018-0535-7. [DOI] [PubMed] [Google Scholar]
- 4.van Karnebeek C.D.M., Stockler S. Treatable inborn errors of metabolism causing intellectual disability: a systematic literature review. Mol. Genet. Metab. 2012;105(3):368–381. doi: 10.1016/j.ymgme.2011.11.191. [DOI] [PubMed] [Google Scholar]
- 5.Saudubray J.M., Mochel F. The phenotype of adult versus pediatric patients with inborn errors of metabolism. J. Inherit. Metab. Dis. 2018;41(5):753–756. doi: 10.1007/s10545-018-0209-9. [DOI] [PubMed] [Google Scholar]
- 6.Sirrs S., Hollak C., Merkel M. The frequencies of different inborn errors of metabolism in adult metabolic centers: report from the SSIEM adult metabolic physicians group. JIMD Rep. 2015:85–93. doi: 10.1007/8904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pérez-López J., Ceberio-Hualde L., García-Morillo J.S. Clinical characteristics of adult patients with inborn errors of metabolism in Spain: a review of 500 cases from university hospitals. Mol. Genet. Metab. Reports. 2017;10:92–95. doi: 10.1016/j.ymgmr.2017.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kemper A.R., Boyle C.A., Brosco J.P., Grosse S.D. Ensuring the life-span benefits of newborn screening. Pediatrics. 2019;144(6):6–10. doi: 10.1542/peds.2019-0904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hope S., Johannessen C.H., Aanonsen N.O., Strømme P. The investigation of inborn errors of metabolism as an underlying cause of idiopathic intellectual disability in adults in Norway. Eur. J. Neurol. 2016;23:36–44. doi: 10.1111/ene.12884. [DOI] [PubMed] [Google Scholar]
- 10.Mochel F., Sedel F. Inborn errors of metabolism in adults: A diagnostic approach to neurological and psychiatric presentations. In: Saudubray J.-M., Baumgartner M.R., Walter J., editors. Inborn Metabolic Diseases. 6th ed. Springer International Publishing; Berlin: 2016. pp. 71–90. [DOI] [Google Scholar]
- 11.Maiese D.R., Keehn A., Lyon M., Flannery D., Watson M. Current conditions in medical genetics practice. Genet. Med. 2019;0(0) doi: 10.1038/s41436-018-0417-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hannah-Shmouni F., Stratakis C.A., Sechi A. Subspecialty training in adult inherited metabolic diseases: a call to action for unmet needs. Lancet Diabetes Endocrinol. 2019;7(2):82–84. doi: 10.1016/S2213-8587(18)30369-3. [DOI] [PubMed] [Google Scholar]
- 13.Gay E.A., Byers P.H., Bennett R.L., Bird T.D., Hisama F.M. Trends over 42 years in the adult medical genetics clinic at the University of Washington. Genet. Med. 2018;0(0):1–5. doi: 10.1038/s41436-018-0329-5. [DOI] [PubMed] [Google Scholar]
- 14.Eble T.N., Nagamani S.C.S., Franco L.M., Plon S.E., Blazo M., Dhar S.U. The practice of adult genetics: a 7-year experience from a single center. Am. J. Med. Genet. A. 2012:89–93. doi: 10.1002/ajmg.a.35684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taylor M.R.G., Edwards J.G., Ku L. Lost in transition: challenges in the expanding field of adult genetics. Am. J. Med. Genet. C. 2006;142C:294–303. doi: 10.1002/ajmg.c. [DOI] [PubMed] [Google Scholar]
- 16.Berry S.A., Brown C., Grant M. Newborn screening 50 years later: access issues faced by adults with PKU. Genet. Med. 2013;15(8):591–599. doi: 10.1038/gim.2013.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Benjamini Y., Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B. 1995;57(1):289–300. doi: 10.1111/j.2517-6161.1995.tb02031.x. [DOI] [Google Scholar]
- 18.Moeschler J.B., Shevell M. Comprehensive evaluation of the child with intellectual disability or global developmental delays. Pediatrics. 2014;134(3):e903–e918. doi: 10.1542/peds.2014-1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Karnebeek C.D.M., Shevell M., Zschocke J., Moeschler J.B., Stockler S. The metabolic evaluation of the child with an intellectual developmental disorder: diagnostic algorithm for identification of treatable causes and new digital resource. Mol. Genet. Metab. 2014;111(4):428–438. doi: 10.1016/j.ymgme.2014.01.011. [DOI] [PubMed] [Google Scholar]
- 20.Engbers H.M., Berger R., Van Hasselt P. Yield of additional metabolic studies in neurodevelopmental disorders. Ann. Neurol. 2008;64(2):212–217. doi: 10.1002/ana.21435. [DOI] [PubMed] [Google Scholar]
- 21.van Karnebeek C.D.M., Scheper F.Y., Abeling N.G. Etiology of mental retardation in children referred to a tertiary care center: a prospective study. Am. J. Ment. Retard. 2005;110(4):253–267. doi: 10.1352/0895-8017(2005)110[253:eomric]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 22.van Karnebeek C.D.M., Jansweijer M.C.E., Leenders A.G.E., Offringa M., Hennekam R.C.M. Diagnostic investigations in individuals with mental retardation: a systematic literature review of their usefulness. Eur. J. Hum. Genet. 2005;13(1):6–25. doi: 10.1038/sj.ejhg.5201279. [DOI] [PubMed] [Google Scholar]
- 23.Sempere A., Arias A., Farré G. Study of inborn errors of metabolism in urine from patients with unexplained mental retardation. J. Inherit. Metab. Dis. 2010;33(1):1–7. doi: 10.1007/s10545-009-9004-y. [DOI] [PubMed] [Google Scholar]
- 24.Papavasiliou A.S., Bazigou H., Paraskevoulakos E., Kotsalis C. Neurometabolic testing in developmental delay. J. Child Neurol. 2000;15(9):620–622. doi: 10.1177/088307380001500909. http://repository.upi.edu/1360/1/s_d5451_0604180_chapter1.pdf [DOI] [PubMed] [Google Scholar]
- 25.Sedel F., Lyon-Caen O., Saudubray J.M. Therapy insight: inborn errors of metabolism in adult neurology - a clinical approach focused on treatable diseases. Nat. Clin. Pract. Neurol. 2007;3(5):279–290. doi: 10.1038/ncpneuro0494. [DOI] [PubMed] [Google Scholar]
- 26.CMS Clinical Laboratory Fee Schedule Files. 2020. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/ClinicalLabFeeSched/Clinical-Laboratory-Fee-Schedule-Files.html Published 2019. Accessed July 5, 2019.
- 27.The American Board of Medical Specialties Cross-Certification Summary, 2017: Diplomates Currently Certified by the ABMGG and other ABMS Member Boards. 2020. http://www.abmgg.org/pdf/Cross-Certification%20Summary%20with%20Other%20ABMS%20Boards.pdf Accessed September 13, 2020.
- 28.Rimoin D.L., Hirschorn H. A history of genetics in pediatrics. Pediatr. Res. 2004;56:150–159. doi: 10.1203/01.PDR.0000129659.32875.84. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material