Abstract
SENP3, a sentrin/SUMO2/3-specific protease, is recognized as a transcriptional factor that accumulates under cellular oxidative stress and plays a significant role in the removal of SUMO2/3 modification. In our study, we examined a TCGA dataset and found that the transcripts per million (TPM) value of SENP3 is high in sarcoma, including osteosarcoma (OS). We found that SENP3 was highly expressed in OS cancer tissues when compared with osteofibrous dysplasia tissues. The survival data of SENP3 in TCGA showed that the sarcoma patients with higher SENP3 expression levels showed poor prognosis. In vitro, SENP3 knockdown in OS cancer cells inhibited cell proliferation, migration, and invasion and induced apoptosis. In contrast, SENP3 overexpression reversed these effects. Next, we found that SENP3 inhibited the expression of E-cadherin (E-Cad) by increasing methylation of the E-Cad promoter. Finally, E-Cad expression was increased in the OS cell line MG63 following methylation, and the cell proliferation, migration, and invasion capacity were decreased. In summary, SENP3 played a significant role in OS carcinogenesis and may act as a potential biomarker in the diagnosis and treatment of OS.
Keywords: osteosarcoma, SENP3, cancer metastasis, DNA methylation, E-cadherin
Introduction
Osteosarcoma is known as the most common aggressive and malignant tumor from bone, with a peak in incidence during childhood and adolescence.1 The incidence of osteosarcoma is estimated at 5 per million people.2 Osteosarcoma accounted for approximately 5% of childhood cancers and also almost 8.9% of childhood cancer-related deaths.3 The high aggressiveness of OS leads to cancer spread within the diaphysis; migration to soft tissues, nerves, and vessels; and tumor metastasis in the lung, indicating the poor prognosis of OS.4 Despite the development of complex technology for treatment in recent decades, early diagnoses and molecule-targeted strategies against metastatic OS have not achieved remarkable progress.5 The clinical outcomes and prognosis of OS have not noticeably improved, and the 5-year survival of OS patients is less than 50%.6 The slow development of diagnostic and targeted therapy strategies is mainly due to the limited understanding of the biology underpinning the high malignancy of OS.7
Epithelial-mesenchymal transition (EMT) of cancer cells plays a significant role in cancer metastasis.8,9 Cadherins exert functions at intercellular level, mainly communicating by cell connections through calcium ion-dependent binding. Instead, at an intracellular level, cadherins bind to catenin molecules establishing links with the cytoskeleton.10 The remarkable increase in N-cadherin and decrease in E-cadherin (E-Cad) is the major biological feature of EMT, resulting in stroma-oriented adhesion and conversion of epithelial cells to a mesenchymal phenotype.11,12 Accumulated evidence has shown that many signaling pathways are involved in accelerating the EMT of OS cells and are essential to OS development and pathological processes. Cadherins is the emerging targets appeared with reference to the OS microenvironment and critical proteins in OS regulation circuits.13 The transforming growth factor-β (TGF-β) signaling pathway can significantly promote invasion and metastasis by inducing EMT in OS cells.14,15 The Wnt-β-catenin pathway plays an oncogenic role in OS by increasing the expression of key molecules of EMT, such as N-cadherin and vimentin.16,17 In addition, the dysregulated expression of the non-coding RNAs involved in regulating EMT progression plays a crucial role in human EMT-induced carcinogenesis.18,19
SENP3 is a sentrin/SUMO2/3-specific protease and a critical redox-sensitive molecule that participates in the removal of SUMO2/3 modification. Recent studies have shown that SENP3 can serve as a core regulator of proliferation, tumorigenesis, angiogenesis, and EMT in cancer cells.20-22 However, the involvement and function of SENP3 in OS carcinogenesis progression and its clinical and prognostic value in OS are unclear. Here, we tested SENP3 expression in OS tissues and examined its function and molecular mechanism in OS cell lines. Our results lend novel insights into the regulation of SENP3, which has key roles in OS progression and may aid in identifying new biomarkers or targeted therapies for OS.
Materials and Methods
Cell Lines and Tissue
The human OS cell line MG63 was purchased from the Cancer Research Institute (Central South University, Hunan, China) and cultured in RPMI 1640 supplemented with 10% fetal bovine serum (FBS, Gibco, Carlsbad, CA, USA) and 1% penicillin under a humidified atmosphere of 5% CO2 at 37°C. Human OS tissues, osteofibrous dysplasia (OFD) tissues and 40 OS Paraffin section were collected from patients who underwent surgical operation and were pathologically diagnosed at the Third Xiangya Hospital of Central South University from January 2017 to December 2018. All patients provided informed consent before undergoing surgical biopsy. All experiments were approved by the Ethics Committee of the Third Xiangya Hospital of Central South University.
Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
Tissue RNA was extracted from cells using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). For qRT-PCR, RNA from the samples was reverse transcribed using a PrimeScript RT Reagent Kit (Takara, Dalian, China). For Twist and E-Cad expression, qRT-PCR was performed using a SYBR Premix Ex Taq II Kit (Takara, Dalian, China) with the CFX96 Real-Time PCR Detection System (Bio-Rad, Hercules, CA, USA). PCR amplification was performed in triplicate. The following qRT-PCR primers were used:
E-Cad Real-time F: 5’-CAGCCTTCTTTTCGGAAGACT-3
E-Cad Real-time R: 5’-GGTAGACAGCTCCCTATGACTG-3
SENP3 Real-time F: 5′ -GGAGTCCGCAGTCTTACGAG-3′
SENP3 Real-time R: 5′ -TCTGGAGGACCTGGTAGAGG-3′
Actin Real-time F: 5’-GCTATGTCGCCCTGGATTTC-3′
Actin Real-time R: 5′ -CACAGGACTCCATACCCAAGAA-3′
Western Blotting
Biopsy tissue cells and cancer cells were washed with PBS and then lysed on ice using RIPA buffer containing 10% proteinase inhibitor (Roche Applied Science, Basel, Switzerland) for 0.5 h. Proteins were quantified using a Total Protein Extraction Kit (ProMab, Richmond, CA, USA), separated using a 10% SDS-polyacrylamide gel, and transferred to a polyvinylidene fluoride membrane. The membranes were incubated with the following primary antibodies: anti-Twist (1:1000, Abcam, Cambridge, UK; ab50887), anti-E-Cad (1:50, Abcam, ab15148), and anti-β-Actin (1:2000, Cell Signaling Technology, Danvers, MA, USA; #3700 S) at 4°C overnight; then, HRP-conjugated secondary antibodies were added at 37°C for 1 h. The signals of the immunoreactive protein bands were assessed using densitometric analysis software and quantified by densitometry using the ImageJ system. β-actin served as a loading control.
Invasion and Migration Assays
The migration capacity of MG63 cells was evaluated using a wound-healing assay. Cells (1 × 105 per mL) were cultivated in 6-well plates and grown to 85–90% confluence. A 1-µL tip was used to scratch a wound in the middle of the monolayer, and the dislodged cellular debris was washed away with 10% PBS. The cells were cultured in a humidified atmosphere of 5% CO2 at 37°C. The wound was photographed at 0 h and 48 h under a microscope.
The invasion capacity of MG63 was detected using a Transwell assay. Cells (2 × 105) in 200 µL serum-free medium were added to the top of an apical chamber; the lower chamber contained 500 µL of 20% FBS. After incubation for 48 h at 37°C, the invaded cells that traversed the membrane were fixed with 100% methanol and stained with 0.1% crystal violet. Cells remaining on the upper chamber surface of the Transwell membrane were wiped off using a cotton bud. The number of invaded tumor cells was captured randomly at 6 visual fields at 20× magnification.
Cell Proliferation Bioassays
The pre-treated MG63 cells were cultivated in 96-well plates (500 cells per well). Cell proliferation after co-culture or transfection was determined using a commercial 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay kit (Sigma Aldrich, St. Louis, MO, USA) according to the manufacturer’s instructions. First, 50 µL MTT was added to cells in each well and incubated for 4 h. Then, 150 µL dimethyl sulfoxide was added to dissolve the MTT crystals. The light absorption value of each well was measured using a spectrometer at 570 nm. All assays were repeated at least 3 times in triplicate wells.
Cell Apoptosis Assays
Cell cycle and apoptosis assays were performed using flow cytometry. The pre-treated MG63 cells were cultivated in 6-well plates for 48 h and then harvested by centrifugation. For apoptosis assays, cells were trypsinized, washed, resuspended in 200 µL binding buffer, and then evaluated for apoptosis by double staining with 5 µL 7-aminoactinomycin D and 5 µL annexin V (all from BD Biosciences, San Jose, CA, USA) using a FACSCalibur Flow Cytometer.
DNA Methylation Analysis
Tissue DNA was extracted from cells using the phenol/chloroform method. The bisulfite detection method was used to examine the methylation status of the E-Cad promoter region.
For bisulfite conversion, the QIAGEN EpiTect Bisulfite Kit (QIAGEN, Hilden, Germany) was used according to the manufacturer’s instructions. DNA methylation was determined by PCR, and the primer sequences used to examine the methylation status of promoter region DNA are as follows:
E-Cad-Met F1: 5’-TGTAGTTACGTATTTATTTTTAGTGGCGTC-3’
E-Cad-Met R1: 5’-CGAATACGATCGAATCGAACCG-3’ (methylation primers)
E-Cad-unMet F1: 5’-TGGTTGTAGTTATGTATTTATTTTTAGTGGTGTT-3’
E-Cad-unMet R1: 5’-ACACCAAATACAATCAAATCAAACCAAA-3’ (non-methylation primers)
Statistical Analysis
The expression levels of SENP3 and E-Cad in OS and OFD tissues were analyzed by unpaired t-test. The statistical software package GraphPrism version 5.0 (GraphPad Software, Inc., La Jolla, CA, USA) was used for all statistical analyses. All experiments were performed in triplicate at the minimum, and the results of the analysis were considered significant in a log-rank test if p < 0.05.
Results
SENP3 Is Highly Expressed in Sarcomas and OS
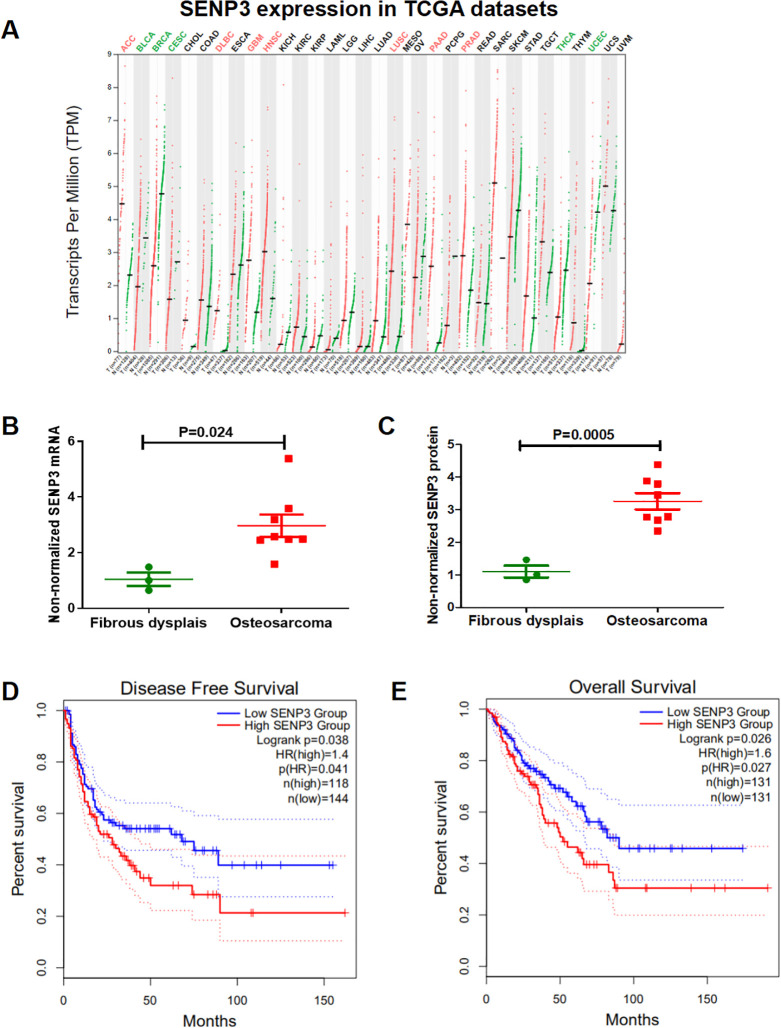
The TCGA dataset showed that the transcripts per million (TPM) value of SENP3 is the highest in many types of human cancers (Figure 1A). To assess SENP3 function in OS, we examined SENP3 expression in 8 OS and 3 OFD tissues using qRT-PCR and western blotting. The results showed that SENP3 was overexpressed in 8 OS tissues when compared with OFD tissues (Figure 1B and C). Based on the TCGA data, the high expression of SENP3 in sarcoma patients indicated a shorter disease-free survival (Figure 1D) and overall survival (Figure 1E). Then we measured the expression clinicopathologic characteristic of SENP3 on paraffin-cut section of 40 OS patients by immunohistochemical.The expression of SENP3 was significantly related with TNM stages (p = 0.0455), and distant metastasis (p = 0.04223; Table 1). In conclusion, SENP3 is potentially an oncogene in sarcoma and may influence the prognosis of sarcoma patients.
Figure 1.
SENP3 is highly expressed in sarcomas and osteosarcoma. (A) The transcripts per million (TPM) value of SENP3 is the highest of many human cancer types. (B) SENP3 mRNA expression was higher in OS tissues (tumor, n = 8; osteofibrous dysplasia, n = 3; p = 0.024). (C) SENP3 protein expression was higher in OS tissues (tumor, n = 8; osteofibrous dysplasia, n = 3; p = 0.0005). (D, E) The TCGA dataset showed shorter disease-free survival (p = 0.038) and overall survival (p = 0.026) with high SENP3 expression in sarcoma.All the TCGA data set was obtained from GEPIA website.
Table 1.
SENP3 Expression and Clinicopathologic Characteristic on 40 Specimens.
| SENP3 expression | ||||
|---|---|---|---|---|
| Characteristics | Total | low | high | p value |
| Age(years) | ||||
| ≥40 | 18 | 8 | 10 | 0.25734 |
| ≤40 | 22 | 6 | 16 | |
| Gender | ||||
| male | 28 | 11 | 17 | 0.26721 |
| female | 12 | 7 | 5 | |
| TNM stage | ||||
| I-II | 16 | 9 | 7 | 0.0455* |
| III-Iv | 24 | 6 | 18 | |
| Distant metastasis | ||||
| negative | 30 | 20 | 10 | 0.04223* |
| positive | 10 | 3 | 7 | |
| Lymph node metastasis | ||||
| negative | 23 | 11 | 12 | 0.96433 |
| positive | 17 | 8 | 9 | |
| Pathological Type | ||||
| conventional OS | 34 | 16 | 18 | 0.37592 |
| Low-Grade Central OS | 6 | 4 | 2 | |
SENP3 expression and clinicopathologic characteristics on 40 OS specimens.
E-Cad Is Downregulated in OS, and the Promoter Region Shows Abnormal Methylation of CpG Islands
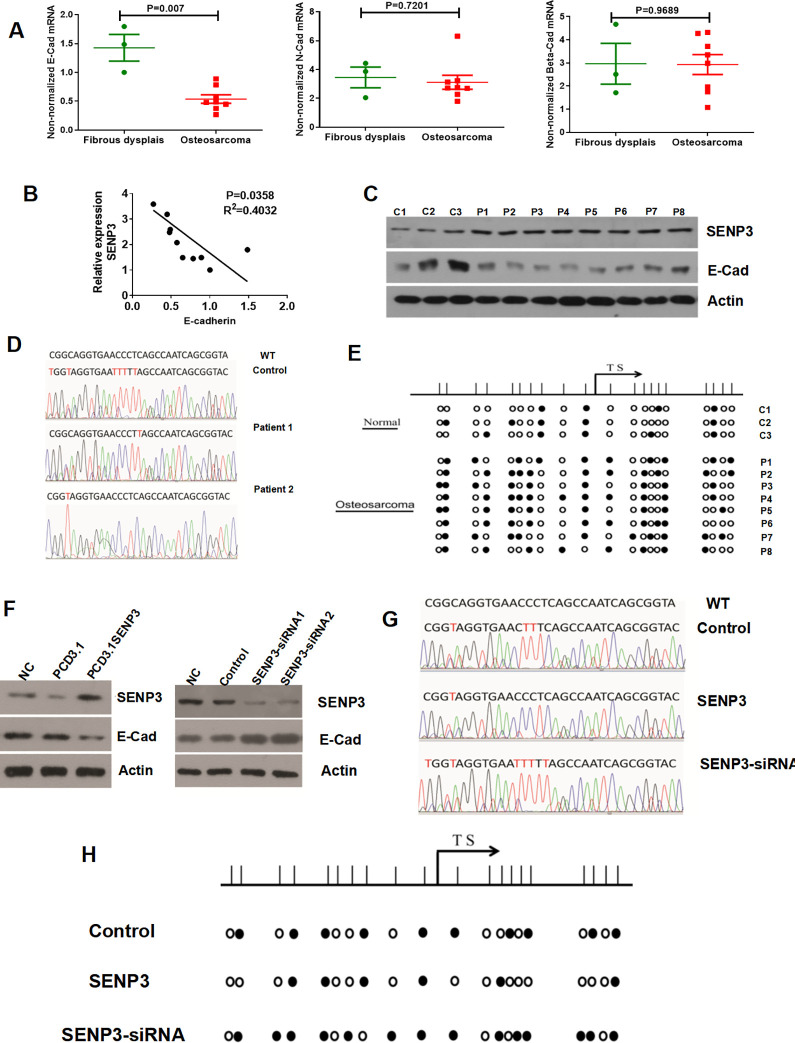
Since SENP3 can serve as a core EMT regulator in both the occurrence and development of cancers,20 we examined the expression of EMT-related factors. The results showed lower E-Cad mRNA expression in 8 OS tissues when compared with OFD tissues the N-Cad and Beta-Cad expression have not shown significant difference (Figure 2A). The correlation analysis demonstrated that the expression of E-Cad mRNA was negatively correlated with SENP3 expression in OS and OFD tissues (p = 0.035, Figure 2B). Western blotting analysis also confirmed these results (Figure 2C). We checked the DNA methylation of E-Cad. The bisulfite conversion results showed that the E-Cad promoter region of patients 1 and 2 had highly methylated bases compared with the control (Figure 2D). The PCR results showed that the E-Cad promoter region had more abnormal methylation of CpG islands in 8 OS patients compared with 3 OFD patients (Figure 2E).
Figure 2.
E-cad is downregulated in osteosarcoma, and the promoter region shows abnormal methylation of CpG islands. (A) E-Cad mRNA expression was lower in OS tissues than in osteofibrous dysplasia (Tumor, n = 8; osteofibrous dysplasia, n = 3; p = 0.007)N-Cad and Beta mRNA expression have not shown significant difference. (B) E-Cad mRNA expression was negatively correlated with SENP3 expression in OS and osteofibrous dysplasia tissues (tumor, n = 8; osteofibrous dysplasia, n = 3; p = 0.035). (C) The expression of SENP3 and E-Cad protein (tumor, n = 8; osteofibrous dysplasia, n = 3). (D) The E-Cad promoter regions of patients 1 and 2 had highly methylated bases compared with the control. (E) The E-Cad promoter region showed more abnormal methylation of CpG islands in 8 OS patients compared with 3 osteofibrous dysplasia patients. (F) SENP3 overexpression in MG63 cells decreased E-Cad protein expression. Silencing of SENP3 expression in MG63 cells increased E-Cad protein expression. (G) Bisulfite conversion showed that SENP3 knockdown and overexpression in MG63 cells decreased and increased the methylated base levels of E-Cad, respectively. (H) The PCR results also confirmed the results of bisulfite conversion.
SENP3 Regulates E-Cad Expression and Induces the DNA Methylation of E-Cad
As SENP3 was highly expressed in OS and its expression was negatively related to E-Cad, we explored the effect of SENP3 on E-Cad expression. Western blotting analysis showed that SENP3 overexpression in MG63 cells decreased the protein expression of E-Cad, and silencing SENP3 expression in MG63 cells increased the protein expression of E-Cad (Figure 2F). To investigate the impact of SENP3 on DNA methylation of E-cad, we detected methylated bases using bisulfite conversion. Knockdown of SENP3 expression decreased the methylated base level of E-Cad, whereas SENP3 overexpression in MG63 cells increased the methylated base level of E-Cad (Figure 2G). The PCR results also confirmed these results (Figure 2H).
SENP3 Regulates Cell Invasion, Migration, Proliferation, and Apoptosis in MG63 Cells
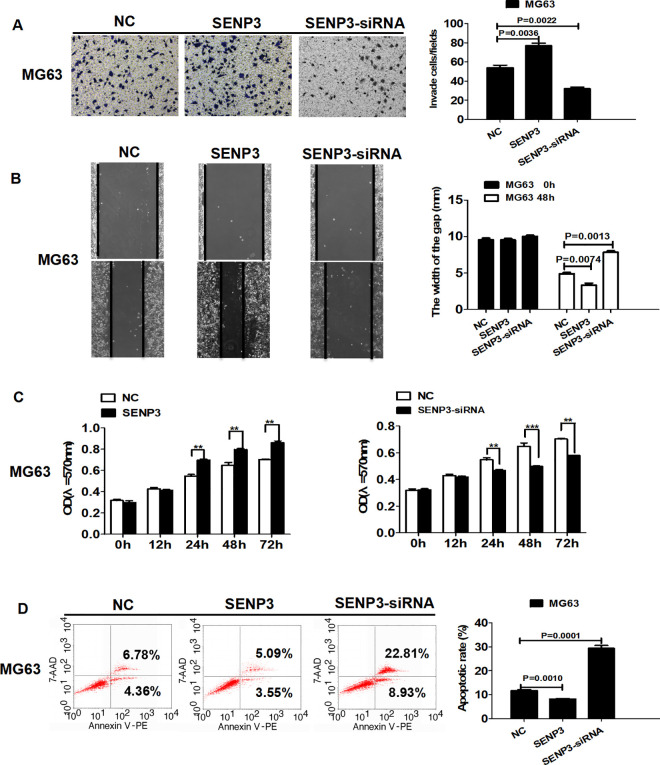
As SENP3 regulates E-Cad expression, we further explored the effect of SENP3 on invasion, migration, proliferation, and apoptosis of MG63 cells. The effects of silencing or overexpression of SENP3 on cell invasion and migration were examined by Transwell and wound-healing assays, respectively. Silencing SENP3 expression decreased cell invasion and migration, whereas overexpressing SENP3 expression increased cell migration (Figure 3A and B). Cell proliferation and apoptosis were detected by MTT and flow cytometry, respectively. Knockdown of SENP3 expression decreased cell proliferation and increased apoptosis, while overexpressing SENP3 increased cell proliferation and decreased apoptosis (Figure 3C and D).
Figure 3.
SENP3 regulates cell invasion, migration, proliferation, and apoptosis in MG63 cells. (A) SENP3 overexpression increased the invasion of MG63 cells (p = 0.0036). SENP3 knockdown decreased cell invasion (p = 0.0022). (B) SENP3 overexpression increased the migration of MG63 cells (p = 0.0074). SENP3 knockdown decreased cell migration (p = 0.0013). (C) SENP3 overexpression increased the proliferation of MG63 cells. SENP3 knockdown decreased cell proliferation. (D) SENP3 overexpression decreased cell apoptosis in MG63 cells (p = 0.0010), and SENP3 knockdown increased cell apoptosis (p = 0.00011).
DNA Methylation Inhibitors Can Reverse SENP3-Induced Methylation Effects on E-Cad and Cell Phenotypes
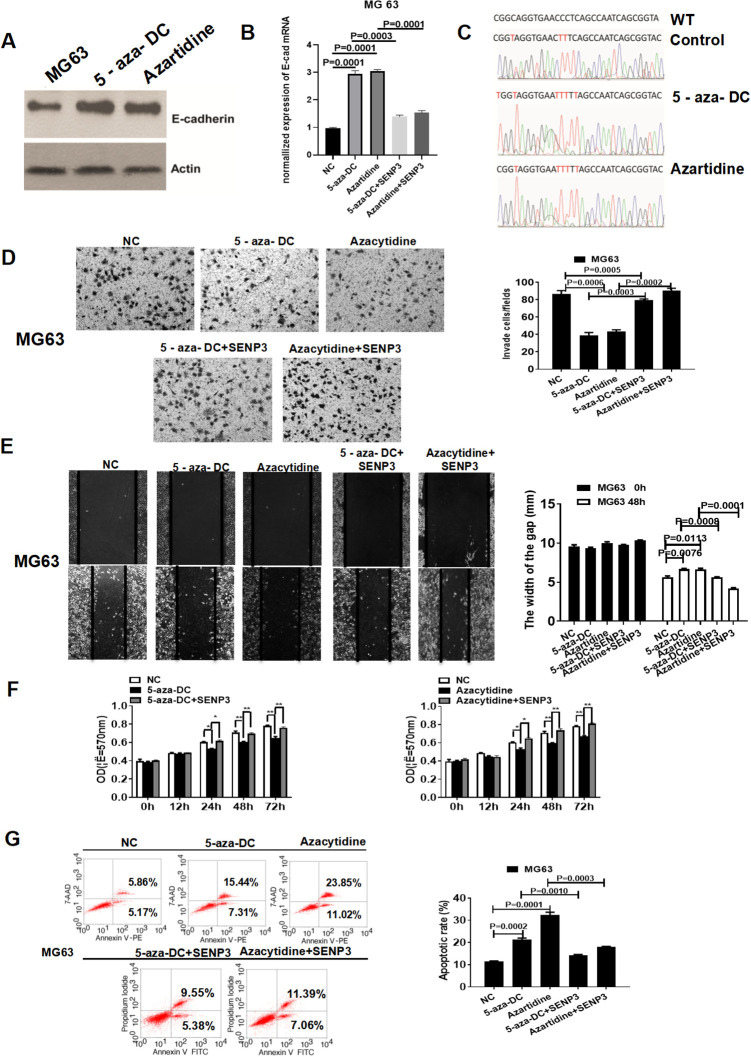
We further examined the effect of DNA methylation inhibitors on E-Cad expression and DNA methylation status. We treated MG63 cells with the DNA methylation inhibitors azartidine and 5-aza-DC. The DNA methylation inhibitors increased E-Cad expression, reversed SENP3-induced E-Cad downregulation (Figure 4A and B), and decreased the DNA methylation of E-Cad (Figure 4C). The Transwell and wound-healing assays indicated that DNA methylation inhibitors reversed the SENP3-induced promotion of the invasion, migration, proliferation, and apoptosis of MG63 cells (Figure 4D-G).
Figure 4.
DNA methylation inhibitors can reverse SENP3-induced methylation effects on E-Cad and cell phenotypes. (A, B) The DNA methylation inhibitors increased the expression of E-cad protein and reversed the SENP3-induced downregulation of E-Cad. (C) The DNA methylation inhibitors decreased the DNA methylation of E-Cad. (D) The DNA methylation inhibitors reversed SENP3-induced promotion of cell invasion of MG63 cells (5-aza-DC-treated group, p = 0.0008; Azartidine-treated group, p = 0.0006; 5-aza-DC + SENP3-treated group, p = 0.0003; Azartidine + SENP3-treated group, p = 0.0002). (E) The DNA methylation inhibitors reversed the SENP3-induced promotion of MG63 cell migration (5-aza-DC-treated group, p = 0.0076; Azartidine-treated group, p = 0.0113; 5-aza-DC + SENP3-treated group, p = 0.0008; Azartidine + SENP3-treated group, p = 0.0001). (F) The DNA methylation inhibitors reversed the SENP3-induced promotion of MG63 cell proliferation. (G) The DNA methylation inhibitors reversed the SENP3-induced apoptosis of MG63 cells (5-aza-DC-treated group, p = 0.0002; Azartidine-treated group, p = 0.0001; 5-aza-DC + SENP3-treated group, p = 0.0010; Azartidine + SENP3-treated group, p = 0.0003).
Discussion
OS is a highly malignant bone tumor that remains the major cause of mortality in bone tumors worldwide.23 The incidence of OS is high in childhood and adolescence, with an annual global incidence rate of about 3 in 1 million.24,25 To enhance OS therapy, new molecular targets for its diagnosis and prognosis must be identified, and new treatments must be developed.
As a member of the SUMO-specific protease family, the function of SENP3 is known to regulate deSUMOylation of chromosome-associated proteins and chromosome stability in multiple cellular biological processes.26,27 Hypoxia is a common phenomenon in human solid tumors. Recent studies have shown that SENP3 is a sensitive redox sensor, as SENP3 upregulation in cells is triggered by oxidative modification and is responsible for HIF-1α transactivation. These studies indicated that SENP3 may play a key role in tumor development.28,29
Growing evidence has confirmed that SENP3 is highly expressed in some types of malignant tumors, such as head and neck cancer,30 ovarian cancer,31 and gastric cancer.22 Our findings are consistent with those of previous studies. Here, we firstly report that the TPM of SENP3 in sarcoma is the highest among many human cancer types, as analysis of a TCGA dataset demonstrated that SENP3 was upregulated in OS compared to that in non-tumor tissues. Additionally, SENP3 upregulation was significantly correlated with poor prognosis in sarcoma, suggesting that SENP3 may serve as a diagnostic biomarker and a novel therapeutic target for OS. Furthermore, upregulating SENP3 significantly promoted the proliferation, migration, and invasion of OS cancer cell lines, downregulating SENP3 by shRNA markedly inhibited MG63 proliferation, migration, and invasion, these indicated that SENP3 can act as an essential character in the development of OS cancer and may be an importantly oncogene of OS.
EMT is one of the core mechanisms for transforming epithelial cells into epithelial cancer cells by imparting migratory and invasive capabilities.32 There is growing evidence that transcription factors participate in the biological processes of EMT and that abnormal expression of some transcription factors can promote malignant tumor occurrence and development by inducing EMT progression, including ZEB1,33 STAT3,34 TWIST1,35 and FOXQ1.36 This paper firstly reports SENP3 as a transcription factor that can regulate the EMT of OS cells by inducing the DNA methylation of E-Cad, and our research provides a novel perspective in understanding the role of the SUMO-specific protease family in OS initiation and promotion.
E-Cad is a hallmark of adherens junctions, which play a pivotal role in maintaining the stability of epithelial cells, and a decrease in E-Cad expression can trigger EMT in cancer.37 The cadherin switch hypothesis indicates that the loss of E-Cad and increases in N-cadherin and vimentin are the major features of EMT.37,38 Downregulation of E-Cad promotes tumor invasion and migration, and post-translational modification is the main manner by which E-cad transcriptional activity is regulated, including acetylation, methylation, and poly- and mono-ubiquitination.39,40 In ovarian cancer-Knockdown of SENP3 decreased the proliferation, migration, and invasion capability of ovarian cancer cells, down-regulated the expression of N-cadherin, and resulted in upregulation of p21 and E-cadherin, which indicated that SENP3 was a redox-sensitive molecule mediating the epithelial-mesenchymal transition.31 Consistent with these results, in our study, SENP3 regulated E-Cad expression and induced the DNA methylation of E-cad in the OS carcinogenesis process, hence providing the first evidence for the SENP3/E-Cad EMT axis in OS cells. However, the exact mechanism by which SENP3 regulates the DNA methylation of E-Cad needs to be investigated further.
In conclusion, SENP3 plays key role in the development of osteosarcoma.SENP3 expression was highly expressed in OS, and SENP3 upregulation significantly increased the invasion, migration, and proliferation abilities and suppressed apoptosis of OS cells. Furthermore, SENP3 is potentially an oncogene in OS and may influence the prognosis of sarcoma patients. The inhibition or overexpression of SENP3 expression can respectively decrease or increase the DNA methylation of E-Cad. Our research indicates that SENP3 may be a prognostic biomarker and an actionable target for novel tumor therapies for OS.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Zhang Hong Lian  https://orcid.org/0000-0002-0143-5757
https://orcid.org/0000-0002-0143-5757
References
- 1. Ritter J, Bielack SS. Osteosarcoma. Ann Oncol. 2010;21(7):320–325. [DOI] [PubMed] [Google Scholar]
- 2. Ottaviani G, Jaffe N. The epidemiology of osteosarcoma. Cancer Treat Res. 2009;152:3–13. doi:10.1007/978-1-4419-0284-9_1 [DOI] [PubMed] [Google Scholar]
- 3. Fan TM, Khanna C. Comparative aspects of osteosarcoma pathogenesis in humans and dogs. Vet Sci. 2015;2(3):210–230. doi:10.3390/vetsci2030210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Savage SA, Mirabello L. Bone cancer: is the osteosarcoma genome targetable? Nat Rev Endocrinol. 2017;13(9):506–508. [DOI] [PubMed] [Google Scholar]
- 5. Lagmay JP, Krailo MD, Dang H, Kim A. Outcome of patients with recurrent osteosarcoma enrolled in seven phase II trials through Children’s Cancer Group, Pediatric Oncology Group, and Children’s Oncology Group: learning from the past to move forward. J Clin Oncol. 2016;34(25):3031–3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Casado-Zapico S, Rodriguez-Blanco J, Garcia-Santos G, et al. Synergistic antitumor effect of melatonin with several chemotherapeutic drugs on human Ewing sarcoma cancer cells: potentiation of the extrinsic apoptotic pathway. J Pineal Res. 2010;48(1):72–80. doi:10.1111/j.1600-079X.2009.00727.x [DOI] [PubMed] [Google Scholar]
- 7. Kansara M, Teng MW, Smyth MJ, Thomas DM. Translational biology of osteosarcoma. Nat Rev Cancer. 2014;14(11):722–735. [DOI] [PubMed] [Google Scholar]
- 8. Mittal V. Epithelial mesenchymal transition in tumor metastasis. Annu Rev Pathol. 2018;13:395–412. [DOI] [PubMed] [Google Scholar]
- 9. Pang A, Carbini M, Moreira AL, Maki RG. Carcinosarcomas and related cancers: tumors caught in the act of epithelial-mesenchymal transition. J Clin Oncol. 2018;36(2):210–216. [DOI] [PubMed] [Google Scholar]
- 10. Jiang Z, Cinti C, Taranta M, et al. Network assessment of demethylation treatment in melanoma: differential transcriptome-methylome and antigen profile signatures. PLoS One. 2018;13(11):e0206686 doi:10.1371/journal.pone [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Braga V. Spatial integration of E-cadherin adhesion, signalling and the epithelial cytoskeleton. Curr Opin Cell Biol. 2016;42:138–145. [DOI] [PubMed] [Google Scholar]
- 12. Frame MC, Inman GJ. NCAM is at the heart of reciprocal regulation of E-cadherin- and integrin-mediated adhesions via signaling modulation. Dev Cell. 2008;15(4):494–496. [DOI] [PubMed] [Google Scholar]
- 13. Sharma A, Cinti C, Capobianco E. Multitype network-guided target controllability in phenotypically characterized osteosarcoma: role of tumor microenvironment. Front Immunol. 2017;8:918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jiang X, Zhang Z, Zhang Q, et al. Glaucocalyxin A reverses EMT and TGF-β1-induced EMT by inhibiting TGF-β1/Smad2/3 signaling pathway in osteosarcoma. Chem Biol Interact. 2019;307:158–166. [DOI] [PubMed] [Google Scholar]
- 15. Chen Y, Zhang K, Li Y, He Q. Estrogen-related receptor α participates transforming growth factor-β (TGF-β) induced epithelial-mesenchymal transition of osteosarcoma cells. Cell Adh Migr. 2017;11(4):338–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tian H, Zhou T, Wang JC, Murray SS. Bone morphogenetic protein-2 promotes osteosarcoma growth by promoting epithelial-mesenchymal transition (EMT) through the Wnt/β-catenin signaling pathway. J Orthop Res. 2019;37(7):1638–1648. [DOI] [PubMed] [Google Scholar]
- 17. Lv YF, Dai H, Yan GN, Meng G, Zhang X, Guo QN. Downregulation of tumor suppressing STF cDNA 3 promotes epithelial-mesenchymal transition and tumor metastasis of osteosarcoma by the Wnt/GSK-3β/β-catenin/Snail signaling pathway. Cancer Lett. 2016;373(2):164–173. [DOI] [PubMed] [Google Scholar]
- 18. Jiang R, Zhang C, Liu G, Gu R, Wu H. MicroRNA-126 inhibits proliferation, migration, invasion, and EMT in osteosarcoma by targeting ZEB1. J Cell Biochem. 2017;118(11):3765–3774. [DOI] [PubMed] [Google Scholar]
- 19. Zhang X, Zhang Y, Mao Y, Ma X. The lncRNA PCAT1 is correlated with poor prognosis and promotes cell proliferation, invasion, migration and EMT in osteosarcoma. Onco Targets Ther. 2018;11:629–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Huang C, Han Y, Wang Y, et al. SENP3 is responsible for HIF-1α transactivation under mild oxidative stress via p300 de-SUMOylation. EMBO J. 2009;28(18):2748–2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Han Y, Huang C, Sun X, et al. SENP3-mediated de-conjugation of SUMO2/3 from promyelocytic leukemia is correlated with accelerated cell proliferation under mild oxidative stress. J Biol Chem. 2010;285(17):12906–12915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ren Y, Liu K, Wang M, et al. De-SUMOylation of FOXC2 by SENP3 promotes the epithelial-mesenchymal transition in gastric cancer cells. Oncotarget. 2014;5(16):7093–7104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. [DOI] [PubMed] [Google Scholar]
- 24. He X, Gao Z, Xu H, Zhang Z, Fu P. A meta-analysis of randomized control trials of surgical methods with osteosarcoma outcomes. J Orthop Surg Res. 2017;12(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lindsey BA, Markel JE, Kleinerman ES. Osteosarcoma overview. Rheumatol Ther. 2017;4(1):25–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liu K, Guo C, Yang J, et al. A fine-tuning mechanism underlying self-control for autophagy: deSUMOylation of BECN1 by SENP3. Autophagy. 2020;6(6):975–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lao Y, Yang K, Yang J, et al. DeSUMOylation of MKK7 kinase by the SUMO2/3 protease SENP3 potentiates lipopolysaccharide-induced inflammatory signaling in macrophages. J Biol Chem. 2018;293(11):3965–3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wang Y, Yang J, Yang K, et al. The biphasic redox sensing of SENP3 accounts for the HIF-1 transcriptional activity shift by oxidative stress. Acta Pharmacol Sin. 2012;33(7):953–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bhattacharjee J, Alahari S, Post M, Caniggia I, Sallais J, Tagliaferro A. Dynamic regulation of HIF1αstability by SUMO2/3 and SENP3 in the human placenta. Placenta. 2016;40:8–17. [DOI] [PubMed] [Google Scholar]
- 30. Zhou Z, Wang M, Li J, et al. SUMOylation and SENP3 regulate STAT3 activation in head and neck cancer. Oncogene. 2016;35(45):5826–5838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cheng J, Su M, Shi N, et al. Upregulation of SENP3/SMT3IP1 promotes epithelial ovarian cancer progression and forecasts poor prognosis. Tumour Biol. 2017;39(3):10. [DOI] [PubMed] [Google Scholar]
- 32. Cho ES, Kang HE, Kim NH, Yook JI. Therapeutic implications of cancer epithelial-mesenchymal transition (EMT). Arch Pharm Res. 2019;42(1):14–24. [DOI] [PubMed] [Google Scholar]
- 33. Zhang Y, Xu L, Li A, Han X. The roles of ZEB1 in tumorigenic progression and epigenetic modifications. Biomed Pharmacother. 2019;110:400–408. [DOI] [PubMed] [Google Scholar]
- 34. Wendt MK, Balanis N, Carlin CR, Schiemann WP. STAT3 and epithelial-mesenchymal transitions in carcinomas. JAKSTAT. 2014;3(1):e28975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wu KJ, Yang MH. Epithelial-mesenchymal transition and cancer stemness: the Twist1-Bmi1 connection. Biosci Rep. 2011;31(6):449–455. [DOI] [PubMed] [Google Scholar]
- 36. Li Y, Zhang Y, Yao Z, Li S, Yin Z, Xu M. Forkhead box Q1: a key player in the pathogenesis of tumors (Review). Int J Oncol. 2016;49(1):51–58. [DOI] [PubMed] [Google Scholar]
- 37. Bruner HC, Derksen PWB. Loss of E-cadherin-dependent cell-cell adhesion and the development and progression of cancer. Cold Spring Harb Perspect Biol. 2018;10(3):a029330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Christofori G, Semb H. The role of the cell-adhesion molecule E-cadherin as a tumour-suppressor gene. Trends Biochem Sci. 1999;24(2):73–76. [DOI] [PubMed] [Google Scholar]
- 39. Park SM, Gaur AB, Lengyel E, Peter ME. The miR-200 family determines the epithelial phenotype of cancer cells by targeting the E-cadherin repressors ZEB1 and ZEB2. Genes Dev. 2008;22(7):894–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Federico A, Sepe R, Monti M, Fusco A. The complex CBX7-PRMT1 has a critical role in regulating E-cadherin gene expression and cell migration. Biochim Biophys Acta Gene Regul Mech. 2019;1862(4):509–521. [DOI] [PubMed] [Google Scholar]