Abstract
Background:
Circulating tumor cells (CTCs) hold huge potential for both clinical applications and basic research into the management of cancer, but the relationship between CTC count and cervical cancer prognosis remains unclear. Therefore, research on this topic is urgently required.
Objective:
This study investigated whether CTCs were detectable in patients with cervical cancer and whether CTC count was an indicator of prognosis.
Methods:
We enrolled 107 patients with pathologically confirmed cervical cancer. CTCs were detected after radiotherapy or concurrent cisplatin-containing chemotherapy in all patients. We evaluated all medical records and imaging data as well as follow-up information to calculate progression-free survival (PFS). PFS was defined as the time until first diagnosis of tumor progression or death. We also analyzed the relationship between CTC count and patient age, disease stage, histological differentiation, tumor size, and pathological type.
Results:
CTCs were identified in 86 of 107 patients (80%), and the CTC count ranged from 0 to 27 cells in 3.2 mL blood. The median progression-free survival (PFS) was 43.1 months. Patients in which CTCs were detected had a significantly shorter PFS than CTC-negative patients (P = 0.018). Multivariate analysis indicated that CTC count was an independent negative prognostic factor for survival. However, no correlation was observed between CTC count and patient age, disease stage, histological differentiation, tumor size, and pathological type.
Conclusion:
CTC count is an independent negative prognostic factor for cervical cancer.
Keywords: circulating tumor cells, cervical cancer, progression free survival, prognostic factor, NEimFISH
Introduction
Cervical cancer is the fourth most common cancer and the fourth leading cause of cancer-related death among women, with an estimated 570,000 cases and 311,000 deaths worldwide in 2018.1 However, it is the most common cancer affecting women in 38 countries, including many countries in sub-Saharan Africa.2 The 1- and 5-year net survival rates were 83.4% and 63.5%, respectively, for 2001–2003 and 82.9% and 62.8% in 2004–2009, respectively.3 This relatively high survival rate among all kinds of malignant cancers was due to the popularization of screening, HPV vaccine, slow progression of the disease as well as the pattern of cervical cancer metastasis.4,5 Local invasion is the earliest and most common route of cervical cancer extension, followed by metastasis via the lymphatic and blood systems. During tumor metastasis, some cancer cells detach from the primary tumor or metastatic solid tumors to reach the blood or lymphatic system, and become circulating tumor cells (CTCs), which may specifically target distant organs and contribute to secondary tumor development.6-9 The presence of CTCs may also be a reflection of the metastatic potential of the tumor, and therefore may be related to survival.10,11 CTCs can predict the response to treatment and clarify prognosis in several tumors such as lung cancer, colorectal cancer, breast cancer, and esophageal cancer.12-15 However, there are limited studies on the significance of CTCs in patients with cervical cancer. The aim of the present study was to evaluate the prognostic relevance of CTCs in patients with cervical cancer. Furthermore, we also studied the relationship between the number of CTCs and the demographics and clinical characteristics of patients with cervical cancer.
Method
Study Design
Our study was based on data from patients treated for cervical cancer at Zhujiang Hospital in 2013 and 2016. A total of 107 patients with pathologically confirmed cervical cancer were enrolled in this retrospective study. Records for each patient included name, personal ID number, age, FIGO stage, date and method of treatment, tumor grade, tumor size, and pathological diagnosis. The patients in our study included those with FIGO stages I, II, III, and IV (limited to stage IVA without distant metastatic tumor) cancer. All treatment plans followed the NCCN guidelines.16 The primary treatment plans of patients with stage I and stage IIA cancer included radical hysterectomy, and patients with positive pelvic nodes and/or positive surgical margins and/or positive parametrium demonstrated by postoperative pathology were given adjuvant pelvic EBRT (external beam radiotherapy) and concurrent cisplatin-containing chemotherapy. The primary treatment plans of patients with stage IIB, IIIA, IIIB, and IVA cancer included pelvic EBRT + concurrent cisplatin-containing chemotherapy + brachytherapy. Blood samples were collected for CTC detection within 30 days of the end of the primary treatment. All patients underwent a pelvic and abdominal CT/MRI scan before treatment and per treatment course to assess whether the tumor had progressed. We evaluated all medical records and imaging data as well as follow-up information to calculate progression-free survival (PFS). PFS was defined as the time to first pathological diagnosis of tumor progression or death. Patient characteristics are summarized in Table 1. All medical examinations and therapies were carried out with patients’ written or verbal consent.
Table 1.
Patient Demographics and Clinical Characteristics and Their Relationship With CTCs.
| Characteristics | n | Proportion (%) | Positive/negative | Positive rate (%) | X 2 | P |
|---|---|---|---|---|---|---|
| Age | 0.005 | 0.946 | ||||
| ≥50 | 72 | 65 | 58/14 | 81.1 | ||
| <50 | 35 | 35 | 28/7 | 82.5 | ||
| FIGO stage | 1.778 | 0.411 | ||||
| I | 17 | 15 | 14/3 | 82.4 | ||
| II | 47 | 45 | 35/12 | 76.5 | ||
| III/IV | 43 | 40 | 37/6 | 87.0 | ||
| Treatment | 3.448 | 0.063 | ||||
| Surgery | 33 | 30 | 23/10 | 70.6 | ||
| Chemoradiotherapy | 74 | 70 | 63/11 | 86.3 | ||
| Pathological type | 0.135 | 0.731 | ||||
| Squamous cell carcinoma | 100 | 94 | 80/20 | 81.3 | ||
| Adenocarcinoma | 7 | 6 | 6/1 | 85.7 | ||
| Histological differentiation | 5.444 | 0.142 | ||||
| Well differentiated | 8 | 9 | 8/0 | 100 | ||
| Moderately differentiated | 55 | 51 | 41/14 | 75.9 | ||
| Poorly or Undifferentiated | 20 | 19 | 15/5 | 77.3 | ||
| Unknown (X) | 24 | 21 | 22/2 | 91.7 | ||
| Tumor Size | 2.124 | 0.145 | ||||
| ≤ 4 cm | 51 | 47 | 38/13 | 75.9 | ||
| > 4 cm | 56 | 53 | 48/8 | 86.7 |
Negative Enrichment and Immune Fluorescence In Situ Hybridization of CTCs
Peripheral blood (3.2 mL) was drawn into an ACD (acid-citrate-dextrose) anti-coagulant tube (MedRay, Shenzhen, China). The volume of the tube was 5 mL, containing 0.8 mL of anticoagulant. After the blood was collected, the mixture was inverted and mixed 8 times, before storing at room temperature (15°C–30°C). All blood samples were processed within 24 h after collection. To avoid bias, all blood sample collection, enrichment, and analysis were performed blind by different people. The strategy of enrichment for cervical cancer CTCs was similar to that of a previous study.17 Peripheral blood samples were processed using the Human Blood Cell Deletion Kit (Cyttel). First, red blood cell lysis was performed. Then, the residue cell pellet was suspended and subsequently incubated with anti-CD45 monoclonal antibody-coated magnetic beads for 20–30 min, followed by the separation of magnetic beads using a magnetic stand (Promega, Madison, WI). The supernatant was subsequently subjected to identification.
The identification of enriched cervical cancer CTCs was performed by immune fluorescence in situ hybridization (imFISH), which combined FISH with chromosome 8 (orange) centromere probes (Abbott Molecular Diagnostics, Des Plaines, IL, USA) and anti-CD45 monoclonal antibody (Red, Cyttel).18 In brief, the probe CEP8 and specimen were hybridized at 37°C for 20–90 min in a hybridizer (DAKO). Subsequently, they were washed in 50% formamide at 43°C for 15 min, and then in a gradient alcohol. Finally, the specimens were incubated with Alexa Flour 594 (Invitrogen) conjugated anti-human CD45 at room temperature for 1 h. Afterward, they were washed again with 0.2% BSA. Finally, the specimens were covered with DAPI containing Vectashield mounting medium. The area of the fixed sample was observed entirely along the “S” track with a microscope (Nikon).
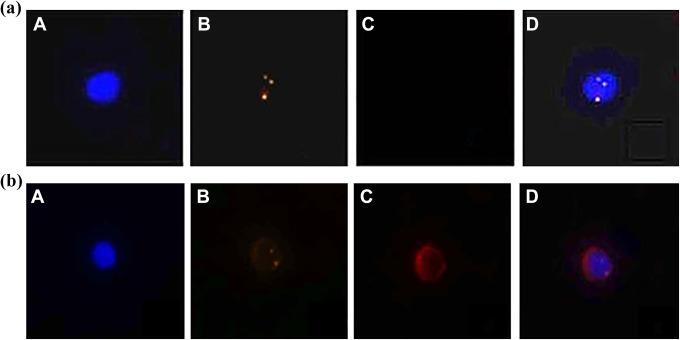
Positive CTCs were defined as hyperdiploid DAPI+/ CEP8+ /CD45-. CEP8+ was defined as more than 2 CEP8 signals in each nucleus, and the corresponding cells were identified as hyperdiploid cells. The distance between 2 signals with less than 1 signal diameter was considered a split and enumerated as 1 signal. Aggregating, overlapping, or smearing cells were excluded. According to the manufacturer’s instructions, less than 2% of cells without a CEP8 signal are considered to have acceptable hybridization efficiency (Figure 1).
Figure 1.
The microscopic identification results of enriched cervical cancer CTCs and white blood cells (WBCs) performed by immune fluorescence in situ hybridization. A: DAPI+, blue; B, CEP8+, orange; C, CD45+, red; D, merge (CEP8 signal point ≥ 3, count as positive; CEP8 signal point≤2, count as negative);(1a) Identification of CTCs: DAPI+ / CEP8+ / CD45-. (1b) Identification of WBCs: DAPI+ / CEP8- / CD45+.
Statistical Analysis
CTC count was correlated to several clinical factors, including the FIGO stage, Pathological type, tumor size, and distant metastasis. Chi-square tests were used to evaluate the relationship between CTC detection and clinical factors. OS is difficult to follow up because of the typically long survival time in cervical cancer, so we investigated the relationship between the number of CTCs and PFS. We performed a correlation analysis to explore the relationship between the number of CTCs and PFS. Survival analysis was performed using the Kaplan–Meier method to analyze the correlation of CTCs count 1, 2, 3 and PFS. The log-rank test was used to statistically compare the curves at the end of the primary treatment. To determine the most appropriate CTC cutoff value, the CTC counts of 1, 2, and 3 were tested for correlation with PFS by the Kaplan–Meier survival analysis, and the most statistically significant cutoff value was 1 based on these results. CTC count and clinical factors were subjected to univariate Cox proportional hazards regression analysis to determine if they were correlated with PFS. Only the clinical factors that were significantly associated with PFS by univariate analysis were included in the multivariate Cox regression analyses. Statistical analyses were performed using SPSS for Windows (SPSS version 22, SPSS Inc., Chicago, IL) and GraphPad Prism (GraphPad Software, San Diego, CA). P-values of ≤0.05 were considered significant.
Results
Patient Characteristics
A total of 107 patients with cervical cancer from Zhujiang Hospital were enrolled in this study from July 2012 to May 2016. At the time of these data analyses, the disease progressed, or resulted in death, in 30 patients. The median age of the patients was 54 (range, 27–78) years; the median follow-up was 25.22 months (range, 12–57). The baseline characteristics of the 107 patients are listed in Table 1.
Relationships Between CTC Count, Patient Demographics, and Clinical Characteristics
CTC count was tested after completing therapy for all 107 patients. The CTC count ranged from 0 to 27 cells/3.2 ml (mean ± standard deviation, 3.39 ± 0.45). About 80.4% (86/107) of patients had a CTC count of ≥1 cells/3.2 ml among them. There were no significant differences in circulating tumor cell positive rates in patients with different demographics and clinical characteristics (Table 1). We further analyzed the relationship between CTC count (0 and ≥1) and tumor size with respect to T-descriptor. We found no difference in CTC count or tumor size.
Table 1. Relationship of CTCs with patient demographics and clinical characteristics: no significant relationship between CTC count and patient age, FIGO stage, treatment, pathological type, histological differentiation, or tumor size was seen.
Relationship Between CTC Count and PFS
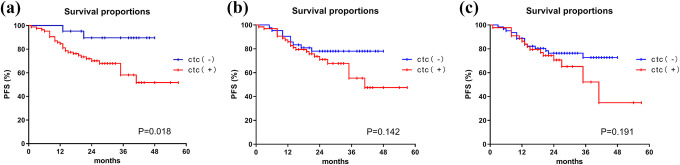
All patients were followed for at least 12 months, and the longest case survived for 57 months. The median follow-up period was 25.22 months (range, 12–57 months). The average PFS was 43.1 months (95% CI: 38.9–47.3 months). We defined the cutoff value as 1 cell/3.2 ml. We defined ≥1 CTCs as “positive” and <1 as “negative.” Patients who were CTC-positive had a significantly shorter PFS (median PFS, 39.8 months; 95% CI: 34.6–45.0 months) compared with patients who were CTC-nagative (44.8 months; 95% CI: 40.6–49.0 months) (P = 0.018) (Figure 2A). The PFS of the patients with detected CTCs was not statistically different at different FIGO stages (Figure 3). However, due to the different times of patient enrollment and follow-up time, the survival results may not be accurate. We set 1 year as the minimum follow-up time to verify our results. Although there was no significant difference in the 1-year PFS between the 2 groups (P = 0.062), the survival curve had already diverged (Figure 4A). We excluded 25 patients whose follow-up time was less than 24 months and calculated the 2-year PFS of the remaining 82 patients. We discovered that patients with a CTC count ≥1/3.2 ml had a shorter PFS (median PFS, 19.4 months; 95% CI: 17.7–21.1 months) than patients CTC-negative (23.2 months; 95% CI: 21.9–24.4 months)(P = 0.047) in 2-year PFS (Figure 4B).
Figure 2.
Kaplan–Meier curves for PFS in 107 cervical cancer patients with different CUT-OFF values of circulating tumor cells. (2a) Kaplan–Meier curve for PFS in patients with 0 and ≥1 circulating tumor cell in 3.2 ml of blood after the primary treatment. (2b) Kaplan–Meier curve for PFS in patients with <2 and ≥2 circulating tumor cells (CUT-OFF value = 2) in 3.2 ml of blood after the primary treatment.(2c)Kaplan–Meier curve for PFS in patients with <3 and ≥3 circulating tumor cells (CUT-OFF value = 3) in 3.2 ml of blood after the primary treatment.
Figure 3.
Kaplan–Meier curves for PFS of patients with detected CTCs or not in FIGO stage I, stage II, and stage III/IV. (3a) Kaplan–Meier curve for PFS of the patients with detected CTCs or not in FIGO stage I; (3b) Kaplan–Meier curve for PFS of the patients with detected CTCs or not in FIGO stage II; (3c) Kaplan–Meier curve for PFS of the patients with detected CTCs or not in FIGO stage III/IV.
Figure 4.
Kaplan–Meier curve for 1-year PFS in 107 and 82 patients with CTC (-) and CTC (+) in 3.2 mL of blood after the primary treatment.(4a) Kaplan–Meier curve for 1-year PFS in 107 patients with CTC (-) and CTC (+) in 3.2 mL of blood after the primary treatment;(4b) Kaplan–Meier curve for 2-year PFS in 82 patients with CTC (-) and CTC (+) in 3.2 mL of blood after the primary treatment.
In the univariate Cox regression analyses, the number of CTCs, Pathological typePathological type, and tumor size were significantly associated with PFS (Table 2). We included factors with a P value < 0.1 from the univariate analysis into the multivariate analysis. In the multivariate Cox proportional hazards regression analysis, the presence of CTCs significantly predicted reduced PFS. (HR, 4.762; 95% CI: 1.127-20.117; P = 0.034) (Table 2).
Table 2.
Univariate and Multivariate Cox Proportional Hazards Regression of Prediction of PFS.
| Parameter | At-risk group | PFS risk | |||
|---|---|---|---|---|---|
| Positive | Negative | P | HR | 95%CI | |
| Statistically significant factors in univariate analysis | |||||
| Age | ≥50 | <50 | 0.276 | 0.669 | 0.325-1.379 |
| FIGO stage | III/IV | I/II | 0.073 | 1.908 | 0.941-3.873 |
| Treatment | Surgery | Chemoradiotherapy | 0.605 | 1.237 | 0.533-2.767 |
| Tumor size | >4cm | ≤4cm | 0.029 | 2.372 | 1.092-5.156 |
| Pathological type | Adenocarcinoma | SCC | 0.021 | 3.509 | 1.209-10.186 |
| CTC count | ≥1 | <1 | 0.030 | 4.931 | 1.166-20.848 |
| Multivariate Cox proportional hazards regression analysis | |||||
| Pathological type | Adenocarcinoma | SCC | 0.029 | 3.271 | 1.127-9.498 |
| CTC count | ≥1 | <1 | 0.034 | 4.762 | 1.127-20.117 |
Table 2. Univariate and multivariate Cox proportional hazards regression analysis for prediction of PFS: CTC count is a strong predictor of PFS in both univariate and multivariate Cox proportional hazards regression analyses.
Note: CTCs were collected in 3.2 ml of peripheral blood.
CTCs, circulating tumor cells; HR, hazard ratio; CI, confidence interval; PFS, progression-free survival; SCC, squamous cell carcinoma
Discussion
At present, the most common approach for capturing CTCs is the cell search system, which is based on the expression of the adhesion molecule EpCAM on the tumor cell surface and cytokeratins (CKs).19-21 However, EpCAM expression can be highly heterogenous with different dynamics among different types of epithelial tumor cells, and epithelial-mesenchymal transition (EMT) may decrease the expression of EpCAM and CKs.22,23 Therefore, this method may not accurately detect CTCs. The new technology named NEimFISH combines negative enrichment and immune fluorescence in situ hybridization (imFISH) to achieve the simultaneous detection of genes and proteins, so it could avoid cell loss caused by second exposure. The negative enrichment technique can largely remove leukocytes in the blood (up to 99.99%), and the remaining cell count is approximately 103. The blood volume was similar to that of previously published reports.17,24 Aneusomy chromosome 8 in cervical cancer has been reported by others25,26 as well as in ovarian carcinoma cells.27 The NEimFISH method can improve the sensitivity and specificity of CTC detection. This innovative technology ensures the accuracy of our experimental data and enhances the reliability of the results.
Several studies have shown promising data on CTCs as a negative prognostic marker in cancer. In lung cancer, at least 1 CTC is seen per 7.5 ml of blood in approximately 70% of patients, but CTCs have not been detected in nonmalignant pulmonary diseases.28 Pei-Pei Wang et al. demonstrated that patients with small cell lung cancer with ≥ 10 CTCs per 4–5 ml of blood had worse prognosis than those with ≤ 9 CTCs.15 In breast cancer, patients with a high total number of CTCs showed significantly shorter OS than those with smaller CTC numbers who were treated with eribulin.29 The correlation between CTC count and patient age, disease stage, histological differentiation, tumor size, and pathological type is different in various cancers.15,30-33 In the present study, the positive rates of CTCs did not correlate with any of the following clinical characteristics: patient age, disease stage, histological differentiation, tumor size, or pathological type. We found that the CTCs positive rate was 80.7% in patients with locally cervical cancer, which was within the CTC detection rates ranging from 66.6% to 98.1% reported by other investigators.24,28,34-36 Most prior investigations employed EpCAM-based CTC analysis.
However, few studies have investigated the prognostic role of CTCs in cervical cancer. We first conducted a correlation analysis between CTC count and PFS in 107 patients with cervical cancer. The result suggests that there is a negative correlation: patients with more CTCs detected may have short progression-free survival. We set different CUT-OFF values (1/2/3)/3.2 ml to further study the relationship between the CTC count and PFS. The Kaplan–Meier survival curves show that the PFS of patients with CTC ≥ 1 /3.2 ml were significantly lower than that of patients without CTCs (CTC = 0) /3.2 ml (Figure 2A). However, when the CUT-OFF value was set to 2 or 3, there was no significant difference between the 2 groups (Figure 2B and C). Interestingly, in contrast to other trials, Lee et al. defined CTC count of ≥2 per 7.5 ml blood as positive, so patients with 1 CTC were qualified as CTC-negative.34 Setting specific cutoff values in CTC-based clinical trials is also common in other tumors. For example, in an early stage NSCLC clinical trial, 5 CTCs per 1 mL blood were used as a threshold to differentiate between patients with favorable and unfavorable outcome,37 whereas 10 CTCs per 5 ml of blood have been shown to be a more suitable cutoff value in breast cancer.14
Due to the different enrollment times and follow-up times, the survival results above may be inaccurate. The shortest follow-up time of the cases enrolled in our study was 12 months, so we set 1 year as the minimum follow-up time to compare the difference between the 2 groups at this timepoint. Although there was no significant difference between the 2 groups (P = 0.062) in the 1-year PFS, the survival curve had already diverged (Figure 4A). Subsequently, we removed 25 patients who were followed up for less than 24 months and calculated the 2-year PFS of the remaining 82 patients. We discovered that patients in which CTCs (≥1) /3.2 ml were detected had a shorter PFS (median PFS, 19.4 months; 95% CI: 17.7–21.1 months) compared with CTC-negative patients (23.2 months; 95% CI: 21.9–24.4 months)(P = 0.047) in the 2-year PFS (Figure 4B). In summary, we found that the difference in survival rates between the CTC-negative and CTC-positive groups became increasingly obvious as the follow-up time increased. We will follow up the prognosis and survival of these cervical cancer patients for the foreseeable future. In the multivariate Cox regression analyses, CTCs (CUT-OFF = 1 cell) were significantly associated with PFS. The results of this analysis demonstrate that in cervical cancer patients, the presence of CTCs in 3.2 ml of peripheral blood after therapy may be associated with shorter PFS. In other words, as long as CTCs can be detected in the peripheral blood of patients after radiotherapy or concurrent cisplatin-containing chemotherapy, this indicates a poor prognosis. We speculate that the shorter PFS is due to tumor metastasis caused by CTCs. In patients with CTC-positive tumors, tumor cells tend to metastasize distantly with peripheral blood or lymph fluid flowing to various organs and tissues throughout the body and may form more micro-metastatic tumor lesions or secondary tumors. This leads to a more advanced stage of the tumor, loss of opportunity for radical treatment, greater spread, and damage to the body, resulting in shorter progression-free survival. Therefore, we conclude that CTC level could be used as a potential prognostic biomarker for progression-free survival in patients with cervical cancer after primary treatment.
Our study has several limitations. First, our study was retrospective in nature and the sample we enrolled was not adequate, at only 107 cases, which may have caused bias in the final results. Second because of the retrospective nature of the study, some medical records were missing, resulting in a few clinical characters being blank and accurate progression-free survival times could not be obtained. Nonetheless, our findings can serve as a foundation for future studies. We can reduce experimental error by performing prospective studies or expanding the number of cases included. Furthermore, we can explore the relationship between PFS and the baseline number of CTCs as well as the degree of decline in CTCs under standardized therapy.
Conclusions
In summary, for cervical cancer patients, CTC count after radiotherapy or concurrent cisplatin-containing chemotherapy is an independent negative prognostic indicator. Patients who with detectable CTCs ≥1 /3.2 ml may have a shorter PFS compared with CTC negative (<1) /3.2 ml. There was no significant relationship between CTC count and patient age, disease stage, treatment, histological differentiation, tumor size, and pathological type in this retrospective study.
Acknowledgments
We would like to thank our colleagues in the Oncology Center of Zhujiang Hospital of Southern Medical University and the Department of Pathology for their cooperation.
Abbreviations
- ACD
acid-citrate-dextrose
- CKs
cytokeratins
- CTCs
circulating tumor cells
- EBRT
external beam radiotherapy
- EMT
epithelial-mesenchymal transition
- imFISH
immune fluorescence in situ hybridization
- NEimFISH
negative enrichment and immune fluorescence in situ hybridization of CTCs
- PFS
progression-free survival.
Authors’ Note: This clinical study was a retrospective study. We only collected clinical data of patients, did not interfere with the patient’s treatment plan, and did not pose any risk to the patient’s physiology. The researchers made every effort to protect the information provided by the patient from disclosing personal privacy-so the ethical permission was not applied for. All medical examinations and therapies have obtained patients’ written or verbal consent.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Qian Huang  https://orcid.org/0000-0002-1044-3361
https://orcid.org/0000-0002-1044-3361
References
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. [DOI] [PubMed] [Google Scholar]
- 2. Ginsburg O, Bray F, Coleman MP, et al. The global burden of women’s cancers: a grand challenge in global health. Lancet (London, England). 2017;389(10071):847–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Benard V, Watson M, Saraiya M, et al. Cervical cancer survival in the United States by race and stage (2001-2009): findings from the CONCORD-2 study. Cancer. 2017;123(suppl 24):5119–5137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Torre LA, Islami F, Siegel RL, Ward EM, Jemal A. Global cancer in women: burden and trend. Cancer Epidemiol Biomarkers Prev. 2017;26(4):444–457. [DOI] [PubMed] [Google Scholar]
- 5. Forman D, de Martel C, Lacey CJ, et al. Global burden of human papillomavirus and related diseases. Vaccine. 2012;30(suppl 5):F12–F23. [DOI] [PubMed] [Google Scholar]
- 6. Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science. 2011;331(6024):1559–1564. [DOI] [PubMed] [Google Scholar]
- 7. Gupta GP, Massague J. Cancer metastasis: building a framework. Cell. 2006;127(4):679–695. [DOI] [PubMed] [Google Scholar]
- 8. Yap TA, Lorente D, Omlin A, Olmos D, de Bono JS. Circulating tumor cells: a multifunctional biomarker. Clin Cancer Res. 2014;20(10):2553–2568. [DOI] [PubMed] [Google Scholar]
- 9. Lianidou ES, Strati A, Markou A. Circulating tumor cells as promising novel biomarkers in solid cancers. Crit Rev Clin Lab Sci. 2014;51(3):160–171. [DOI] [PubMed] [Google Scholar]
- 10. Scarlotta M, Simsek C, Kim AK. Liquid biopsy in solid malignancy. Genet Test Mol Biomarkers. 2019;23(4):284–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pawlikowska P, Faugeroux V, Oulhen M, et al. Circulating tumor cells (CTCs) for the noninvasive monitoring and personalization of non-small cell lung cancer (NSCLC) therapies. Journal Thorac Dis. 2019;11(suppl 1):S45–S56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cohen SJ, Punt C, Iannotti N, et al. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer (vol 26, pg 3213, 2008). J Clin Oncol. 2009;27(11):1923–1923. [DOI] [PubMed] [Google Scholar]
- 13. Qiao Y, Li J, Shi C, et al. Prognostic value of circulating tumor cells in the peripheral blood of patients with esophageal squamous cell carcinoma. Onco Targets Ther. 2017;10:1363–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Guan X, Ma F, Li C, et al. The prognostic and therapeutic implications of circulating tumor cell phenotype detection based on epithelial-mesenchymal transition markers in the first-line chemotherapy of HER2-negative metastatic breast cancer. Cancer Commun (London, England). 2019;39(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang PP, Liu SH, Chen CT, et al. Circulating tumor cells as a new predictive and prognostic factor in patients with small cell lung cancer. J Cancer. 2020;11(8):2113–2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Koh WJ, Abu-Rustum NR, Bean S, et al. Cervical cancer, version 3.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2019;17(1):64–84. [DOI] [PubMed] [Google Scholar]
- 17. Zhang Z, Xiao Y, Zhao J, et al. Relationship between circulating tumour cell count and prognosis following chemotherapy in patients with advanced non-small-cell lung cancer. Respirology (Carlton, Vic). 2016;21(3):519–525. [DOI] [PubMed] [Google Scholar]
- 18. Ning N, Zhan T, Zhang Y, et al. Improvement of specific detection of circulating tumor cells using combined CD45 staining and fluorescence in situ hybridization. Clin Chim Acta. 2014;433:69–75. [DOI] [PubMed] [Google Scholar]
- 19. de Bono JS, Scher HI, Montgomery RB, et al. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin Cancer Res. 2008;14(19):6302–6309. [DOI] [PubMed] [Google Scholar]
- 20. Cristofanilli M. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. Semin Oncol. 2006;33(3): S9–S14. [DOI] [PubMed] [Google Scholar]
- 21. Mohtar MA, Syafruddin SE, Nasir SN, Yew LT. Revisiting the roles of pro-metastatic EpCAM in cancer. Biomolecules. 2020;10(2):255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gorges TM, Tinhofer I, Drosch M, et al. Circulating tumour cells escape from EpCAM-based detection due to epithelial-to-mesenchymal transition. BMC Cancer. 2012;12:178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hyun KA, Koo GB, Han H, et al. Epithelial-to-mesenchymal transition leads to loss of EpCAM and different physical properties in circulating tumor cells from metastatic breast cancer. Oncotarget. 2016;7(17):24677–24687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chen YY, Xu GB. Effect of circulating tumor cells combined with negative enrichment and CD45-FISH identification in diagnosis, therapy monitoring and prognosis of primary lung cancer. Med Oncol (Northwood, London, England). 2014;31(12):240. [DOI] [PubMed] [Google Scholar]
- 25. Mark HF, Feldman D, Samy M, et al. Assessment of chromosome 8 copy number in cervical cancer by fluorescent in situ hybridization. Exp Mol Pathol. 1999;66(2):157–162. [DOI] [PubMed] [Google Scholar]
- 26. Earley A, Lamont JL, Dahabreh IJ, Cowan J, Feldman S, Uhlig K. Fluorescence in situ hybridization testing for the diagnosis of high-grade cervical abnormalities: a systematic review. J Low Genit Tract Dis. 2014;18(3):218–227. [DOI] [PubMed] [Google Scholar]
- 27. Fiegl M, Massoner A, Haun M, et al. Sensitive detection of tumour cells in effusions by combining cytology and fluorescence in situ hybridisation (FISH). Br J Cancer. 2004;91(3):558–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mascalchi M, Maddau C, Sali L, et al. Circulating tumor cells and microemboli can differentiate malignant and benign pulmonary lesions. J Cancer. 2017;8(12):2223–2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ito M, Horimoto Y, Tokuda E, et al. Impact of circulating tumour cells on survival of eribulin-treated patients with metastatic breast cancer. Med Oncol. 2019;36(10):89. [DOI] [PubMed] [Google Scholar]
- 30. Hofman V, Bonnetaud C, Ilie MI, et al. Preoperative circulating tumor cell detection using the isolation by size of epithelial tumor cell method for patients with lung cancer is a new prognostic biomarker. Clin Cancer Res. 2011;17(4):827–835. [DOI] [PubMed] [Google Scholar]
- 31. Nair VS, Keu KV, Luttgen MS, et al. An observational study of circulating tumor cells and (18)F-FDG PET uptake in patients with treatment-naive non-small cell lung cancer. PloS One. 2013;8(7): e67733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Qiao GL, Qi WX, Jiang WH, Chen Y, Ma LJ. Prognostic significance of circulating tumor cells in esophageal carcinoma: a meta-analysis. Onco Targets Ther. 2016;9:1889–1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Xie Z, Gao X, Cheng K, Yu L. Correlation between the presence of circulating tumor cells and the pathologic type and staging of non-small cell lung cancer during the early postoperative period. Oncol Lett. 2017;14(5):5825–5830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Liu JF, Kindelberger D, Doyle C, Lowe A, Barry WT, Matulonis UA. Predictive value of circulating tumor cells (CTCs) in newly-diagnosed and recurrent ovarian cancer patients. Gynecol Oncol. 2013;131(2):352–356. [DOI] [PubMed] [Google Scholar]
- 35. Lee M, Kim EJ, Cho Y, et al. Predictive value of circulating tumor cells (CTCs) captured by microfluidic device in patients with epithelial ovarian cancer. Gynecol Oncol. 2017;145(2):361–365. [DOI] [PubMed] [Google Scholar]
- 36. Pfitzner C, Schröder I, Scheungraber C, et al. Digital-direct-RT-PCR: a sensitive and specific method for quantification of CTC in patients with cervical carcinoma. Sci Rep. 2014;4:3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Frick MA, Feigenberg SJ, Jean-Baptiste SR, et al. Circulating tumor cells are associated with recurrent disease in patients with early-stage non-small cell lung cancer treated with stereotactic body radiotherapy. Clin Cancer Res. 2020;26(10):2372–2380. [DOI] [PMC free article] [PubMed] [Google Scholar]