Abstract
Contrast-induced acute kidney injury (CI-AKI) is a serious complication of percutaneous coronary intervention (PCI) in patients with acute ST-segment elevation myocardial infarction (STEMI). Early identification of high-risk patients has an essential role in preventing CI-AKI. This study was designed to evaluate the predictive value of d-dimer, a marker of thrombosis and hypercoagulable state, for CI-AKI and prognosis in patients with STEMI. We included 400 patients with STEMI who underwent PCI. The patients were subdivided into 4 groups according to d-dimer level using the 4-quantile method. Contrast-induced acute kidney injury occurred in 66 (16.5%) patients. The incidence of CI-AKI in the highest quartile of the d-dimer groups (29.0%) was higher than that in the other 3 groups. Multivariable logistic regression showed that a low d-dimer level was significantly associated with a decreased risk of CI-AKI independent of confounding factors, with an odds ratio (OR) of 0.487 (95% CI: 0.178-0.931, P = 0.041) for those in the first quartile compared with those in the highest quartile. Age (OR: 1.047, 95% CI: 1.003-1.092), diabetes mellitus (OR: 5.896, 95% CI: 2.496-13.927), anemia (OR: 3.488, 95% CI: 1.308-9.306), and total bilirubin (OR: 0.946, 95% CI: 0.904-0.992) were independent predictors of CI-AKI. The incidence of major adverse cardiovascular and cerebral events and all-cause mortality within 30 days, 6 months, and 1 year after PCI in the highest quartile of the d-dimer groups were higher than those in the other 3 groups. In conclusion, increasing d-dimer levels were independently associated with the incidence of CI-AKI and adverse outcomes in patients with STEMI after PCI.
Keywords: d-dimer, acute myocardial infarction, percutaneous coronary intervention, contrast-induced acute kidney injury, major adverse cardiovascular and cerebral events
Introduction
Acute ST-segment elevation myocardial infarction (STEMI) is one of the leading causes of death globally. Early restoration of coronary antegrade flow by percutaneous coronary intervention (PCI) plays a critical role in improving the prognosis of patients with STEMI. Contrast-induced acute kidney injury (CI-AKI) is defined as acute renal damage that develops secondary to contrast media exposure and is the third leading cause of in-hospital acute kidney injury.1 Contrast-induced acute kidney injury occurs frequently in patients with STEMI undergoing PCI due to hemodynamic instability and inadequate prophylaxis2,3 and is related to long-term morbidity and mortality.4–6 Vascular endothelial dysfunction, vasoconstriction, inflammation, free radical damage, tubular cell toxicity, reactive oxygen species, and oxidative stress have been proposed as pathophysiological mechanisms of CI-AKI.7 At present, there are no operative prophylactic–therapeutic measures for CI-AKI.8 Therefore, early identification of high-risk groups and active intervention are essential to prevent CI-AKI. d-dimer is a product of the degradation of cross-linked fibrin, an indicator of ongoing fibrinolysis and the severity of a hypercoagulable state. Previous studies have shown that when vascular endothelial cells are damaged, tissue factors are released to activate the coagulation fibrinolysis system, resulting in hypercoagulation and hyperfibrinolysis of the blood.9 Wakabayashi and Masuda10 showed that a hypercoagulable state of blood in patients with diabetes mellitus severely affected glomerular filtration function. In addition, Simes et al demonstrated that d-dimer was correlated with the level of high-sensitivity C-reactive protein, and high d-dimer levels reflected an inflammatory state.11 However, few studies have explored the correlation between d-dimer, a marker of thrombosis and hypercoagulable state, and CI-AKI. Furthermore, d-dimer testing might be useful in the diagnosis and prognosis of patients with acute coronary syndromes (ACSs).12,13 However, the prognostic value of d-dimer in patients with ACS has long been controversial. Some previous studies revealed that d-dimer levels were not predictive of cardiovascular events.14,15 In contrast, Fiotti et al found that d-dimer levels on admission were associated with worse in-hospital prognosis in patients with unstable angina pectoris.16 Thus, the purpose of this study was to investigate whether d-dimer levels on admission predict CI-AKI after PCI, as well as to evaluate the prognostic value of d-dimer levels in patients with STEMI and to provide ideas for improving STEMI and CI-AKI risk stratification.
Methods
Study Population
This is a retrospective observational cohort study. From December 2013 to January 2017, consecutive patients with STEMI admitted to Zhongda Hospital and treated with PCI were enrolled. The inclusion criteria were as follows: (1) age between 18 and 80 years old and (2) patients with STEMI who were diagnosed based on the Guidelines for the Diagnosis and Treatment of Acute ST-segment Elevation Myocardial Infarction in 201517 and underwent PCI. The exclusion criteria were as follows: (1) allergy to iodine or iodine contrast medium, (2) end-stage renal failure requiring peritoneal dialysis, hemodialysis, and kidney transplantation, (3) severe cardiac insufficiency and valvular heart disease with unstable hemodynamics, (4) definite bacterial and fungal infections, acute and chronic inflammatory diseases, autoimmune diseases, or tumors, (5) antibiotics use before admission, (6) contrast medium administration within 2 weeks before admission, and (7) nephrotoxic drug application during hospitalization. The study was approved by the medical ethics committee of Zhongda Hospital, and all methods were performed in accordance with the relevant guidelines and regulations. All patients included volunteered to participate in this clinical study and signed an informed consent form.
Grouping
The patients were divided into 4 groups according to d-dimer by using the 4-quantile method: group 1 (n = 100, d-dimer ≤109 µg/L), group 2 (n = 100, 110 ≤ d-dimer ≤ 195 µg/L), group 3 (n = 100, 196 ≤ d-dimer ≤ 377 µg/L), and group 4 (n = 100, d-dimer ≥ 378 µg/L).
Laboratory Investigations
Serum creatinine (SCr) concentration was measured before and 2 to 3 days after contrast media exposure. Renal function was measured using the estimated glomerular filtration rate (eGFR) with the modified MDRD formula (eGFR = 175 × creatinine−1.234 × age−0.179 × [0.79 (female)]) according to data from Chinese patients with CKD. Plasma d-dimer was tested via an enzyme immunoassay using INNOVANCE d-dimer (SIEMENS), and the d-dimer level was reported in fibrinogen equivalent units. Total bilirubin, alanine aminotransferase, aspartate aminotransferase, blood glucose, total cholesterol, triglycerides (TGs), high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, albumin, and other biochemical indicators were measured after an overnight fast ≥12 hours.
Coronary Angiography
All patients were given aspirin (300 mg), ticagrelor (180 mg), or clopidogrel (300 mg) before the operation. Selective coronary arteriography was performed by cardiologists specializing in interventional treatments. The corresponding diseased vessels were treated according to the specific results of the coronary angiography. Nonionic and low-osmotic contrast agents (Iopromide injection, Bayer Vital GmbH) were used during the operation, and the amounts were recorded.
Percutaneous Coronary Intervention
Percutaneous coronary intervention included balloon dilation and/or stent implantation for infarct-related vessels and was performed by experienced operators according to standard techniques. Hydration therapy was given to patients with an eGFR <60 mL/min/1.73 m2 and consisted of intravenous administration of isotonic saline (1.0-1.5 mL/kg/h) at 3 to 12 hours before the operation and 6 to 24 hours after the operation. Isotonic saline solution was intravenously given at 0.5 mL/kg/h if left ventricular ejection fraction (LVEF) <35% or NYHA >2. Aspirin (100 mg, one daily), ticagrelor (90 mg, twice daily), or clopidogrel (75 mg, once daily) were administered after surgery. Statins, β-blockers, nitrates, and angiotensin-converting enzyme inhibitors were commonly used in all patients without contraindications.
Study Protocol: End Points and Follow-Up
The primary end point was CI-AKI. Contrast-induced acute kidney injury was defined as a rise in SCr of 44.2 μmol/L or a 25% increase from the baseline value within 2 to 3 days after contrast media exposure.18 The secondary end points were the main adverse cardiovascular and cerebrovascular events (MACCEs) and hospitalization for kidney failure during the follow-up period (30 days, 6 months, 1 year after PCI). The MACCEs included all-cause death, target vessel revascularization, myocardial infarction during follow-up, unstable angina pectoris requiring hospitalization, heart failure, stroke, or transient cerebral ischemia. Each patient’s baseline clinical data (including demographic data, previous medical history, and vital signs on admission), biochemical and angiographic variables, and echocardiography results were recorded. The patients made a follow-up visit 30 days, 6 months, and 1 year after the operation. Follow-up data were obtained from hospital records or via interviews (in person or by telephone) with patients and their families conducted by at least 2 cardiologists.
Statistical Analysis
Analyses were performed using SPSS software, version 16.0 (SPSS, Inc). Continuous variables are expressed as the means ± SDs or medians (interquartile ranges). Categorical variables are expressed as frequencies with percentages. Univariable and multivariable logistic regression analyses were used to determine CI-AKI predictors. Variables with univariable P values <.10 were selected for multivariable analysis and expressed as odds ratios (ORs) with 95% CIs. Survival was graphically represented using Kaplan-Meier curves. Differences in survival rates were compared using the log-rank test. The area under the receiver operating characteristic (ROC) curves (AUCs) were used to indicate the predictive value of d-dimer level on CI-AKI. All tests were 2 tailed, and statistical significance was defined as P < .05.
Results
Patient Characteristics
This study included 400 patients with STEMI who underwent PCI, and CI-AKI occurred in 66 (16.5%) patients. All patients were subdivided into 4 groups according to d-dimer level. Baseline clinical data and therapy at admission for the 4 groups are shown in Table 1. The biochemical and angiographic variables and echocardiography results of the 4 groups are shown in Table 2. There were statistically significant differences among the 4 groups in terms of the incidence of CI-AKI (P = .001), age (P < .001), proportion of female patients (P = .001), smoking history (P = .001), anemia (P < .001), atrial fibrillation (AF; P = .001), hemoglobin (P < .001), albumin (P < .001), glucose (P = .034), TGs (P = .004), SCr (P < .001), eGFR (P < .001), and LVEF (P < .001), and there were no statistically significant differences in the other indicators. The incidence of CI-AKI after PCI was higher among patients with STEMI, with d-dimer levels in the highest quartile (group 4). Patients in the high dimer group were older, had higher glucose and creatinine levels, and had lower hemoglobin, albumin, eGFR, and LVEF. d-dimer levels were higher in anemic and AF patients.
Table 1.
Baseline Clinical Data and Therapy at Admission of the 4 Groups.a
| Variable | Group 1 (n = 100) | Group 2 (n = 100) | Group 3 (n = 100) | Group 4 (n = 100) | P value |
|---|---|---|---|---|---|
| Age, years | 55.2 ± 11.0 | 59.2 ± 11.6 | 65.1 ± 11.8 | 70.5 ± 9.5 | <.001 |
| Male | 90 (90.0) | 83 (83.0) | 75 (75.0) | 68 (68.0) | .001 |
| SBP (mm Hg) | 129.8 ± 19.4 | 128.3 ± 21.3 | 128.3 ± 21.9 | 127.8 ± 23.8 | .924 |
| Smoker | 62 (62.0) | 51 (51.0) | 42 (42.0) | 35 (35.0) | .001 |
| Hypertension | 55 (55.0) | 59 (59.0) | 63 (63.0) | 71 (71.0) | .111 |
| Diabetes mellitus | 23 (23.0) | 20 (20.0) | 25 (25.0) | 33 (33.0) | .178 |
| Anemia | 4 (4.0) | 8 (8.0) | 13 (13.0) | 26 (26.0) | <.001 |
| Previous AMI | 1 (1.0) | 1 (1.0) | 2 (2.0) | 4 (4.0) | .382 |
| Atrial fibrillation | 1 (1.0) | 2 (2.0) | 4 (4.0) | 12 (12.0) | .001 |
| CI-AKI | 11 (11.0) | 14 (14.0) | 12 (12.0) | 29 (29.0) | .001 |
| Therapy at admission | |||||
| Aspirin | 98 (98.0) | 96 (96.0) | 99 (99.0) | 91 (91.0) | .028 |
| β-blockers | 81 (81.0) | 72 (72.0) | 80 (80.0) | 68 (68.0) | .325 |
| Statins | 94 (94.0) | 98 (98.0) | 97 (97.0) | 97 (97.0) | .681 |
| ACEI/ARB | 74 (74.0) | 61 (61.0) | 60 (60.0) | 59 (59.0) | .051 |
Abbreviations: ACEI, angiotensin-converting enzyme inhibitor; AMI, acute myocardial infarction; ARB, angiotensin receptor blocker; CI-AKI, contrast induced acute kidney injury; SBP, systolic blood pressure.
a Data are presented as the mean ± SD or n (%).
Table 2.
Biochemical and Angiographic Variables of the 4 Groups.a
| Variable | Group 1 (n = 100) | Group 2 (n = 100) | Group 3 (n = 100) | Group 4 (n = 100) | P value |
|---|---|---|---|---|---|
| Biochemical indicators | |||||
| d-dimer, µg/L (IQR) | 74.0 (58.0-89.5) | 146.0 (128.0-162.5) | 256.5 (215.0-320.5) | 576.0 (458.5-762.5) | <.001 |
| NT-proBNP, pg/mL (IQR) | 504.5 (102.1-2725.3) | 636.3 (82.9-3102.5) | 562.5 (182.7-2325.9) | 693.0 (137.2-3503.2) | .205 |
| Hemoglobin, g/L | 142.3 ± 15.7 | 142.1 ± 19.9 | 136.8 ± 18.3 | 129.6 ± 20.9 | <.001 |
| White blood cells, 109/L | 10.3 ± 3.3 | 10.5 ± 4.0 | 10.1 ± 3.4 | 10.2 ± 4.1 | .902 |
| Neutrophil ratio | 75.6 ± 12.2 | 75.9 ± 13.3 | 75.2 ± 12.0 | 75.8 ± 12.4 | .981 |
| Platelet, 109/L | 219.7 ± 54.3 | 215.5 ± 64.3 | 209.7 ± 60.8 | 204.0 ± 72.3 | .325 |
| Albumin, g/L | 38.5 ± 4.1 | 38.1 ± 4.9 | 36.7 ± 4.9 | 34.5 ± 5.3 | <.001 |
| Total bilirubin, µmol/L | 13.9 ± 7.7 | 13.3 ± 7.0 | 14.1 ± 6.7 | 13.8 ± 7.9 | .891 |
| Glucose, mmol/L | 8.1 ± 3.5 | 8.2 ± 3.4 | 8.6 ± 4.0 | 9.7 ± 5.3 | .034 |
| TC, mmol/L | 4.7 ± 1.3 | 4.6 ± 1.3 | 4.7 ± 1.0 | 4.3 ± 1.2 | .059 |
| Triglycerides, mmol/L | 2.1 ± 1.8 | 2.0 ± 1.7 | 1.7 ± 1.1 | 1.4 ± 0.9 | .004 |
| HDL-C, mmol/L | 1.1 ± 0.2 | 1.1 ± 0.3 | 1.1 ± 0.3 | 1.1 ± 0.3 | .609 |
| LDL-C, mmol/L | 2.9 ± 0.9 | 2.9 ± 0.9 | 2.9 ± 0.8 | 2.7 ± 0.8 | .274 |
| Uric acid, µmol/L | 325.0 ± 92.8 | 333.8 ± 99.0 | 312.4 ± 110.6 | 340.2 ± 113.6 | .263 |
| SCr, µmol/L | 77.3 ± 19.1 | 85.7 ± 27.0 | 90.6 ± 31.6 | 112.5 ± 85.3 | <.001 |
| eGFR, mL/min | 101.5 ± 39.6 | 87.7 ± 35.5 | 76.3 ± 26.2 | 66.2 ± 34.1 | <.001 |
| Coronary angiography | |||||
| Contrast agent, mL | 112.3 ± 26.5 | 111.0 ± 21.5 | 110.4 ± 22.6 | 108.4 ± 19.7 | .676 |
| Lesion vessels | 3.0 ± 1.6 | 3.1 ± 1.4 | 3.0 ± 1.5 | 3.2 ± 1.6 | .599 |
| Three-vessel disease | 51 (51.0) | 52 (52.0) | 41 (41.0) | 55 (55.0) | .550 |
| LVEF | 0.58 ± 0.10 | 0.54 ± 0.11 | 0.51 ± 0.11 | 0.52 ± 0.14 | <.001 |
Abbreviations: eGFR, estimated glomerular filtration rate; HDL-C, high-density lipoprotein cholesterol; IQR, interquartile range; LDL-C, low-density lipoprotein cholesterol; LVEF, left ventricular ejection fraction; NT-proBNP, NT-proB-type natriuretic peptide; SCr, serum creatinine concentration; TC, total cholesterol.
a Data are presented as the IQR, mean ± SD, or n (%).
Risk Factors for CI-AKI
Baseline clinical data and therapy at admission of the CI-AKI and non-CI-AKI groups are given in Table 3. The biochemical and angiographic variables and echocardiography results of the CI-AKI and non-CI-AKI groups are shown in Table 4. The proportions of females (31.8% vs 18.9%, P, .018), diabetes mellitus (47.0% vs 21.0%, P < .001), and anemia (31.8% vs 9.0%, P < .001) in the CI-AKI group were higher than those in the non-CI-AKI group. There were statistically significant differences (P < .05) between the CI-AKI and non-CI-AKI groups in terms of d-dimer, age, hemoglobin, neutrophil ratio, albumin, total bilirubin, glucose, SCr, and eGFR.
Table 3.
Baseline Clinical Data and Therapy at Admission of the CI-AKI and Non-CI-AKI Groups.a
| Variable | CI-AKI (n = 66) | Non-CI-AKI (n = 334) | P value |
|---|---|---|---|
| Male | 45 (68.2) | 271 (81.1) | .018 |
| Smoker | 28 (42.4) | 162 (48.5) | .366 |
| Hypertension | 42 (63.6) | 206 (61.9) | .786 |
| Diabetes mellitus | 31 (47.0) | 70 (21.0) | <.001 |
| Anemia | 21 (31.8) | 30 (9.0) | <.001 |
| Previous AMI | 3 (4.5) | 5 (1.5) | .106 |
| Atrial fibrillation | 4 (6.1) | 15 (4.5) | .584 |
| Three-vessel disease | 39 (59.1) | 164 (49.1) | .138 |
| Therapy at admission | |||
| Aspirin | 63 (95.5) | 321 (96.1) | .805 |
| β-blockers | 53 (80.3) | 264 (79.0) | .817 |
| Statins | 65 (98.5) | 321 (96.1) | .337 |
| ACEI/ARB | 43 (65.2) | 211 (63.2) | .760 |
Abbreviations: ACEI, angiotensin-converting enzyme inhibitor; AMI, acute myocardial infarction; ARB, angiotensin receptor blocker; CI-AKI, contrast-induced acute kidney injury.
a Data are presented as n (%).
Table 4.
Biochemical and Angiographic Variables of the CI-AKI and Non-CI-AKI Groups.a
| Variable | Overall (N = 400) | CI-AKI (n = 66) | Non-CI-AKI (n = 334) | P value |
|---|---|---|---|---|
| Biochemical indicators | ||||
| d-dimer, µg/L(IQR) | 194.5 (108.5-377.5) | 273.0 (130.0-611.8) | 184.5 (104.0-335.0) | .011 |
| Age, years | 62.5 ± 12.4 | 67.1 ± 10.4 | 61.6 ± 12.6 | .001 |
| SBP (mm Hg) | 128.5 ± 21.6 | 127.6 ± 21.8 | 128.7 ± 21.6 | .687 |
| NT-proBNP, pg/mL (IQR) | 586.5 (88.5-2985.7) | 637.4 (92.5-2795.4) | 549.6 (98.9-2602.3) | .145 |
| Hemoglobin, g/L | 137.7 ± 19.4 | 127.5 ± 23.8 | 139.8 ± 17.8 | <.001 |
| White blood cells, 109/L | 10.3 ± 3.7 | 10.1 ± 3.5 | 10.3 ± 3.7 | .638 |
| Neutrophil ratio | 75.6 ± 12.4 | 79.1 ± 10.4 | 74.9 ± 12.7 | .012 |
| Platelet, 109/L | 212.2 ± 63.2 | 215.9 ± 74.1 | 211.5 ± 60.9 | .607 |
| Albumin, g/L | 37.0 ± 5.0 | 35.8 ± 5.8 | 37.2 ± 4.8 | .040 |
| Total bilirubin, µmol/L | 13.8 ± 7.3 | 11.6 ± 5.7 | 14.2 ± 7.5 | .008 |
| Glucose, mmol/L | 8.6 ± 4.1 | 9.8 ± 5.5 | 8.4 ± 3.8 | .014 |
| TC, mmol/L | 4.6 ± 1.2 | 4.5 ± 1.2 | 4.6 ± 1.2 | .474 |
| Triglycerides, mmol/L | 1.8 ± 1.5 | 1.6 ± 0.9 | 1.9 ± 1.6 | .213 |
| HDL-C, mmol/L | 1.1 ± 0.3 | 1.1 ± 0.3 | 1.1 ± 0.2 | .773 |
| LDL-C, mmol/L | 2.8 ± 0.9 | 2.8 ± 0.9 | 2.8 ± 0.8 | .813 |
| Uric acid, µmol/L | 327.9 ± 104.5 | 343.9 ± 110.3 | 324.7 ± 103.2 | .172 |
| SCr, µmol/L | 91.5 ± 50.0 | 117.0 ± 101.0 | 86.5 ± 30.0 | <.001 |
| eGFR, mL/min | 83.6 ± 36.8 | 69.7 ± 53.1 | 86.1 ± 32.4 | .004 |
| Coronary angiography | ||||
| Contrast agent, mL | 110.5 ± 22.7 | 109.2 ± 21.4 | 110.8 ± 23.0 | .616 |
| Lesion vessels | 3.0 ± 1.5 | 3.3 ± 1.5 | 3.0 ± 1.5 | .187 |
| LVEF | 0.54 ± 0.12 | 0.52 ± 0.12 | 0.54 ± 0.12 | .328 |
Abbreviations: CI-AKI, contrast-induced acute kidney injury; eGFR, estimated glomerular filtration rate; HDL-C, high-density lipoprotein cholesterol; IQR, interquartile range; LDL-C, low-density lipoprotein cholesterol; LVEF, left ventricular ejection fraction; NT-proBNP, NT-proB-type natriuretic peptide; SBP, systolic blood pressure; SCr, serum creatinine concentration; TC, total cholesterol.
a Data are presented as the IQR or mean ± SD.
Univariable and multivariable analyses and predictors for CI-AKI are presented in Table 5. Univariable logistic regression showed that d-dimer, age, sex, diabetes mellitus, anemia, neutrophil ratio, albumin, blood glucose, total bilirubin, and eGFR were risk factors for CI-AKI in patients with STEMI after PCI. Multivariable logistic regression showed that a low d-dimer level was significantly associated with a decreased risk of CI-AKI in patients with STEMI after PCI independent of confounding factors, with an OR of 0.487 (95% CI: 0.178-0.931, P = .041) for those in the first quartile compared with those in the highest quartile. In addition, multivariable logistic regression also showed that age (OR: 1.047, 95% CI: 1.003-1.092), diabetes mellitus (OR: 5.896, 95% CI: 2.496-13.927), anemia (OR: 3.488, 95% CI: 1.308-9.306), and total bilirubin (OR: 0.946, 95% CI: 0.904-0.992) were independent predictors of CI-AKI in patients with STEMI after PCI (all P < .05). Multivariable logistic regression models were created which included d-dimer, age, sex, diabetes mellitus, anemia, neutrophil ratio, albumin, blood glucose, total bilirubin, and eGFR (P < .10).
Table 5.
Univariable and Multivariable Analysis and Predictors for CI-AKI.
| Variable | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | |
| d-dimer grouping | ||||||
| Group 4 | 1 | 1 | ||||
| Group 1 | 0.334 | 0.159-0.701 | .004a | 0.487 | 0.178-0.931 | .041b |
| Group 2 | 0.399 | 0.196-0.812 | .011a | 0.585 | 0.258-1.131 | .157 |
| Group 3 | 0.303 | 0.141-0.648 | .002a | 0.354 | 0.213-1.097 | .078 |
| Age | 1.039 | 1.015-1.064 | .001a | 1.047 | 1.003-1.092 | .036b |
| Male | 0.498 | 0.277-0.895 | .020a | 0.737 | 0.306-1.777 | .497 |
| Smoker | 0.782 | 0.459-1.333 | .367 | |||
| Hypertension | 1.079 | 0.624-1.866 | .786 | |||
| Diabetes mellitus | 3.340 | 1.926-5.793 | <.001a | 5.896 | 2.496-13.927 | <.001b |
| Anemia | 4.729 | 2.495-8.964 | <.001a | 3.488 | 1.308-9.306 | .013b |
| Previous AMI | 3.133 | 0.730-13.445 | .124 | |||
| Atrial fibrillation | 1.372 | 0.441-4.273 | .585 | |||
| NT-proBNP, pg/mL | 1.027 | 0.298-3.016 | .383 | |||
| White blood cells, 109/L | 0.982 | 0.913-1.057 | .637 | |||
| Neutrophil ratio | 1.031 | 1.006-1.056 | .013a | 1.031 | 0.998-1.066 | .070 |
| Platelet, 109/L | 1.001 | 0.997-1.005 | .606 | |||
| Albumin, g/L | 0.947 | 0.898-0.998 | .041a | 1.002 | 0.923-1.087 | .965 |
| Glucose, mmol/L | 1.069 | 1.012-1.130 | .018a | 0.979 | 0.895-1.072 | .647 |
| Total bilirubin, µmol/L | 0.938 | 0.894-0.984 | .009a | 0.946 | 0.904-0.992 | .040b |
| TC, mmol/L | 0.919 | 0.730-1.157 | .473 | |||
| Triglycerides, mmol/L | 0.854 | 0.664-1.098 | .217 | |||
| HDL-C, mmol/L | 0.853 | 0.289-2.515 | .773 | |||
| LDL-C, mmol/L | 0.962 | 0.697-1.328 | .812 | |||
| Uric acid, umol/L | 1.003 | 0.998-1.005 | .173 | |||
| eGFR, mL/min | 0.985 | 0.975-0.995 | .004a | 0.994 | 0.979-1.021 | .277 |
| Contrast agent, mL | 0.997 | 0.985-1.009 | .615 | |||
| Lesion vessels | 1.121 | 0.946-1.329 | .188 | |||
| Three-vessel disease | 1.497 | 0.876-2.558 | .140 | |||
| LVEF | 0.310 | 0.030-3.236 | .328 | |||
Abbreviations: AMI, acute myocardial infarction; CI-AKI, contrast-induced acute kidney injury; eGFR, estimated glomerular filtration rate; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; LVEF, left ventricular ejection fraction; NT-proBNP, NT-proB-type natriuretic peptide; SBP, systolic blood pressure; TC, total cholesterol.
a Univariate logistic regression showed that d-dimer, age, sex, diabetes mellitus, anemia, neutrophil ratio, albumin, blood glucose, total bilirubin, and eGFR were risk factors for CI-AKI in patients with ST-segment elevation myocardial infarction (STEMI) after percutaneous coronary intervention (PCI).
b Multivariate logistic regression showed that d-dimer, age, diabetes mellitus, anemia, and total bilirubin were independent predictors of CI-AKI in patients with STEMI after PCI.
The ROC curves for d-dimer as a marker to differentiate CI-AKI in patients with STEMI after PCI are illustrated in Figure 1. The AUC of d-dimer for predicting the occurrence of CI-AKI in patients with STEMI after PCI was 0.702 (95% CI: 0.627-0.778; P = .039). The Youden index was used to find the best cutoff in the ROC curve. The cutoff of normality used by the specific laboratory was 437 µg/L. When the detection cutoff point of d-dimer was >437 µg/L for the diagnosis of CI-AKI, the sensitivity and specificity of d-dimer in predicting CI-AKI were 59.6% and 83.5%, respectively.
Figure 1.
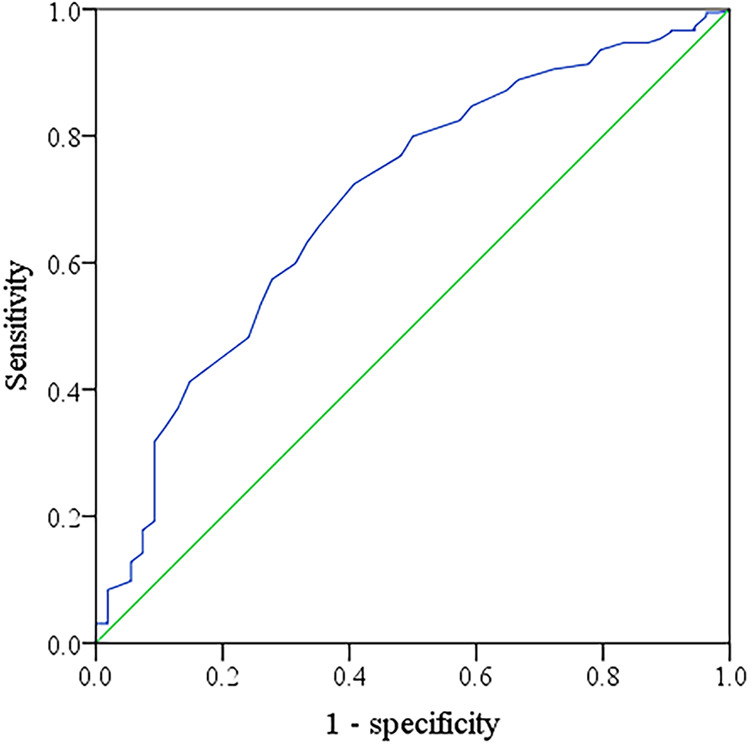
Receiver operating characteristic (ROC) curves for d-dimer as a marker to differentiate contrast-induced acute kidney injury (CI-AKI) in patients with ST-segment elevation myocardial infarction (STEMI) after percutaneous coronary intervention (PCI). The area under the ROC curves (AUCs) of d-dimer predicting the occurrence of CI-AKI in patients with STEMI after PCI was 0.702 (95% CI: 0.627-0.778; P = .039).
The occurrence of MACCE in each d-dimer group is shown in Table 6. The Kaplan-Meier survival curves that depict follow-up without an MACCE (MACCE-free) for the CI-AKI group and non-CI-AKI group are illustrated in Figure 2. The incidence of MACCEs within 30 days (21%), 6 months (32%), and 1 year (47%) after PCI were higher among patients with STEMI, with d-dimer levels in the highest quartile (group 4). The cumulative probability of the overall survival of the 4 groups at the 1-year follow-up is illustrated in Figure 3. The incidence of all-cause mortality within 30 days (7%), 6 months (9%), and 1 year (10%) after PCI were higher among patients with STEMI, with d-dimer levels in the highest quartile (group 4). As shown in Table 7, the incidence of hospitalization for kidney failure within 6 months and 1 year after PCI in group 4 were higher than that in the other 3 groups.
Table 6.
MACCE Occurrence in Each d-dimer Group.a
| Variable | Group 1 (n = 100) | Group 2 (n = 100) | Group 3 (n = 100) | Group 4 (n = 100) | P value |
|---|---|---|---|---|---|
| MACCE occurrence (30 days after PCI) | 4 (4.0%) | 4 (4.0%) | 12 (12.0%) | 21 (21.0%) | <.001b |
| MACCE occurrence (6 months after PCI) | 12 (12.0%) | 14 (14.0%) | 22 (22.0%) | 32 (32.0%) | .001b |
| MACCE occurrence (1 year after PCI) | 26 (26.0%) | 33 (33.0%) | 36 (36.0%) | 47 (47.0%) | .001b |
Abbreviations: MACCEs, main adverse cardiac and cerebrovascular events; PCI, percutaneous coronary intervention.
a Data are presented as n (%).
b The incidence of MACCE within 30 days, 6 months, and 1 year after PCI in group 4 were higher than that in the other 3 groups.
Figure 2.
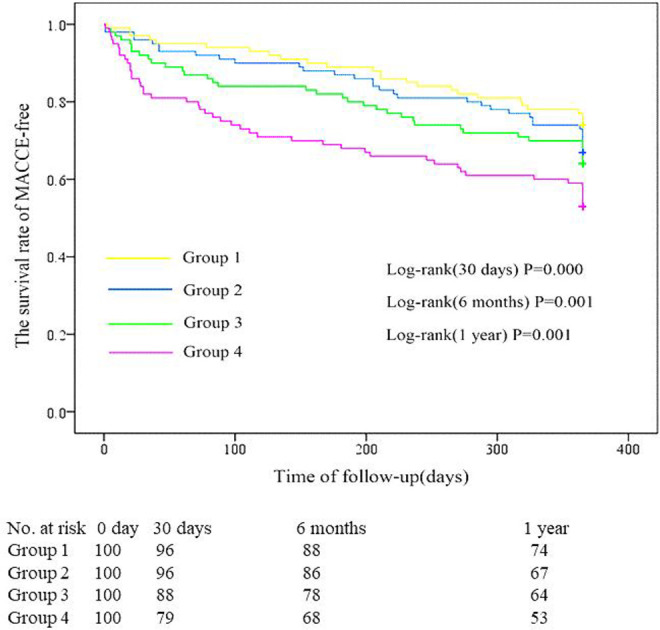
Survival curves for follow-up without an main adverse cardiovascular and cerebrovascular event (MACCE-free) for the 4 groups. The incidence of MACCEs within 30 days, 6 months, and 1 year after percutaneous coronary intervention (PCI) in group 4 were higher than that in the other 3 groups (P < .01).
Figure 3.
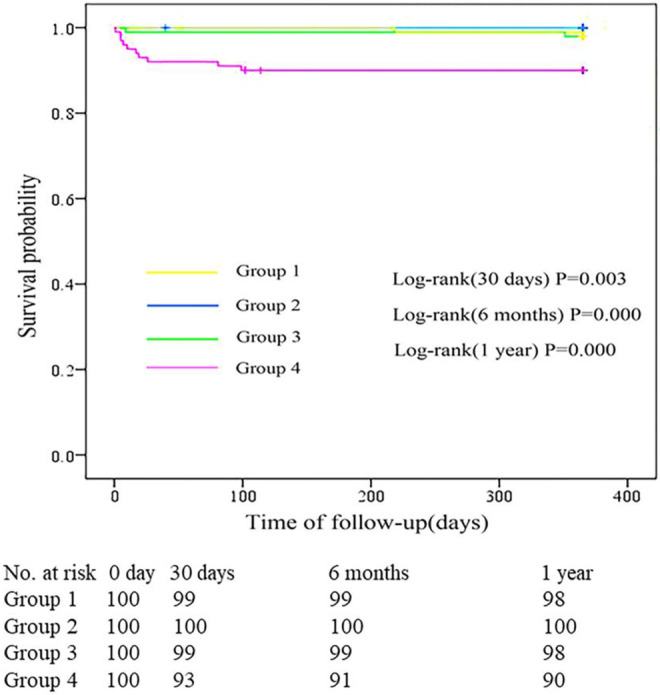
Cumulative probability of the overall survival for the 4 groups at the 1-year follow-up. The incidence of all-cause mortality within 30 days, 6 months, and 1 year after percutaneous coronary intervention (PCI) in group 4 were higher than that in the other 3 groups (P < 0.01).
Table 7.
Hospitalization for Kidney Failure in Each d-Dimer Group.a
| Variable | Group 1 (n = 100) | Group 2 (n = 100) | Group 3 (n = 100) | Group 4 (n = 100) | P value |
|---|---|---|---|---|---|
| Hospitalization for kidney failure (30 days after PCI) | 0 (0.0%) | 1 (1.0%) | 1 (1.0%) | 3 (3.0%) | .278 |
| Hospitalization for kidney failure (6 months after PCI) | 1 (1.0%) | 1 (1.0%) | 2 (2.0%) | 8 (8.0%) | .009b |
| Hospitalization for kidney failure (1 year after PCI) | 3 (3.0%) | 4 (4.0%) | 6 (6.0%) | 12 (12.0%) | .040b |
Abbreviation: PCI, percutaneous coronary intervention.
a Data are presented as n (%).
b The incidence of hospitalization for kidney failure within 6 months and 1 year after PCI in group 4 were higher than that in the other 3 groups (P < .05).
Discussion
Contrast-induced acute kidney injury is characterized by acute impairment of renal function following exposure to contrast agents,19 is an independent predictor of worse outcomes, and is significantly associated with long-term mortality.20 Previous studies have shown that congestive heart failure, chronic renal insufficiency, diabetes mellitus, intravascular volume depletion, and the use of a large amount of contrast agent were considered important predisposing factors.21 The diagnosis of CI-AKI has traditionally relied on SCr, which is a delayed indicator of CI-AKI.22 Early identification of high-risk groups and active intervention are indispensable to prevent CI-AKI.
Several risk scores and risk factors have been established for the prediction of CI-AKI, but none is recommended for use in daily practice due to a lack of sufficient verification in the literature.7,23 Advances in biomarkers for predicting CI-AKI have been made recently. Neutrophil gelatinase-associated lipocalin has been strongly correlated with SCr levels and have been considered in the diagnosis of CI-AKI.24 Cystatin C levels have been shown to be a more accurate early marker of GFR reduction and were less influenced by nonrenal variables than SCr levels.25,26 Procalcitonin, a novel marker of systemic inflammatory conditions, has been recently demonstrated to predict CI-AKI in patients with ACS.27 Unfortunately, although such biomarkers are promising, no standard cutoffs for the diagnosis of CI-AKI have been established, and existing data on clinical outcomes are inadequate.
d-dimer is the ultimate product of fibrin degradation by plasmin, the plasma concentrations of which are increased during ongoing or recent thrombosis. The measurement of d-dimer is routinely used in combination with clinical parameters in the initial assessment of suspected venous thromboembolism.28 Furthermore, there is an increasing trend toward using d-dimer to exclude aortic dissection.29
This study showed that d-dimer, a marker of thrombosis and hypercoagulable state, was negatively correlated with eGFR and that d-dimer levels were higher in anemic and AF patients. In addition, patients with higher d-dimer levels were significantly older and more frequently female. The incidence of CI-AKI after PCI was higher among patients with STEMI, with d-dimer levels in the highest quartile. Multivariable logistic regression showed that d-dimer was an independent predictor of CI-AKI in patients with STEMI after PCI (P < .05). The AUC of d-dimer for predicting the occurrence of CI-AKI in patients with STEMI after PCI was 0.702. When the detection cutoff point of d-dimer for the diagnosis of CI-AKI was >437 µg/L, the sensitivity and specificity of d-dimer in predicting CI-AKI were 59.6% and 83.5%, respectively. Moreover, multivariable logistic regression also showed that age (OR: 1.047, 95% CI: 1.003-1.092), diabetes mellitus (OR: 5.896, 95% CI: 2.496-13.927), anemia (OR: 3.488, 95% CI: 1.308-9.306), and total bilirubin (OR: 0.946, 95% CI: 0.904-0.992) were independent predictors of CI-AKI in patients with STEMI after PCI.
The prognostic value of d-dimer in patients with ACSs has long been debated. In our study, Kaplan-Meier analysis showed a significantly increased cumulative risk of MACCEs within 30 days, 6 months, and 1 year after PCI for patients with d-dimer levels in the fourth quartile (Figure 2). Likewise, Kaplan-Meier survival analysis revealed significantly lower survival rates within 30 days, 6 months, and 1 year after PCI for patients with d-dimer levels in the fourth quartile (Figure 3). Overall, this study indicated that the incidence of MACCEs and all-cause mortality within 30 days, 6 months, and 1 year after PCI were higher among patients with STEMI, with d-dimer levels in the highest quartile.
Study Limitations
The following limitations of the present study should be addressed. First, this study was not a standard randomized controlled trial. There was a selection bias, and whether these defects will affect the results also requires a prospective study with larger samples. Second, it was more reasonable to measure d-dimer several times during hospitalization. In addition, the changes in d-dimer during the follow-up period were not measured or analyzed. Third, the role of potential preventive strategies for CI-AKI, such as hydration and drug therapy, was not assessed. Although we adjusted for several known confounding variables in the multiple logistic regression model, other unknown factors might have played roles in CI-AKI. A greater sample size, more d-dimer subgroups, more thrombosis parameters, a longer follow-up time, and multicenter trials are necessary to further determine the cutoff point of d-dimer and other thrombosis parameters for patients to provide ideas for improving STEMI and CI-AKI risk stratification.
Conclusion
In conclusion, the current study demonstrated that higher d-dimer levels represent a strong independent indicator of increased risk of CI-AKI in patients with STEMI after PCI. Age, diabetes mellitus, anemia, and total bilirubin were independent predictors of CIN in patients with STEMI after PCI. In addition, increasing d-dimer levels were associated with the incidence of MACCE and all-cause mortality within 30 days, 6 months, and 1 year in patients with STEMI after PCI. Based on these solid results, d-dimer levels may help to screen patients with STEMI with a relatively high risk of CI-AKI and MACCEs on admission and help physicians select the best treatment options.
Footnotes
Authors’ Note: Erfei Luo and Dong Wang contributed equally to this work and should be considered co-first authors.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by grants to Chengchun Tang and Dong Wang from the National Natural Science Foundation of China (Research Grant #81670237 and 81800244).
ORCID iD: Chengchun Tang  https://orcid.org/0000-0003-3767-3551
https://orcid.org/0000-0003-3767-3551
References
- 1. Wichmann JL, Katzberg RW, Litwin SE, et al. Contrast-induced nephropathy. Circulation. 2015;132(20):1931–1936. doi:10.1161/CIRCULATIONAHA.115.014672 [DOI] [PubMed] [Google Scholar]
- 2. Nakahashi H, Kosuge M, Sakamaki K, et al. Combined impact of chronic kidney disease and contrast-induced nephropathy on long-term outcomes in patients with ST-segment elevation acute myocardial infarction who undergo primary percutaneous coronary intervention. Heart Vessels. 2017;32(1):22–29. doi:10.1007/s00380-016-0836-8 [DOI] [PubMed] [Google Scholar]
- 3. Dangas G, Iakovou I, Nikolsky E, et al. Contrast-induced nephropathy after percutaneous coronary interventions in relation to chronic kidney disease and hemodynamic variables. Am J Cardiol. 2005;95(1):13–19. doi:10.1016/j.amjcard.2004.08.056 [DOI] [PubMed] [Google Scholar]
- 4. Marenzi G, Lauri G, Assanelli E, et al. Contrast-induced nephropathy in patients undergoing primary angioplasty for acute myocardial infarction. J Am Coll Cardiol. 2004;44(9):1780–1785. doi:10.1016/j.jacc.2004.07.043 [DOI] [PubMed] [Google Scholar]
- 5. Amin AP, Spertus JA, Reid KJ, et al. The prognostic importance of worsening renal function during an acute myocardial infarction on long-term mortality. Am Heart J. 2010;160(6):1065–1071. doi:10.1016/j.ahj.2010.08.007 [DOI] [PubMed] [Google Scholar]
- 6. McCullough PA, Adam A, Becker CR, et al. Epidemiology and prognostic implications of contrast-induced nephropathy. Am J Cardiol. 2006;98(6A):5K–13K. doi:10.1016/j.amjcard.2006.01.019 [DOI] [PubMed] [Google Scholar]
- 7. Azzalini L, Spagnoli V, Ly HQ. Contrast-induced nephropathy: from pathophysiology to preventive strategies. Can J Cardiol. 2016;32(2):247–255. doi:10.1016/j.cjca.2015.05.013 [DOI] [PubMed] [Google Scholar]
- 8. Golshahi J, Nasri H, Gharipour M. Contrast-induced nephropathy; a literature review. J Nephropathol. 2014;3(2):51–56. doi:10.12860/jnp.2014.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Futrakul N, Futrakul P. Renal microvascular disease predicts renal function in diabetes. Ren Fail. 2012;34(1):126–129. doi:10.3109/0886022X.2011.623490 [DOI] [PubMed] [Google Scholar]
- 10. Wakabayashi I, Masuda H. Association of D-dimer with microalbuminuria in patients with type 2 diabetes mellitus. J Thromb Thrombolysis. 2009;27(1):29–35. doi:10.1007/s11239-007-0155-0 [DOI] [PubMed] [Google Scholar]
- 11. Simes J, Robledo KP, White HD, et al. D-dimer predicts long-term cause-specific mortality, cardiovascular events, and cancer in patients with stable coronary heart disease. Circulation. 2018;138(7):712–723. doi:10.1161/CIRCULATIONAHA.117.029901 [DOI] [PubMed] [Google Scholar]
- 12. Turker Y, Dogan A, Ozaydin M, et al. Association of thrombotic and fibrinolytic factors with severity of culprit lesion in patients with acute coronary syndromes without ST elevation. South Med J. 2010;103(4):289–294. doi:10.1097/SMJ.0b013e3181ccb3d7 [DOI] [PubMed] [Google Scholar]
- 13. Saigo M, Hsue PY, Waters DD. Role of thrombotic and fibrinolytic factors in acute coronary syndromes. Prog Cardiovasc Dis. 2004;46(6):524–538. [DOI] [PubMed] [Google Scholar]
- 14. McCann CJ, Glover BM, Menown IB, et al. Prognostic value of a multimarker approach for patients presenting to hospital with acute chest pain. Am J Cardiol. 2009;103(1):22–28. doi:10.1016/j.amjcard.2008.08.026 [DOI] [PubMed] [Google Scholar]
- 15. Brugger-Andersen T, Ponitz V, Staines H, Grundt H, Hetland O, Nilsen DW. The prognostic utility of D-dimer and fibrin monomer at long-term follow-up after hospitalization with coronary chest pain. Blood Coagul Fibrinolysis. 2008;19(7):701–707. doi:10.1097/MBC.0b013e32830b1512 [DOI] [PubMed] [Google Scholar]
- 16. Fiotti N, Di Chiara A, Altamura N, et al. Coagulation indicators in chronic stable effort angina and unstable angina: relationship with acute phase reactants and clinical outcome. Blood Coagul Fibrinolysis. 2002;13(3):247–255. [DOI] [PubMed] [Google Scholar]
- 17. Chinese Society of Cardiology. Guidelines for the diagnosis and treatment of acute ST segment elevation myocardial infarction in 2015 (China). Chin J Cardiol. 2015;43(5):380–393. doi:10.3760/cma.j.issn.0253-3758.2015.05.003 [Google Scholar]
- 18. Stacul F, van der Molen AJ, Reimer P, et al. Contrast induced nephropathy: updated ESUR contrast media safety committee guidelines. Eur Radiol. 2011;21(12):2527–2541. doi:10.1007/s00330-011-2225-0 [DOI] [PubMed] [Google Scholar]
- 19. Shaker OG, El-Shehaby A, El-Khatib M. Early diagnostic markers for contrast nephropathy in patients undergoing coronary angiography. Angiology. 2010;61(8):731–736. doi:10.1177/0003319710373093 [DOI] [PubMed] [Google Scholar]
- 20. Sun G, Chen P, Wang K, et al. Contrast-induced nephropathy and long-term mortality after percutaneous coronary intervention in patients with acute myocardial infarction. Angiology. 2019;70(7):621–626. doi:10.1177/0003319718803677 [DOI] [PubMed] [Google Scholar]
- 21. Mehran R, Aymong ED, Nikolsky E, et al. A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention: development and initial validation. J Am Coll Cardiol. 2004;44(7):1393–1399. doi:10.1016/j.jacc.2004.06.068 [DOI] [PubMed] [Google Scholar]
- 22. Devarajan P. Neutrophil gelatinase-associated lipocalin: a promising biomarker for human acute kidney injury. Biomark Med. 2010;4(2):265–280. doi:10.2217/bmm.10.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McCullough PA, Adam A, Becker CR, et al. Risk prediction of contrast-induced nephropathy. Am J Cardiol. 2006;98(6A):27K–36K. doi:10.1016/j.amjcard.2006.01.022 [DOI] [PubMed] [Google Scholar]
- 24. Haase M, Bellomo R, Devarajan P, Schlattmann P, Haase-Fielitz A. Accuracy of neutrophil gelatinase-associated lipocalin (NGAL) in diagnosis and prognosis in acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis. 2009;54(6):1012–1024. doi:10.1053/j.ajkd.2009.07.020 [DOI] [PubMed] [Google Scholar]
- 25. Khan E, Batuman V, Lertora JJ. Emergence of biomarkers in nephropharmacology. Biomark Med. 2010;4(6):805–814. doi:10.2217/bmm.10.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dharnidharka VR, Kwon C, Stevens G. Serum cystatin C is superior to serum creatinine as a marker of kidney function: a meta-analysis. Am J Kidney Dis. 2002;40(2):221–226. doi:10.1053/ajkd.2002.34487 [DOI] [PubMed] [Google Scholar]
- 27. Kurtul A, Murat SN, Yarlioglues M, et al. Procalcitonin as an early predictor of contrast-induced acute kidney injury in patients with acute coronary syndromes who underwent percutaneous coronary intervention. Angiology. 2015;66(10):957–963. doi:10.1177/0003319715572218 [DOI] [PubMed] [Google Scholar]
- 28. Pabinger I, Ay C. Biomarkers and venous thromboembolism. Arterioscler Thromb Vasc Biol. 2009;29(3):332–336. doi:10.1161/ATVBAHA.108.182188 [DOI] [PubMed] [Google Scholar]
- 29. Marill KA. Serum D-dimer is a sensitive test for the detection of acute aortic dissection: a pooled meta-analysis. J Emerg Med. 2008;34(4):367–376. doi:10.1016/j.jemermed.2007.06.030 [DOI] [PubMed] [Google Scholar]


