Abstract
Background and Aims:
The long-term health effects of the use of electronic cigarettes (ECs) in patients with chronic obstructive pulmonary disease (COPD) are largely unexplored. We present findings from a 5-year prospective assessment of respiratory parameters in a cohort of COPD patients who substantially reduced conventional smoking or achieved abstinence by switching to ECs.
Methods:
Patients were evaluated prospectively for their measurements of respiratory exacerbations, spirometric indices, quality of life using the COPD assessment tool (CAT), 6-min walk distance (6MWD), as well as conventional cigarette consumption. Baseline measurements prior to switching to EC use were compared with follow-up visits at 12-, 24-, 48- and 60-months. Age- and sex-matched COPD patients reporting to be regular smokers (not using ECs) were the reference group for the analysis.
Results:
Complete data were available from 39 patients. Those in the EC user group achieved a marked decline in cigarette smoking or abstinence. COPD EC users had a significant diminution in COPD exacerbations; with the mean (±SD) exacerbation rate falling from 2.3 (±0.9) at baseline to 1.1 (±1.0) at 5 years (p < 0.001), whereas no significant changes were observed in the control group.
Significant and constant improvements in lung function, CAT scores and 6MWD were reported in the EC user group over the 5-year observation period compared with the reference group (p < 0.05).
Conclusion:
The present study suggests that EC use may ameliorate objective and subjective COPD outcomes, and that the benefits gained appear to persist long term. EC use for abstinence and smoking reduction may ameliorate some of the harm resulting from tobacco smoking in COPD patients.
Keywords: COPD, electronic cigarette, smoking cessation, tobacco harm reduction
Introduction
Tobacco smoking is a major cause of preventable premature mortality worldwide, caused primarily by lung cancer, cardiovascular disease and chronic obstructive pulmonary disease (COPD).1,2
COPD is a condition epitomised by ongoing inflammation and remodelling of the airways, culminating in respiratory symptoms, progressive lung function deterioration, respiratory failure and death.3–5 Long-term exposure to a variety of smoke intoxicants is assumed to be the cause of this distinct airway inflammatory response.6,7 Abstinence from conventional tobacco use is the only reported evidence-based strategy that improves the prognosis for COPD.8,9 Besides, quitting has been shown to attenuate the decline in lung function and to enhance overall health status.10–12 In addition, smoking cessation attenuates the risk of developing other tobacco-related illnesses.2
While addressing smoking cessation is a priority for smokers with COPD, these patients experience high failure rates in their quit attempts.13,14 Licensed quitting therapies [i.e. nicotine replacement therapy (NRT), bupropion and varenicline] appear to have only fairly small or variable effects on sustained cessation in patients with COPD who smoke.15–17 Patients experience difficulty in completely ceasing nicotine use, and may require prolonged treatment and/or sustained nicotine use to attain continued abstinence from smoking. Obviously, a better understanding of predictors of quitting attempts and quitting success in smoking cessation could help routine clinical consultation and may improve outcomes,18,19 though this knowledge is lacking for COPD patients who smoke. An alternative for patients with COPD who are having difficulty quitting is one of the pragmatic strategies of tobacco harm reduction (THR), substituting combustion-free nicotine delivery strategies [i.e. electronic cigarettes (ECs)] for cigarette smoking to achieve significant health gains. The harm reduction potential mechanism is to reduce combustible tobacco chemicals that are responsible for the devastating aetiology of tobacco-related morbidity and mortality. As pulmonologists, we must be cognisant of the damage caused by tobacco smoke, and be aware of the limited negative health effects of nicotine when consumed at low concentrations.20 Of course, the use of electronic cigarettes is a strategy for treating patients who smoke, and not an endorsement of its use by those who do not smoke and youth, who should be discouraged from consuming nicotine in any form.
ECs have been gaining acceptance by smokers for decreasing their cigarette consumption, saving money compared with cigarettes, and seeking a much less harmful alternative to smoking that allows them to have a ‘smoking encounter without smoking’.21–24 The advantage of ECs is that they do not contain tobacco, nor do they operate with the combustion temperatures that generate smoke toxicants. However, they are not completely safe, though in routine conditions of use, laboratory testing has demonstrated that the amount of chemical constituents in emissions from EC aerosols is considerably lower than smoking conventional cigarettes.23,25,26 The significant reductions in toxic exposures from substituting EC for conventional cigarette consumption is expected to bring about substantial health gains. ECs as a THR strategy may save more lives more swiftly than possible previously. However, the odds of completely abstaining from conventional cigarettes for EC users are variable.27–29 Most studies suggesting low quit rates for ECs have investigated earlier poor quality vaping products with inadequate nicotine delivery profile.30,31 On the contrary, more recent (and better designed) randomised controlled trials (RCTs) using high-quality vaping products are now showing remarkable quit rates – even compared with NRTs.32,33 Nevertheless, data on the risk–benefit ratio of EC use in COPD smokers is limited.
United States (US) surveys from 2014 and 2015 indicate that former smokers with COPD may be using ECs to avoid relapse.34 A large cross-sectional survey of 1190 COPD EC users found that 75.7% stated that they had benefits in respiratory symptoms after switching, and only 0.8% reported a worsening of symptoms.24
In a retrospective analyses of smokers with COPD who had been ‘vaping’ (the acting of inhaling from ECs) routinely for at least 24 months reported no negative effects.35 Furthermore, the same study found a marked reduction in yearly exacerbations of COPD and overall health status improvements assessed with the COPD assessment tool (CAT) and physical activity assessed using the 6-min walk distance test (6MWT). A subsequent prospective follow up at 3 years of the same cohort of COPD patients using ECs regularly, by the same group of researchers, confirmed that these objective and subjective benefits persist long term.36
Nonetheless, more knowledge on the long-term health impacts of routine ‘vaping’ in this patient population is essential in order to provide sound advice to COPD patients who cannot quit or who are not interested in quitting.
The aim of the current study was to validate and expand these initial findings by reporting objective and subjective health parameters of subsequent follow ups in the same cohort of COPD patients who continued to vape daily for up to 5 years. Findings were compared with age- and sex-matched COPD patients who smoked regularly.
Methods
Patient population
A review of medical records of COPD patients followed up routinely in outpatient clinics of four Italian hospitals was conducted. A diagnosis of COPD was made in accordance to the criteria set out by the Global initiative for Chronic Obstructive Lung Disease (GOLD).37 Regarding pharmacological treatment, patients were taking various combinations of inhaled corticosteroids with bronchodilators (including long-acting β2 agonists and long-acting anti-cholinergics, individually or in combination) as recommended by GOLD guidelines.
Over a period of approximately 6 years (September 2013–October 2019), data were extracted from patients’ medical records from baseline and follow-up visits. Details of these patients’ population have been described previously.35,36 In brief, eligible COPD patients reported regular daily EC use at their scheduled outpatients appointments. They were using their own vaping product as part of their choice in embracing a new tobacco-smoke-free lifestyle. Those who reported daily ECs use on at least two successive outpatients appointments (no less than 12 months apart) were included in the study (EC users group). The baseline visit was considered as the clinic visit prior to the first of the two consecutive follow-up visits when the patients were not using ECs. EC devices and corresponding nicotine e-liquid strengths were noted. Datasets from age- and sex-matched COPD patients who regularly smoked conventional cigarettes (and not using ECs) over the same observation period and attending the same clinics were included as a reference group (cigarette smokers group). Outpatients in this study group were not keen to stop smoking, in spite of brief cessation advice routinely offered at every contact. The study objectives and design were not known to the hospital staff; data extraction was carried out from patients’ medical record.
In the current study, data analyses was conducted for follow-up visits that were timed at approximately 12, 24, 48 and 60 months from baseline. Approval for the study was acquired by the coordinating centre’s ethics review board at ‘Policlinico-Vittorio Emanuele Hospitals’ (approval number 647 on 14 May 2013) and each patient provided written informed consent.
Study design and study assessments
Study design and assessments of the study have been detailed previously.35,36 In brief, a review of patients’ clinical notes at baseline (when COPD patients in the EC group first reported EC use), at 12 ± 1.5 (follow-up visit 1; F/up1) and at 24 ± 2.5 (follow-up visit 2; F/up2) months to obtain data about (i) their respiratory symptoms, (ii) smoking status [biochemically confirmed by exhaled breath carbon monoxide (eCO)], cigarette consumption per day (cig/day), as well as EC use, (iii) the annual number of severe COPD exacerbations, (iv) lung function parameters post-bronchodilator [forced expiratory flow in 1 s (FEV1); forced vital capacity (FVC); expiratory ratio (FEV1/FVC)]; (v) CAT scores and (vi) 6MWD.
In the current study, COPD EC user and COPD control groups were evaluated prospectively for changes in the same objective and subjective parameters at follow-up visits at 48 ± 3 months (follow-up visit 3; F/up3) and 60 ± 3 months (follow-up visit 4; F/up4).
Additionally, we also assessed variations in the relative proportion of COPD GOLD stages over the 5-year study period.
For the purposes of the study severe exacerbations were defined as changes in the patients’ pulmonary symptoms necessitating antibiotics and/or oral corticosteroids via the primary care physician, emergency department attendance and/or admission to hospital. Nebulised therapy may have been administered to attain improvements in the patients’ condition for the latter two instances.
The CAT is a health status validated questionnaire used in COPD patients. A two unit difference is considered a minimal clinical important difference in patients’ symptomatology.38,39
The 6MWD is an assessment of patients’ overall ability to conduct everyday activities. This test was offered only to patients who were agreeable and physically able to complete the test.40
Smoking/vaping status
Abstinence from smoking was defined as a complete self-reported termination of conventional smoking (not even a puff) from the prior study visit; which was confirmed biochemically at F/up3 and 4 by eCO levels of ⩽7 ppm.
COPD EC users who completely ceased conventional tobacco smoking were defined as quitters (single users), and patients who reported using ECs in combination with conventional tobacco smoking were defined as dual users.
Data management and statistical analyses
Demographic and clinical data for all patients recruited onto the study were recorded in their case noted at the time of the outpatient visit. Patient data were extracted for the current study from case records onto an electronic spreadsheet before statistical computation. Of note, the investigators engaged in the study analyses were not involved in the medical management of the study participants or in the extraction of the data from the case records.
In the current analyses patient parameters are presented as means [± standard deviation (SD)] and medians [interquartile range (IQR)] for parametric and non-parametric data, respectively. Data from single and dual users were also extracted for secondary analyses. Statistical analyses using the student’s t-test and Wilcoxon-signed rank test were conducted for parametric or non-parametric data, respectively. Within-group dual and single users had similar statistical analyses conducted from baseline, and these analyses were excluded in the overall analyses. Analysis of repeated measures with Bonferroni correction between the two study groups was conducted for repeated parameter measurements over the 5-year period. A two-tailed p value of less than 0.05 was considered to signify statistical significance. All statistical evaluations were performed with the Statistical Package for Social Science (SPSS for Windows, version 20.0, Chicago, IL, USA).
Results
Patient characteristics
At 60 months, complete datasets were available for 39 COPD patients (33 male, 6 female) of the 48 individuals enrolled at baseline, 19 in the COPD smokers group and 20 in the COPD EC user group. Datasets from four patients (16.7%) were excluded in the EC user group due to relapse to cigarette smoking or quitting vaping. In the COPD smokers group, dataset from five patients were excluded or unavailable because two quit smoking (8.3%), one moved to another city, one developed a malignancy and was transferred to another clinic and one died. The baseline demographics, parameters assessed and COPD GOLD staging are outlined in Table 1. There were no statistical differences between the two study groups for any of the parameters. Patients had mild-to-severe airway obstruction as per the COPD GOLD guidelines, and were managed accordingly.37
Table 1.
Baseline demographics of participants who completed 60-months of evaluation (before switching to ECs).
| COPD controls (n = 19) | COPD EC users (n = 20) | Baseline p-value between groups | |
|---|---|---|---|
| Age¥ | 65 (±5.7) | 66.9 (±5.8) | 0.338 |
| Sex | 16M, 3F | 17M, 3F | – |
| COPD GOLD Staging | |||
| Stage 1 | 2 | 2 | – |
| Stage 2 | 5 | 6 | – |
| Stage 3 | 8 | 9 | – |
| Stage 4 | 4 | 3 | – |
| post-BD FEV1* (l) | 1.46 (1.19, 1.67) | 1.25 (0.98, 1.78) | 0.508 |
| post-BD FVC* (l) | 2.31 (2.10, 2.54) | 2.49 (2.08, 2.65) | 0.785 |
| %FEV1/FVC¥ | 60.9 (±6.8) | 55.8 (±10.8) | 0.088 |
| Pack years of smoking¥ | 49.7 (±6.8) | 52.8 (±11.0) | 0.304 |
| Cig/day¥ | 20.2 (±2.9) | 22.1 (±4.7) | 0.140 |
| FTND | 5.8 (±3.1) | 5.9 (±3.3) | 0.355 |
| CAT score* | 20 (17, 24.5) | 21 (17, 25.3) | 0.714 |
| COPD exacerbations¥ | 2 (±1.1) | 2.3 (±0.9) | 0.350 |
| 6MWD* (m) | 285 (219.3, 361.8) | 278 (186, 313) | 0.463 |
| Co-morbidities | |||
| Respiratory failure | 4 (21%) | 5 (25%) | – |
| Chronic heart failure | 4 (21%) | 4 (20%) | – |
| Coronary heart disease | 2 (10.5%) | 3 (15%) | – |
| Hypertension | 9 (47.4%) | 8 (40%) | – |
| Diabetes | 4 (21%) | 5 (25%) | – |
| Obstructive sleep apnoea | 6 (31.6%) | 6 (30%) | – |
| Chronic kidney failure | 1 (5.3%) | 0 | – |
| Liver cirrhosis | 1 (5.3%) | 0 | – |
| Lung cancer | 0 | 1 (5%) | – |
| Pulmonary hypertension | 0 | 1 (5%) | – |
| Gastroesophageal reflux | 5 (26.3%) | 4 (20%) | – |
| Degenerative joint disease | 4 (21%) | 5 (25%) | – |
| Osteoporosis | 3 (15.8%) | 3 (15%) | – |
| Depression/anxiety | 4 (21%) | 5 (25%) | – |
| Others | 3 (15.8%) | 3 (15%) | – |
6MWD, 6-min walk distance; BD, bronchodilator; CAT, COPD assessment tool; Cig, conventional cigarettes; COPD, chronic obstructive pulmonary disease; EC, electronic cigarettes; F, Female; FEV1, forced expiratory volume in 1 s; FTND, Fagerstrom test nicotine dependence; FVC, forced vital capacity; GOLD, global initiative for chronic obstructive lung disease; IQR, interquartile range; M, Male; SD, standard deviation.
Median (IQR).
Mean (±SD).
Smoking consumption and EC use
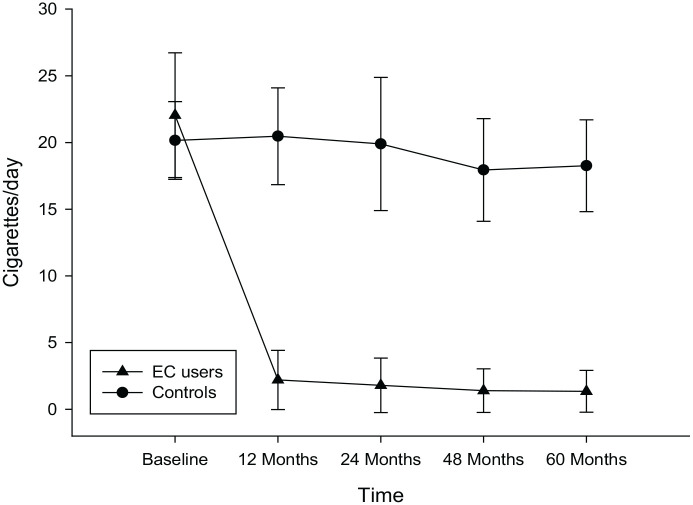
In the COPD EC users, a significant reduction in conventional cigarette use was noted with a mean (±SD) cigarettes/day of 22.1 (±4.7) at baseline, falling to 2.2 (±2.2), 1.8 (±2), 1.4 (±1.6) and 1.4 (±1.6) at F/up1, F/up 2, F/up 3 and F/up 4, respectively (p < 0.001 for all four visits) (Table 2; Figure 1). No significant changes were observed in the COPD controls in the number of cigarettes smoked per day. In the COPD EC user group, complete abstinence (quitters; exclusive EC users or single users) in cigarettes smoked per day was reported in 9/20 (45%) at F/up 4; and in those continuing to smoke (dual users) in 11/20 (55%) (Table 3). There was a considerable decline in conventional cigarette consumption in dual users with the mean (±SD) cigarettes/day at baseline decreasing from 23.7 (±5.4) to 4 (±1.2), 3.6 (±1.3), 3.1 (±0.6) and to 3.0 (±0.5) at F/up 1, F/up 2, F/up 3 and F/up 4, respectively (p < 0.001 for all four visits) (Table 3). Of note, all the dual users consistently reduced their daily smoking by at least 80% of their baseline consumption throughout the whole duration of the study. Overall, there was a significant reduction in conventional cigarettes smoked per day between the study groups over the 60-month observation period (p < 0.001).
Table 2.
Changes in study parameters from baseline at 12-, 24-, 36-, 48- and 60-month follow-up visits in COPD controls and COPD EC users.
| Baseline | 12-month follow-up | Within group p value versus baseline | 24-month follow-up | Within group p value versus baseline | 48-month follow-up | Within group p value versus baseline | 60-month follow-up | Within group p value versus baseline | Overall between group p value from baseline | |
|---|---|---|---|---|---|---|---|---|---|---|
| COPD Controls (n = 19) | ||||||||||
| post-BD FEV1* (l) | 1.46 (1.19, 1.67) | 1.41 (1.17, 1.61) | 0.532 | 1.36 (1.18, 1.61) | 0.747 | 1.34 (1.17, 1.61) | 0.732 | 1.33 (1.13, 1.53) | 0.387 | 0.004 |
| post-BD FVC* (l) | 2.31 (2.10, 2.54) | 2.27 (2.20, 2.57) | 0.135 | 2.31 (2.18, 2.66) | 0.268 | 2.33 (2.11, 2.45) | 0.286 | 2.34 (2.20, 2.58) | 0.840 | 0.016 |
| %FEV1/FVC | 60.9 (±6.8) | 59.7 (±6.9) | 0.008 | 59.6 (±6.9) | 0.026 | 60.2 (±8.7) | 0.637 | 57.9 (±9.1) | 0.074 | 0.038 |
| Cig/day¥ | 20.2 (±2.9) | 20.5 (±3.6) | 0.618 | 19.9 (±5.0) | 0.810 | 17.9 (±3.9) | 0.061 | 18.3 (±3.4) | 0.091 | <0.001 |
| CAT score* | 20 (17, 24.5) | 20 (17.5, 23) | 0.294 | 20 (15, 24) | 0.367 | 20 (18, 23) | 0.618 | 20 (17.5, 23.5) | 0.962 | 0.158 |
| COPD Exacerbations¥ | 2 (±1.1) | 2.2 (±1) | 0.494 | 2 (±1.1) | 1.000 | 1.8 (±1.0) | 0.385 | 1.7 (±1.1) | 0.331 | 0.046 |
| 6MWD*∑ (m) | 285 (219.3, 361.8) | 270 (227, 372.5) | 0.286 | 277.5 (243, 374.8) | 0.213 | 306 (245.3, 355.5) | 0.328 | 305 (243, 342.5) | 0.722 | 0.012 |
| COPD EC users (n = 20) | ||||||||||
| post-BD FEV1* (l) | 1.25 (0.98, 1.78) | 1.23 (0.96, 1.73) | 0.038 | 1.29 (0.95, 1.69) | 0.093 | 1.40 (1.10, 1.80) | 0.008 | 1.42 (1.22, 1.95) | 0.001 | |
| post-BD FVC* (l) | 2.49 (2.08, 2.65) | 2.51 (2.15, 2.73) | 0.046 | 2.46 (2.07, 2.86) | 0.156 | 2.54 (2.14, 3.00) | 0.008 | 2.70 (2.17, 3.03) | 0.002 | |
| %FEV1/FVC¥ | 55.8 (±10.8) | 55.6 (±10.8) | 0.806 | 56.1 (±10.7) | 0.558 | 57.5 (±9.0) | 0.147 | 58.2 (±9.2) | 0.054 | |
| Cig/day¥ | 22.1 (±4.7) | 2.2 (±2.2) | <0.001 | 1.8 (±2) | <0.001 | 1.4 (±1.6) | <0.001 | 1.4 (±1.6) | <0.001 | |
| CAT score* | 21.0 (17, 25.3) | 18 (16, 20.5) | <0.001 | 18 (14.8, 20) | 0.002 | 17.5 (14.8, 22.5) | 0.033 | 17 (14.8, 20.8) | 0.020 | |
| COPD exacerbations¥ | 2.3 (±0.9) | 1.8 (±1) | 0.004 | 1.4 (±0.9) | <0.001 | 1.2 (±1.0) | <0.001 | 1.1 (±1.0) | <0.001 | |
| 6MWD*∑ (m) | 278 (186, 313) | 305 (217.6, 337.8) | 0.007 | 319.3 (225.6, 355.5) | 0.007 | 347.5 (232.5, 404.5) | 0.005 | 344.5 (239, 394.8) | 0.005 | |
6MWD, 6 min walk distance; BD, bronchodilator; CAT, COPD assessment tool; Cig, conventional cigarettes; COPD, chronic obstructive pulmonary disease; EC, electronic cigarettes; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; IQR, interquartile range; SD, standard deviation.
Median (IQR).
Mean (±SD).
10 subjects in the COPD EC user group and 11 in the COPD control group at all time points.
Figure 1.
Number of cigarettes smoker per day at baseline, follow-up visit 1 (12 ± 1.5 months), visit 2 (24 ± 2.5 months), visit 3 (48 ± 3 months) and visit 4 (60 ± 3 months) separately for COPD electronic cigarettes users (closed triangles) and COPD controls (closed circles). All data expressed as mean and error bars are standard deviation of the mean.
COPD, chronic obstructive pulmonary disease; EC, electronic cigarette.
Table 3.
Changes in study parameters from baseline at 12-, 24-, 36-, 48- and 60-month follow-up visits in single and dual users.
| Parameter | Baseline | 12-month follow-up | Within group p value versus baseline | 24-month follow-up | Within group p value versus baseline | 48-month follow-up | Within group p value versus baseline | 60-month follow-up | Within group p value versus baseline |
|---|---|---|---|---|---|---|---|---|---|
| COPD EC users reducing cig use (dual users) | (n = 11) | (n = 11) | – | (n = 10) | – | (n = 9) | – | (n = 9) | – |
| Sex | 10M, 1F | 10M, 1F | – | 10M | – | 9M | – | 9M | – |
| % Smoking reduction compared with baseline | – | 82.7 (±4.8) | – | 84.7 (±4.7) | – | 88.4 (±4.2) | – | 91.0 (±6.9) | – |
| post-BD FEV1* (l) | 1.23 (0.94, 1.6) | 1.2 (0.91, 1.7) | 0.026 | 1.18 (0.91, 2.01) | 0.108 | 1.18 (1.0, 1.8) | 0.086 | 1.29 (1.0, 1.9) | 0.055 |
| post-BD FVC* (l) | 2.34 (2.04, 2.86) | 2.35 (2.07, 2.89) | 0.074 | 2.52 (2.2, 2.93) | 0.274 | 2.41 (2.2, 3.04) | 0.105 | 2.50 (2.3, 3.1) | 0.075 |
| %FEV/FVC* | 53.8 (±9.5) | 53.8 (±9.9) | 1.000 | 54.0 (±12.1) | 0.940 | 52.7 (±8) | 0.759 | 53.6 (±7.9) | 0.461 |
| Cig/day¥ | 23.7 (±5.4) | 4 (±1.2) | <0.001 | 3.6 (±1.3) | <0.001 | 3.1 (±0.6) | <0.001 | 3.0 (±0.5) | <0.001 |
| CAT score* | 25 (19.5, 26.5) | 20 (18, 22) | <0.001 | 19 (15.8, 22) | 0.011 | 20 (16, 25) | 0.156 | 20 (15, 24) | 0.176 |
| COPD exacerbations¥ | 2.6 (±0.8) | 2.3 (±0.8) | 0.104 | 1.5 (± 0.9) | 0.004 | 1.4 (± 0.9) | 0.010 | 1.6 (±1.0) | 0.021 |
| COPD EC users ceasing cig use (single users) | (n = 9) | (n = 9) | (n = 10) | (n = 11) | (n = 11) | ||||
| Sex | 7M, 2F | 7M, 2F | 7M, 3F | 8M, 3F | 8M, 3F | ||||
| Smoking reduction compared with baseline | – | – | – | – | – | ||||
| post-BD FEV1* (l) | 1.32 (1.05, 1.76) | 1.26 (1.09, 1.72) | 0.632 | 1.34 (1.22, 1.57) | 0.388 | 1.45 (1.2, 1.77) | 0.026 | 1.5 (1.32, 1.93) | 0.003 |
| post-BD FVC* (l) | 2.57 (2.27, 2.65) | 2.6 (2.22, 2.72) | 0.444 | 2.44 (1.92, 2.81) | 0.384 | 2.57 (2.08, 2.91) | 0.034 | 2.7 (2.11, 2.93) | 0.003 |
| %FEV/FVC* | 58.2 (±12.4) | 57.9 (±12.1) | 0.351 | 58.2 (±9.2) | 0.338 | 61.4 (±8.1) | 0.110 | 62.0 (±8.7) | 0.063 |
| Cig/day¥ | 20 (±2.7) | – | – | – | – | ||||
| CAT score* | 18 (17, 22) | 16 (14, 18) | 0.009 | 16 (14.3, 19.5) | 0.014 | 16 (14.5, 19.5) | 0.073 | 16 (14.5, 18.5) | 0.065 |
| COPD exacerbations¥ | 1.9 (±0.9) | 1.1 (±0.8) | <0.001 | 1.2 (±0.9) | 0.019 | 1.0 (±1) | 0.019 | 0.7 (±0.9) | 0.008 |
BD, bronchodilator; CAT, COPD assessment tool; cig, conventional cigarettes; COPD, Chronic obstructive pulmonary disease; EC, electronic cigarettes; F, female; FEV1, forced expiratory volume in 1 sec; FVC, forced vital capacity; M, male; IQR, interquartile range; SD, standard deviation.
Median (IQR).
Mean (±SD).
A mix of different vaping products were used by COPD patients in the COPD EC users group over the 5-year duration of the study. Devices and/or e-liquids were changed quite frequently over time. An increasing percentage of users switched from standard refillable e-cigs to more advanced devices during the study (from 9% at baseline to 18% at first wave). Further details were not recorded in subsequent waves. However, nicotine strength was more accurately tracked. Most users started with 12–18 mg/ml (medium/high) nicotine strength at baseline and then gradually reduced their nicotine strength over time; by 5-year follow up, only 2 out of 20 users were still using medium/high nicotine strength e-liquids, whereas the others were consuming 3–9 mg/ml (low) nicotine strength.
COPD exacerbations
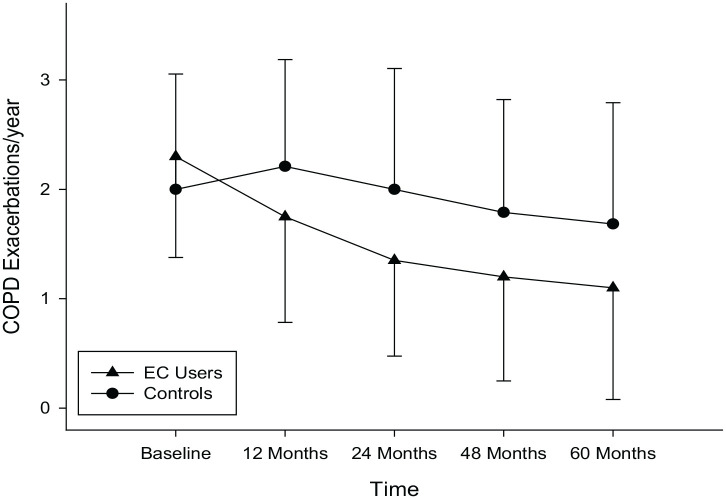
COPD EC users had a marked reduction in COPD exacerbations; with the mean (±SD) annual exacerbation rate declining from 2.3 (±0.9) at baseline to 1.8 (±1) at F/up1 (p = 0.004), 1.4 (±0.9) at F/up2 (p < 0.001), 1.2 (±1.0) at F/up3 (p < 0.001) and 1.1 (±1.0) at F/up 4 (p < 0.001), respectively (Table 2). Conversely there were no significant changes from baseline in the annual COPD exacerbation rates in the control group over the 5 years. An overall significant (p = 0.046) between group reduction in COPD exacerbations was noted over the 5-year observation period (Table 2; Figure 2). Consistent decline in COPD exacerbations were noted in the dual users from baseline; with the mean (±SD) annual exacerbation rate of 2.6 (±0.8) at baseline significantly reducing to 1.5 (±0.9) at F/up2 (p = 0.004), 1.4 (±0.9) at F/up 3 (p = 0.010) and 1.6 (±1.0) at F/up4 (p = 0.021), respectively (Table 3). Significant reductions in the annual COPD exacerbations were noted at all the follow-up visits compared with baseline in the exclusive EC users (single users) (Table 3).
Figure 2.
Changes in the number of COPD exacerbations per year from baseline, at follow-up visit 1 (12 ± 1.5 months), visit 2 (24 ± 2.5 months), visit 3 (48 ± 3 months) and visit 4 (60 ± 3 months) separately for COPD electronic cigarettes users (closed triangles) and COPD controls (closed circles). Data expressed as mean and error bars are standard deviation of the mean.
COPD, chronic obstructive pulmonary disease; EC, electronic cigarette.
Lung function assessments and COPD staging
There were substantial improvements in post-bronchodilator FEV1 and FVC in the EC users compared with baseline at all follow-up visits except for F/up 2 (Table 2; Figure 3a–b). Reductions in spirometric indices compared with baseline at all follow-up visits in the control group were noted, though this was not significant (Table 2; Figure 3a–b). There were overall marked differences between the two study groups in the spirometric assessments were observed in favour of the EC users (Table 2).
Figure 3.
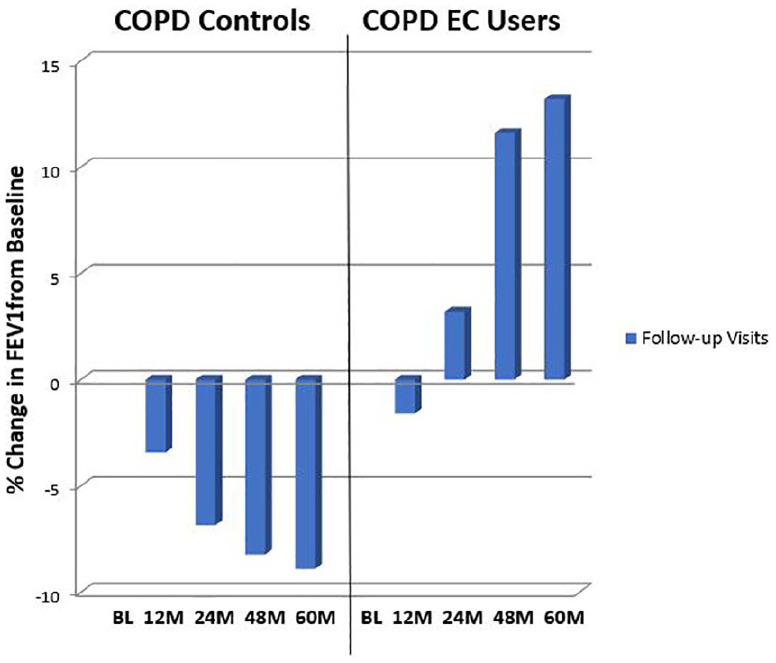
Percentage change in median FEV1 (a) and FVC (b) from baseline, at follow-up visit 1 (12 ± 1.5 months), visit 2 (24 ± 2.5 months), visit 3 (48 ± 3 months) and visit 4 (60 ± 3 months) separately for COPD EC users and COPD controls.
BL, baseline; COPD, chronic obstructive pulmonary disease; EC, electronic cigarette; FEV1, forced expiratory volume in 1 second; FVC, Forced vital capacity; L, litres; M, months.
GOLD COPD staging changes throughout the study are illustrated in Figure 4. Over the 60-month observation period, a number of patients in the EC group down-staged (i.e. improved) from GOLD COPD Stages 4 and 3 to Stages 3 and 2, respectively. In contrast, minimal changes in COPD GOLD stages were observed in the control group.
Figure 4.
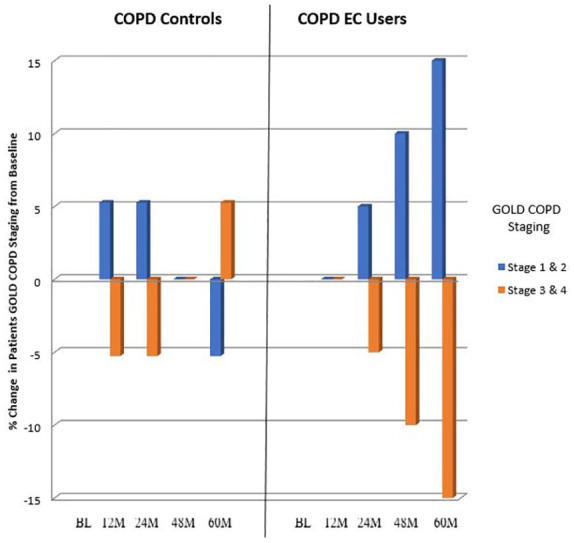
Percentage change in patients COPD GOLD stage over the study period.
BL, baseline; COPD, chronic obstructive pulmonary disease; GOLD, global initiative for chronic obstructive lung disease; EC, electronic cigarette; M, months.
CAT scores and 6MWD
CAT scores, which is a subjective COPD assessment, significantly improved in the EC COPD group at all four follow-up time points compared with baseline (p < 0.05 at all follow-up visits) (Table 2). Similarly, throughout the study relevant clinical improvements in median CAT scores were observed from baseline (Table 2). In the control group, no significant or clinically relevant improvements were noted at any of the follow-up visits from baseline. There was no overall significant improvement in CAT scores between groups over the 5-year study period (p = 0.158) (Table 2; Figure 5).
Figure 5.
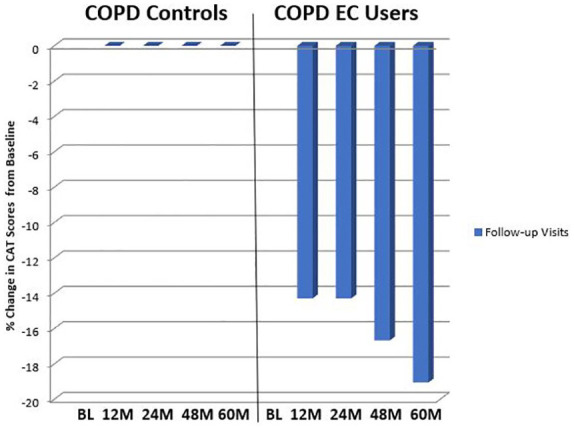
Percentage change in the median CAT scores from baseline, at follow-up visit 1 (12 ± 1.5 months), visit 2 (24 ± 2.5 months), visit 3 (48 ± 3 months) and visit 4 (60 ± 3 months) separately for COPD EC users and COPD.
BL, baseline; CAT, COPD assessment tool; COPD, chronic obstructive pulmonary disease; EC, electronic cigarette; M, Months.
Results of 6MWD at all four follow-up visits were available for 10 patients in the COPD EC group, whilst those from the COPD control group were available for 11 subjects. In the EC user group, significant improvements from baseline in 6MWD was observed all time points (p < 0.01); there were no notable improvements in 6MWD in the control group (Table 2). At 5 years from baseline the 6MWD improved by a median of 66.5m (p = 0.005) in the COPD EC user group, whereas it increased by a median of 20 m (p = 0.722) in the COPD control group (Table 2). A significant (p = 0.012) improvement in 6MWD was observed between the study groups over the 5-year period of follow-up (Table 2).
Discussion
In our study, offering the longest clinical follow up ever reported in this field, patients with COPD who abstained from smoking or substantially reduced their cigarette consumption by swapping to vaping experienced improvements in objective and subjective health outcomes. These positive health effects persisted long term, for up to 5 years. Switching from smoking to vaping leads to improved COPD outcomes, which is expected because quitting smoking is known to slow COPD progression and to improve patients’ respiratory health.8,10–12
A major finding of the study is that COPD exacerbations were reduced by approximately 50% in patients who stopped or considerably reduced their smoking consumption after switching to vaping. The magnitude of the number of COPD exacerbations prevented in these patients is of clinical significance and similar to that observed with pharmacological interventions.41 Prolonged exposure to cigarette smoke has been demonstrated to increase susceptibility to airway infection and respiratory exacerbations so that quitting smoking may reduce these conditions and related symptoms.42–44 Former smokers in one study reported a 43% lower risk for COPD-related hospitalizations compared with active smokers.45 Another study found a 22% risk reduction of COPD exacerbation in former smokers compared with active smokers; this was confirmed after adjusting for co-morbidity, COPD severity indices, and socioeconomic status.46 Studies that have not found differences in hospital admissions between smokers and former smokers with COPD failed to account for critical confounders for COPD exacerbation risk, such as duration of smoking abstinence duration, disease severity, presence of co-morbidities, and age.47,48 Therefore, switching from smoking to vaping would be expected to result in the marked attenuation of respiratory infections and COPD exacerbations.
In agreement with our previous observations,35,36 lung function, respiratory health (i.e. CAT) and physical activity (i.e. 6MWD) improved consistently in COPD patients who quit or reduced substantially cigarette consumption after switching to vaping products. These results are similar to those of COPD patients undergoing intensive rehabilitation programs.39,49
Small improvements in post-bronchodilator FEV1 were noted over the 5-year observation period in COPD EC users, but the differential impact of medication usage between the two study groups cannot be excluded. Alternatively, due to the prolonged absence/reduction of exposure to cigarette smoke in the COPD EC users, we might speculate that the airways could have improved their responsiveness to salbutamol. In further support of this hypothesis, a post hoc analysis of pre- versus post-bronchodilator difference in FEV1 values between the two groups indicated that post-bronchodilator values at F/up 3 and 4 were higher than pre-bronchodilator values in the COPD EC user group. Nonetheless, the benefits of EC use in determining improvements of lung function have been disputed in some studies. The association of EC use and self-reported chronic respiratory conditions have been reported in cross-sectional surveys of adults in the US,34,50 but these cross-sectional studies cannot demonstrate causation, and are not adjusted for smoking history, an obvious critical confounder. A recent paper analysing data from two large prospective cohorts concluded that e-cigarette users had more rapid decline in lung function,51 but this trend did not persist after adjustment for conventional cigarette smoking – which is of course the key factor driving the accelerated decline in lung function – and the study did not measure frequency of EC use. Also, e-cigarette users had heavier conventional cigarette smoking history, thus explaining why they also had poorer respiratory health, and were more likely to report chronic bronchitis and exacerbations as clarified in a detailed critique of this study.52
Down staging from GOLD COPD classes 3/4–1/2 in the COPD EC user group over the 5-year duration of the study was not a surprising observation given the above-mentioned improvements in exacerbation rates, overall health status and lung function.
Another important finding of the study is that only 8.3% patients from the COPD EC user group relapsed to cigarette smoking over the 5-year duration of the study, thus suggesting that relapse prevention may be an important mechanism by which vaping contributes to long-term smoking abstinence. Vaping mimics the experience of smoking and related rituals and provides powerful compensatory physical and behavioural effects, possibly serving as an effective relapse prevention method contributing to the low relapse rates observed in this study. Similar lower relapse rates with vaping have also been observed in studies of smokers with schizophrenia, asthma and hypertension.53–55 The reduction in relapse rates is vital because smokers with COPD do not respond very successfully to smoking cessation programs.15,56,57
The decline in carbon monoxide exposure and in carboxyhaemoglobin levels following smoking abstinence and the associated time-dependent improvement in exercise tolerance that occurs after quitting smoking may account for the reported improved health outcomes.44,58
Our study has a number of limitations. First, our findings are based on a small cohort of COPD patients must be interpreted with care. Notwith-standing, beneficial effects in several COPD health indicators were consistently observed over the entire 5-year duration of the study. Second, patients in the index study may represent a self-selected sample, and as such may not be indicative of the archetypical COPD smoker. Finally, the 6MWD test was not performed in approximately half of the study participants.
The present study confirms our previous research that switching from smoking to vaping ameliorates respiratory health in COPD patients and that these positive health effects may persist long term.35,36 By substantially reducing cigarette smoking or achieving abstinence with EC use, thereby curtailing exposure to several toxic chemicals, may have resulted in the ameliorated respiratory outcomes and bestowed an overall health advantage
The findings of this study are valuable because many COPD patients show little interest in quitting or reducing cigarettes in spite of their symptoms. Switching to much less harmful substitutes may limit the suffering of many patients by reducing some of the otherwise unavoidable burden of respiratory morbidity and mortality caused by cigarette smoking. Physicians in charge of the smoking patient with COPD should consider all the options available and opt for the ones that provide the greatest probability of stopping exposure to tobacco smoke, including ECs.59
Larger studies will be required to clarify the role of the e-vapour category for smoking cessation and/or harm reversal in smokers with COPD. Although these findings are preliminary, the evidence presented in our study about the long-term health impacts of vaping on COPD can be considered by health professionals when providing specific advice to their COPD patients who cannot or do not want to quit smoking.59,60
Footnotes
Author contributions: RP – study design, data interpretation, drafting and manuscript drafting and revision.
JBM – data analyses, data interpretation and manuscript drafting and revision.
UP - review of medical records and data collection.
BB - review of medical records and data collection.
AP – review of medical records, data collection, data interpretation and manuscript revision.
M Malerba - data interpretation and manuscript revision.
M Maglia - review of medical records and data collection.
PC – study design, data interpretation and manuscript revision.
All authors have read and approved the final manuscript.
Conflict of interest statement: In relation to his work in the area of tobacco control and respiratory diseases, RP has received lecture fees and research funding from Pfizer, GlaxoSmithKline, CV Therapeutics, NeuroSearch A/S, Sandoz, MSD, Boehringer Ingelheim, Novartis, Duska Therapeutics and Forest Laboratories. He has also served as a consultant for Pfizer, Global Health Alliance for treatment of tobacco dependence, CV Therapeutics, Boehringer Ingelheim, Novartis, Duska Therapeutics, ECITA (Electronic Cigarette Industry Trade Association, in the UK), Arbi Group Srl. and Health Diplomats. RP has been the Director of the Center of Excellence for the acceleration of Harm Reduction at the University of Catania (CoEHAR), which has received a grant from Foundation for a Smoke Free World to develop and carry out eight research projects. RP is also currently involved in the following pro bono activities: scientific advisor for LIAF (Lega Italiana Anti Fumo – Italian acronym for Italian Anti-Smoking League), the Consumer Advocates for Smoke-free Alternatives (CASAA) and the International Network of Nicotine Consumers Organizations (INNCO); Chair of the European Technical Committee for standardization on ‘Requirements and test methods for emissions of electronic cigarettes’ (CEN/TC 437; WG4). All other authors have no relevant conflict of interest to declare in relation to this study. The Associate Editor of Therapeutic Advances in Chronic Disease is an author of this paper, therefore, the peer review process was managed by alternative members of the Board and the submitting Editor had no involvement in the decision-making process
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by university grant no. 21040100 of ‘Ricerca Scientifica Finanziata dall’Ateneo di Catania’. RP is a full-time employee of the University of Catania, Italy. JBM is full-time employee of the Royal Brompton and Harefield Hospital NHS Trust, UK. UP is a full-time employee of the ASP Messina (Ospedale ‘San Vincenzo’ – Taormina, Italy). BB is a full-time employee of the Catania Hospital Trust (ARNAS Garibaldi, Italy). AP is a full-time employee of the Casa di Cura Musumeci-Gecas, Gravina di Catania, Italy. MM is a full-time employee of the University of Piemonte Orientale, Novara, Italy.
ORCID iD: Ricardo Polosa  https://orcid.org/0000-0002-8450-5721
https://orcid.org/0000-0002-8450-5721
Jaymin B Morjaria  https://orcid.org/0000-0003-1212-3190
https://orcid.org/0000-0003-1212-3190
Contributor Information
Ricardo Polosa, Department of Clinical and Experimental Medicine, University of Catania, Catania, Italy; Centre for the Prevention and Treatment of Tobacco Addiction (CPCT), Teaching Hospital ‘Policlinico – V. Emanuele’, University of Catania, Catania, Italy; Center of Excellence for the Acceleration of Harm Reduction (CoEHAR), Università di Catania, Catania, Italy.
Jaymin B Morjaria, Department of Respiratory Medicine, Royal Brompton and Harefield Hospital Foundation Trust, Harefield Hospital, Hill End Road, Harefield UB9 6JH, UK.
Umberto Prosperini, Hospital ‘San Vincenzo’, Taormina, Italy.
Barbara Busà, UOC Farmacia Ospedaliera, Hospital ARNAS Garibaldi, Catania, Italy.
Alfio Pennisi, Department of Respiratory Medicine, Hospital Clinics ‘Musumeci-Gecas’, Catania, Italy.
Mario Malerba, Center of Excellence for the Acceleration of Harm Reduction (CoEHAR), Università di Catania, Catania, Italy; Department of Translational Medicine, University of Piemonte Orientale, Novara, Italy.
Marilena Maglia, Department of Clinical and Experimental Medicine, University of Catania, Catania, Italy; Centre for the Prevention and Treatment of Tobacco Addiction (CPCT), Teaching Hospital ‘Policlinico – V. Emanuele’, University of Catania, Catania, Italy.
Pasquale Caponnetto, Department of Clinical and Experimental Medicine, University of Catania, Catania, Italy; Centre for the Prevention and Treatment of Tobacco Addiction (CPCT), Teaching Hospital ‘Policlinico – V. Emanuele’, University of Catania, Catania, Italy; Center of Excellence for the Acceleration of Harm Reduction (CoEHAR), Università di Catania, Catania, Italy.
References
- 1. World Health Organization. WHO report on the global tobacco epidemic 2017. World Health Organization, 2017. [Google Scholar]
- 2. U.S. Department of Health and Human Services. The health consequences of smoking: 50 years of progress: a report of the sugeon general. Atlanta, GA: US Department of Health and Human Services, Centres for Disease Control and Prevention, National Centre for Chronic Disease Prevetnion and Health Promotion, Office on Smoking and Health, 2014. [Google Scholar]
- 3. Falk JA, Kadiev S, Criner GJ, et al. Cardiac disease in chronic obstructive pulmonary disease. Proc Am Thorac Soc 2008; 5: 543–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. MacNee W. Pathogenesis of chronic obstructive pulmonary disease. Proc Am Thorac Soc 2005; 2: 258–266; discussion 290–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Morjaria JB, Malerba M, Polosa R. Biologic and pharmacologic therapies in clinical development for the inflammatory response in COPD. Drug Discov Today 2010; 15: 396–405. [DOI] [PubMed] [Google Scholar]
- 6. U.S. Department of Health and Human Services. How tobacco smoke causes disease: the biology and behavioral basis for smoking-attributable disease: a report of the surgeon general. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2010. [PubMed] [Google Scholar]
- 7. Stratton K, Shetty P, Wallace R, et al. Clearing the smoke: the science base for tobacco harm reduction–executive summary. Tob Control 2001; 10: 189–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. U.S. Department of Health and Human Services. The health benefits of smoking cessation. Rockville, Maryland: U.S. Department of Health and Human Services, Public Health Service, Centers for Disease Control, Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 1990. [Google Scholar]
- 9. Hersh CP, DeMeo DL, Al-Ansari E, et al. Predictors of survival in severe, early onset COPD. Chest 2004; 126: 1443–1451. [DOI] [PubMed] [Google Scholar]
- 10. Anthonisen NR, Connett JE, Kiley JP, et al. Effects of smoking intervention and the use of an inhaled anticholinergic bronchodilator on the rate of decline of FEV1. The lung health study. JAMA 1994; 272: 1497–1505. [PubMed] [Google Scholar]
- 11. Burchfiel CM, Marcus EB, Curb JD, et al. Effects of smoking and smoking cessation on longitudinal decline in pulmonary function. Am J Respir Crit Care Med 1995; 151: 1778–1785. [DOI] [PubMed] [Google Scholar]
- 12. Kanner RE, Connett JE, Williams DE, et al. Effects of randomized assignment to a smoking cessation intervention and changes in smoking habits on respiratory symptoms in smokers with early chronic obstructive pulmonary disease: the lung health study. Am J Med 1999; 106: 410–416. [DOI] [PubMed] [Google Scholar]
- 13. Jimenez-Ruiz CA, Masa F, Miravitlles M, et al. Smoking characteristics: differences in attitudes and dependence between healthy smokers and smokers with COPD. Chest 2001; 119: 1365–1370. [DOI] [PubMed] [Google Scholar]
- 14. van der Meer RM, Wagena EJ, Ostelo RW, et al. Smoking cessation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2003: CD002999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tashkin DP. Smoking cessation in chronic obstructive pulmonary disease. Semin Respir Crit Care Med 2015; 36: 491–507. [DOI] [PubMed] [Google Scholar]
- 16. Pezzuto A, D’Ascanio M, Grieco A, et al. Functional benefit of smoking cessation in severe COPD patients undergoing bronchial valve implantation. Eur J Intern Med 2019; 68: 55–59. [DOI] [PubMed] [Google Scholar]
- 17. Pezzuto A, Spoto C, Vincenzi B, et al. Short-term effectiveness of smoking-cessation treatment on respiratory function and CEA level. J Comp Eff Res 2013; 2: 335–343. [DOI] [PubMed] [Google Scholar]
- 18. Klemperer EM, Mermelstein R, Baker TB, et al. Predictors of smoking cessation attempts and success following motivation-phase interventions among people initially unwilling to quit smoking. Nicotine Tob Res 2020; 22: 1446–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Caponnetto P, Polosa R. Common predictors of smoking cessation in clinical practice. Respir Med 2008; 102: 1182–1192. [DOI] [PubMed] [Google Scholar]
- 20. Greenland S, Satterfield MH, Lanes SF. A meta-analysis to assess the incidence of adverse effects associated with the transdermal nicotine patch. Drug Saf 1998; 18: 297–308. [DOI] [PubMed] [Google Scholar]
- 21. Polosa R, Rodu B, Caponnetto P, et al. A fresh look at tobacco harm reduction: the case for the electronic cigarette. Harm Reduct J 2013; 10: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Caponnetto P, Russo C, Bruno CM, et al. Electronic cigarette: a possible substitute for cigarette dependence. Monaldi Arch Chest Dis 2013; 79: 12–19. [DOI] [PubMed] [Google Scholar]
- 23. Farsalinos KE, Polosa R. Safety evaluation and risk assessment of electronic cigarettes as tobacco cigarette substitutes: a systematic review. Ther Adv Drug Saf 2014; 5: 67–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Farsalinos KE, Romagna G, Tsiapras D, et al. Characteristics, perceived side effects and benefits of electronic cigarette use: a worldwide survey of more than 19,000 consumers. Int J Environ Res Public Health 2014; 11: 4356–4373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Goniewicz ML, Knysak J, Gawron M, et al. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob Control 2014; 23: 133–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Margham J, McAdam K, Forster M, et al. Chemical composition of aerosol from an E-cigarette: a quantitative comparison with cigarette smoke. Chem Res Toxicol 2016; 29: 1662–1678. [DOI] [PubMed] [Google Scholar]
- 27. McRobbie H, Bullen C, Hartmann-Boyce J, et al. Electronic cigarettes for smoking cessation and reduction. Cochrane Database Syst Rev 2014: CD010216. [DOI] [PubMed] [Google Scholar]
- 28. Kalkhoran S, Glantz SA. E-cigarettes and smoking cessation in real-world and clinical settings: a systematic review and meta-analysis. Lancet Respir Med 2016; 4: 116–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. El Dib R, Suzumura EA, Akl EA, et al. Electronic nicotine delivery systems and/or electronic non-nicotine delivery systems for tobacco smoking cessation or reduction: a systematic review and meta-analysis. BMJ Open 2017; 7: e012680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Caponnetto P, Campagna D, Cibella F, et al. EffiCiency and Safety of an eLectronic cigAreTte (ECLAT) as tobacco cigarettes substitute: a prospective 12-month randomized control design study. PLoS One 2013; 8: e66317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bullen C, Howe C, Laugesen M, et al. Electronic cigarettes for smoking cessation: a randomised controlled trial. Lancet 2013; 382: 1629–1637. [DOI] [PubMed] [Google Scholar]
- 32. Hajek P, Phillips-Waller A, Przulj D, et al. E-cigarettes compared with nicotine replacement therapy within the UK stop smoking services: the TEC RCT. Health Technol Assess 2019; 23: 1–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hajek P, Phillips-Waller A, Przulj D, et al. A randomized trial of E-cigarettes versus nicotine-replacement therapy. N Engl J Med 2019; 380: 629–637. [DOI] [PubMed] [Google Scholar]
- 34. Kruse GR, Kalkhoran S, Rigotti NA. Use of electronic cigarettes among U.S. adults with medical comorbidities. Am J Prev Med 2017; 52: 798–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Polosa R, Morjaria JB, Caponnetto P, et al. Evidence for harm reduction in COPD smokers who switch to electronic cigarettes. Respir Res 2016; 17: 166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Polosa R, Morjaria JB, Prosperini U, et al. Health effects in COPD smokers who switch to electronic cigarettes: a retrospective-prospective 3-year follow-up. Int J Chron Obstruct Pulmon Dis 2018; 13: 2533–2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Global Initiative For Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease (Updated 2008). Published 2008, http://goldcopd.org [Google Scholar]
- 38. Jones PW, Harding G, Berry P, et al. Development and first validation of the COPD assessment test. Eur Respir J 2009; 34: 648–654. [DOI] [PubMed] [Google Scholar]
- 39. Kon SS, Canavan JL, Jones SE, et al. Minimum clinically important difference for the COPD assessment test: a prospective analysis. Lancet Respir Med 2014; 2: 195–203. [DOI] [PubMed] [Google Scholar]
- 40. ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002; 166: 111–117. [DOI] [PubMed] [Google Scholar]
- 41. Aaron SD. Management and prevention of exacerbations of COPD. BMJ 2014; 349: g5237. [DOI] [PubMed] [Google Scholar]
- 42. Feldman C, Anderson R. Cigarette smoking and mechanisms of susceptibility to infections of the respiratory tract and other organ systems. J Infect 2013; 67: 169–184. [DOI] [PubMed] [Google Scholar]
- 43. Sopori M. Effects of cigarette smoke on the immune system. Nat Rev Immunol 2002; 2: 372–377. [DOI] [PubMed] [Google Scholar]
- 44. Campagna D, Cibella F, Caponnetto P, et al. Changes in breathomics from a 1-year randomized smoking cessation trial of electronic cigarettes. Eur J Clin Invest 2016; 46: 698–706. [DOI] [PubMed] [Google Scholar]
- 45. Godtfredsen NS, Vestbo J, Osler M, et al. Risk of hospital admission for COPD following smoking cessation and reduction: a Danish population study. Thorax 2002; 57: 967–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Au DH, Bryson CL, Chien JW, et al. The effects of smoking cessation on the risk of chronic obstructive pulmonary disease exacerbations. J Gen Intern Med 2009; 24: 457–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Anthonisen NR, Connett JE, Enright PL, et al. Hospitalizations and mortality in the lung health study. Am J Respir Crit Care Med 2002; 166: 333–339. [DOI] [PubMed] [Google Scholar]
- 48. Kessler R, Faller M, Fourgaut G, et al. Predictive factors of hospitalization for acute exacerbation in a series of 64 patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1999; 159: 158–164. [DOI] [PubMed] [Google Scholar]
- 49. Greulich T, Koczulla AR, Nell C, et al. Effect of a three-week inpatient rehabilitation program on 544 consecutive patients with very severe COPD: a retrospective analysis. Respiration 2015; 90: 287–292. [DOI] [PubMed] [Google Scholar]
- 50. Wills TA, Pagano I, Williams RJ, et al. E-cigarette use and respiratory disorder in an adult sample. Drug Alcohol Depend 2019; 194: 363–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bowler RP, Hansel NN, Jacobson S, et al. Electronic cigarette use in us adults at risk for or with COPD: analysis from two observational cohorts. J Gen Intern Med 2017; 32: 1315–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cummings KM, Polosa R. E-cigarette and COPD: unreliable conclusion about health risks. J Gen Intern Med 2018; 33: 784–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Caponnetto P, Auditore R, Russo C, et al. Impact of an electronic cigarette on smoking reduction and cessation in schizophrenic smokers: a prospective 12-month pilot study. Int J Environ Res Public Health 2013; 10: 446–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Polosa R, Morjaria JB, Caponnetto P, et al. Persisting long term benefits of smoking abstinence and reduction in asthmatic smokers who have switched to electronic cigarettes. Discov Med 2016; 21: 99–108. [PubMed] [Google Scholar]
- 55. Farsalinos K, Cibella F, Caponnetto P, et al. Effect of continuous smoking reduction and abstinence on blood pressure and heart rate in smokers switching to electronic cigarettes. Intern Emerg Med 2016; 11: 85–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Morjaria JB, Mondati E, Polosa R. E-cigarettes in patients with COPD: current perspectives. Int J Chron Obstruct Pulmon Dis 2017; 12: 3203–3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zhang MW, Ho RC, Cheung MW, et al. Prevalence of depressive symptoms in patients with chronic obstructive pulmonary disease: a systematic review, meta-analysis and meta-regression. Gen Hosp Psychiatry 2011; 33: 217–223. [DOI] [PubMed] [Google Scholar]
- 58. Berkovitch A, Kivity S, Klempfner R, et al. Time-dependent relation between smoking cessation and improved exercise tolerance in apparently healthy middle-age men and women. Eur J Prev Cardiol 2015; 22: 807–814. [DOI] [PubMed] [Google Scholar]
- 59. Polosa R, Campagna D, Caponnetto P. What to advise to respiratory patients intending to use electronic cigarettes. Discov Med 2015; 20: 155–161. [PubMed] [Google Scholar]
- 60. Polosa R, Caponnetto P. E-cigarettes and smoking cessation: a critique of a New England Journal Medicine-commissioned case study. Intern Emerg Med 2017; 12: 129–131. [DOI] [PMC free article] [PubMed] [Google Scholar]