Abstract
Background:
Compared to the general population, kidney transplant recipients are at increased risk of hemorrhage and thrombosis. Whether this risk is affected by graft function and albuminuria is unknown.
Objective:
To determine the association between graft function and albuminuria and the risk of post-transplant hemorrhage and thrombosis.
Design:
Retrospective cohort study.
Setting:
We used linked health care databases in Alberta, Canada.
Patients/sample/participants:
We included adult kidney transplant recipients from 2002 to 2015 with a functioning graft at 1 year.
Measurements:
Estimated glomerular filtration rate (eGFR) and albuminuria measurements at 1 year post-transplant were used to categorize recipients (eGFR: ≥45 vs. <45 mL/min/1.73 m2; albuminuria: absence vs. presence). We determined the rates of post-transplant hemorrhage and venous thrombosis based on validated diagnostic and procedural codes.
Methods:
We determined the association between categories of eGFR and albuminuria and post-transplant hemorrhage and venous thrombosis using Poisson regression with log link.
Results:
Of 1284 kidney transplant recipients, 21% had an eGFR <45 mL/min/1.73 m2 and 40% had presence of albuminuria at 1 year post-transplant. Over a median follow-up of 6 years, there were 100 hemorrhages (12.6 events per 1000 person-years) and 57 venous thrombosis events (7.1 events per 1000 person-years). The age- and sex-adjusted rate of hemorrhage and thrombosis was over 2-fold higher in recipients with lower eGFR and presence of albuminuria compared to higher eGFR and no albuminuria (hemorrhage: incidence rate ratio, IRR, 2.6, 95% confidence interval [CI]: 1.5-4.4, P = .001; thrombosis: IRR, 2.3, 95% CI: 1.1-5.0, P = .046).
Limitations:
Complete relevant medication information, such as anticoagulants, were not available in our datasets. Due to sample size, this study was underpowered to conduct a fully adjusted analysis.
Conclusion:
Among kidney transplant recipients, lower eGFR and presence of albuminuria at 1 year post-transplant were associated with an over 2-fold higher risk of hemorrhage and venous thrombosis. Graft function and albuminuria at 1 year post-transplant are important prognostic factors in determining risk of post-transplant hemorrhage and venous thrombosis. Further research, including medication data, are needed to further delineate outcomes and safety.
Trial registration:
Not applicable (cohort study).
Keywords: albuminuria, glomerular filtration rate, hemorrhage, kidney transplantation, thrombosis
Abrégé
Contexte:
Les receveurs d’une greffe rénale sont plus sujets aux hémorragies et aux thromboses que l’ensemble de la population. Nous ignorons cependant si ce risque est affecté par l’albuminurie et la fonction du greffon.
Objectif:
Déterminer s’il existe une association entre l’albuminurie et le risque de subir une hémorragie ou une thrombose à la suite d’une greffe.
Type d’étude:
Étude de cohorte rétrospective.
Sources:
Les bases de données du système de santé albertain (Canada).
Sujets:
Nous avons inclus les adultes ayant reçu une greffe rénale entre 2002 et 2015, et dont le greffon était toujours fonctionnel un an après l’intervention.
Mesures:
Nous avons utilisé les mesures d’albuminurie et de débit de filtration glomérulaire estimé (DFGe) un an après la greffe pour classer les sujets (absence ou présence d’albuminurie; DFGe: ≥45 ou <45 mL/min/1,73 m2). Les taux d’hémorragies et de thromboses veineuses post-transplantation ont été établis à l’aide de codes de diagnostic et de codes de procédure validés.
Méthodologie:
L’association entre le DFGe ou l’albuminurie et un risque d’hémorragie ou de thrombose veineuse post-greffe a été établie par une régression de Poisson avec lien logarithmique.
Résultats:
Un an après l’intervention, 40 % des 1 284 receveurs d’une greffe rénale inclus dans l’étude présentaient de l’albuminurie et 21 % présentaient un DFGe inférieur à 45 mL/min/1,73 m2. Au cours d’un suivi médian de six ans, 100 hémorragies (12,6 événements par 1 000 années-personnes) et 57 thromboses veineuses (7,1 événements par 1 000 années-personnes) ont été rapportées. Les taux d’hémorragies et de thromboses, corrigés selon l’âge et le sexe des sujets, étaient au moins deux fois plus élevés chez les receveurs présentant une albuminurie et un faible DFGe comparativement à ceux qui ne présentaient pas d’albuminurie et dont le DFGe était d’au moins 45 mL/min/1,73 m2 (rapport des taux d’incidence [RTI] pour les hémorragies = 2,6; IC à 95 %: 1,5-4,4; p=0,001; RTI pour les thromboses veineuses: 2,3; IC à 95 %: 1,1-5,0; p=0,046).
Limites:
Les informations complètes sur les médicaments, notamment sur les anticoagulants, n’étaient pas disponibles dans nos jeux de données. En raison de la taille de l’échantillon, cette étude s’est avérée insuffisante pour mener une analyze entièrement ajustée.
Conclusion:
Un an après l’intervention, la présence d’albuminurie et un faible DFGe ont été associés à un risque au moins deux fois plus élevé d’hémorragies et de thromboses veineuses chez les receveurs d’une greffe rénale. La fonction du greffon et l’albuminurie s’avèrent donc d’importants facteurs de risque d’hémorragie et de thrombose veineuse pour ces patients. Des recherches supplémentaires, y compris des données sur les médicaments, sont toutefois nécessaires pour mieux définir les résultats et l’innocuité.
Enregistrement de l’essai:
Sans objet (étude de cohorte).
Introduction
Kidney transplant recipients are at increased risk of hemorrhage and venous thromboembolism (VTE) when compared to the general population. The 3-year incidence rate of major hemorrhage in kidney transplant recipients has been reported to be 12.7 events per 1000 person-years and 8-fold higher than the general population.1 Similarly, the incidence of VTE (comprising pulmonary embolism [PE] and deep vein thrombosis [DVT]) in kidney transplant recipients has been reported to be 16.3 events per 1000 person-years and 7-fold higher than the general population.2 In addition, recipients who experience a post-transplant VTE have a 2-fold higher risk of graft loss and 4-fold higher risk of death compared to recipients who do not experience a VTE.2
Patients with chronic kidney disease (CKD) are also at an increased risk of hemorrhage and VTE, and lower estimated glomerular filtration rate (eGFR) and heavier albuminuria have been associated with this increased risk.3-5 Lower eGFR and heavier albuminuria in the non-transplant population have been independently associated with other important outcomes including mortality, kidney replacement therapy (KRT), and cardiovascular events.6-10 We have shown similar associations in the kidney transplant population.11,12 Thus, eGFR and albuminuria are thought to be important prognostic factors post-transplant. Whether eGFR and albuminuria are associated with the risk of hemorrhage and VTE in kidney transplant recipients is unknown.
Methods
Design and Setting
We conducted a population-based, retrospective cohort study using linked health care databases within the Alberta Kidney Disease Network (AKDN) that incorporates data from Alberta Health, the provincial health ministry.13 More than 99% of Alberta residents are registered with Alberta Health and have universal access to hospital care and physician services. We followed guidelines for the reporting of observational studies (Table S1) and a protocol approved by the research ethics boards at the University of Alberta and the University of Calgary, with a waiver of patient consent.
Data Sources
We ascertained baseline patient characteristics, covariate information, and outcome data from the AKDN records (Table S2). The Alberta Health database contains information on demographic data, vital statistics, and diagnostic and procedural information for inpatient and outpatient physician services. We identified kidney transplant recipients using the Northern and Southern Alberta Renal Program databases, which provide care to all patients treated with maintenance dialysis or kidney transplant in the province. We linked these data sources to a provincial laboratory repository (which captures all laboratory data in Alberta) via unique, encoded, patient identifiers held by the AKDN. These databases have been used extensively for research on health outcomes and services.6,11,12,14,15
Population
We included all adult kidney transplant recipients (≥18 years old), who received their first kidney-only transplant between May 1, 2002 and March 31, 2015 in Alberta (Figure S1). We excluded pediatric recipients (<18 years old) and those who had received a previous organ transplant or a simultaneous multi-organ transplant (eg, kidney-pancreas). Eligible recipients had at least one outpatient serum creatinine measurement and at least one outpatient measurement of albuminuria at 1 year following transplantation. We chose to classify kidney function at this time to ensure stability of kidney function and immunosuppression regimen and because this metric has been shown to be predictive of other clinical outcomes, such as mortality and graft failure.11,16-18 In addition, we wanted to exclude perioperative hemorrhage or thrombosis events within the first year following transplantation. Thus, to be included in the study, recipients must have survived at least 1 year with a functioning graft. We excluded transplant recipients who had graft failure (death or return to dialysis) in the first year post-transplant or whose eGFR was <15 mL/min/1.73 m2. The start date for follow-up (index date) was the 1 year post-transplant date.
Measurement of Kidney Function and Albuminuria
The eGFR at 1 year post-transplant was estimated using the Chronic Kidney Disease-Epidemiology Collaboration (CKD-EPI) equation.19 Although data on race were not available, misclassification of eGFR was expected to be minimal as ~3% of the Alberta population are Black.20 Only outpatient values were used to determine baseline eGFR and albuminuria to ensure representation of baseline kidney function and albuminuria. Baseline kidney function (index eGFR) was estimated using all outpatient serum creatinine measurements taken within a 3-month look-forward period of the creatinine measurement closest to the 1 year post-transplant date (index creatinine; Figure S2). The 3-month look-forward period was used to ensure creatinine values were after the 1 year post-transplant date. The index eGFR was calculated as the mean of these measurements within the 3-month period and categorized arbitrarily as ≥45 or <45 mL/min/1.73 m2. The KDIGO (Kidney Disease: Improving Global Outcomes) CKD categories 1 to 5 were not used as we anticipated too few events in each category to perform meaningful analyses.21
Albuminuria was ascertained from outpatient, random, spot urine measurements of albumin-creatinine ratio (ACR), protein-creatinine ratio (PCR), or urine dipstick and categorized as absent (ACR <30 mg/g, PCR <15 mg/mmol, or dipstick negative) or present (ACR ≥30 mg/g, PCR ≥15 mg/mmol, dipstick ≥trace).13,21-23 The KDIGO categories of albuminuria were not used as we anticipated too few events in each category to perform meaningful analyses. Albumin-creatinine ratio was the primary measure of albuminuria and if unavailable was supplemented with PCR measurements. When both ACR and PCR were unavailable, dipstick urinalysis was used. All outpatient ACR or PCR measurements or urine dipsticks in the 3-month periods before and after the index creatinine value were used to establish baseline albuminuria. The 3-month before and after periods were used, as outpatient urine protein measurements may not be routinely ordered with serum creatinine measurements, and multiple measurements have been shown to be more representative.24 For recipients with multiple albuminuria measurements within the 3 months of the index creatinine value, the median value was calculated.
Baseline Characteristics
Baseline demographic data, including age and sex, were determined from the Alberta Health administrative data files. The presence of one or more diagnostic codes in the 3 years prior to the index date was used for identification of comorbidities according to validated International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) and International Statistical Classification of Diseases, Tenth Revision (ICD-10) coding algorithms applied to physician claims and hospitalization data.25,26 Comorbidities of interest included previous hemorrhage, VTE, atrial fibrillation, and cancer. Hypertension and diabetes mellitus were identified from hospital discharge records and physician claims based on validated algorithms.27,28 Data were complete except for income quintile (<1% missing), which were imputed based on the most frequent age-sex-specific value.
Outcomes
Recipients were followed from the first post-transplant anniversary date (index date) until death, emigration from the province, end of study (March 31, 2017), or outcome of interest (first event only). Post-transplant hemorrhage included intracranial bleeds and gastrointestinal (GI) bleeds using validated diagnostic codes, similar to previous studies.1,4,29 VTE was defined using a validated algorithm comprising of one diagnostic code and one imaging code for either DVT or PE within 30 days of each other or during the same hospitalization for inpatients.30 A combination of diagnostic and imaging codes was used as the presence of a single code for VTE has a poor positive predictive value.31
Statistical Analyses
Differences between groups were compared using chi-square test for categorical variables and Kruskal-Wallis tests for all continues variables. Poisson regression with log link was used to evaluate the association between the baseline factors and each outcome of interest, with rates expressed per 1000 person-years.32 The 95% confidence interval (CI) was estimated using bootstrap techniques. If the primary assumption that variance equals the mean was not met, a negative binomial model or a generalized Poisson model (with a log link) was used. We calculated unadjusted rates for each of the outcomes by level of eGFR and presence or absence of albuminuria. We then calculated the event rates for each outcome, adjusting for age and sex. Two-way interactions between eGFR and albuminuria were assessed and included in all clinical outcomes, as they were shown to be significant in the non-transplant population for both hemorrhage and VTE.3,4 Finally, we calculated incidence rate ratios (IRR) using the recipients with eGFR ≥45 mL/min/1.73 m2 and no albuminuria as the referent group. In sensitivity analyses, we treated death as a competing risk event rather than censoring for death. Cumulative incidence functions were estimated using the Fine and Gray approach.
For the primary analysis, we included all patients with at least one albuminuria measurement based on ACR, PCR, or urine dipstick. In sensitivity analyses, we included measurements based on ACR and PCR alone (not including urine dipstick). We also performed analyses that adjusted for additional baseline variables, including peripheral vascular disease and diabetes mellitus. We added one variable at a time to the age- and sex-adjusted model. Statistical analyses were performed using Statistical Analysis Software STATA version 15 (STATA Corporation, College Station, TX). A P value of <.05 was used to define statistical significance.
Results
Baseline Characteristics
There were 1284 adult kidney-only transplant recipients with a functioning graft and at least one serum creatinine and urine protein measurement at 1 year post-transplant (Figure S1). Baseline characteristics according to level of kidney function and albuminuria are shown in Table 1. In our cohort, 21% had eGFR <45 mL/min/1.73 m2 and 40% had presence of albuminuria. The median age of the cohort was 53 years (interquartile range [IQR]: 41-62 years). Compared to recipients with eGFR ≥45 mL/min/1.73 m2 and no albuminuria, recipients with eGFR <45 mL/min/1.73 m2 and presence of albuminuria were more likely to be older and have a longer pre-transplant dialysis duration. In addition, recipients with lower eGFR and presence of albuminuria had more comorbidities, such as diabetes mellitus and peripheral vascular disease. Other comorbidities including previous hemorrhage, VTE, cancer, and atrial fibrillation were similar across all groups.
Table 1.
Demographic Characteristics of Recipients at 1 Year Post-Transplant by Level of Kidney Function and Albuminuria.
| Characteristic | Overall |
Estimated glomerular filtration
rate (mL/min per 1.73 m2) |
Albuminuria |
||
|---|---|---|---|---|---|
| n (%) | ≥45 | <45 | Absence | Presence | |
| Recipients (n) | 1284 (100) | 1015 (79.0) | 269 (21.0) | 768 (59.8) | 516 (40.2) |
| Age (years) | 53.2 (40.9-62.2) | 51.6 (39.6-61.3) | 58.3 (47.9-66.3) | 52.1 (40.1-61.8) | 55.0 (42.9-63.0) |
| >65 years | 240 (18.7) | 163 (16.1) | 77 (28.6) | 140 (18.2) | 100 (19.4) |
| Female sex | 463 (36.1) | 364 (35.9) | 99 (36.8) | 290 (37.8) | 173 (33.5) |
| Socio-economic statusa | |||||
| Lowest | 284 (22.1) | 222 (21.9) | 62 (23.0) | 163 (21.2) | 121 (23.4) |
| Middle | 260 (20.2) | 210 (20.7) | 50 (18.6) | 150 (19.5) | 110 (21.3) |
| Highest | 221 (17.2) | 178 (17.5) | 43 (16.0) | 144 (18.8) | 77 (14.9) |
| Urban residenceb | 1146 (89.3) | 912 (89.9) | 234 (87.0) | 697 (90.8) | 449 (87.0) |
| Pre-transplant dialysis modalityc | |||||
| Hemodialysis | 768 (59.8) | 593 (58.4) | 175 (65.1) | 454 (59.1) | 314 (60.9) |
| Peritoneal | 344 (26.8) | 283 (27.9) | 61 (22.7) | 209 (27.2) | 135 (26.2) |
| Pre-emptive | 172 (13.4) | 139 (13.7) | 33 (12.3) | 105 (13.7) | 67 (13.0) |
| Dialysis duration (years) | 2.3 (1.3-3.6) | 2.2 (1.2-3.5) | 2.7 (1.6-4.1) | 2.1 (1.1-3.3) | 2.6 (1.5-4.0) |
| Comorbiditiesd | |||||
| Hypertension | 1146 (89.3) | 909 (89.6) | 237 (88.1) | 684 (89.1) | 462 (89.5) |
| Diabetes mellitus | 475 (37.0) | 357 (35.2) | 118 (43.9) | 244 (31.8) | 231 (44.8) |
| Myocardial infarction | 22 (1.7) | 14 (1.4) | 8 (3.0) | 11 (1.4) | 11 (2.1) |
| Percutaneous coronary intervention/coronary artery bypass graft | 49 (3.8) | 38 (3.7) | 11 (4.1) | 29 (3.8) | 20 (3.9) |
| Heart failure | 135 (10.5) | 104 (10.2) | 31 (11.5) | 72 (9.4) | 63 (12.2) |
| Atrial fibrillation | 67 (5.2) | 47 (4.6) | 20 (7.4) | 36 (4.7) | 31 (6.0) |
| Stroke/transient ischemic attack | 63 (4.9) | 49 (4.8) | 14 (5.2) | 40 (5.2) | 23 (4.5) |
| Peripheral vascular disease | 99 (7.7) | 66 (6.5) | 33 (12.3) | 48 (6.3) | 51 (9.9) |
| Cancer | 25 (1.9) | 22 (2.2) | 3 (1.1) | 16 (2.1) | 9 (1.7) |
| Hemorrhage | 105 (8.2) | 77 (7.6) | 28 (10.4) | 66 (8.6) | 39 (7.6) |
| Venous thromboembolism | 61 (4.8) | 46 (4.5) | 15 (5.6) | 31 (4.0) | 30 (5.8) |
Note. Data are presented as number (%) except for age and dialysis duration, which are presented as median (interquartile range).
Income was categorized according to fifths of average neighborhood income (first quintile is the lowest and the fifth quintile is the highest).
Urban location indicates a population >10 000 or a population >1000 with population density >400/km2.
Recipients identified as pre-emptive were assessed for the presence of dialysis codes and re-classified as hemodialysis (n = 22) or peritoneal dialysis (n = 14).
Outcomes
Over a median follow-up of 6.1 years, there were 100 hemorrhages (12.6 events per 1000 person-years) and 57 VTE events (7.1 events per 1000 person-years). Of the 57 VTE events, 49 were de novo (86%) and 8 were recurrent (14%). The median time from 1 year post-transplant to the first hemorrhage and VTE event was 4.5 and 2.3 years, respectively.
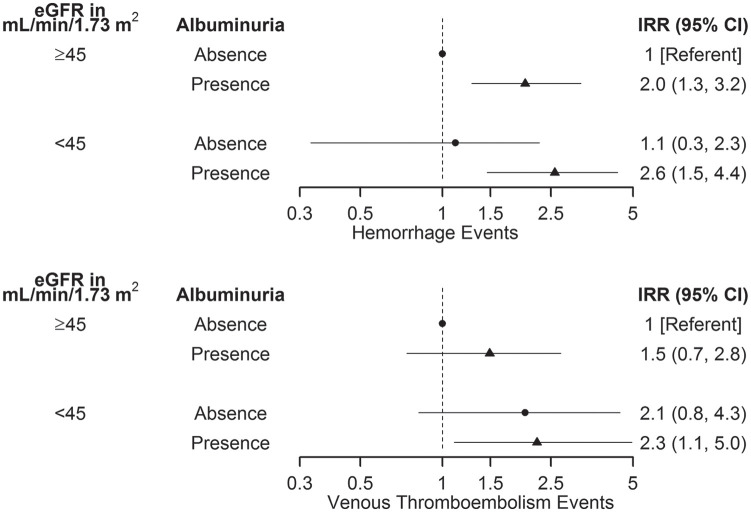
The rate of hemorrhage was over 2-fold higher in recipients with eGFR <45 mL/min/1.73 m2 and presence of albuminuria compared to recipients with eGFR ≥45 mL/min/1.73 m2 and no albuminuria (23.1 vs. 9.0 events per 1000 person-years; IRR: 2.6, 95% CI: 1.5-4.4; P = .001; Table 2, Figure 1). The rate of hemorrhage was nearly 4-fold higher when only ACR and PCR measurements (excluding urine dipstick) were used in sensitivity analyses (IRR: 3.9, 95% CI: 1.8-7.5; P < .001). In recipients with eGFR ≥45 mL/min/1.73 m2, the rate of hemorrhage was significantly higher with the presence of albuminuria compared to no albuminuria (IRR: 2.0, 95% CI: 1.3-3.2; P = .003). Sensitivity analyses using only ACR and PCR measurements showed similar findings (IRR: 2.7, 95% CI: 1.5-5.3; P = .002). In recipients with no albuminuria, the rate of hemorrhage was not significantly higher with eGFR <45 mL/min/1.73 m2 compared to eGFR ≥45 mL/min/1.73 m2 (10.0 vs. 9.0 events per 1000 person-years; IRR: 1.1, 95% CI: 0.3-2.3; P = .8).
Table 2.
Rates of Hemorrhage by Level of eGFR and Albuminuria in Kidney Transplant Recipients, Per 1000 Person-Years.
| eGFR | Albuminuria |
Albuminuria
(albumin-to-creatinine ratio, protein-to-creatinine
ratio) |
||||
|---|---|---|---|---|---|---|
| Overall | Absence | Presence | Overall | Absence | Presence | |
| ≥45 mL/min/1.73 m2 | ||||||
| Events, n | 74 | 38 | 36 | 44 | 15 | 29 |
| Patients, n | 1015 | 659 | 356 | 743 | 424 | 319 |
| Unadjusted | 11.6 (9.2, 14.4) | 8.7 (6.1, 11.5) | 17.8 (12.8, 24.6) | 10.6 (7.7, 13.8) | 6.2 (3.8, 10.1) | 16.8 (11.1, 23.7) |
| Adjusteda | 11.9 (9.5, 14.8) | 9.0 (6.4, 11.8) | 18.0 (12.9, 25.1) | 10.7 (7.7, 13.9) | 6.3 (3.9, 10.2) | 17.0 (11.2, 24.0) |
| <45 mL/min/1.73 m2 | ||||||
| Events, n | 26 | 7 | 19 | 22 | 5 | 17 |
| Patients, n | 269 | 109 | 160 | 196 | 55 | 141 |
| Unadjusted | 17.2 (11.1, 23.8) | 10.0 (3.0, 18.9) | 23.5 (14.0, 35.3) | 22.4 (12.7, 31.4) | 16.9 (4.4, 38.5) | 24.7 (13.3, 36.0) |
| Adjusteda | 17.1 (10.9, 23.4) | 10.0 (3.0, 19.2) | 23.1 (13.8, 35.0) | 22.1 (12.6, 31.2) | 16.6 (4.6, 38.1) | 24.6 (12.7, 36.0) |
Note. Data are presented as rate (95% confidence interval). eGFR = estimated glomerular filtration rate.
Adjusted for age and sex.
Figure 1.
Adjusted incidence rate ratios of hemorrhage and venous thromboembolism by level of eGFR and albuminuria in kidney transplant recipients.
Note. All values, including point estimates and confidence limits, are plotted on log scale. eGFR = estimated glomerular filtration rate; IRR = incidence rate ratio; CI = confidence interval.
The rate of VTE was also significantly higher in recipients with eGFR <45 mL/min/1.73 m2 and presence of albuminuria compared to recipients with eGFR ≥45 mL/min/1.73 m2 and no albuminuria (11.8 vs. 5.2 events per 1000 person-years; IRR: 2.3, 95% CI: 1.1-5.0; P = .046; Table 3, Figure 1). Sensitivity analyses using only ACR and PCR measurements showed similar findings (IRR: 3.0, 95% CI: 1.0-8.8; P = .046). In recipients with eGFR ≥45 mL/min/1.73 m2, the rate of VTE was not significantly higher with the presence of albuminuria compared to no albuminuria (IRR: 1.5, 95% CI: 0.7-2.8; P = .2). In recipients with no albuminuria, the rate of VTE was not significantly higher with eGFR <45 mL/min/1.73 m2 compared to eGFR ≥45 mL/min/1.73 m2 (IRR: 2.1, 95% CI: 0.8-4.3; P = .1).
Table 3.
Rates of Venous Thromboembolism by Level of eGFR and Albuminuria in Kidney Transplant Recipients, Per 1000 Person-Years.
| eGFR | Albuminuria |
Albuminuria
(albumin-to-creatinine ratio, protein-to-creatinine
ratio) |
||||
|---|---|---|---|---|---|---|
| Overall | Absence | Presence | Overall | Absence | Presence | |
| ≥45 mL/min/1.73 m2 | ||||||
| Events, n | 39 | 23 | 16 | 23 | 9 | 14 |
| Patients, n | 1,015 | 659 | 356 | 743 | 424 | 319 |
| Unadjusted | 5.5 (3.5, 8.0) | 5.2 (3.2, 7.4) | 7.7 (4.4, 11.9) | 6.0 (4.2, 7.9) | 3.7 (1.6, 6.4) | 7.9 (4.3, 12.6) |
| Adjusteda | 6.1 (4.1, 8.0) | 5.2 (3.2, 7.3) | 7.8 (4.4, 12.1) | 5.5 (3.5, 7.9) | 3.7 (1.6, 6.5) | 7.9 (4.4, 12.4) |
| <45 mL/min/1.73 m2 | ||||||
| Events, n | 18 | 8 | 10 | 11 | 3 | 8 |
| Patients, n | 269 | 109 | 160 | 196 | 55 | 141 |
| Unadjusted | 10.8 (4.9, 17.8) | 11.2 (4.6, 21.5) | 12.0 (5.6, 21.6) | 11.6 (6.6, 18.0) | 9.8 (0.0, 23.2) | 11.2 (3.1, 20.0) |
| Adjusteda | 11.4 (6.5, 17.6) | 10.9 (4.3, 20.2) | 11.8 (5.4, 21.0) | 10.7 (4.6, 17.8) | 9.8 (0.0, 25.9) | 11.1 (4.3, 20.8) |
Note. Data are presented as rate (95% confidence interval). eGFR = estimated glomerular filtration rate.
Adjusted for age and sex.
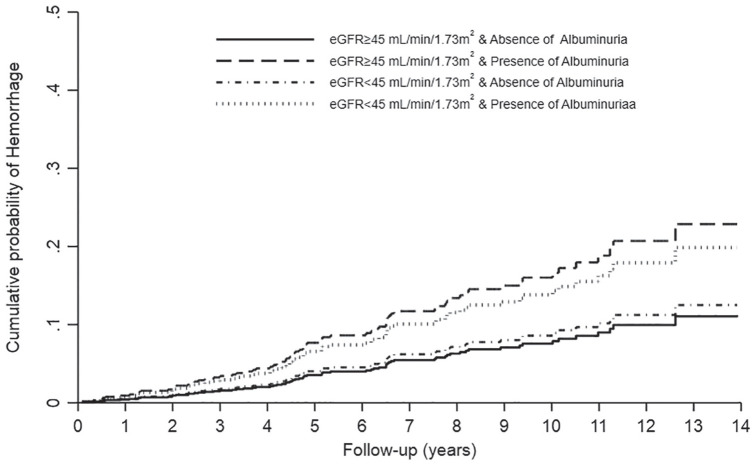
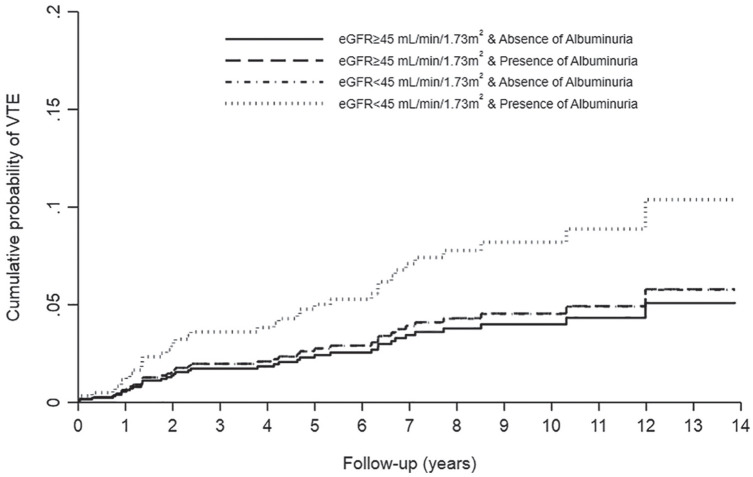
Interactions between albuminuria and eGFR were not statistically significant for hemorrhage (P = .81) and VTE (P = .62). However, the difference in risk associated with presence of albuminuria (as compared with no albuminuria) appeared clinically relevant with every eGFR stratum for both hemorrhage and VTE. Results for both hemorrhage and VTE were similar in sensitivity analyses that adjusted for peripheral vascular disease and diabetes mellitus. Age- and sex-adjusted cumulative incidence curves for hemorrhage and VTE are shown in Figures 2 and 3, respectively.
Figure 2.
Cumulative incidence curves of hemorrhage by level of eGFR and albuminuria in kidney transplant recipients.
Note. eGFR = estimated glomerular filtration rate.
Figure 3.
Cumulative incidence curves of venous thromboembolism (VTE) by level of eGFR and albuminuria in kidney transplant recipients.
Note. eGFR = estimated glomerular filtration rate.
Discussion
In this retrospective study of 1284 adult kidney transplant recipients, we found a 2-fold increase in rates of hemorrhage and VTE in recipients with lower eGFR and presence of albuminuria compared to higher eGFR and no albuminuria. In recipients with higher eGFR, the rates of hemorrhage were higher with the presence of albuminuria compared to no albuminuria. However, in recipients with no albuminuria, the rates of hemorrhage were not higher with lower eGFR. Rates of VTE were not higher with the presence of albuminuria or lower eGFR independently. To our knowledge, this is the first study of its kind in kidney transplant recipients.
Our findings in kidney transplant recipients are similar to that of the CKD population, where there is an increased risk of major hemorrhage and GI bleeding with lower eGFR and higher albuminuria.4,33 In an Ontario study of 516 197 patients, the highest risk of hemorrhage over 3 years was found in patients with eGFR <15 mL/min/1.73 m2 and ACR >300 mg/g when compared to eGFR >90 mL/min/1.73 m2 and ACR <30 mg/g.4 Additionally, albuminuria had an eGFR-independent effect on the risk of hemorrhage. As albuminuria is a marker of endothelial dysfunction, hypertensive vascular injury could lead to endothelial dysfunction and hemorrhage.4 Similarly, we found increased rates of hemorrhage with the presence of albuminuria vs. no albuminuria in the higher eGFR group, but not with eGFR <45 vs. ≥45 mL/min/1.73 m2 in the no albuminuria group.
Risk of VTE is also increased with lower eGFR and higher albuminuria in patients with CKD.3,5,34 Cheung et al reported that the risk of VTE increased more than 2-fold in patients with eGFR <45 mL/min/1.73 m2 compared to patients with eGFR ≥90 mL/min/1.73 m2.34 In another Canadian study, the risk of VTE progressively increased with worsening albuminuria, but this effect was attenuated in lower eGFR categories such that at <60 mL/min/1.73 m2, worsening albuminuria had no effect on VTE risk.3 In contrast, though our study showed higher rates of VTE in the lower eGFR and presence of albuminuria group, there was no independent effect of either presence of albuminuria or lower eGFR on the risk of VTE. This may due to our small sample size in a transplant population and low VTE event rate.
The higher rates of hemorrhage we found in recipients with lower eGFR and presence of albuminuria could be due to underlying anticoagulant use. Diabetes mellitus and peripheral vascular disease were also higher in this group, which could indicate higher antiplatelet use, increasing their overall hemorrhage risk. However, myocardial infarction, coronary revascularization, stroke or transient ischemic attack, and atrial fibrillation, which are common indications for antiplatelet or anticoagulant use, were not significantly different between the groups. The higher rates of VTE in recipients with lower eGFR and presence of albuminuria may be due to the development of a hypercoagulable state post-transplant, such as nephrotic syndrome. The majority of VTE were de novo with no prior history of VTE. Therefore, it is less likely to be due to a pre-existing hypercoagulable disorder and unlikely to be related to the transplant surgery as we followed patients starting from their 1 year post-transplant anniversary date.
The association of graft function and albuminuria with rates of hemorrhage and VTE is clinically important as this adds significant morbidity to kidney transplant recipients. Graft function and albuminuria at 1 year post-transplant is associated with mortality and graft loss, as well as cardiovascular events and heart failure.11,12 In addition, post-transplant VTE is associated with a 4-fold increase in all-cause mortality and 2-fold increase in graft loss.2 Therefore, graft function and albuminuria at 1 year post-transplant can be helpful in evaluating kidney transplant recipients and their risk of post-transplant hemorrhage and VTE. The mechanism for increased risk of hemorrhage and VTE in kidney disease is likely due to abnormalities of the complex interactions between uremic toxins and changes in the endothelium with the coagulation cascade and platelets.35 In kidney failure, there are disturbances in the composition of platelet α-granules that contain multiple proteins including von Willebrand factor (vWF), fibrinogen, and coagulation factors V and XIII.36,37 Oxidative stress and inflammation in kidney failure can affect platelet function, and uremic toxins appear to decrease glycoprotein Ib receptor for adhesion molecules on endothelial cells and interfere with the binding of vWF and fibrinogen to activated platelets.38 Anemia can also reduce platelet-vessel wall interaction through adenosine diphosphate release and inactivation of prostacyclin and through decreased scavenging of nitric oxide by hemoglobin.35 Prothrombotic and antithrombotic imbalances have been described in nephrotic syndrome where high albuminuria is present, and lower eGFR is associated with increased procoagulants and proinflammatory substances, such as factor VII, factor VIII, C-reactive protein, fibrinogen, interleukin, and D-dimer.3
Our study has several strengths. It is the first study in kidney transplant recipients that describes the association of albuminuria and eGFR at 1 year post-transplant with the risk of post-transplant hemorrhage and VTE. It represents a large Canadian cohort of more than 1000 kidney transplant recipients followed over a 13-year period, using provincial, multicenter data. It is clinically important as it builds on previous studies to help prognosticate health outcomes for kidney transplant recipients. The serum creatinine measurements used in our databases are standardized across provincial laboratories, reducing interlaboratory variation in measurements. We used only outpatient laboratory data and multiple measurements of serum creatinine and albuminuria, reducing the risk of misclassification. We performed sensitivity analyses using ACR and PCR only for albuminuria measurements and showed similar findings. We also incorporated use of validated diagnostic codes to define comorbidities and outcomes.25,27-30,39-44
Our study does have some limitations. Due to the sample size, our study was underpowered to conduct a fully adjusted analysis. Although we did have information regarding relevant comorbidities, we did not have complete medication information such as anticoagulants, antiplatelets, and hormone therapies. Other risk factors for VTE such as obesity, smoking, and hypercoagulable disorders were not captured in our datasets. We did not have other laboratory data such as serum albumin and lipid profile to correlate with the risk of VTE. We did not exclude recipients with recent hospitalizations or surgeries which could have increased their risk of VTE from immobilization or increased their risk of bleeding if the hospitalization was for thrombolysis or anticoagulation. There is also risk for survival and selection bias, as we included recipients with a functioning graft 1 year post-transplant with at least one outpatient serum creatinine and albuminuria measurement. Therefore, our findings are limited to kidney transplant recipients who have survived to 1 year post-transplant. Finally, as it is a retrospective study, there is risk of confounding variables that we were not able to account for.
Conclusions
In a Canadian cohort of 1284 adult kidney transplant recipient, lower eGFR and presence of albuminuria at 1 year post-transplant were associated with an over 2-fold increase in rates of hemorrhage and VTE. These results further add to known associations with all-cause mortality, graft loss, and cardiovascular events in kidney transplant recipients. Thus, eGFR and albuminuria at 1 year post-transplant are important prognostic factors in determining risk of post-transplant hemorrhage and VTE. Future research, including complete medication data, would further delineate the effects of graft function and albuminuria on post-transplant hemorrhage and VTE, as well as provide information on medication safety in transplant recipients.
Supplemental Material
Supplemental material, RTx_GFR-Prot_Bleed-VTE_-_Appendix_-_20200608 for Graft Function, Albuminuria, and the Risk of Hemorrhage and Thrombosis After Kidney Transplantation by Rachel Jeong, Robert R. Quinn, Pietro Ravani, Feng Ye, Manish M. Sood, David Massicotte-Azarniouch, Marcello Tonelli, Brenda R. Hemmelgarn and Ngan N. Lam in Canadian Journal of Kidney Health and Disease
Acknowledgments
This study is based in part, on data provided by Alberta Health and Alberta Health Services. The interpretation and conclusions contained herein are those of the researchers and do not necessarily represent the views of the Government of Alberta or Alberta Health Services. Neither the Government of Alberta, nor Alberta Health or Alberta Health Services express any opinion in relation to this study.
Footnotes
List of Abbreviations: ACR, albumin-creatinine ratio; AKDN, Alberta Kidney Disease Network; CI, confidence interval; CKD, chronic kidney disease; CKD-EPI, Chronic Kidney Disease-Epidemiology Collaboration; DVT, deep vein thrombosis; eGFR, estimated glomerular filtration rate; GI, gastrointestinal; ICD-9-CM, International Classification of Diseases, Ninth Revision, Clinical Modification; ICD-10, International Statistical Classification of Diseases, Tenth Revision; IQR, interquartile range; IRR, incidence rate ratio; KDIGO, Kidney Disease: Improving Global Outcomes; KRT, kidney replacement therapy; PCR, protein-creatinine ratio; PE, pulmonary embolism; VTE, venous thromboembolism; vWF, von Willebrand factor.
Ethics Approval and Consent to Participate: Ethics approval was obtained from the research ethics boards at the University of Alberta and the University of Calgary, with a waiver of patient consent granted.
Consent for Publication: All authors reviewed the final manuscript and consented for publication.
Availability of Data and Materials: Data and materials may be made available upon written request to the corresponding author.
Author Contributions: N.N.L. conceived the study. R.J. and N.N.L. drafted the manuscript. F.Y. performed the statistical analyses and created the figures. All authors interpreted the results, revised the manuscript, and approved the final version of the manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Pietro Ravani  https://orcid.org/0000-0001-6973-8570
https://orcid.org/0000-0001-6973-8570
Manish M. Sood  https://orcid.org/0000-0002-9146-2344
https://orcid.org/0000-0002-9146-2344
David Massicotte-Azarniouch  https://orcid.org/0000-0002-6954-2030
https://orcid.org/0000-0002-6954-2030
Ngan N. Lam  https://orcid.org/0000-0002-0129-7091
https://orcid.org/0000-0002-0129-7091
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Sood MM, Garg AX, Bota SE, et al. Risk of major hemorrhage after kidney transplantation. Am J Nephrol. 2015;41(1):73-80. [DOI] [PubMed] [Google Scholar]
- 2. Lam NN, Garg AX, Knoll GA, et al. Venous thromboembolism and the risk of death and graft loss in kidney transplant recipients. Am J Nephrol. 2017;46(4):343-354. [DOI] [PubMed] [Google Scholar]
- 3. Massicotte-Azarniouch D, Bader Eddeen A, LazoLanger A, et al. Risk of venous thromboembolism in patients by albuminuria and estimated GFR. Am J Kidney Dis. 2017;70:826-833. [DOI] [PubMed] [Google Scholar]
- 4. Molnar AO, Bota SE, Garg AX, et al. The risk of major hemorrhage with CKD. J Am Soc Nephrol. 2016;27(9):2825-2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mahmoodi BK, Gansevoort RT, Næss IA, et al. Association of mild to moderate chronic kidney disease with venous thromboembolism: pooled analysis of five prospective general population cohorts. Circulation. 2012;126:1964-1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hemmelgarn BR, Manns BJ, Lloyd A, et al. Relation between kidney function, proteinuria, and adverse outcomes. JAMA. 2010;303:423-429. [DOI] [PubMed] [Google Scholar]
- 7. Bello AK, Hemmelgarn B, Lloyd A, et al. Associations among estimated glomerular filtration rate, proteinuria, and adverse cardiovascular outcomes. Clin J Am Soc Nephrol. 2011;6(6):1418-1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Matsushita K, van der Velde M, Astor BC, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375:2073-2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Astor BC, Matsushita K, Gansevoort RT, et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with mortality and end-stage renal disease. Kidney Int. 2011;79(12):1331-1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. van der Velde M, Matsushita K, Coresh J, et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with all-cause and cardiovascular mortality. A collaborative meta-analysis of high-risk population cohorts. Kidney Int. 2011;79(12):1341-1352. [DOI] [PubMed] [Google Scholar]
- 11. Lam NN, Tonelli M, Lentine KL, et al. Albuminuria and posttransplant chronic kidney disease stage predict transplant outcomes. Kidney Int. 2017;92(2):470-478. [DOI] [PubMed] [Google Scholar]
- 12. Lam NN, Klarenbach S, Quinn RR, et al. Renal function, albuminuria, and the risk of cardiovascular events after kidney transplantation. Transplant Direct. 2018;4(10):e389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hemmelgarn BR, Clement F, Manns BJ, et al. Overview of the Alberta Kidney Disease Network. BMC Nephrol. 2009;10:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lam NN, Lentine KL, Hemmelgarn B, et al. Follow-up care of living kidney donors in Alberta, Canada. Can J Kidney Health Dis. 2018;5:1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jeong R, Quinn RR, Lentine KL, et al. Outcomes following macrolide use in kidney transplant recipients. Can J Kidney Health Dis. 2019;6:1-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hariharan S, McBride MA, Cherikh WS, Tolleris CB, Bresnahan BA, Johnson CP. Post-transplant renal function in the first year predicts long-term kidney transplant survival. Kidney Int. 2002;62(1):311-318. [DOI] [PubMed] [Google Scholar]
- 17. Lenihan CR, O’Kelly P, Mohan P, et al. MDRD-estimated GFR at one year post-renal transplant is a predictor of long-term graft function. Ren Fail. 2008;30:345-352. [DOI] [PubMed] [Google Scholar]
- 18. Schnitzler MA, Johnston K, Axelrod D, et al. Associations of renal function at 1-year after kidney transplantation with subsequent return to dialysis, mortality, and healthcare costs. Transplantation. 2011;91:1347-1356. [DOI] [PubMed] [Google Scholar]
- 19. Shaffi K, Uhlig K, Perrone RD, et al. Performance of creatinine-based GFR estimating equations in solid-organ transplant recipients. Am J Kidney Dis. 2014;63(6):1007-1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Statistics Canada. 2016 census of population. https://www12.statcan.gc.ca/census-recensement/2016/dp-pd/prof/details/page.cfm?Lang=E&Geo1=PR&Code1=48&Geo2=PR&Code2=01&Data=Count&SearchText=alberta&SearchType=Begins&SearchPR=01&B1=All&TABID=1. Accessed September 23, 2018.
- 21. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2013;3:1-150. [Google Scholar]
- 22. Lamb EJ, MacKenzie F, Stevens PE. How should proteinuria be detected and measured? Ann Clin Biochem. 2009;46(pt 3):205-217. [DOI] [PubMed] [Google Scholar]
- 23. Tonelli M, Muntner P, Lloyd A, et al. Impact of age on the association between CKD and the risk of future coronary events. Am J Kidney Dis. 2014;64(3):375-382. [DOI] [PubMed] [Google Scholar]
- 24. Bello A, Thompson S, Lloyd A, et al. Multiple versus single and other estimates of baseline proteinuria status as predictors of adverse outcomes in the general population. Am J Kidney Dis. 2012;59(3):364-371. [DOI] [PubMed] [Google Scholar]
- 25. Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130-1139. [DOI] [PubMed] [Google Scholar]
- 26. Tonelli M, Wiebe N, Fortin M, et al. Methods for identifying 30 chronic conditions: application to administrative data. BMC Med Inform Decis Mak. 2015;15:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hux JE, Ivis F, Flintoft V, Bica A. Diabetes in Ontario: determination of prevalence and incidence using a validated administrative data algorithm. Diabetes Care. 2002;25(3):512-516. [DOI] [PubMed] [Google Scholar]
- 28. Quan H, Khan N, Hemmelgarn BR, et al. Validation of a case definition to define hypertension using administrative data. Hypertension. 2009;54(6):1423-1428. [DOI] [PubMed] [Google Scholar]
- 29. Arnason T, Wells PS, van Walraven C, Forster AJ. Accuracy of coding for possible warfarin complications in hospital discharge abstracts. Thromb Res. 2006;118(2):253-262. [DOI] [PubMed] [Google Scholar]
- 30. Alotaibi GS, Wu C, Senthilselvan A, McMurtry MS. The validity of ICD codes coupled with imaging procedure codes for identifying acute venous thromboembolism using administrative data. Vasc Med. 2015;20(4):364-368. [DOI] [PubMed] [Google Scholar]
- 31. Al-Ani F, Shariff S, Siqueira L, Seyam A, Lazo-Langner A. Identifying venous thromboembolism and major bleeding in emergency room discharges using administrative data. Thromb Res. 2015;136(6):1195-1198. [DOI] [PubMed] [Google Scholar]
- 32. Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702-706. [DOI] [PubMed] [Google Scholar]
- 33. Ishigami J, Grams ME, Naik RP, et al. Chronic kidney disease and risk for gastrointestinal bleeding in the community: the atherosclerosis risk in communities (ARIC) study. Clin J Am Soc Nephrol. 2016;11:1735-1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cheung KL, Zakai NA, Folsom AR, et al. Measures of kidney disease and the risk of venous thromboembolism in the REGARDS (Reasons for Geographic and Racial Differences in Stroke) study. Am J Kidney Dis. 2017;70(2):182-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lutz J, Menke J, Sollinger D, Schinzel H, Thürmel K. Haemostasis in chronic kidney disease. Nephrol Dial Transplant. 2014;29(1):29-40. [DOI] [PubMed] [Google Scholar]
- 36. Di Minno G, Martinez J, McKean ML, et al. Platelet dysfunction in uremia. Multifaceted defect partially corrected by dialysis. Am J Med. 1985;79:552-559. [DOI] [PubMed] [Google Scholar]
- 37. Eknoyan G, Brown CH., III Biochemical abnormalities of platelets in renal failure. Am J Nephrol. 1981;1(1):17-23. [DOI] [PubMed] [Google Scholar]
- 38. Brunini TMC, Mendes-Ribeiro AC, Ellory JC, et al. Platelet nitric oxide synthesis in uremia and malnutrition: a role for L-arginine supplementation in vascular protection? Cardiovascular Research. 2007;73:359-367. [DOI] [PubMed] [Google Scholar]
- 39. Fan J, Arruda-Olson AM, Leibson CL, et al. Billing code algorithms to identify cases of peripheral artery disease from administrative data. J Am Med Inform Assoc. 2013;20(e2):e349-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Austin PC, Daly PA, Tu JV. A multicenter study of the coding accuracy of hospital discharge administrative data for patients admitted to cardiac care units in Ontario. Am Heart J. 2002;144(2):290-296. [DOI] [PubMed] [Google Scholar]
- 41. Lee DS, Stitt A, Wang X, et al. Administrative hospitalization database validation of cardiac procedure codes. Med Care. 2013;51(4):e22-e26. [DOI] [PubMed] [Google Scholar]
- 42. Quan H, Li B, Saunders LD, et al. Assessing validity of ICD-9-CM and ICD-10 administrative data in recording clinical conditions in a unique dually coded database. Health Serv Res. 2008;43(4):1424-1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Alonso A, Agarwal SK, Soliman EZ, et al. Incidence of atrial fibrillation in whites and African-Americans: the Atherosclerosis Risk in Communities (ARIC) study. Am Heart J. 2009;158:111-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kokotailo RA, Hill MD. Coding of stroke and stroke risk factors using International Classification of Diseases, Revisions 9 and 10. Stroke. 2005;36(8):1776-1781. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, RTx_GFR-Prot_Bleed-VTE_-_Appendix_-_20200608 for Graft Function, Albuminuria, and the Risk of Hemorrhage and Thrombosis After Kidney Transplantation by Rachel Jeong, Robert R. Quinn, Pietro Ravani, Feng Ye, Manish M. Sood, David Massicotte-Azarniouch, Marcello Tonelli, Brenda R. Hemmelgarn and Ngan N. Lam in Canadian Journal of Kidney Health and Disease