Abstract
Introduction:
Sulodexide represents a mixture of fast-moving heparin (FMH) and dermatan sulfate (DS) and has been used for the management of venous diseases such as DVT and related disorders. The purpose of this study is to compare sulodexide and its components with unfractionated heparin (UFH) to determine its suitability for the indications in which UFH is used.
Materials and Method:
Active pharmaceutical ingredients (API) versions of sulodexide, FMH and DS were obtained from Alfasigma. API versions of UFH were obtained from Medefil Inc. Normal human citrated plasma was obtained from blood bank of the Loyola University Medical Center. Each of the individual agents were supplemented in plasma at a graded concentration of 0.0-10 µg/mL. Clotting assays (PiCT, aPTT, PT and TT), anti-Xa and anti-IIa and thrombin generation studies were carried out. Results were compiled as mean ± SD of 3 individual determination.
Result:
In the clot based (PiCT, aPTT and TT), anti-Xa and IIa assays, both the UFH and FMH produced stronger activities in these assays followed by sulodexide. DS did not show any anticoagulant activity. In the thrombin generation assay, FMH and UFH produced comparable inhibition of thrombin generation as measured by various parameters. Sulodexide was slightly weaker in this assay, whereas DS produced relatively weaker effects.
Conclusion:
In comparison to sulodexide, both UFH and FMH exhibit comparable anticoagulant activity despite differences in their molecular weight. These results suggest that sulodexide can be developed as a parenteral anticoagulant for indications in which UFH is used.
Keywords: unfractionated heparin, dermatan sulfate, fast moving heparin, sulodexide, thrombin generation inhibition, COVID-19
Introduction
Since the discovery of unfractionated heparin (UFH) this drug remains to be the anticoagulant of choice for various indications including medical, surgical and interventional procedures.1 UFH is the only anticoagulant with an effective antidote, protamine sulfate. UFH belongs to the glycosaminoglycans (GAGs) group and is extracted from mammalian sources including bovine (cow), ovine (sheep) and porcine (pig) mucosal tissues. The currently approved UFH preparations are obtained from porcine mucosal tissue, while other UFHs prepared from bovine and ovine sources are not available at this time. The UFH obtained from bovine mucosal tissue was initially used clinically, but it was withdrawn from the market due to the bovine spongiform encephalopathy (BSE).2,3 Since then improved methods for viral de-loading and safer bovine heparin preparations have become available.2 In 2008, global contamination of UFH resulted in the withdrawal of this anticoagulant from the market resulting in the shortage of this drug.4–6 Since then, the regulatory agencies have provided stringent measures to quality assure this drug. Other factors impacted the supply of this anticoagulant including the availability of the porcine mucosal raw material which is primarily supplied by China. In 2018, the outbreak of African swine flu (ASF) in China and other countries resulted in a severe shortage of porcine tissues impacting the global supply of this drug.7 Several investigators have reported on the imminent risk of a global heparin shortage because of the impact of ASF in Europe and Asia on the pig population.8 Almost 50% of the population of pigs in China are affected which represent almost 70% of the anticoagulant used clinically.9 Recent improvements in manufacturing, viral de-load and other quality measures, have resulted in high quality bovine heparin that has become widely available.2 The potential shortage of porcine mucosal heparin has prompted several initiatives at industrial and regulatory levels.10 The reintroduction of bovine heparin, and development of ovine mucosal heparin are now considered as viable options. Additionally, synthetic and biotechnological approaches have been used to develop UFH and related drugs. Such agents as sulodexide, danaparoid and pentosan polysulfate (PPS) may be considered as substitute parenteral anticoagulant for UFH.
Both the heparin and sulodexide represent glycosaminoglycan (GAGs) mixture of known composition which is extracted from porcine intestinal tissue.11 However, both agents can also be produced by using other mammalian tissues of bovine and ovine origin. Sulodexide is composed of low molecular weight heparin (LMWH) and dermatan sulfate (DS). This mixture contains 80% iduronyl glycosaminoglycan sulfate (IGS, known as fast-moving heparin [FMH] because of its electrophoretic mobility in the barium propane diamine system), and 20% DS.12,13 Sulodexide can be used clinically as an oral or parenteral (intravenous, IV and intramuscular IM) agent. The pharmacologic properties of sulodexide are comparable to UFH and are mediated by antithrombin (AT) and heparin cofactor II (HCII).14 Sulodexide is also capable of inhibiting thrombin generation which is a result of the synergistic effects of both FMH and DS.15 Sulodexide has been used for various clinical indications including chronic venous disease (CVD), prevention of recurrent venous thromboembolism (VTE) and treatment of other vascular disorders.16,17 As sulodexide is similar to heparin and its composition, it may possess certain undesirable attributes such as the interaction with platelet factor 4 and the potential generation of anti-heparin platelet factor 4 antibodies. The parenteral versions of sulodexide possess stronger anticoagulant effects which may lead to bleeding and uncontrolled situations, thus an antidote such as protamine sulfate may be required to control potential hemorrhagic complications. Additionally, the parenteral form of sulodexide may need standardization in terms of anticoagulant activity as referenced against standard.
Sulodexide exhibits poly-pharmacologic actions targeting thrombo-inflammation which makes this drug very useful for the management of venous disease. As COVID-19 associated pathophysiology results in thrombo-inflammation, this agent may be useful in the modulation of complex pathophysiology in COVID-19 associated coagulopathy. Sulodexide is a multicomponent drug and produces additional pharmacological actions including antiviral effects. Thus, beside an anticoagulant, sulodexide may have similar pleiotropic therapeutic actions as UFH.
The average molecular weight of FMH is 7 kDa and its pharmacological actions may be comparable to the LMWH.18 The anticoagulant and antiprotease effects of FMH are dependent on its interaction with AT and HCII, however it is reported to have higher affinity to AT.19,20 Dermatan sulfate is composed of the disaccharide unit of l-iduronic acid and D-N-acetyl galactosamine. The anticoagulant effects of DS are dependent on binding to the HCII, targeting factor Xa and IIa.14 Recent evidence suggests a novel biological action of DS, namely inhibition of matrix metalloproteinases (MMP), which plays a key role in extracellular matrix (ECM) remodeling, thus also conferring protective effects to sulodexide against vascular wall damage and inflammation in chronic venous diseases (CVD).21,22 It has also been reported that DS may act as an adjuvant factor for initiating and accelerating wound healing.23 Extensive studies on the effect of sulodexide and its components on the conventional clotting processes have been carried out including both the clot-based and protease inhibitory assays. However, a parallel study comparing these assays with thrombin generation inhibitory potential of sulodexide is not available. The purpose of this study is to determine the anticoagulant, antiprotease and thrombin generation profile of sulodexide and its components with reference to UFH.
Material and Method
Testing Agents
Active pharmaceutical ingredients (API) versions of sulodexide, fast moving heparin and dermatan sulfate were obtained from Alfasigma (Bologna, Italy). Pharmaceutical grade of unfractionated heparin (190 U/mg) was obtained from Medefil, Inc., (Glendale Heights, Illinois, USA). All agents were dissolved in 0.9% NaCl to make stock solution of 10 mg/ml. Working dilutions were prepared at 100 µg/mL.
Anticoagulant Assay
Each of the agents were supplemented in citrated plasma over a concentration range of 0.0-10.0 µg/mL. Saline was used for referencing purpose. For prothrombinase-induced clotting time (PiCT), reagents were obtained from PentaPharm (Basel, Switzerland) and samples were analyzed by using 2-stage technique. For activated partial thromboplastin time (aPTT) testing, the TriniCLOT aPTT reagent was obtained from Diagnostica Stago (Parsippany, New Jersey, USA). Alfa human thrombin was obtained for thrombin time (TT) from Enzyme Research Laboratories (South Bend, Indiana, USA). The PT, HemosIL reagent was obtained from Instrumentation Laboratory (Bedford, Massachusetts, USA). Thrombin was reconstituted with 0.025 M CaCl2 at a concentration of 5 U/ml which was used to measure TT. ACL-Elite (Instrumentation Laboratory, Bedford, Massachusetts, USA) was used to analyze aPTT, TT and PT. The PiCT test was measured on the ST4 clot analyzer (Diagnostica Stago, Paris, France). Results were compiled in terms of average mean ± SD of 3 runs.
Chromogenic Assay
Anti-factor Xa and anti-factor IIa activities were measured by using a kinetic amidolytic method on the ACL-Elite instrument (Instrumentation Laboratory, Bedford, Massachusetts, USA). Alfa human thrombin and bovine factor-Xa were obtained from Enzyme Research Laboratories (South Bend, Indiana, USA) and both the factor-Xa agent and thrombin were diluted in 50 nM Tris buffer (pH = 8.4) to a concentration of 1.25 IU/mL. Factor Xa and IIa substrate used in this assay were obtained from BioMedica Diagnostics (Connecticut, USA). Factor Xa substrate was reconstituted in sterile water to make 2.5 µM and factor IIa substrate at 1.0 µM. Each of the agents were supplemented in citrated normal human plasma over a concentration range of 0.0-10.0 µg/mL. Saline was used for referencing purpose. Results were compiled in terms of average mean ± SD of 3 runs.
Inhibition of Thrombin Generation
Inhibition of thrombin generation was measured by using a Fluoroskan Ascent Fluorimeter, calibrated automated thrombogram (CAT), (Diagnostica Stago, Parsippany, New Jersey, USA). Reagents used in this assay included the fluo-substrate, fluo-buffer, tissue factor high reagent (mixture of tissue factor and phospholipids) and a thrombin calibrator. The thrombin generation studies were carried out in 96-well Immulon 2HB transparent round bottom plates. Drugs were supplemented in citrated normal human pooled plasma in a concentration range from 0.0-10.0 µg/mL. The thrombin generation potential was measured in terms of the peak thrombin concentration, lag time and endogenous thrombin potential (ETP)/area under the curve (AUC). Results were compiled in terms of average mean ± SD of 3 runs.
Results
Anticoagulant Assay
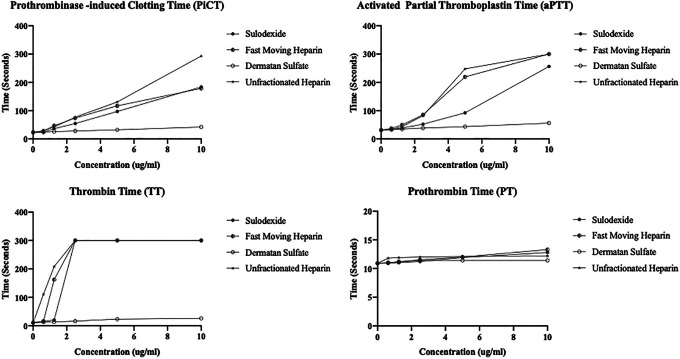
The detailed analysis of the anticoagulation studies with the sulodexide and its components and their comparison with UFH is represented in Figure 1A to D. All agents produced concentration dependent effects with the exception of DS. In the PiCT test sulodexide, FMH and UFH showed comparable response at lower concentration’s, however UFH showed maximal response at the higher concentration. In the aPTT assay, FMH and UFH showed strong and almost comparable responses, however sulodexide showed slightly weaker anticoagulant activities. In TT assay, sulodexide, FMH and UFH all showed comparable and maximum response at higher concentration. In the PT assay, all of these agents showed no anticoagulant effects in the assay range 0.0-10.0 µg/mL.
Figure 1.
Anticoagulation profiling of the sulodexide and its components in comparison to UFH. A. Prothrombinase-induced clotting time (PiCT), B. Activated partial thromboplastin time (aPTT), C. Thrombin time (TT), D. Prothrombin time (PT).
Chromogenic Assay
The amidolytic anti-Xa and anti-IIa activities of these agents are depicted in Figure 2A and B. All agents produced concentration dependent inhibitory responses toward factor Xa and IIa activities with the exception of DS. In both the anti-Xa and anti-IIa assay, UFH and FMH produced strong antiprotease effects. Sulodexide showed slightly weaker effects compared to UFH and FMH.
Figure 2.
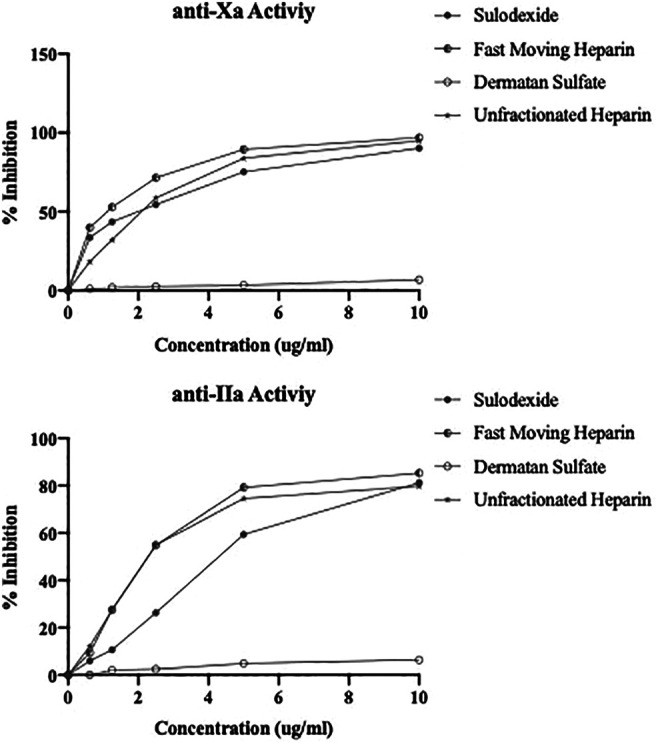
Antiprotease activity of the sulodexide and its components in comparison to UFH. A. Anti-Xa activity, B. Anti-IIa activity.
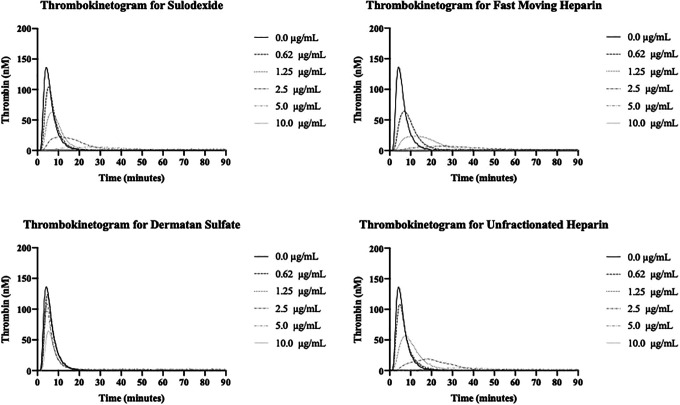
Inhibition of Thrombin Generation
The results of the thrombin generation inhibition of UFH, FMH, sulodexide and DS are shown in Figure 3A to D and Figure 4A to C. The effect of sulodexide, its components and UFH on the inhibition of thrombin generation was measured in terms of peak thrombin, ETP/AUC and lag time. The kinetics of thrombin generation of each of the agent is shown in Figure 3A to D. All agents produced concentration dependent effects however DS was relatively weak. FMH and sulodexide produced strong inhibition of thrombin generation in a comparable fashion and the thrombin generation was completely inhibited at a concentration of 2.5 µg/mL. UFH completely inhibited the thrombin generation at 5.0 µg/mL. A comparison of the individual thrombin generation parameters for all of these drugs is shown in Figure 4A to C. In peak thrombin and AUC measurements, FMH showed stronger inhibition of thrombin generation followed by UFH and sulodexide. The same trend was observed in the lag time. FMH showed an increase in the time for the generation of thrombin followed by UFH and sulodexide, however the difference between each of the agents was minimal.
Figure 3.
Inhibition of thrombin generation of sulodexide and its components in comparison to UFH. A. Thrombokinetogram for sulodexide B. Thrombokinetogram for fast moving heparin (FMH), C. Thrombokinetogram for dermatan sulfate (DS) D. Thrombokinetogram for unfractionated heparin (UFH).
Figure 4.
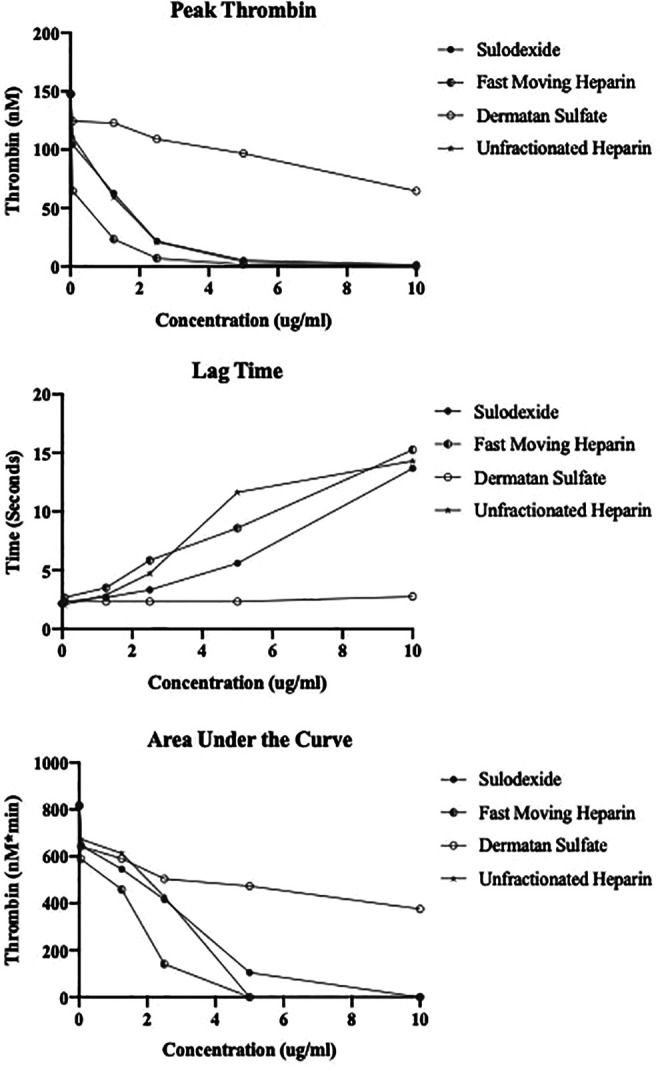
Parameter of thrombin generation for sulodexide and its components in comparison to UFH. A. Peak thrombin, B. Lag time, C. Area under the curve (AUC).
Discussion
Unlike UFH, sulodexide represent a balance mixture of FMH and DS, while the FMH component mediates it effects with AT and HCII.14,15 DS mediates its effects primarily via HCII.11 Conventional clotting assays such as aPTT and TT have been used to profile the anticoagulant effects of sulodexide and its components. However, in the current studies we have also used PiCT test to profile sulodexide and its components in comparison to UFH. Amidolytic assays to measure the anti-Xa and anti-IIa properties of UFH have been conventionally used. These assays also reflect the interaction of sulodexide and its components with HCII and AT. Thrombin generation potential of plasma is now considered to be an important measure of the overall protease activity reflecting the endogenous thrombin generation potential of plasma.24 This test has been widely used in the evaluation of anticoagulant drugs. The composition of plasma and other physiological factors also contribute to the thrombin generation potential. In our studies, we have compared the effect of sulodexide and its components with UFH for their inhibitory effects on thrombin generation potential in terms of various parameters. These studies were carried out at mass adjusted concentrations.
In the clot-based assays, all agents produced concentration dependent anticoagulant effects in the range of 0.0-10.0 µg/mL in the PiCT, aPTT and TT assays however no effect was observed on the PT assay. FMH and UFH consistently produced near comparable effects in the aPTT, PiCT and TT assay in comparison to sulodexide. However, DS didn’t have any sizable effects on these assays. Despite its lower molecular weight, the FMH exhibited comparable or slightly higher anticoagulant effects, this may be due to stronger interaction of FMH with AT. Sulodexide was relatively weaker compared to the UFH and FMH in the aPTT and PiCT assay, however it produced the comparable effect on TT.
The antiprotease activities were measured by the anti-Xa and anti-IIa activities. FMH produced higher anti-Xa activity with an IC50 value of 1.1 µg/mL, whereas sulodexide and UFH exhibited comparable IC50 values in the range of 2.1 µg/mL. However, DS did not have any anti-Xa activity. These results are slightly different than those noted in the clot-based assays, where sulodexide was differentiated from UFH and FMH. Interestingly, the anti-IIa activity of UFH and FMH was almost comparable with the IC50 of 2.3 µg/mL. Sulodexide was relatively weaker with the IC50 of 4.4 µg/mL. This may be due to the compositional differences in sulodexide. These results suggest that both the anti-Xa and anti-IIa activities can be used to compare sulodexide and FMH to UFH.
Sulodexide, FMH and UFH produced strong inhibition of thrombin generation as measured by various parameters. DS on the other hand produced relatively weaker inhibition. Interestingly the peak thrombin values revealed FMH to have a stronger effect than UFH with a lower IC50 of 0.5 µg/mL, in contrast to UFH 1.55 µg/mL. Sulodexide exhibited a relatively higher IC50 value for peak thrombin inhibition at 2.65 µg/mL. DS had an IC50 >10 µg/mL. All agents produced a concentration dependent increase in the lag time value with the exception of DS. This suggest that sulodexide and its components can delay the generation of thrombin in a concentration dependent manner. Therefore, not only the amount of thrombin generated but the initiation of its formation is affected by sulodexide and its components in a similar fashion to UFH. ETP as measured by AUC values, represents total thrombin generation and was comparable to UFH and FMH, however sulodexide has a lower AUC value. DS produced relatively weaker effects on this parameter.
These integrated studies of the thrombin generation inhibitory profile of sulodexide in comparison to the anticoagulant and antiprotease activities clearly demonstrate that the FMH component of sulodexide is comparable to UFH in terms of various measured activities. As depicted in Table 1, where sulodexide and its components are compared in different assays at fixed concentrations of 10 µg/mL, FMH and UFH produced nearly comparable results, whereas sulodexide showed slightly weaker activities in some of the assays. DS at 10 µg/mL produced much weaker activities. Taken on a cumulative basis, the UFH and FMH exhibit comparable activities. It is interesting that the FMH reportedly exhibits much lower molecular weight (7-8 kDa) in contrast to UFH which has a much higher molecular weight in the range of 15-17 kDa. This data also demonstrates that DS has relatively weaker biochemical properties in comparison to sulodexide, FMH and UFH. However, the presence of DS in sulodexide may have other endogenous modulatory effects which are not measurable in the in vitro assays.
Table 1.
Effects of Sulodexide and its Components in Comparison to UFH in Different Laboratory assay.
| Laboratory assays (10 µg/mL) | Sulodexide | FMH | DS | UFH | |
|---|---|---|---|---|---|
|
Clotting
Assays |
PiCT (seconds) | 184.10 | 178.90 | 42.30 | 293.30 |
| aPTT (seconds) | 256.50 ± 61.52 | 300.00 ± 0.00 | 56.10 ± 2.26 | 300.00 ± 0.00 | |
| TT (seconds) | 300.00 ± 0.00 | 300.00 ± 0.00 | 26.05 ± 3.89 | 300.00 ± 0.00 | |
| PT (seconds) | 12.80 | 13.30 | 11.40 | 12.20 | |
| Chromogenic Assays | anti-Xa (% Inhibition) | 90.17 ± 8.30 | 96.88 ± 2.15 | 6.84 ± 2.60 | 94.98 ± 0.60 |
| anti-IIa (% Inhibition) | 81.20 ± 6.51 | 85.27 ± 2.92 | 6.37 ± 1.03 | 79.83 ± 5.87 | |
|
Thrombin Generation
Assay |
Peak thrombin (nM) | 1.58 ± 0.19 | 0.44 ± 0.15 | 64.66 ± 3.16 | 0.63 ± 2.23 |
| Lag Time (seconds) | 13.67 ± 1.41 | 15.25 ± 5.77 | 2.75 ± 0.11 | 14.30 ± 0.00 | |
| AUC (nM*min) | 0.00 ± 0.00 | 0.00 ± 0.00 | 376.06 ± 2.77 | 0.00 ± 0.00 | |
PiCT, Prothrombinase-induced Clotting Time; aPTT, Activated Partial Thromboplastin Time; TT, Thrombin Time; PT, Prothrombin Time; AUC, Area Under the Curve.
All results are expressed as mean of 3 determination with ± 1 SD with the exception of PiCT and PT test.
The data presented in this communication is highly suggestive of sulodexide as a potential alternate to UFH. Parenteral versions of sulodexide have been available and can be used as an alternate to UFH. It should be noted that their safety profile may be different for sulodexide in comparison to UFH due to the much lower molecular weight of FMH. Currently available sulodexide preparations are not standardized in terms of their anticoagulant activity in reference to UFH, therefore the parenteral version of sulodexide should be cross referenced against the pharmacopial standards such as the USP and the EP reference. In our studies, we have used plasma-based assays which can provide a comparative anticoagulant profile of sulodexide. Additional studies in both animal models and clinical settings are required to validate the pharmacologic effects of sulodexide in comparison to UFH and LMWHs. Similarly, the pharmacologic validation of the efficacy of this agent, such indication as cancer associated thrombosis, auto-immune diseases and other non-thrombotic usage will require additional basic and clinical investigations.
As UFH and LMWHs are now widely used in the management of COVID-19 associated thrombotic complications, sulodexide may also be valuable for this indication.25 The pleiotropic effects of sulodexide including anti-inflammatory, profibrinolytic, cytoprotective, endothelial sparing among others have been discussed in several previous reports.16,26–30 Besides an anticoagulant, sulodexide has also been shown to have other pharmacological properties, which may be beneficial to the COVID-19 patients. The pharmacologic agents targeting thrombo-inflammation in COVID-19 have been recently reviewed by Bikdeli et al.31 As thrombo-inflammation is considered to be one of the major pathophysiologic focus, this manuscript has provided a comprehensive account of pharmacologic agents which can be used in the management of COVID-19. Sulodexide is discussed briefly with qualifying statement that despite potential interest in sulodexide due to its poly-pharmacologic nature there is no information on the clinical use of sulodexide in this indication. As this agent possess both the anticoagulant and anti-inflammatory properties which have been extensively investigated in both clinical and experimental settings, sulodexide may be a useful alternate for UFH and LMWHs. A meta-analysis of the randomized trial on sulodexide is reported, highlighting the broad-spectrum nature of this agent covering a variety of cardiovascular indications and the safety considerations.32 Thus sulodexide may have beneficial effects, not only in the control of thrombo-inflammation but in managing the cardiovascular complications observed in COVID-19 patients.
Conclusion
While the anticoagulant properties of sulodexide and its components have been reported before, our studies provide a direct comparison of sulodexide and its components in clot-based, protease inhibitory and thrombin generation assays, suggesting that this agent exhibits comparable anticoagulant effects to UFH. Therefore, sulodexide can be developed for parenteral usage for such indications as surgical, medical anticoagulation, hemodialysis and interventional procedures. The pharmacokinetic and pharmacodynamic properties of sulodexide may differ from UFH and LMWHs and should be taken into account for dosage selection in different indications. Clinical validation for the use of sulodexide in indications for UFH is warranted at this time.
Acknowledgments
The authors gratefully acknowledge the skillful assistance of the staff of the Hemostasis and Thrombosis Research Unit, Loyola University Health Science Division. We are also thankful to Dr. Giuseppe D’Ambrosio and Dr. Benedetta Montera for their helpful advice and suggestions in preparing this manuscript. We gratefully acknowledge Dr. Paul Riley of Diagnostica Stago (Paris, France) for providing us the instrument and reagents for the thrombin generation studies. The PiCT reagent used in the study was the generous gift of Dr. Michael Janssen from PentaPharm (Basel, Switzerland). We are also thankful to Ms. Erin Healy Erickson for her expert assistant in preparing this manuscript. We also acknowledge the support of Dr. Eva Wojcik, Chairperson, Department of Pathology and Laboratory Medicine and Dr. Seth Robia, Co-director of the Cardiovascular Institute for facilitating these studies.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Fakiha Siddiqui  https://orcid.org/0000-0002-2219-7049
https://orcid.org/0000-0002-2219-7049
Debra Hoppensteadt  https://orcid.org/0000-0001-9342-4213
https://orcid.org/0000-0001-9342-4213
Jawed Fareed  https://orcid.org/0000-0003-3465-2499
https://orcid.org/0000-0003-3465-2499
References
- 1. Bick RL, Frenkel EP, Walenga J, Fareed J, Hoppensteadt DA. Unfractionated heparin, low molecular weight heparins, and pentasaccharide: basic mechanism of actions, pharmacology, and clinical use. Hematol Oncol Clin North Am. 2005;19(1):1-51. doi:10.1016/j.hoc.2004.09.003 [DOI] [PubMed] [Google Scholar]
- 2. Bett C, Grgac K, Long D, et al. A heparin purification process removes spiked transmissible spongiform encephalopathy agent. AAPS J. 2017;19(3):765–771. doi:10.1208/s12248-017-0047-y [DOI] [PubMed] [Google Scholar]
- 3. Torri G, Naggi A. Heparin centenary—an ever-young life-saving drug. Int J Cardiol. 2016;212(suppl 1):S1-S4. doi:10.1016/S0167-5273(16)12001-7 [DOI] [PubMed] [Google Scholar]
- 4. Mitka M. Contaminated heparin seized by FDA. JAMA. 2008;300(22):2597 doi:10.1001/jama.2008.786 [DOI] [PubMed] [Google Scholar]
- 5. Tanne JH. Four deaths and 350 adverse events lead to US recall of heparin. BMJ. 2008;336(7641):412-413. doi:10.1136/bmj.39496.419248.DB [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fareed J, Jeske W, Ramacciotti E. Porcine mucosal heparin shortage crisis! What are the options? Clin Appl Thromb Hemost. 2019;25:1076029619878786 doi:10.1177/1076029619878786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Normile D. Arrival of deadly pig disease could spell disaster for China. Science. 2018;361(6404):741 doi:10.1126/science.361.6404.741 [DOI] [PubMed] [Google Scholar]
- 8. Vilanova E, Tovar AMF, Mourão PAS. Imminent risk of a global shortage of heparin caused by the African Swine Fever afflicting the Chinese pig herd. J Thromb Haemost. 2019;17(2):254-256. doi:10.1111/jth.14372 [DOI] [PubMed] [Google Scholar]
- 9. Bipartisan E&C leaders request FDA briefing on threat to U.S. heparin supply. Press Release. July 30, 2019 https://energycommerce.house.gov/newsroom/press-releases/bipartisan-ec-leaders-request-fda-briefing-on-threat-to-us-heparin-supply
- 10. Keire D, Mulloy B, Chase C, et al. Diversifying the global heparin supply chain: reintroduction of bovine heparin in the United States? Pharmaceutical Technol. 2015;39(11):2–8. [Google Scholar]
- 11. Bianchini P, inventor; Opocrin SRL, assignee. Method for preparing glucoronyl-glucosamino-glycan sulphates exhibiting antilipasaemic activity. United States patent US 3936351 February 3, 1976.
- 12. Callas DD, Hoppensteadt DA, Jeske W, et al. Comparative pharmacologic profile of a glycosaminoglycan mixture, sulodexide, and a chemically modified heparin derivative, suleparoide. Semin Thromb Hemost. 1993;19(suppl 1):49-57. [PubMed] [Google Scholar]
- 13. Casu B, Johnson EA, Mantovani M, et al. Correlation between structure, fat-clearing and anticoagulant properties of heparins and heparan sulphates. Arzneimittelforschung. 1983;33(1):135-142. [PubMed] [Google Scholar]
- 14. Tollefsen DM. Vascular dermatan sulfate and heparin cofactor II. Prog Mol Biol Transl Sci. 2010;93:351-372. doi:10.1016/S1877-1173(10)93015-9 [DOI] [PubMed] [Google Scholar]
- 15. Cosmi B, Cini M, Legnani C, Pancani C, Calanni F, Coccheri S. Additive thrombin inhibition by fast moving heparin and dermatan sulfate explains the anticoagulant effect of sulodexide, a natural mixture of glycosaminoglycans. Thromb Res. 2003;109(5-6):333-339. doi:10.1016/s0049-3848(03)00246-9 [DOI] [PubMed] [Google Scholar]
- 16. Carroll BJ, Piazza G, Goldhaber SZ. Sulodexide in venous disease. J Thromb Haemost. 2019;17(1):31-38. doi:10.1111/jth.14324 [DOI] [PubMed] [Google Scholar]
- 17. Andreozzi GM, Bignamini AA, Davì G, et al. Sulodexide for the prevention of recurrent venous thromboembolism: the Sulodexide in Secondary Prevention of Recurrent Deep Vein Thrombosis (SURVET) study: a multicenter, randomized, double-blind, placebo-controlled trial. Circulation. 2015;132(20):1891-1897. doi:10.1161/CIRCULATIONAHA.115.016930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Silvestro L, Lanzarotti E, Marchi E, et al. Human pharmacokinetics of glycosaminoglycans using deuterium-labeled and unlabeled substances: evidence for oral absorption. Semin Thromb Hemost. 1994;20(3):281-292. doi:10.1055/s-2007-1001914 [DOI] [PubMed] [Google Scholar]
- 19. Veraldi N, Guerrini M, Urso E, et al. Fine structural characterization of sulodexide. J Pharm Biomed Anal. 2018;156:67-79. doi:10.1016/j.jpba.2018.04.012 [DOI] [PubMed] [Google Scholar]
- 20. Mosier PD, Krishnasamy C, Kellogg GE, Desai UR. On the specificity of heparin/heparan sulfate binding to proteins. Anion-binding sites on antithrombin and thrombin are fundamentally different. PLoS One. 2012;7(11):e48632 doi:10.1371/journal.pone.0048632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mannello F, Raffetto JD. Matrix metalloproteinase activity and glycosaminoglycans in chronic venous disease: the linkage among cell biology, pathology and translational research. Am J Transl Res. 2011;3(2):149-158. [PMC free article] [PubMed] [Google Scholar]
- 22. Mattana P, Mannello F, Ferrari P, Agus GB. Vascular pathologies and inflammation: the antinflammatory properties of sulodexide. J Vasc Endovasc Surg. 2012;19(2(3)):1–7. [Google Scholar]
- 23. Plichta JK, Radek KA. Sugar-coating wound repair: a review of FGF-10 and dermatan sulfate in wound healing and their potential application in burn wounds. J Burn Care Res. 2012;33(3):299-310. doi:10.1097/BCR.0b013e318240540a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Duarte RCF, Ferreira CN, Rios DRA, Reis HJD, Carvalho MDG. Thrombin generation assays for global evaluation of the hemostatic system: perspectives and limitations. Rev Bras Hematol Hemoter. 2017;39(3):259-265. doi:10.1016/j.bjhh.2017.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bikdeli B, Madhavan MV, Jimenez D, et al. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up. J Am Coll Cardiol. 2020;75(23):2950–2973. doi:10.1016/j.jacc.2020.04.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Połubińska A, Staniszewski R, Baum E, Sumińska-Jasińska K, Bręborowicz A. Sulodexide modifies intravascular homeostasis what affects function of the endothelium. Adv Med Sci. 2013;58(2):304-310. doi:10.2478/ams-2013-0016 [DOI] [PubMed] [Google Scholar]
- 27. Mannello F, Ligi D, Raffetto JD. Glycosaminoglycan sulodexide modulates inflammatory pathways in chronic venous disease. Int Angiol. 2014;33(3):236-242. [PubMed] [Google Scholar]
- 28. Ciszewicz M, Polubinska A, Antoniewicz A, Suminska-Jasinska K, Breborowicz A. Sulodexide suppresses inflammation in human endothelial cells and prevents glucose cytotoxicity. Transl Res. 2009;153(3):118-123. doi:10.1016/j.trsl.2008.12.007 [DOI] [PubMed] [Google Scholar]
- 29. Urbanek T, Krasinski Z, Sumińska-Jasińska K, et al. Sulodexide reduces the inflammatory reaction and senescence of endothelial cells in conditions involving chronic venous disease. Int Angiol. 2016;35(2):140-147. [PubMed] [Google Scholar]
- 30. Ligi D, Croce L, Mosti G, Raffetto JD, Mannello F. Chronic venous insufficiency: transforming growth Factor-β isoforms and soluble endoglin concentration in different states of wound healing. Int J Mol Sci. 2017;18(10):2206 doi:10.3390/ijms18102206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bikdeli B, Madhavan MV, Gupta A, et al. Pharmacological agents targeting thromboinflammation in COVID-19: review and implications for future research. Thromb Haemost. 2020;120(7):1004–1024. doi:10.1055/s-0040-1713152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bikdeli B, Chatterjee S, Kirtane AJ, et al. Sulodexide versus control and the risk of thrombotic and hemorrhagic events: meta-analysis of randomized trials. Semin Thromb Hemost. 2020. In press. [DOI] [PubMed] [Google Scholar]