Abstract
Berberine (BBR), a major active component of Rhizoma coptidis, is one of the most promising agents for breast cancer adjuvant therapy. It is well accepted that BBR could exhibit remarkable anticancer efficacy with few side effects, and when treated with chemotherapeutic agents in combination, BBR could enhance the chemosensitivity of cancer cells. Our previous study reported that low-dose BBR (LDB) induced hormetic effect and attenuated the anticancer activity of chemotherapeutic agents. However, the underlying mechanisms are still unclear. In this study, we confirmed that LDB could promote cancer cell proliferation and antagonize the anti-breast cancer activities of chemotherapeutic agents. And the mechanisms were proved to be induction of autophagy and antioxidation by LDB. Our results showed that LDB could mildly induce reactive oxygen species, raise the level of autophagy by promoting the phosphorylation of adenosine monophosphate-activated protein kinase, and promote antioxidant enzymes expression through activating nuclear factor erythroid 2-related factor 2 in breast cancer cells. These findings revealed a potential negative impact of BBR on its adjuvant anti-breast cancer therapy, providing guidance for a safe and effective use of naturally originated medicines in the clinic.
Keywords: berberine, breast cancer, chemotherapy, hormesis, ROS, autophagy
Introduction
Breast cancer is one of the leading causes of death from cancer among women.1 According to the American Society of Clinical Oncology, it is estimated that more than 40 thousand breast cancer-related death will occur in 2020 just in the United States. As chemotherapy is still the main treatment for breast cancer in the clinic, though progress has been made in early detection and prognosis, the resistance and side effects of conventional chemotherapeutic agents remain major causes for the therapeutic failure.2-4 Therefore, the strategies that could overcome resistance and have few side effects are urgently needed.
Berberine (BBR), a natural nitrogenous cyclic compound generally distributed in the stems and the roots of several herbs, including Coptis chinensis Franch. and Phellodendron chinense Schneid.,5 is accepted to have the potential in cancer therapy, especially for breast cancer.6-8 Studies have proved its potential in chemosensitivity enhancement both in vitro and in vivo, which have made BBR a promising agent in breast cancer adjuvant therapy.6,8-10 However, the dose range of BBR used in those studies was all over 2.5 μM and the effect of lower dose of BBR (≤2.5 μM) were still unknown. To fully describe the dose-response curve of BBR, a previous study in our group has studied the effect of BBR on breast cancer cells and revealed a prosurvival effect of low-dose BBR (LDB), which attenuated the toxicity of chemotherapeutic agents to cancer cells.11 This is consistent with the theory of hormesis, which is characterized as beneficial effects of low doses of stimulations and toxic effects at high doses, and suggested the potential side effects of BBR used as an adjuvant. However, the underlying molecular mechanisms are largely unknown.
Autophagy is a conserved mechanism to maintain cellular homeostasis by cleaning misfolded proteins and damaged organelles.12 In cancer therapy, autophagy is considered as a double-edged sword for that they could prevent tumorigenesis in early period while protect cancer cell in later period,13 among which the latter normally contributes to the resistance in chemotherapy. Thus, the combinations of autophagy inhibitors and chemotherapy are expected as a promising strategy for cancer therapy and multiple autophagy inhibitors have already been in the preclinical stage.14 However, BBR was reported as a potent natural autophagic enhancer,10,15-17 and it is pending validation whether the BBR induced autophagy in breast cancer cells and whether autophagy involves in the hormesis mechanism of BBR. In our current studies, we tested the effect of BBR on the anti-breast cancer activities of chemotherapeutic agents as well as on the level of autophagy and oxidative stress in order to provide important information for a safer clinical use of BBR.
Materials and Methods
Chemicals and Reagents
Berberine was obtained from Sigma-Aldrich. 5-Fluorouracil (5-FU) and camptothecin (CPT) were purchased from Aladdin Industrial Corporation. Chloroquine (CQ) diphosphate salt, 3-methyladenine (3-MA), thiazolyl blue tetrazolium bromide (MTT), and dimethyl sulfoxide (DMSO) were obtained from Sigma Chemical Co. Dulbecco’ modified Eagle’s medium (DMEM), penicillin, streptomycin, and fetal bovine serum (FBS) were purchased from Gibco. Polyclonal antibodies against microtubule-associated protein 1 light chain 3 B (LC3B), sequestosome 1 (SQSTM1/p62), autophagy protein Beclin1, unc-51 like autophagy activating kinase 1 (ULK1), p-ULK1, heme oxygenase-1 (HO-1), superoxide dismutase 2 (SOD2), nuclear factor erythroid 2-related factor 2 (Nrf2), Kelch-like ECH-associated protein 1 (Keap1), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were purchased from Signaling Technology. Polyclonal antibodies adenosine monophosphate–activated protein kinase (AMPK) and p-AMPK were purchased from Santa Cruz Biotechnology. HRP-labeled goat anti-rabbit IgG (H+L) and nuclear Lamin B1 rabbit polyclonal antibody were supplied by Beyotime Institute of Biotechnology and Proteintech, respectively. Nrf2 small interfering RNA (siRNA; sc-37030) and control siRNA (sc-37007) were supplied by Santa Cruz Biotechnology. The plasmid-enhanced microtubule-associated protein 1 light-chain 3 β (pEGFP-LC3) was purchased from Addgene. Annexin V-FITC/propidium iodide (PI) apoptosis detection kit was purchased from KeyGEN BioTECH. Bicinchoninic acid (BCA) protein quantitation kit was supplied by Thermo. Lipofectamine 3000 was supplied by Invitrogen. Reactive oxygen species (ROS) Assay Kit, Nuclear and Cytoplasmic Protein Extraction Kit, and crystal violet dye were obtained from Beyotime Institute of Biotechnology.
Cell Culture and Drug Treatments
Human breast cancer cells (MCF-7 and MDA-MB-231) were purchased from American Type Culture Collection. According to American Type Culture Collection guidelines, cells were cultured in DMEM with 10% FBS and 1% penicillin/streptomycin in a humidified atmosphere with 5% CO2 at 37 °C. In terms of drugs, BBR (50 mM), 5-FU (100 mM), CPT (20 mM), CQ (10 mM), and 3-MA (50 mM), in powder form, were dissolved in DMSO as stock solutions, before which were diluted with DMEM containing 10% FBS upon use.
Cell Viability and Combination Index Analysis
Cell viabilities were determined by MTT assay. Briefly, cells were seeded into 96-well plates at the density of 5 × 103 cells/well, and after the cell attached to a surface, treated the cells with the prepared drugs, BBR (0-10 μM) with 5-FU (0-250 μM) or CPT (0-2.5 μM), or with CQ (20 μM) or 3-MA (10 μM), for 48 hours. Berberine lower than 2.5 μM were considered as LDB according to our previous study.11 The doses of 5-FU and CPT were used according to doubling dilution method at highest dose 250 and 2.5 μM, respectively. Then, 10 µL of MTT working solution (0.50 mg/mL) was added to the wells and incubated in the dark for 4 hours. After the removal of the medium, 100-µL DMSO was added to the wells. The optical density value was measured at 570 nm using SpectraMax M5 microplate reader (Molecular Devices). The combination index (CI) values were analyzed by CompuSyn software, version 34.
Analysis of Apoptosis by Flow Cytometry
Apoptosis was evaluated by Annexin V-FITC/PI double staining. Briefly, MCF-7 and MDA-MB-231 cells were seeded into 6-well plates at the density of 8 × 104 cells/well and treated with CPT (1.25 μM) or with 2 concentrations of LDB (0.31 and 0.62 μM) for 48 hours. Then, the collected cells were stained with Annexin V-FITC/PI and incubated in the dark for 15 minutes, before which the cells were detected by FACS Canto. The distributions of apoptotic cells were calculated using FlowJo software, version 7.6.1.
Transient Transfection With EGFP-LC3B Plasmid
Cells were transfected with EGFP-LC3B plasmid with Lipofectamine 3000 reagent. Briefly, cells were seeded into 6-well plates at the density of 3 × 105 cells/well, and then the DNA-lipid complex, which were prepared with diluted Lipofectamine 3000 reagent and diluted plasmid, were added to the plates. Cells were selected by 500 µg/mL G418 exposure before treated with increasing concentrations of BBR for 24 hours. Quantitative analysis was performed using INCell analyzer 2000.
Western Blotting
Cells were lysed in ice-cold RIPA buffer containing 1% protease inhibitor cocktail and 1% phenylmethylsulfonyl fluoride, and the concentration of the extracted protein were measured using BCA protein quantitation kit. Proteins were separated by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and blotted onto methanol activated poly vinylidene fluoride (PVDF) membranes at 25 V, 0.5 A for 30 minutes. After blocking with 5% skim milk for 1 hour at room temperature, the PVDF membranes were probed with primary antibodies at 4 °C overnight and were incubated with secondary antibodies for 1 hour at room temperature. Protein bands were visualized using Bio-Rad ChemiDoc (Hercules, California).
Reactive Oxygen Species Detection
Reactive oxygen species production was evaluated with dichloro-dihydro-fluorescein diacetate (DCFH-DA) dye. Briefly, cells were seeded in 6-well plates at a density of 8 × 104 cells/well and cultured for 24 hours. Then, the cells were treated with increasing doses of BBR for 2 hours, after which the cells were collected and cultured with DCFH-DA at 37 °C in the dark for 30 minutes. The intracellular DCFH-DA fluorescence was detected by FACS Canto.
Immunofluorescence
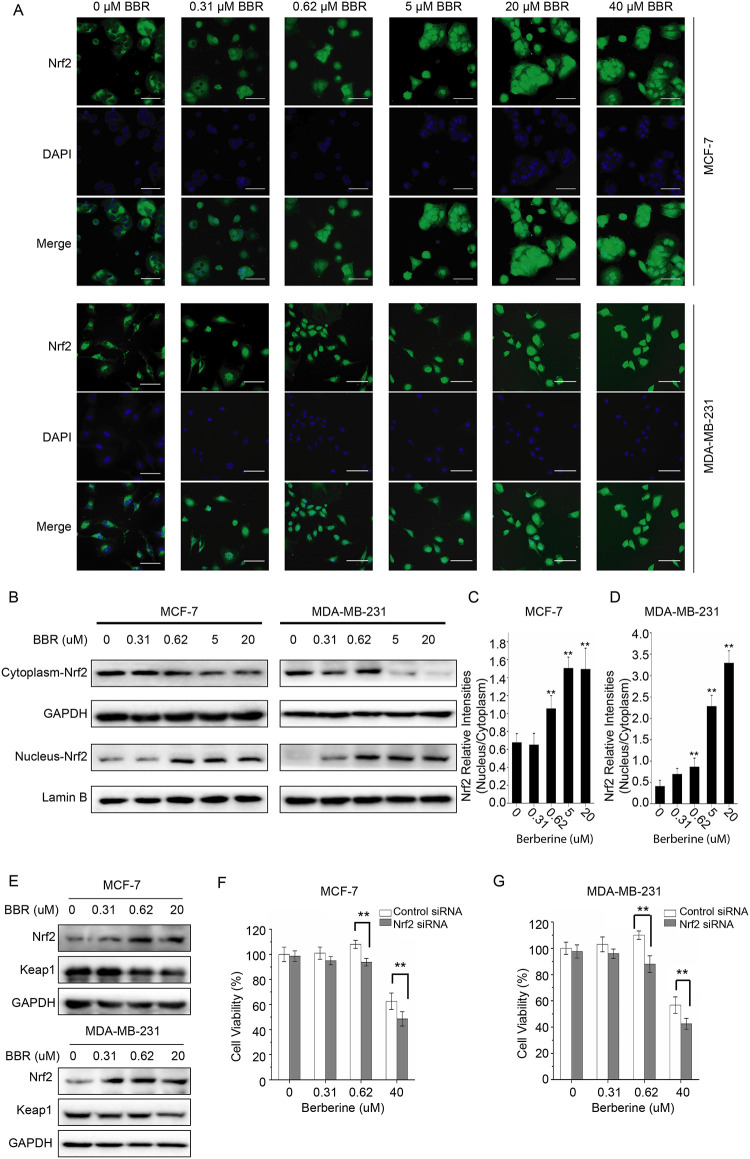
Cells were cultured in 96-well plates for 24 hours and treated with increasing doses of BBR for 24 hours and then fixed in 4% paraformaldehyde for 10 minutes at room temperature. After the blockage of the nonspecific binding with 3% bovine serum albumin in PBS for 1 hour, the cells were incubated overnight with Nrf2 primary antibodies (1:100) and labeled with FITC-conjugated secondary antibodies for 1 hour at room temperature in the dark. 4’,6-diamidino-2-phenylindole (DAPI) was used to dye the nucleus. Related localization was captured using INCell analyzer 2000 system.
Identification of Nrf2 in Nucleus and Cytoplasm
Proteins of the cells cultured and treated as immunofluorescence assay were extracted from nucleus and cytoplasm, respectively, using Nuclear and Cytoplasmic Protein Extraction Kit, according to manufacturer’s instructions. Further, the collected proteins were tested for further experiments.
RNA Interference
Cells were transfected with 4 µg siRNA against Nrf2 and negative control siRNA using Lipofectamine 3000 for 24 hours. After the removal of the supernatants, the cells were seeded to 96-well plates and treated with distinct concentrations of BBR for 48 hours for further experiments.
Statistical Analysis
All data are expressed as means ± SD of 3 independent experiments. One-way analysis of variance and Turkey multiple comparison test were used in the GraphPad Prism 5.0 software (GraphPad Software, Inc). A value of P < .05 was considered statistically significant.
Results
Low-Dose BBR Antagonized the Cytotoxicity of Chemotherapeutic Agents in Human Breast Cancer Cell Lines
A previous study in our group has proved that the hormetic effect of BBR (prosurvival effect of LDB and cytotoxic effect of high-dose BBR [HDB]) on a variety of human cancer cells (melanoma, breast, and colon) and revealed that such effect attenuated the anticancer activities of chemotherapeutic agents at least in human melanoma cells.11 As a follow-up study which aimed to elucidate the mechanism behind that, this study employed the breast cancer cells (MCF-7 and MDA-MB-231) to do the further study.
To determine the effect of BBR on the anti-breast cancer activities of chemotherapeutic agents, BBR (0-10 μM) with 5-FU (0-250 μM) or CPT (0-2.5 μM) were used to treat MCF-7 cells and MDA-MB-231 cells. Similar to the results in melanoma cells we have observed before, breast cancer cells could also be protected by the hormetic effect of BBR against chemotherapeutic agents. For MCF-7 cells, compared with the group treated with 5-FU alone (IC50 = 85.85 ± 3.78 μM), though HDB (10 μM) enhances the cytotoxicity of 5-FU (to IC50 = 63.13 ± 4.15 μM), the cytotoxicity of the group co-treated with LDB (0.31, 0.62, 1.25 μM) decreased significantly (to IC50 = 148.98 ± 2.84, 204.54 ± 3.25, 118.68 ± 3.94 μM, respectively), which was in line with the typical character of hormesis (Figure 1A). Moreover, the calculated CI value of LDB and 5-FU (>1) indicated an antagonistic effect of LDB toward 5-FU in human breast cancer cell lines (Figure 1B). Likewise, LDB exhibited hormetic responses and antagonistic effects on the cytotoxicity of 5-FU and CPT in human breast MCF-7 and MDA-MB-231 cells (Figure 1C-H).
Figure 1.
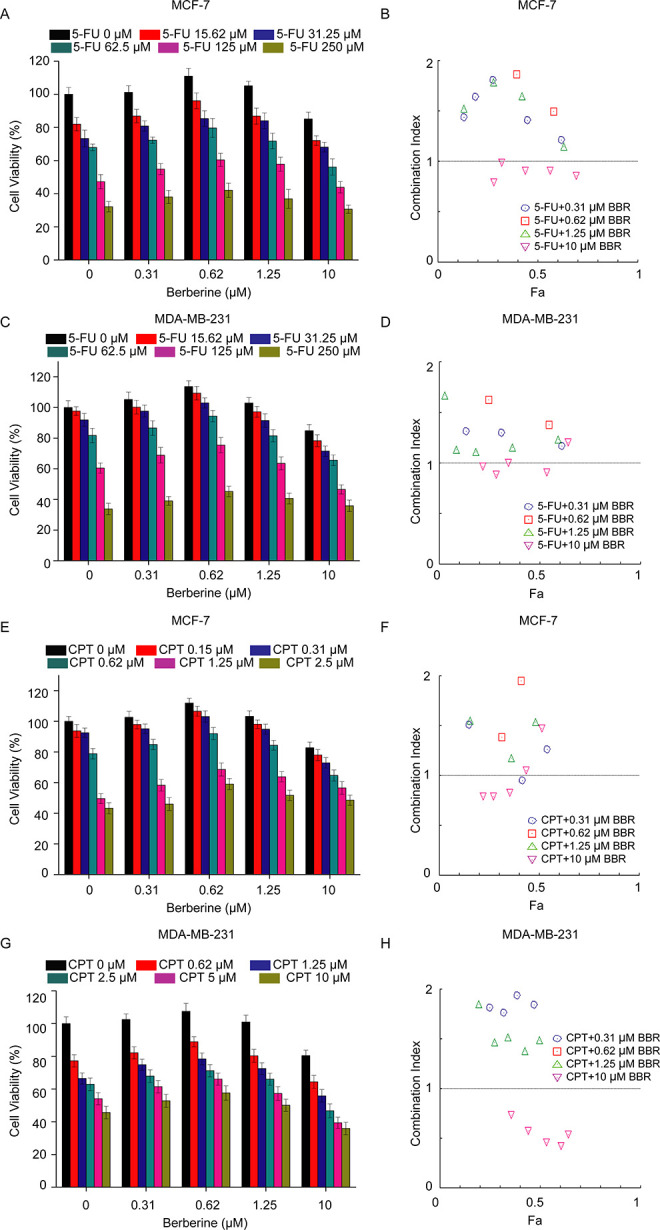
Effect of BBR on chemotherapeutic agents induced cytotoxicity in breast cancer cells. MCF-7 and MDA-MB-231 cells were treated with different concentrations of BBR and 5-FU (A-D) or CPT (E-G), before the determination of cell viability by MTT assay (A, C, E, G) and the combination index analysis by CompuSyn software 34 (B, D, F, H). Data are expressed as mean ± SD (n = 3). CI < 1 indicates synergy, CI = 1 indicates an additive effect, and CI > 1 indicates antagonism. BBR indicates berberine; 5-FU, 5-fluorouracil; CPT, camptothecin; MTT, thiazolyl blue tetrazolium bromide; CI, combination index.
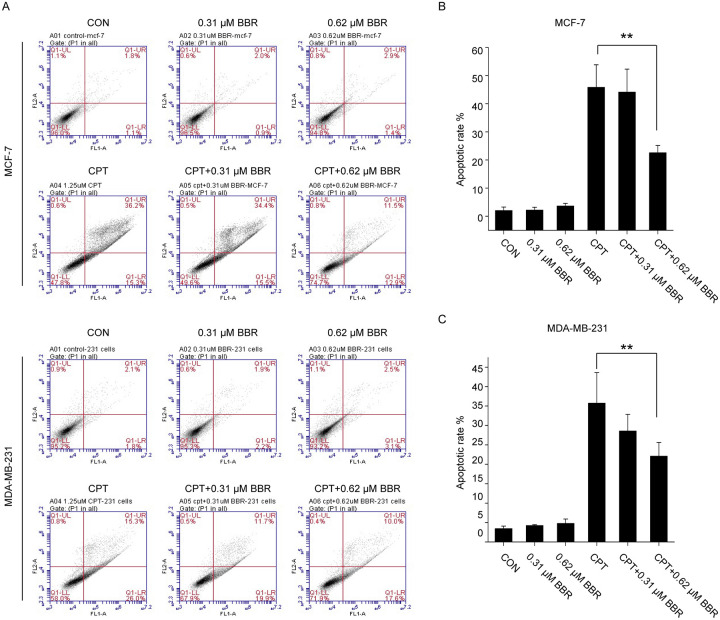
To validate the antagonism induced by LDB, we tested the apoptotic rate of MCF-7 and MDA-MB-231 cells treated with CPT and LDB, either separately or in combination. For MCF-7 cells, compared with the control group (1.50% ± 0.42%), CPT-induced cell apoptosis significantly (to 30.55% ± 7.99%), and the co-treatment of LDB (0.31 and 0.62 μM) restored the rate of apoptosis remarkably (to 27.75% ± 9.40% and 12.5% ± 1.56%). Likewise, LDB could also reduce the apoptotic rate induced by CPT in MDA-MB-231 cells (Figure 2). Such results supported the antagonism of LDB against the cytotoxic effect of chemotherapeutic agents.
Figure 2.
Effect of LDB on CPT induced apoptosis in breast cancer cells. MCF-7 (A, B) and MDA-MB-231 (C, D) cells were treated with CPT or LDB, either alone or in combination, before apoptosis evaluation by Annexin V-FITC/PI double staining. Data are expressed as mean ± SD (n = 3). *P < .05, **P < .01, compared with the CPT group. CPT indicates camptothecin; LDB, low dose BBR; PI, propidium iodide.
Low-Dose BBR Promoted the Proliferation of Human Breast Cancer Cells Through Autophagy Induction
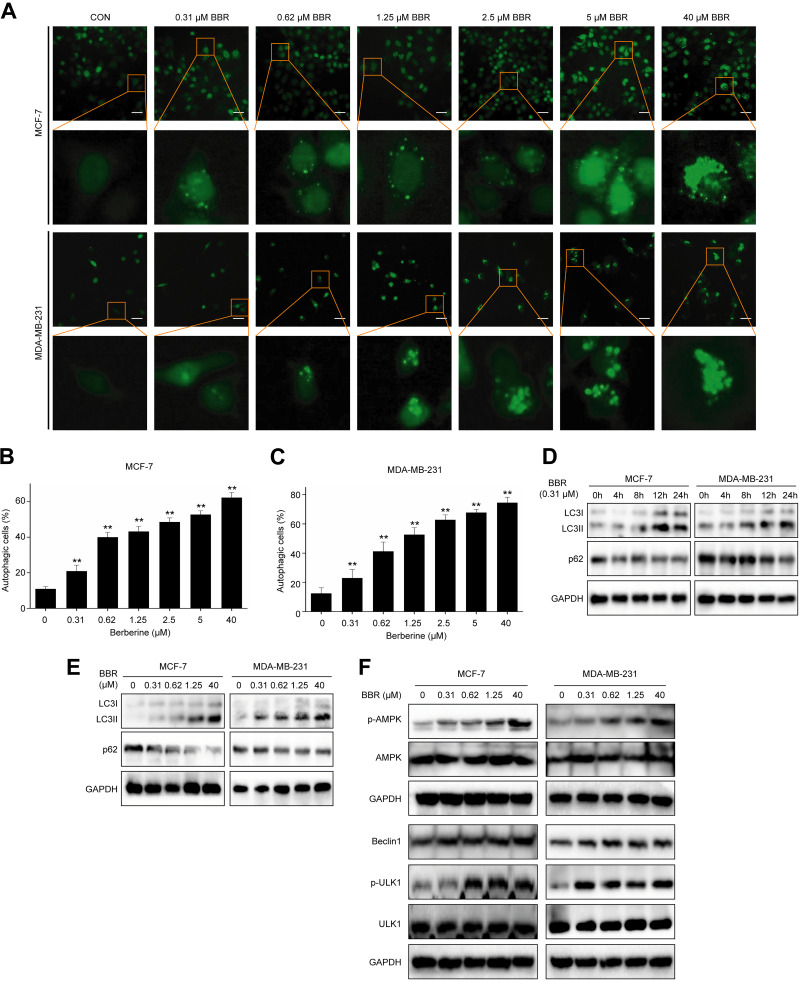
Since autophagy is a cellular process that generally involves in cytoprotective role in response to a variety of stresses,18 and BBR was reported to induce autophagy,19,20 we assume that the BBR-induced autophagy is responsible for the antagonistic effect of LDB. We directly monitored autophagy using EGFP-LC3B stably transfected human breast cancer cell lines, which labels autophagosomes in green. The results showed that BBR could lead to LC3 punctate dots accumulation dose dependently (Figure 3A-C), suggesting that BBR induced autophagy in breast cancer cells. To further determine the autophagy induction by BBR, we tested the level of LC3Ⅱ, which is converted from LC3I to initiate the formation and lengthening of the autophagosome, and p62, one of the best-known autophagic degradation substrates. Our results showed that BBR increased the level of LC3Ⅱ and decreased p62 protein level time and concentration dependently (Figure 3D-E), indicating BBR could enhance autophagic flux in both MCF-7 and MDA-MB-231 cells.
Figure 3.
BBR induced autophagy in breast cancer cells. A, The EGFP-LC3B stably transfected MCF-7 and MDA-MB-231 cells were treated with different concentrations of BBR, before quantitative analysis performed by INCell analyzer 2000. B, C, The autophagic cells (%) = total number of EGFP-LC3 positive cells/total number of viable cells × 100%. * P < .05, ** P < .01, compared with the CON (0 μM) group. D, MCF-7 and MDA-MB-231 cells were treated with LDB (0.31 μM) for 0, 4, 8, 12, and 24 hours. E, F, MCF-7 and MDA-MB-231 cells were treated with different concentrations of BBR (0.31-40 μM) for 24 hours. Total protein of cell lysates was extracted and subjected to Western blot analysis. GAPDH was used as the internal control. Data are expressed as mean ± SD (n = 3). BBR indicates berberine; EGFP-LC3B, enhanced GFP-microtubule-associated protein 1A/1B light chain 3 beta; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; LDB, low-dose BBR, AMPK, adenosine monophosphate-activated protein kinase; ULK1, unc-51 like autophagy activating kinase 1.
Since AMPK-ULK1-Beclin1 signaling pathway plays a critical role in autophagy induction,21-23 we tested the activity of this pathway in breast cancer cells treated with BBR. The results showed that BBR promoted the phosphorylation of AMPK and upregulated the levels of p-ULK1 and beclin1 in MCF-7 and MDA-MB-231 cells (Figure 3F), suggesting that BBR induced autophagy through AMPK-ULK1-Beclin1 signaling pathway.
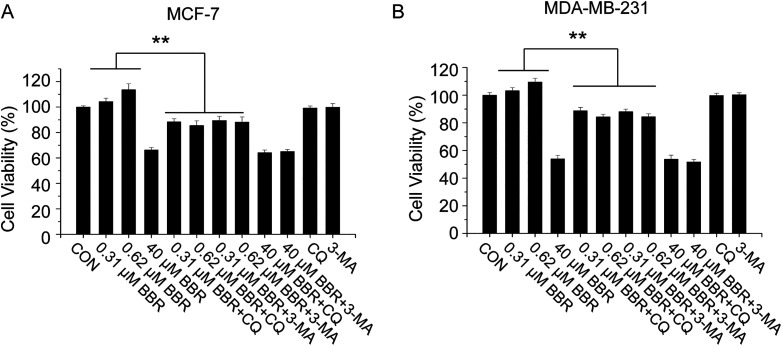
To test whether the BBR-induced autophagy is responsible for the prosurvival effect of LDB, we employed the autophagy inhibitors (3-MA, CQ) to determine whether the effect of BBR would be reversed by blocking autophagic flux. Consistently, the cell viabilities of LDB-treated cells were higher and HDB-treated cells were lower than the control group. After the co-treatment of autophagy inhibitors, though the killing effect of HDB was not further enhanced, the proliferation promoting effect of LDB was reversed (Figure 4), revealing a requirement for autophagy induction in the prosurvival effect.
Figure 4.
LDB promoted the proliferation of human breast cancer cells through autophagy induction. MCF-7 and MDA-MB-231cells were treated with different concentrations of BBR with or without autophagy inhibitors (CQ or 3-MA), before the determination of cell viability by MTT assay. Data are expressed as mean ± SD (n = 3). * P < .05, ** P < .01, compared with the autophagy inhibitors free groups. BBR indicates berberine; LDB, low-dose BBR; CQ, chloroquine; 3-MA, 3-methyladenine, MTT, thiazolyl blue tetrazolium bromide.
Low-Dose BBR Promoted the Proliferation of Human Breast Cancer Cells Through Nrf2 Activation
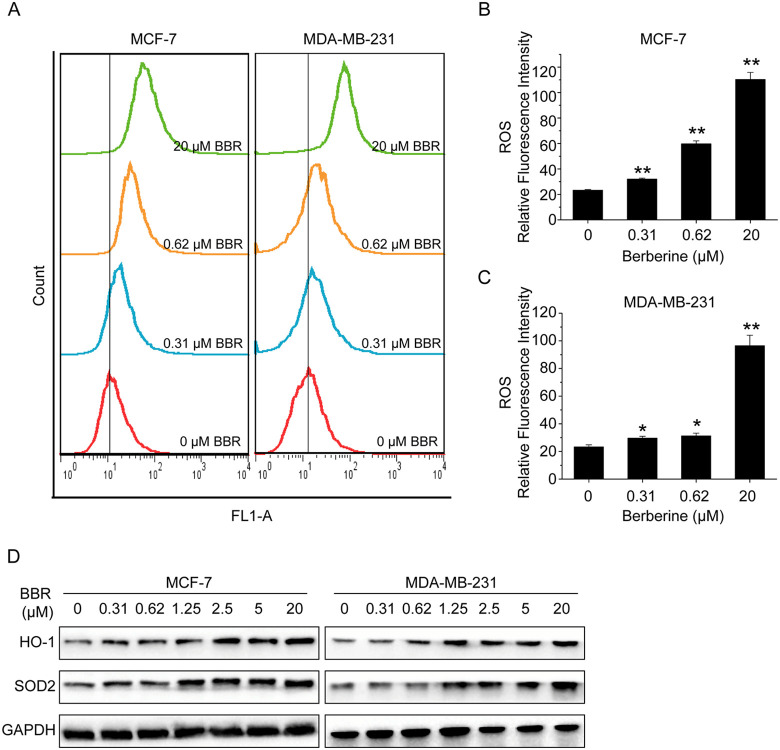
Previous studies reported that oxidative stress was one of the causes to induce autophagy in breast cancer cells.24-26 To test our hypothesis that oxidative stress could involve in the mechanism behind the prosurvival effect of LDB, we measured ROS production in the cells exposed to BBR. The results showed that LDB mildly while HDB greatly stimulated ROS generation (Figure 5A-C). We then detected the expression levels of some of antioxidant proteins and found BBR could upregulate the expression of HO-1 and SOD2 remarkably in both MCF-7 and MDA-MB-231 cells (Figure 5A-D), suggesting that BBR-induced ROS generation may activate the antioxidant response therefore to promote cancer cell proliferation.
Figure 5.
BBR stimulated ROS generation and upregulated antioxidant proteins. A, MCF-7 and MDA-MB-231 cells were treated with different concentrations of BBR, before ROS production evaluation with DCFH-DA dye. B and C, The quantitative analysis of ROS relative fluorescence intensity. Data are expressed as mean ± SD (n = 3). * P < .05, ** P < .01, compared with the CON (0 μM) group. D, MCF-7 and MDA-MB-231 cells were treated with different concentrations of BBR. Total protein of cell lysates was extracted and subjected to Western blot analysis. GAPDH was used as the internal control. BBR indicates berberine; ROS, reactive oxygen species; HO-1, heme oxygenase-1; SOD2, superoxide dismutase 2; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Depending on cellular redox balance, Nrf2-Keap1-antioxidant response element (ARE) signaling system is widely accepted to regulate expression of genes containing ARE.27 To determine the relationship between oxidative stress and the prosurvival effect of LDB, we monitored Nrf2 in the cells treated with BBR. The results showed that BBR promoted the Nrf2 nuclear translocation significantly in MCF-7 and MDA-MB-231 cells (Figure 6A), revealing the activation of Nrf2 induced by BBR. To further validate Nrf2 activation, we extracted the protein in nucleus and cytoplasm and tested the level of Nrf2, respectively. The results showed that with the concentration of BBR getting higher, cytoplasm-Nrf2 decreased and nucleus-Nrf2 increased (Figure 6B-D). Then, we tested Keap1 and Nrf2 simultaneously in the breast cancer cells exposed to BBR and found that compared with the control group, BBR raised the level of Nrf2 but reduced the level of Keap1 in a dose-dependent manner, indicating that BBR-induced oxidative stress activated antioxidant response through Nrf2-Keap1-ARE pathway.
Figure 6.
LDB promoted the proliferation of human breast cancer cells through Nrf2 activation. A, MCF-7 and MDA-MB-231 cells were treated with different concentrations of BBR, before immunofluorescence and localization analysis by INCell analyzer 2000 system. Top panel: Green fluorescence showing Nrf2 localization; middle panel: stained nucleus with DAPI. B, MCF-7 and MDA-MB-231 cells were treated with different concentrations of BBR. Desired proteins were extracted from the nucleus and cytoplasm, respectively, and subjected to Western blot analysis. GAPDH was used as the internal control for cytoplasm-Nrf2, and Lamin B was used as the internal control for nucleus-Nrf2. C and D, The quantitative analysis of Nrf2 relative intensities in (B). The density of each band was quantified by Image J, and the relative density ratio of each protein was calculated accordingly. Data are expressed as mean ± SD (n = 3). * P < .05, ** P < .01, compared with the CON (0 μM) group. E, MCF-7 and MDA-MB-231 cells were treated with different concentrations of BBR for 24 hours. Total protein of cell lysates was extracted and subjected to Western blot analysis. GAPDH was used as the internal control. F, MCF-7 and MDA-MB-231 cells were transfected with siRNA against Nrf2 and negative control siRNA. The cells were treated with different concentrations of BBR, before the determination of cell viability by MTT assay. Data are expressed as mean ± SD (n = 3). * P < .05, ** P < .01, compared with the Control siRNA groups. BBR indicates berberine; LDB, low-dose BBR; Nrf2, nuclear factor erythroid 2-related factor 2; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; MTT, thiazolyl blue tetrazolium bromide; siRNA, small interfering RNA.
To investigate the requirement of Nrf2 activation in the proliferation promoting effect, we knocked down the expression of Nrf2 in the breast cancer cells and detected the viability of cells treated with BBR. The results showed that the knockdown of Nrf2 leads to a reversed trend compared with the control group in both MCF-7 and MDA-MB-231 cells, indicating that the activation of Nrf2 is required for prosurvival effect of LDB (Figure 6F and 6G).
Discussion
Our previous study has demonstrated that hormetic effect of BBR attenuated the anticancer activity of chemotherapeutic agents.11 However, the underlying mechanisms are still largely unknown. In this study, we took particular emphasis on the hormetic effect of BBR on anti-breast cancer activities, trying to elucidate the mechanisms of the hormetic effect and antagonistic effect on chemotherapy of LDB. Our results indicated that LDB induced hormetic effect and antagonized the anticancer efficacy of chemotherapeutic agents in breast cancer cells, and these effects were found to be closely related to the autophagy and antioxidation induced by LDB.
Breast cancer, the most common cancer among females, is threatening the health of woman. Clinical treatment for breast cancer mainly includes surgery, chemotherapy, radiotherapy, endocrine therapy, and photodynamic therapy, among which chemotherapy is still the main strategy in the clinic.28 However, due to the high occurrence of recurrence and metastasis, the therapeutic effect of chemotherapy cannot meet the clinical needs, necessitating the modification of the conventional strategies that aims to be more effective and with fewer undesirable side effects. As one of the alternate remedies, phytochemicals are widely developed in cancer treatment. Not just for treating, but also preventing, and not just as individual molecules, but in combination with conventional treatments, phytochemicals are proved to be promising in cancer therapy.29 But natural agents are not free of hidden danger. Taken curcumin for example, accumulating studies indicated that it involved in multiple mechanisms of anticancer efficacy, including, but not limit to, interfering cell cycle and inhibiting key transcriptional factors of cancer cells, and could be used with chemotherapeutics for more significant curative effects.30 Nevertheless, it has been reported that the curcumin taken orally could attenuate the efficacy of chemotherapy, and it was suggested that patients who would receive chemotherapy should avoid curcumin-containing foods,31 which suggested the potential adverse side effects of natural originated drugs.
Berberine, another widely accepted promising agent in adjuvant therapy, is proved to have definite curative efficacy in cancer treatment either.6,8,32-35 Previous study in our group has analyzed the dose-response curve of BBR on several cancer cells and revealed a hormetic effect of BBR, which attenuated the anticancer activity of some chemotherapeutic agents.11 Hormesis is a kind of dose response that is characterized by the benign stimulations or effects of some deleterious agents at low doses. Many anticancer drugs including chemotherapeutic agents and natural products are found to exhibit hormetic effect to promote cancer cell proliferation, which is definitely unexpected during cancer therapy.36,37 In our current study, we firstly demonstrated that though HDB could enhance the anticancer efficacy of 5-FU and CPT in breast cancer therapy to some extent, LDB (0.31, 0.62, 1.25 μM) did antagonize them on the contrary (Figures 1 and 2). Such finding suggested that caution must be exercised for BBR during breast cancer chemotherapy.
Autophagy is an emerging target for cancer therapy since it plays a prosurvival role not just in normal organs but also tumor tissues in response to endogenous and exogenous stress.12,13 Tumor in a certain stage would be confronted with severe survival stress, such as starvation, when autophagy could help to restore the growth and metabolism by controlling the quality and degrading the damaged proteins and organelles. Therefore, autophagy inhibitors are expected to assist common chemotherapeutic agents in cancer therapy.38,39 However, BBR was widely reported to induce autophagy,10,15-17 and our results confirmed BBR as an autophagy-inducing agent in breast cancer cells (Figure 3). We then asked if the BBR-induced autophagy is responsible for the hormetic effect of BBR. The results showed that autophagy inhibitors could reverse the prosurvival effect of LDB (Figure 4), which indicated that the cell-promoting effect of LDB was attributable, at least partially, to the autophagy induction. Of note, HDB, which was also proved to be cytotoxic (Figure 1) to the cells, did induce autophagy either (Figure 3), while the autophagy inhibitor did not further enhance the toxicity (Figure 4), suggesting that autophagy induced by HDB barely exhibits protection.
Oxidative stress is reported to be one of the reasons to induce autophagy in breast cancer cells. Under oxidative stress, when ROS accumulated to a certain amount, exceeding the antioxidant capacity, the cell contents including proteins and lipids would be oxidized and autophagy would be induced.25,26 Berberine was previously reported to induce ROS generation in human cells, including breast cancer cells,40-43 and our results confirmed that BBR could stimulate the ROS generation and upregulate the levels of antioxidant proteins (HO-1 and SOD) in MCF-7 and MDA-MB-231 cells (Figure 5). And it is widely accepted that autophagy could be regulated by mitochondrial ROS through AMPK activation.44 Our data showed that BBR promoted the phosphorylation of AMPK and upregulated the levels of p-ULK1 and beclin1 (Figure 3F), suggesting that the oxidative stress stimulated by BBR could lead to autophagy through AMPK-ULK1-Beclin1 pathway.
Nrf2-Keap1-ARE signaling system, which regulates the expression of genes containing ARE, is one of the targets for cancer therapy.27 As a master transcription factor responsible for coordinating antioxidant activities, Nrf2 involves in maintenance of intracellular homeostasis, cellular defense, identification of damaged macromolecules, and so on.27 In brief, Nrf2 is primarily regulated by Keap1. Under basal conditions, Nrf2 mostly exists as a complex with Keap1, before which ROS oxidizes Keap1 and allows Nrf2 to evade Keap1-mediated ubiquitination and to translocate to nucleus. Nrf2 would bind to ARE in nucleus, resulting in the transcription and translation of antioxidant enzymes. The enhanced antioxidant enzymes such as HO-1 and SOD would facilitate cell survival by several mechanisms, including inhibiting cell apoptosis.45 Our data showed that BBR promoted Keap1 degradation and Nrf2 nuclear translocation dose independently (Figure 6A-E). Additionally, the results showed that the prosurvival effect of LDB disappeared after knocking down the expression of Nrf2, necessitating the activation of Nrf2 in cell survival (Figure 6F and 6G).
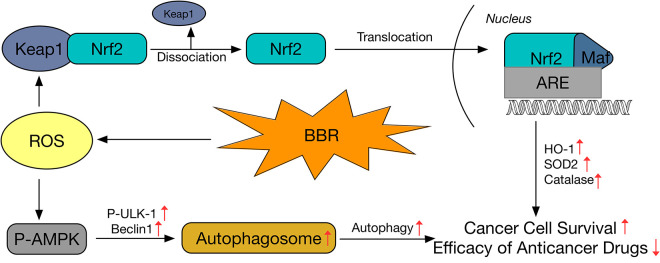
In summary, our study demonstrated that LDB (0.31, 0.62, 1.25 μM) antagonized the anticancer effect of chemotherapeutic agents (5-FU and CPT). These effects were in accord with hormetic theory and were associated with the activation of adaptive responses, including autophagy and antioxidation. By inducing autophagy and activating Nrf2, LDB could assist the cancer cells survive from the chemotherapeutic drugs through several routes as depicted in Figure 7. In brief, moderate stress caused by LDB induced the generation of ROS, which elicited autophagy through AMPK-ULK1-Beclin1 pathway in adverse circumstances, and at the same time, ROS activated Nrf2/ARE pathway by oxidizing Keap1 and dissociating Nrf2/Keap1 complex, leading to regulation of antioxidant proteins, which provide duplicate protection for breast cancer cells. Our study suggested cautious application of promising natural adjuvant agents, to which the clinical treatment should pay attention.
Figure 7.
Schematic diagram showing the proposed mechanisms for the enhancement of cancer cell survival and antagonism of anticancer drugs by low-dose berberine.
Footnotes
Authors’ Note: Bing Han and Kai Wang contributed equally to this work. BH, KW, and CH conceived and designed the study. BH and KW performed the experiments. YT and LT analyzed the data. BH and CH drafted the manuscript. All the authors have read and approved the final manuscript prior to submission.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Macao Science and Technology Development Fund (FDCT/070/2017/A2) and the Research Fund of the University of Macau (MYRG2018-00176-ICMS).
ORCID iD: Chengwei He  https://orcid.org/0000-0003-4701-2984
https://orcid.org/0000-0003-4701-2984
References
- 1. Bai XP, Ni J, Beretov J, Graham P, Li Y. Cancer stem cell in breast cancer therapeutic resistance. Cancer Treat Rev. 2018;69:152–163. doi:10.1016/j.ctrv.2018.07.004 [DOI] [PubMed] [Google Scholar]
- 2. Zheng HC. The molecular mechanisms of chemoresistance in cancers. Oncotarget. 2017;8(35):59950–59964. doi:10.18632/oncotarget.19048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ou HT, Chung WP, Su PF, et al. Health-related quality of life associated with different cancer treatments in Chinese breast cancer survivors in Taiwan. Eur J Cancer Care. 2019;28(4):e13069 doi:10.1111/ecc.13069 [DOI] [PubMed] [Google Scholar]
- 4. Zhang XP, Yang HJ, Zhang RP. Challenges and future of precision medicine strategies for breast cancer based on a database on drug reactions. Biosci Rep. 2019;39(9):10 doi:10.1042/bsr20190230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Imenshahidi M, Hosseinzadeh H. Berberine and barberry (Berberis vulgaris): a clinical review. Phytother Res. 2019;33(3):504–523. doi:10.1002/ptr.6252 [DOI] [PubMed] [Google Scholar]
- 6. Kaboli PJ, Leong MP-Y, Ismail P, Ling K-H. Antitumor effects of berberine against EGFR, ERK1/2, P38 and AKT in MDA-MB231 and MCF-7 breast cancer cells using molecular modelling and in vitro study. Pharmacol Rep. 2019;71(1):13–23. doi:10.1016/j.pharep.2018.07.005 [DOI] [PubMed] [Google Scholar]
- 7. El Khalki L, Maire V, Dubois T, Zyad A. Berberine impairs the survival of triple negative breast cancer cells: cellular and molecular analyses. Molecules. 2020;25(3):506 doi:10.3390/molecules25030506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pan Y, Zhang F, Zhao Y, et al. Berberine enhances chemosensitivity and induces apoptosis through dose-orchestrated AMPK signaling in breast cancer. J Cancer. 2017;8(9):1679–1689. doi:10.7150/jca.19106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Refaat A, Abdelhamed S, Yagita H, et al. Berberine enhances tumor necrosis factor-related apoptosis-inducing ligand-mediated apoptosis in breast cancer. Oncol Lett. 2013;6(3):840–844. doi:10.3892/ol.2013.1434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang K, Zhang C, Bao J, et al. Synergistic chemopreventive effects of curcumin and berberine on human breast cancer cells through induction of apoptosis and autophagic cell death. Sci Rep. 2016;6:26064 doi: 10.1038/srep26064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bao JL, Huang BR, Zou LD, et al. Hormetic effect of berberine attenuates the anticancer activity of chemotherapeutic agents. PLoS One. 2015;10(9):e0139298 doi:10.1371/journal.pone.0139298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Agathokleous E, Kitao M, Calabrese EJ. Environmental hormesis and its fundamental biological basis: rewriting the history of toxicology. Environ Res. 2018;165:274–278. doi:10.1016/j.envres.2018.04.034 [DOI] [PubMed] [Google Scholar]
- 13. Liu J, Fan L, Wang H, Sun G. Autophagy, a double-edged sword in anti-angiogenesis therapy. Med Oncol. 2016;33(1):10 doi:10.1007/s12032-015-0721-9 [DOI] [PubMed] [Google Scholar]
- 14. Chude CI, Amaravadi RK. Targeting autophagy in cancer: update on clinical trials and novel inhibitors. Int J Mol Sci. 2017;18(6):1279 doi:10.3390/ijms18061279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fan X, Wang J, Hou J, et al. Berberine alleviates ox-LDL induced inflammatory factors by up-regulation of autophagy via AMPK/mTOR signaling pathway. J Transl Med. 2015;13(1):92 doi:10.1186/s12967-015-0450-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yao Z, Wan Y, Li B, et al. Berberine induces mitochondrial-mediated apoptosis and protective autophagy in human malignant pleural mesothelioma NCI-H2452 cells. Oncol Rep. 2018;40(6):3603–3610. doi:10.3892/or.2018.6757 [DOI] [PubMed] [Google Scholar]
- 17. Li MH, Zhang YJ, Yu YH, et al. Berberine improves pressure overload-induced cardiac hypertrophy and dysfunction through enhanced autophagy. Eur J Pharmacol. 2014;728:67–76. doi:10.1016/j.ejphar.2014.01.06 [DOI] [PubMed] [Google Scholar]
- 18. Cheng Y, Ren X, Hait WN, Yang JM. Therapeutic targeting of autophagy in disease: biology and pharmacology. Pharmacol Rev. 2013;65(4):1162–1197. doi:10.1124/pr.112.007120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yu R, Zhang ZQ, Wang B, et al. Berberine-induced apoptotic and autophagic death of HepG2 cells requires AMPK activation. Cancer Cell Int. 2014;14:49 doi:10.1186/1475-2867-14-49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sun YX, Yu J, Liu XR, et al. Oncosis-like cell death is induced by berberine through ERK1/2-mediated impairment of mitochondrial aerobic respiration in gliomas. Biomed Pharmacother. 2018;102:699–710. doi:10.1016/j.biopha.2018.03.132 [DOI] [PubMed] [Google Scholar]
- 21. Carling D, Thornton C, Woods A, Sanders MJ. AMP-activated protein kinase: new regulation, new roles? Biochem J. 2012;445(1):11–27. doi:10.1042/BJ20120546 [DOI] [PubMed] [Google Scholar]
- 22. Gallagher LE, Williamson LE, Chan EY. Advances in autophagy regulatory mechanisms. Cells. 2016;5(2):24 doi:10.3390/cells5020024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lin MG, Hurley JH. Structure and function of the ULK1 complex in autophagy. Curr Opin Cell Biol. 2016;39:61–68. doi:10.1016/j.ceb.2016.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gonzalez Y, Aryal B, Chehab L, Rao VA. Atg7- and Keap1-dependent autophagy protects breast cancer cell lines against mitoquinone-induced oxidative stress. Oncotarget. 2014;5(6):1526–1537. doi:10.18632/oncotarget.1715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hwang JJ, Kim HN, Kim J, et al. Zinc (II) ion mediates tamoxifen-induced autophagy and cell death in MCF-7 breast cancer cell line. Biometals. 2010;23(6):997–1013. doi:10.1007/s10534-010-9346-9 [DOI] [PubMed] [Google Scholar]
- 26. Zhang Q, Zhang Y, Zhang P, et al. Hexokinase II inhibitor, 3-BrPA induced autophagy by stimulating ROS formation in human breast cancer cells. Genes Cancer. 2014;5(3-4):100 doi:10.18632/genesandcancer.9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tkachev VO, Menshchikova EB, Zenkov NK. Mechanism of the Nrf2/Keap1/ARE signaling system. Biochemistry (Mosc). 2011;76(4):407–422. doi:10.1134/s0006297911040031 [DOI] [PubMed] [Google Scholar]
- 28. Aniogo EC, George BPA, Abrahamse H. The role of photodynamic therapy on multidrug resistant breast cancer. Cancer Cell Int. 2019;19:91 doi:10.1186/s12935-019-0815-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Banudevi S, Swaminathan S, Maheswari KU. Pleiotropic role of dietary phytochemicals in cancer: emerging perspectives for combinational therapy. Nutr Cancer. 2015;67(7):1021–1048. doi:10.1080/01635581.2015.1073762 [DOI] [PubMed] [Google Scholar]
- 30. Kumar P, Kadakol A, Shasthrula PK, et al. Curcumin as an adjuvant to breast cancer treatment. Anti-Cancer Agents Med Chem. 2015;15(5):647–656. doi:10.2174/1871520615666150101125918 [DOI] [PubMed] [Google Scholar]
- 31. Somasundaram S, Edmund NA, Moore DT, et al. Dietary curcumin inhibits chemotherapy-induced apoptosis in models of human breast cancer. Cancer Res. 2002;62(13):3868–3875. https://cancerres.aacrjournals.org/content/62/13/3868.long. Accessed July 1, 2002. [PubMed] [Google Scholar]
- 32. Ortiz LMG, Lombardi P, Tillhon M, Scovassi AI. Berberine, an epiphany against cancer. Molecules. 2014;19(8):12349–12367. doi:10.3390/molecules190812349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yao MJ, Fan XD, Yuan B, et al. Berberine inhibits NLRP3 inflammasome pathway in human triple-negative breast cancer MDA-MB-231 cell. BMC Complement Med Ther. 2019;19(1):216 doi:10.1186/s12906-019-2615-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tak J, Sabarwal A, Shyanti RK, Singh RP. Berberine enhances posttranslational protein stability of p21/cip1 in breast cancer cells via down-regulation of Akt. Mol Cell Biochem. 2019;458(1-2):49–59. doi:10.1007/s11010-019-03529-4 [DOI] [PubMed] [Google Scholar]
- 35. Gao XJ, Wang J, Li MQ, et al. Berberine attenuates XRCC1-mediated base excision repair and sensitizes breast cancer cells to the chemotherapeutic drugs. J Cell Mol Med. 2019;23(10):6797–6804. doi:10.1111/jcmm.14560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Borriello A, Bencivenga D, Caldarelli I, et al. Resveratrol and cancer treatment: is hormesis a yet unsolved matter? Curr Pharm Design. 2013;19(30):5384–5393. doi:10.2174/1381612811319300007 [DOI] [PubMed] [Google Scholar]
- 37. Lu X, Zhou D, Hou B, et al. Dichloroacetate enhances the antitumor efficacy of chemotherapeutic agents via inhibiting autophagy in non-small-cell lung cancer. Cancer Manag Res. 2018;10:1231 doi:10.2147/CMAR.S156530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Deng S, Shanmugam MK, Kumar AP, et al. Targeting autophagy using natural compounds for cancer prevention and therapy. Cancer. 2019;125(8):1228–1246. doi:10.1002/cncr.31978 [DOI] [PubMed] [Google Scholar]
- 39. Zhan Y, Wang K, Li Q, et al. The novel autophagy inhibitor alpha-hederin promoted paclitaxel cytotoxicity by increasing reactive oxygen species accumulation in non-small cell lung cancer cells. Int J Mol Sci. 2018;19(10):3221 doi:10.3390/ijms19103221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Meeran SM, Katiyar S, Katiyar SK. Berberine-induced apoptosis in human prostate cancer cells is initiated by reactive oxygen species generation. Toxicol Appl Pharmacol. 2008;229(1):33–43. doi:10.1016/j.taap.2007.12.027 [DOI] [PubMed] [Google Scholar]
- 41. Wang L, Liu L, Shi Y, et al. Berberine induces caspase-independent cell death in colon tumor cells through activation of apoptosis-inducing factor. PLoS One. 2012;7(5):e36418 doi:10.1371/journal.pone.0036418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hur JM, Hyun MS, Lim SY, Lee WY, Kim D. The combination of berberine and irradiation enhances anti-cancer effects via activation of p38 MAPK pathway and ROS generation in human hepatoma cells. J Cell Biochem. 2009;107(5):955–964. doi:10.1002/jcb.22198 [DOI] [PubMed] [Google Scholar]
- 43. Xie J, Xu Y, Huang X, et al. Berberine-induced apoptosis in human breast cancer cells is mediated by reactive oxygen species generation and mitochondrial-related apoptotic pathway. Tumor Biol. 2015;36(2):1279–1288. doi:10.1007/s13277-014-2754-7 [DOI] [PubMed] [Google Scholar]
- 44. Li L, Chen Y, Gibson SB. Starvation-induced autophagy is regulated by mitochondrial reactive oxygen species leading to AMPK activation. Cell Signal. 2013;25(1):50–65. doi:10.1016/j.cellsig.2012.09.020 [DOI] [PubMed] [Google Scholar]
- 45. Zhu C, Dong Y, Liu H, Ren H, Cui Z. Hesperetin protects against H2O2-triggered oxidative damage via upregulation of the Keap1-Nrf2/HO-1 signal pathway in ARPE-19 cells. Biomed Pharmacother. 2017;88:124–133. doi:10.1016/j.biopha.2016.11.089 [DOI] [PubMed] [Google Scholar]