Abstract
Background & Aims:
The contribution of surveillance colonoscopy, as opposed to that of initial colonoscopy examination, to prevention of colorectal cancer (CRC) is uncertain. We estimated the preventive effect of surveillance colonoscopy by applying the recently developed metric of adenoma dwell time avoided needed to prevent 1 CRC case (DTA).
Methods:
We followed subjects in the prostate, lung, colorectal and ovarian (PLCO) cancer screening trial who underwent colonoscopies following positive findings from sigmoidoscopies (colonoscopy cohort, n=15,935) for CRC incidence for 10 years. The number and timing of adenomas removed during surveillance were measured in a subset (n=3492) of patients and extrapolated to the entire cohort to estimate the total avoided adenoma dwell time. A previously determined DTA value of 612 dwell years was applied to estimate the number of CRC cases prevented by surveillance. Proportional reduction in CRC was computed as CP/(CO+CP), where CO and CP are observed and estimated prevented cases, respectively.
Results:
In the colonoscopy cohort of the PLCO, 2882 subjects had advanced adenomas (AAs), 572 had 3 or more non-advanced adenomas (NAA3+), 4496 had 1–2 non-advanced adenomas (NAA1-2), and 7985 had no adenoma (NA). The mean number of subsequent colonoscopy examinations over 10 years were 1.80 for subjects with AAs, 1.63 for subjects with NAA3+, and 1.46 for subjects with NAA1–2. Average years of avoided adenoma dwell time per subject were 4.0 for subjects with AAs, 5.5 for subjects with NAA3+, and 2.4 for subjects with NAA1–2. There were 56 cases of CRC in subjects with AAs, 4 cases of CRC in subjects with NAA3+, and 33 cases of CRC in subjects with NAA1–2. Estimated proportional reductions in CRC incidence were 25.0% in subjects with AAs (95% CI, 16%–34%), 34.4% in subjects with NAA1–2 (95% CI, 25%–40%), and 30.4% overall (in subjects with AAs, NAA3+, or NAA1–2; 95% CI, 25%–40%). Absolute CRC incidence reductions were 7.1 per 10,000 PY in subjects with AAs and 4.1 per 10,000 PY in subjects with NAA1-2.
Conclusions:
Using the recently developed metric of DTA, we estimated that surveillance colonoscopy during 10 years of follow up (in the PLCO colonoscopy cohort) prevented 30% of CRC cases. Because the methodology for estimation is indirect, the true effect is uncertain.
Keywords: colon cancer, tumor precursor, progression, screening
Introduction:
Randomized trials demonstrate that endoscopic screening with flexible sigmoidoscopy reduces colorectal cancer (CRC) incidence and mortality (1-4). CRC incidence declines because screening identifies pre-cancerous adenomatous polyps, whose removal prevents subsequent progression to cancer. Reducing cancer incidence has an even greater impact on cancer mortality than detecting cancer at an early stage, as estimates suggest that reducing cancer incidence accounts for 2/3 of the mortality reduction produced by screening (5).
Although screening for CRC is effective, the contribution to CRC prevention of colonoscopy and adenoma removal after the diagnosis of adenomatous polyps, or surveillance colonoscopy, is uncertain (6). A U.K. study demonstrated that patients undergoing surveillance colonoscopy after diagnosis of an advanced adenoma or 3-4 non-advanced adenomas had lower CRC risk compared to subjects not undergoing surveillance, but the study was not randomized, and subjects not undergoing surveillance may have had a different underlying risk (7).
In clinical practice, colonoscopy after diagnosis of adenomatous polyps is not limited to routine surveillance exams, but also includes exams for symptoms or family history (8). About 25% of all U.S. colonoscopies are performed for surveillance (9). U.S. data suggest that compared to guidelines, there is both under- and over-utilization of surveillance colonoscopy (8,10). A randomized trial to assess the impact of surveillance colonoscopy on cancer incidence is underway in Europe (11) and another will begin accruing shortly in the United States (12), but the results of those studies will not be available for many years. In the interim, methods for determining and evaluating the effectiveness and impact of surveillance colonoscopy are needed.
The number of adenomas needed to be removed to prevent one CRC case (NNR) and the adenoma dwell time avoided needed to prevent one CRC case (DTA) are recently developed metrics for quantifying the relationship between adenoma removal and CRC cases prevented (13). The NNR is analogous to the number needed to screen (NNS) for estimation of the benefit of screening, and is defined as the number of adenomas needed to be removed (on average) to prevent one incident CRC case. The DTA metric incorporates a time horizon and is defined as the amount of adenoma dwell time avoided, by adenoma removal, required to prevent one incident CRC case. Values for these metrics were estimated from data from four randomized sigmoidoscopy trials, three from Europe as well as the U.S.-based Prostate, Lung, Colorectal and Ovarian (PLCO) cancer screening trial (13).
In this investigation, we apply the DTA metric to the PLCO colonoscopy cohort, a cohort nested within PLCO that received colonoscopy in follow-up to a positive screening flexible sigmoidoscopy (FSG). We seek to measure the contribution of surveillance colonoscopy, or colonoscopy exams subsequent to the initial colonoscopy, on CRC prevention. Specifically, we estimate the reduction in CRC incidence achieved with the observed level of adenoma removal in this cohort.
A previous analysis in this cohort showed that those with non-advanced adenomas at baseline colonoscopy had similar long-term CRC risk to those with no adenomas (14). A caveat to that analysis was that the non-advanced adenoma group had more surveillance colonoscopy use and adenoma removal after the baseline exam than the no adenoma group. The excess adenoma removal in the non-advanced adenoma group may have reduced their CRC risk and masked a true difference in CRC risk between the two groups. By applying the DTA metric, we compare CRC risk between the two groups while accounting for differences in adenoma removal.
Methods:
PLCO Design
The PLCO trial was a multiphasic trial evaluating screening for four cancers (1). Flexible sigmoidoscopy (FSG) was the modality employed for CRC screening. The trial enrolled 154,900 men and women aged 55- 74 from 1993-2001 at ten U.S. centers and randomized them to screening or usual care. Intervention arm subjects received FSG exams at year 0 and again at year 3 (for those randomized before April 1995) or year 5. Participants with positive FSGs (findings of a polyp or mass) were referred to their primary care physicians for decisions regarding diagnostic follow-up; about 75% underwent colonoscopy (1). Participating centers obtained written informed consent and the institutional review board approved the protocol at each center.
Subjects were followed for cancer incidence by annual study questionnaires and medical record abstracting, as well as by linkage with state cancer registries. Most subjects were followed for incidence through 12/31/2014, although 12% refused extended follow-up and were only followed through 12/31/2009 (15). Participants were followed for mortality generally through 2015.
PLCO Colonoscopy Cohort
Figure 1 shows the flowchart for inclusion into the PLCO colonoscopy cohort. Eligible subjects had a first positive FSG followed within one year by a (index) colonoscopy, with no CRC diagnosed within 60 days of that colonoscopy (13). The cohort was divided into 4 groups based on the adenoma findings at index colonoscopy - advanced adenoma (≥10 mm or tubulovillous/villous histology or high-grade dysplasia) (AA), 1-2 non-advanced adenoma (NAA1-2), 3+ non-advanced adenomas (NAA3+) or no adenoma (NA). Of 77,442 intervention arm subjects, 22,082 had ≥1 positive FSG screen and 16,162 had a colonoscopy within a year of their first positive FSG screen. Of the 16,162, those with CRC diagnosis (N=217) or end of follow-up within one year of the positive screen (N=10) were excluded, leaving 15,935 included subjects.
Figure 1. Flowchart of inclusion into PLCO colonoscopy cohort.
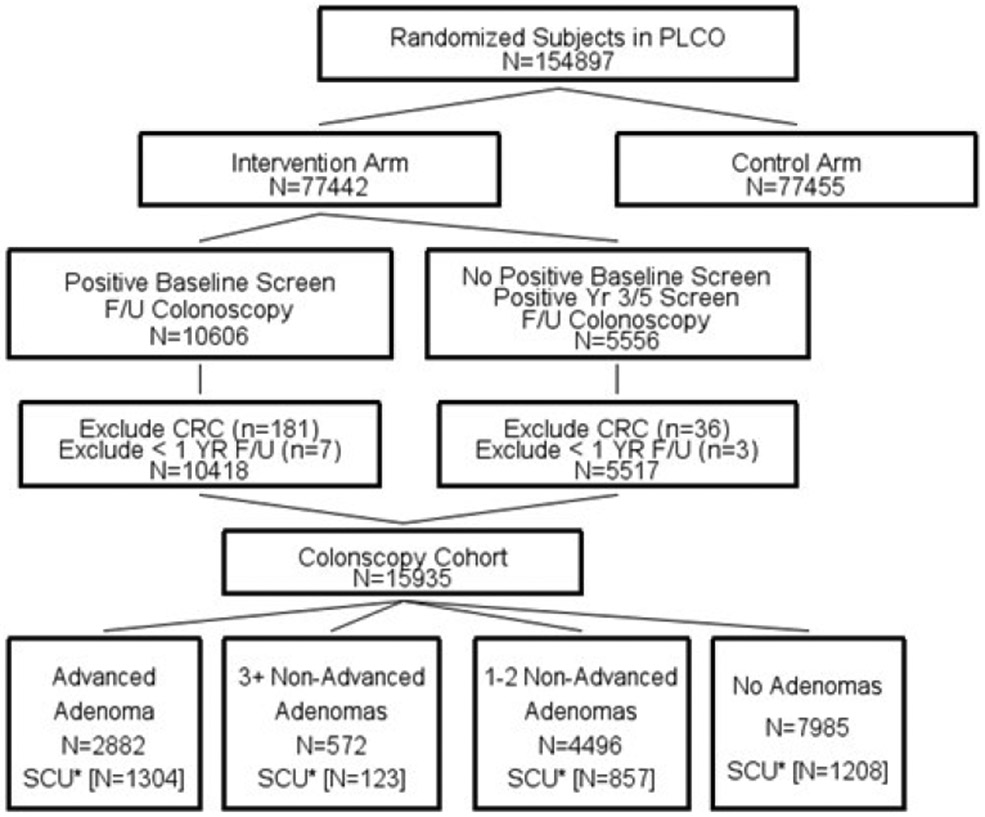
*SCU is the Study of Colonoscopy Utilization. Data on surveillance colonoscopy use and adenoma removal were available only from the SCU subset and were extrapolated to the remainder of the cohort. Numbers in brackets are size of SCU subset (e.g., 1304 for advanced adenoma).
F/U is follow-up.
Within the PLCO colonoscopy cohort, a subset (N=3492) participated in an ancillary study, the Study of Colonoscopy Utilization (SCU) (8, 10). SCU collected detailed information on the timing of and findings from subsequent colonoscopy exams in subjects after an initial colonoscopy following a positive FSG screen. SCU utilized differential sampling frequencies among eligible subjects based on their baseline adenoma status, with those with advanced adenomas oversampled. Median (25th/75th percentile) follow-up in SCU from the index colonoscopy was 8.8 (7.8/9.5) years, with a maximum of about 10 years.
Avoided Adenoma Dwell Time and CRC Cases Prevented
Data on adenomas removed during surveillance colonoscopy exams, which are necessary to compute avoided adenoma dwell time and to utilize the DTA metric, were available only from the SCU subset. Figure 2 illustrates how avoided dwell time was computed for SCU subjects. The average avoided dwell time was measured for each baseline adenoma group and this average applied to the entire colonoscopy cohort to obtain the estimated total avoided dwell time for each group. Data on CRC incidence was based on the entire cohort, not only the SCU subsample. Because maximum SCU follow-up was 10 years, avoided dwell time and CRC incidence were computed for a maximum of 10 years from initial colonoscopy.
Figure 2: Schematic of avoided dwell time.
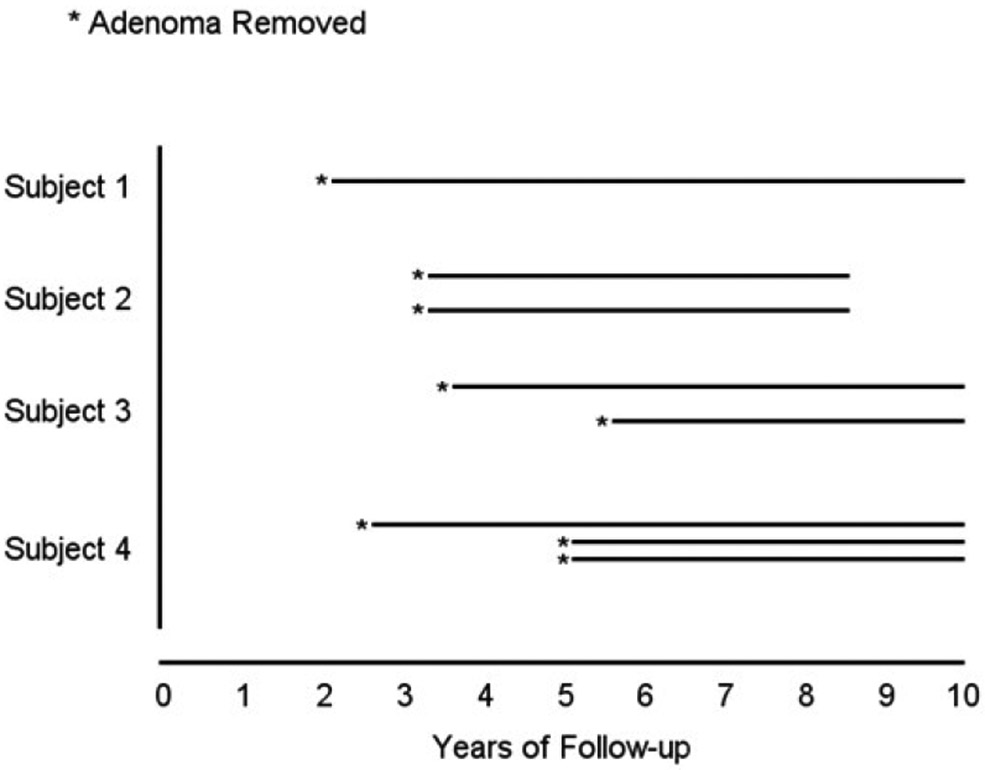
Total time from all removed adenomas comprises the avoided adenoma dwell time for the given subject. For example, Subject 3 had an adenoma removed at year 3.5 and also at year 5.5; therefore, the total avoided dwell years equals 11 (10 − 3.5 + 10 − 5.5 ). Subjects with no adenomas removed during follow-up had a zero value for avoided dwell time.
To estimate the number of CRC cases prevented through adenoma removal within each baseline adenoma group, the estimated total avoided dwell time for the group was divided by the DTA metric value, which reflects the amount of avoided dwell time needed to prevent one CRC case. The DTA value of 612 years was derived from PLCO; however, for a sensitivity analysis, we also evaluated a DTA of 500 years derived from analysis of all 4 randomized FSG trials (12). The DTA metric is computed from randomized trial data by examining the difference in the number of CRC cases between trial arms, which provides the CRC cases prevented, and by measuring the number and timing of adenomas removed in the intervention arm (Appendix 1).
The number of CRC cases prevented compared to those observed provides the relative reduction in CRC incidence due to adenoma removal subsequent to the baseline colonoscopy. The proportional reduction was computed as CP/(CO+CP) where CO and CP are observed and prevented cases, respectively.
Kaplan-Meier analysis was used to compute the cumulative probability of receiving at least one colonoscopy exam as extrapolated from the SCU subset to the entire colonoscopy cohort; these rates were previously reported (13). A bootstrapping approach was used to compute confidence intervals for the incidence reductions (Appendix 2).
Effect of Adenoma Removal Subsequent to the baseline exam on CRC risk in the PLCO Colonoscopy Cohort
A prior analysis of the PLCO colonoscopy cohort showed no statistically significant difference in long-term CRC risk between those with non-advanced adenomas compared to those with no adenomas, but the non-advanced adenoma group had more surveillance colonoscopy exams and adenomas removed than the no adenoma group (13). To assess the potential effect on CRC incidence of differences in adenoma removal rates between the groups, we used DTA to estimate the number of CRC cases prevented in each group. We then computed adjusted rate-ratios (RRs) across groups using the sum of the observed plus prevented cases to determine the underlying CRC risk accounting for the effects of adenoma removal. Confidence intervals were generated using bootstrapping.
Results
In the PLCO colonoscopy cohort, there were 2882, 572, 4496 and 7985 subjects in the advanced adenoma (AA), 3+ non-advanced adenoma (NAA3+), 1-2 non-advanced adenoma (NAA1-2), and no adenoma (NA) groups, respectively (Table 1). Median age was similar across groups. Subjects with adenomas were more likely to be male. Mean follow-up for CRC incidence (through 10 years) was similar across groups, about 9.2 years. Of the total, the number followed by the Study of Colonoscopy Utilization (SCU) were 1304 (45%), 123 (22%), 857 (19%) and 1208 (15%) in the AA, NAA3+, NAA1-2 and NA groups, respectively. Within each adenoma group, the subset in the SCU study were similar demographically to the group as a whole (Table 1).
Table 1.
Demographics of PLCO Colonoscopy Cohort and SCU Subset by Baseline Adenoma Group
| Baseline Adenoma Group | ||||||||
|---|---|---|---|---|---|---|---|---|
| Advanced Adenoma (AA) |
3+ Non-advanced adenomas (NAA3+) |
1-2 Non-advanced adenomas (NAA1-2) |
No Adenoma (NA) |
|||||
| All | SCU Subset |
All | SCU Subset |
All | SCU Subset |
All | SCU Subset |
|
| N | 2882 | 1304 | 572 | 123 | 4496 | 857 | 7985 | 1208 |
| Median (25th/75th) Age at Baseline Colonoscopy | 65 (61/69) | 63 (60/68) | 64 (61/69) | 65 (60/69) | 64 (61/68) | 63 (59/67) | 64 (61/68) | 63 (59/67) |
| N (%) | N (%) | N(%) | N(%) | N(%) | N(%) | N (%) | N (%) | |
| Male | 1937 (67.2) | 858 (65.8) | 432 (75.5) | 95 (77.2) | 2875 (64.0) | 540 (63.0) | 4263 (53.4) | 606 (50.2) |
| Race/ethnicity | ||||||||
| White (non-Hispanic) | 2608 (90.5) | 1226 (94.0) | 518 (90.6) | 114 (92.7) | 4033 (89.7) | 789 (92.0) | 7289 (91.3) | 1131 (93.6) |
| Black (non-Hispanic) | 140 (4.9) | 34 (2.6) | 19 (3.3) | 6 (4.9) | 183 (4.1) | 34 (2.6) | 415 (5.2) | 43 (3.6) |
| Hispanic | 37 (1.3) | 15 (1.2) | 2 (0.4) | 1 (0.8) | 87 (1.9) | 15 (1.2) | 122 (1.5) | 18 (1.5) |
| Asian | 58 (2.0) | 21 (1.6) | 21 (3.7) | 2 (1.6) | 135 (3.0) | 21 (1.6) | 78 (1.0) | 9 (0.8) |
| Other/Unknown | 39 (1.4) | 8 (0.6) | 12 (2.1) | 0 | 58 (1.3) | 8 (0.6) | 81 (1.0) | 7 (0.6) |
| Family History of CRC (1st degree relative) | 355 (12.3) | 174 (13.9) | 70 (12.8) | 17 (14.5) | 487 11.3) | 91 (11.1) | 858 (10.7) | 159 (13.7) |
| Mean follow-up (years) for CRC incidence, through 10 years | 9.24 | 9.94 | 9.25 | 9.98 | 9.25 | 9.98 | 9.28 | 9.95 |
Surveillance Colonoscopy Usage and Avoided Adenoma Dwell Time
The cumulative proportions estimated to have received colonoscopy subsequent to baseline are shown in Table 2A. Cumulative proportions receiving colonoscopy at 5 years were 62.5% (AA), 59.9% (NAA3+,), and 53.0% (NAA1-2). The mean number of colonoscopy exams per subject over the ten-year period was 1.80, 1.63 and 1.46 for AA, NAA3+ and NAA1-2 groups, respectively.
Table 2.
Surveillance Colonoscopy: Utilization and Avoided Adenoma Dwell Time
| Baseline Adenoma Group | ||||
|---|---|---|---|---|
| Advanced Adenoma (AA) (n=2882) |
3+ Non-advanced Adenomas (NAA3+) (n=572) |
1-2 Non- advanced Adenomas (NAA1-2) (n=4496) |
No Adenoma (NA) (n=7985) |
|
| A. Surveillance Colonoscopy Utilization | ||||
| Cumulative % with any colonoscopy exam, 3 years 1 | 33.4 | 25.7 | 19.8 | 11.4 |
| Cumulative % with any colonoscopy exam, 5 years 1 | 62.5 | 59.9 | 53.0 | 36.9 |
| Cumulative % with any colonoscopy exam, 7 years 1 | 76.3 | 77.0 | 71.5 | 59.9 |
| Mean # of Colonoscopy Exams 1 | 1.80 | 1.63 | 1.46 | 1.20 |
| B. Adenomas removed at surveillance exams | ||||
| Average # (Total) Adenomas removed 2 | 0.81 (2328) | 1.15 (625) | 0.58 (2588) | 0.31 (2411) |
| Average # (Total) Advanced adenomas removed 2 | 0.17 (496) | 0.20 (110) | 0.09 (400) | 0.07 (531) |
| Average (Total) Avoided Adenoma Dwell Time (years) 2 | 4.0 (11442) | 5.5 (2954) | 2.4 (10617) | 1.3 (9879) |
| Average (Total) Avoided Advanced Adenoma Dwell Time (years) 2 | 0.9 (2577) | 1.0 (542) | 0.4 (1632) | 0.2 (1861) |
Estimate based on extrapolation from SCU subset. Mean # is through 10 years.
Estimate based on extrapolation from SCU subset; covers follow-up period of 10 years from baseline exam. Averages include zero values for subjects with no adenomas removed.
The average number of adenomas removed per subject ranged from 0.81 (AA) to 0.31 (NA); the average avoided dwell time per subject was 4.0, 5.5, 2.4 and 1.3 years for the AA, NAA3+, NAA1-2 and NA groups, respectively (Table 2B). AA removal constituted 22.5% (2577/11442) of the avoided dwell time in the AA and 15.4% (1632/10617) in the NAA1-2 group (Table 2).
CRC Cases Prevented
Table 3 shows the number of observed CRC cases and the estimated number prevented due to adenoma removal subsequent to the baseline exam. For the AA group, 18.7 cases were estimated to be prevented. Based on 56 observed cases, the proportion of cases prevented by adenoma removal was 25.0% (18.7/[56+18.7]), (95% CI, 16%-34%). In the NAA1-2 group, the estimated number of cases prevented was 17.3 or 34.4% (95% CI, 21%-48%). The NAA3+ group had too few CRC cases, N=4, to reliably estimate the proportion prevented. For all subjects with adenomas (AA, NAA3+, NAA1-2), adenoma removal subsequent to the baseline colonoscopy resulted in an overall 30.5% (95% CI, 22% – 39%) reduction in CRC incidence (Table 3). The percentage reductions for AA (25.0%) and NAA1-2 (34.4%) were not statistically different, p=0.29.
Table 3.
Prevented CRC Cases and Percentage Reduction due to Adenoma Removal Subsequent to the Baseline colonoscopy
| Baseline Adenoma Group | |||||
|---|---|---|---|---|---|
| Advanced Adenoma (AA) |
3+ Non- advanced adenomas (NAA3+) |
1-2 Non- advanced adenomas (NAA1-2) |
Any adenoma (AA, NAA3+, NAA1-2) |
No adenoma (N) |
|
| Person Years of CRC incidence follow-up | 26540 | 5263 | 41772 | 73575 | 73846 |
| # (rate per 10,000 PY) | # (rate per 10,000 PY) | # (rate per 10,000 PY) | # (rate per 10,000 PY) | # (rate per 10,000 PY) | |
| Observed number of CRC cases (rate per 10,000 PY) |
56 (21.1) | 4 (7.9) | 33 (7.9) | 93 (12.6) | 48 (6.5) |
| Number of prevented cases (rate per 10,000 PY) 1 | 18.7 (7.1) | 4.8 (9.5) | 17.3 (4.1) | 40.9 (5.6) | 16.1 (2.2) |
| Percentage Incidence Reduction (95% CI) 2 | 25.0 (16-34) | ** | 34.4 (21-48) | 30.5 (22-39) | 25.1(13-39) |
Cases prevented were derived by dividing total dwell time avoided (Table 2) by the estimate of DTA (dwell time avoided per CRC case prevented) of 612 years.
Percentage incidence reduction equals 100*( Cp/(CP+CO) ) where CO is observed cases and CP is prevented cases.
- Number of cases too small to reliably calculate
For a sensitivity analysis, CRC incidence reductions were computed using the DTA value derived from all four sigmoidoscopy trials of 500 years, rather than 612 years, derived from PLCO. The results were similar. Prevention in the AA and NAA1-2 groups was 29.0% and 39.1% respectively, compared to 25.0% and 34.4% in the primary analysis.
Adjusted Rate Ratios (RRs) for Comparisons Among Adenoma Groups
Table 4 shows original RRs for CRC incidence across adenoma groups, compared to the no adenoma group, as well as adjusted RRs accounting for the effect of cases prevented due to adenoma removal. For the AA group, the original 10-year RR in comparison to the NA group was 3.3 (95% CI, 2.2 – 4.8); the adjusted RR was similar at 3.2. For the NAA3+ and NAA1-2 groups combined, accounting for prevented cases increased the original RR of 1.2 (95% CI, 0.8 - 1.9) to 1.5 (95% CI, 0.99-2.1) compared to the NA group. For the NAA1-2 group, the adjusted RR was 1.4 (95% CI, 0.93-2.0), compared to the original 1.2 (0.8-1.9).
Table 4.
Original and adjusted CRC incidence rate ratios (RR) for colonoscopy cohort
| Baseline Adenoma Group | ||||
|---|---|---|---|---|
| Advanced Adenoma (AA) |
Non-advanced adenoma (NAA1-2 + NAA3+) |
1-2 Non- advanced adenomas (NAA1-2) |
No adenoma (NA) |
|
| RR (95% CI) | RR (95% CI) | RR (95% CI) | ||
| Within 10 Years | ||||
| Original RR 1 | 3.3 (2.2-4.8) | 1.2 (0.8-1.9) | 1.2 (0.8-1.9) | Referent |
| Adjusted RR 2 | 3.2 (2.3-4.5) | 1.5 (0.99-2.1) | 1.4 (0.93-2.0) | Referent |
Reported in Click et al., (reference 13).
Adjusted for estimated CRC cases prevented due to adenoma removal (see text for details).
Discussion
Colonoscopy subsequent to detection and removal of adenomas is emerging as a critically important issue in the CRC screening process. The increase in the prevalence of screening (16), coupled with the increased emphasis on detection of adenomas at screening, measured as the adenoma detection rate (17), will necessarily identify more subjects with adenomas and funnel more subjects into subsequent colonoscopy examinations for surveillance (18). However, the contribution to CRC prevention of adenoma removal subsequent to the initial colonoscopy exam is uncertain. By applying the metric of adenoma Dwell Time Avoided (DTA), we estimated that among subjects with adenomas at baseline in the PLCO colonoscopy cohort and no CRC diagnosis within a year, colonoscopy with adenoma removal subsequent to the baseline exam was responsible for about a 30% reduction in CRC incidence over ten years. Since surveillance colonoscopy was not dictated by the trial, the intensity of surveillance reflects U.S. practice during that time period.
This is one of the first quantitative attempts at assessing the contribution of surveillance colonoscopy and adenoma removal to CRC prevention. The DTA metric brings a physiologic approach to determining the benefit of colonoscopy, by directly linking adenoma removal to CRC incidence reduction. Furthermore, by quantifying avoided adenoma dwell time, the preventive effect of subsequent adenoma removal can be measured independently from the baseline exam.
The NNR and DTA metrics attach impact to every adenoma removed. Based on the DTA estimate from PLCO, the number of avoided adenoma dwell years needed to prevent one CRC case is 612 years. Adenomatous polyps require multiple genetic mutations to advance to cancer, and progression can take years (19, 20). The high prevalence of adenomas relative to cancers indicates that most adenomas do not progress. Accordingly, not every adenoma removed contributes to cancer prevention, but the metric provides an average estimate for progression.
Determining the optimal timing of surveillance will benefit from randomized trials of surveillance intervals, to see whether delayed surveillance, although allowing adenomas to remain in-situ for longer periods of time, will still be adequate enough to prevent cancers from developing (17). This is the rationale for the European EPOS trial, which is randomizing subjects with non-advanced adenomas into 5 and 10 year vs 10 year intervals for colonoscopy surveillance (11). A similarly designed U.S. study, FORTE, is underway (12).
The magnitude of incidence reduction depends on both colonoscopy frequency and the effectiveness of adenoma removal in preventing CRC. Colonoscopy rates in the AA and NAA1-2 groups were high, with 62.5% (AA) and 53.0% (NAA1-2) receiving at least one colonoscopy by year 5 and a mean of 1.80 (AA) and 1.46 (NAA1-2) exams during the 10-year period. Thus, it required a relatively large amount of colonoscopy to achieve the overall 30% CRC incidence reduction. From a public health perspective, the absolute number of CRC cases prevented is of interest. In the AA group, there were 7.1 CRC cases prevented per 10,000 PY, or about 7 cases per 1,000 participants over ten years. This can be weighed against the mean of 1.8 colonoscopy exams per AA subject, which computes to about 260 exams to prevent one CRC case. For the NAA1-2 group, the roughly 4 cases prevented per 1,000 participants and the mean of 1.46 colonoscopy exams results in about 360 exams to prevent one CRC case.
No significant difference in long-term cancer incidence was observed between individuals with non-advanced adenomas compared to those with no adenoma (13). However, those with non-advanced adenoma had additional colonoscopy use (12%) and adenoma removal (8%) compared to those with no adenoma. By accounting for the additional subsequent adenoma removal, adjusted RRs for subjects with non-advanced adenomas and 1-2 non-advanced adenomas, compared to those with no adenoma, increased to 1.5 and 1.4, respectively, from the original RRs of 1.2. Low rates of progression to CRC or AAs among those with non-advanced adenomas have been observed (21).
The clinical implications of our findings are uncertain. Studies are accumulating to suggest that individuals with low-risk adenomas do not have significantly increased risks of advanced neoplasia (CRC or AA) compared to those with no adenomas (14, 22, 23). However, these studies are retrospective, and it is not known whether the underlying cancer risk was similar among those who did or did not receive surveillance. Moreover, there was high use of surveillance in subjects with both low-risk and with no adenomas. Prospective studies will permit accurate assessment of CRC risk in subjects with low risk adenomas, and more importantly, an unbiased assessment of the impact of surveillance on reducing subsequent CRC incidence and mortality.
Limitations of our study should be acknowledged. We only measured surveillance colonoscopy use in the SCU subset (3492 of 15,935 subjects; 21.9%), and extrapolated from that sample to the entire cohort. However, the SCU sample was large enough to produce stable estimates and was representative demographically of the whole cohort. Sessile serrated lesions were not a recognized entity at the time of PLCO; thus, the impact of the current practice of identifying and removing them cannot be assessed. The DTA metric was used to translate avoided adenoma dwell time into prevented cases. The exact value of DTA is uncertain, but using two DTA estimates, one derived from PLCO and one from the four randomized FSG trials, gave similar results. The DTA estimate from PLCO was based on avoided adenoma dwell time, of which 27% was due to AA removal (13). In this analysis, the avoided dwell time due to AA removal was only 22.5%. Presumably, avoided dwell time has greater preventive impact when it derives from removal of AAs as opposed to non-advanced adenomas because AAs are more likely to transform into cancer. Since adenoma removal in this cohort was more weighted towards non-advanced adenomas, this analysis may overestimate the effect of adenoma removal and DTA may be greater than 612 years. In contrast, the DTA value of 612 years could be overestimated due to colonoscopy use in the control arm, which makes the difference in CRC cases across arms smaller than it would have been otherwise. The net effect of these two opposing factors on the estimate of CRC incidence reduction attributed to surveillance is uncertain.
Colonoscopy exams after the initial diagnosis of adenomas were sometimes performed for reasons other than surveillance, such as diagnostic exams for symptoms. Our estimates are therefore not representative of pure, routine surveillance colonoscopy. However, in any cohort, symptoms and circumstances will trigger colonoscopy exams, hence our cohort is representative of a real-world experience. Finally, the population was overwhelmingly (91%) non-Hispanic white, so it is unclear how these results extrapolate to other races/ethnicities.
It is of interest to apply the methods of this analysis to other randomized trials of endoscopic screening, including the three other sigmoidoscopy trials for which the DTA metric was previously estimated. However, the expected proportion of incident CRC prevented by surveillance will vary according to the intensity of that surveillance, and this intensity may differ across trials. In addition, as stated above, the DTA estimate could have been biased upward in PLCO due to control arm contamination; contamination was lower in the other three sigmoidoscopy trials.
In conclusion, with 10 years of follow-up in the PLCO colonoscopy cohort, about 30% of CRC cases are estimated to have been prevented by adenoma removal subsequent to the baseline colonoscopy exam. Accounting for the increased adenoma removal in those with 1-2 non-advanced adenomas increased the RR estimate for incident CRC from 1.2 to 1.4 compared to those with no adenomas. Thus, PLCO colonoscopy cohort subjects with non-advanced adenomas may have an increased underlying CRC risk relative to those with no adenomas. More research is needed on long-term CRC risk in subjects with non-advanced adenoma, on the benefit of subsequent colonoscopy exams, and on how to optimize the timing of surveillance colonoscopy subsequent to the identification of adenomatous polyps.
Need to Know.
Background:
The contribution of surveillance colonoscopy, independent of the initial colonoscopy examination, to prevention of colorectal cancer (CRC) is uncertain.
Findings:
Subjects in the prostate, lung, colorectal and ovarian cancer screening trial who underwent colonoscopies following positive findings from sigmoidoscopies were followed for 10 years. The authors estimate that surveillance colonoscopy prevented about 30% of CRC cases.
Implications for patient care:
Surveillance colonoscopy contributes to prevention of CRC, but more research is needed to optimize frequency and timing of examinations.
Acknowledgments:
Cancer incidence data have been provided by the following state cancer registries: Alabama, Arizona, California, Colorado, District of Columbia, Hawaii, Idaho, Maryland, Michigan, Minnesota, Missouri, Nevada, Ohio, Pennsylvania, Texas, Utah, Virginia and Wisconsin. All are supported in part by funds from the Centers for Disease Control and Prevention, National Program for Central Registries, local states, or by the National Cancer Institute, Surveillance, Epidemiology, and End Results Program. The results reported here and the conclusions derived are the sole responsibility of the authors.
Appendix 1. Deriving the estimate of DTA
The metric of DTA, or adenoma dwell time avoided required to prevent one case of CRC, is a measure of the effectiveness of adenoma removal in preventing incident CRC. The value of DTA was estimated from randomized trials of sigmoidoscopy screening. To estimate DTA, the difference in incidence across trial arms (control arm minus screening arm) is calculated to compute the number of CRC cases prevented. For simplicity, we assume here equal sized trial arms; then this is just the difference in the number of CRC cases across arms (denoted NDiff). Since the trial arms, by randomization, should have equal CRC risk initially, the difference in incident cases is presumed due to the endoscopy intervention and subsequent adenoma removal. If the adenomas hadn’t been removed, the additional time they would have existed in the colorectum would have resulted in enough extra CRC cases to make the two arms equal in incidence. Therefore, the total avoided adenoma dwell time due to adenoma removal, say T, resulted in NDiff CRC cases prevented, so the ratio T/NDiff equals the estimate of DTA, or dwell time avoided to prevent one CRC case
Appendix 2. Bootstrapping confidence intervals for incidence reduction estimates
Subjects were sampled with replacement to obtain replicates for the observed number of CRC cases and total avoided adenoma dwell time. The expected number of CRC cases prevented for each replicate was computed using the avoided adenoma dwell time for the replicate and the DTA value; the actual number of cases prevented for the replicate was then generated as a Poisson random variable with the above expected value as the mean. The bootstrapping accounted for the fact that avoided dwell time was only available on the SCU subset by sampling that subset with replacement.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: The authors report no conflicts.
References
- 1.Schoen RE, Pinsky PF, Weissfeld JL, et al. Colorectal-cancer incidence and mortality with screening flexible sigmoidoscopy. New England Journal of Medicine. 2012;366(25):2345–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Atkin W, Wooldrage K, Parkin DM, et al. Long term effects of once-only flexible sigmoidoscopy screening after 17 years of follow-up: the UK Flexible Sigmoidoscopy Screening randomised controlled trial. Lancet. 2017;389(10076):1299–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Segnan N, Armaroli P, Bonelli L, et al. Once-Only Sigmoidoscopy in Colorectal Cancer Screening: Follow-up Findings of the Italian Randomized Controlled Trial--SCORE. Journal of the National Cancer Institute. 2011;103(17):1310–22. [DOI] [PubMed] [Google Scholar]
- 4.Holme O, Loberg M, Kalager M, et al. Effect of flexible sigmoidoscopy screening on colorectal cancer incidence and mortality: a randomized clinical trial.[Erratum appears in JAMA. 2014 Sep 3;312(9):964]. JAMA. 2014;312(6):606–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doroudi M, Schoen RE, Pinsky PF. Early detection versus primary prevention in the PLCO flexible sigmoidoscopy screening trial: Which has the greatest impact on mortality? Cancer. 2017;123(24):4815–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lieberman DA, Rex DK, Winawer SJ, et al. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2012;143(3):844–57. [DOI] [PubMed] [Google Scholar]
- 7.Atkin W, Wooldrage K, Brenner A, et al. Adenoma surveillance and colorectal cancer incidence: a retrospective, multicentre, cohort study. Lancet Oncol. 2017;18(6):823–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schoen RE, Pinsky PF, Weissfeld JL, et al. Utilization of surveillance colonoscopy in community practice. Gastroenterology. 2010;138(1):73–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lieberman DA, Williams JL, Holub JL, et al. Colonoscopy utilization and outcomes 2000 to 2011. Gastrointest Endosc. 2014;80(1):133–43. [DOI] [PubMed] [Google Scholar]
- 10.Pinsky PF, Schoen RE, Weissfeld JL, et al. The yield of surveillance colonoscopy by adenoma history and time to examination. Clinical Gastroenterology & Hepatology. 2009;7(1):86–92. [DOI] [PubMed] [Google Scholar]
- 11.Jover R, Bretthauer M, Dekker E, et al. Rationale and design of the European Polyp Surveillance (EPoS) trials. Endoscopy. 2016;48(6):571–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weinberg DS, Schoen RE. Preneoplastic Colorectal Polyps: "I Found Them and Removed Them-Now What?". Ann Intern Med. 2019. [DOI] [PubMed] [Google Scholar]
- 13.Pinsky PF, Loberg M, Senore C, et al. Number of Adenomas Removed and Colorectal Cancers Prevented in Randomized Trials of Flexible Sigmoidoscopy Screening. Gastroenterology. 2018;155(4):1059–68.e2. [DOI] [PubMed] [Google Scholar]
- 14.Click B, Pinsky PF, Hickey T, et al. Association of Colonoscopy Adenoma Findings With Long-term Colorectal Cancer Incidence. Jama-Journal of the American Medical Association. 2018;319(19):2021–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller EA, Pinsky PF, Schoen RE, et al. Effect of flexible sigmoidoscopy screening on colorectal cancer incidence and mortality: long-term follow-up of the randomised US PLCO cancer screening trial. The Lancet Gastroenterology & Hepatology. 2018;28:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Centers for Disease C, Prevention. Vital signs: colorectal cancer screening test use--United States, 2012. MMWR - Morbidity & Mortality Weekly Report. 2013;62(44):881–8. [PMC free article] [PubMed] [Google Scholar]
- 17.Mehrotra A, Morris M, Gourevitch RA, et al. Physician characteristics associated with higher adenoma detection rate. Gastrointestinal Endoscopy. 2018;87(3):778–86.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ladabaum U, Schoen RE. Post-Polypectomy Surveillance That Would Please Goldilocks--Not Too Much, Not Too Little, but Just Right. Gastroenterology. 2016;150(4):791–6. [DOI] [PubMed] [Google Scholar]
- 19.Vogelstein B, Kinzler KW. The Path to Cancer --Three Strikes and You're Out. New England Journal of Medicine. 2015;373(20):1895–8. [DOI] [PubMed] [Google Scholar]
- 20.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74. [DOI] [PubMed] [Google Scholar]
- 21.Dube C, Yakubu M, McCurdy BR, et al. Risk of Advanced Adenoma, Colorectal Cancer, and Colorectal Cancer Mortality in People With Low-Risk Adenomas at Baseline Colonoscopy: A Systematic Review and Meta-Analysis. Am J Gastroenterol. 2017;112(12):1790–801. [DOI] [PubMed] [Google Scholar]
- 22.Lieberman D, Sullivan BA, Hauser ER, et al. Baseline Colonoscopy Findings Associated with 10-Year Outcomes in a Screening Cohort Undergoing Colonoscopy Surveillance. Gastroenterology. 2019. [DOI] [PubMed] [Google Scholar]
- 23.Lee JK, Jensen C, Levin TR, et al. Risk of colorectal cancer and related-mortality following detection and removal of low- and high-risk adenomas. Gastroenterology. 2019;156(6):S150–S. [Google Scholar]


