Abstract
Porphyromonas gingivalis is a Gram-negative anaerobic pathogen that can trigger oral dysbiosis as an early event in the pathogenesis of periodontal disease. The FDA-approved drug zafirlukast (ZAF) was recently shown to display antibacterial activity against P. gingivalis. Here, 15 novel ZAF derivatives were synthesized and evaluated for their antibacterial activity against P. gingivalis and for their cytotoxic effects. Most derivatives displayed superior antibacterial activity against P. gingivalis compared to ZAF and its first generation derivatives along with little to no growth inhibition of other oral bacterial species. The most active compounds displayed bactericidal activity against P. gingivalis and less cytotoxicity than ZAF. The superior and selective antibacterial activity of ZAF derivatives against P. gingivalis along with an increased safety profile compared to ZAF suggest these new compounds, especially 14b and 14e, show promise as antibacterials for future studies aimed to test their potential for preventing/treating P. gingivalis-induced periodontal disease.
Keywords: Oral microbiome species, dental plaque, oral dysbiosis, periodontal disease
Periodontal diseases are bacterial infections characterized by unresolved inflammation that leads to the destruction of the gum and bone that support the teeth.1 In the United States, >47% of adults 30 years and older suffer from periodontal diseases.2 An early event in the pathogenesis of periodontal diseases is oral dysbiosis, a phenomenon that describes changes in the abundance of specific bacterial species that are normally found in oral health. Among the triggers of oral dysbiosis, “key stone” pathogens such as Porphyromonas gingivalis have been identified. In addition to periodontal disease, P. gingivalis has been correlated to diseases such as cardiovascular diseases, diabetes, rheumatoid arthritis, and Alzheimer’s disease.3−6 Therefore, P. gingivalis is a plausible bacterial target for preventing/treating periodontal diseases with antibacterials. The lack of clear guidelines for selecting the antibiotic regimen for treating generalized aggressive forms of periodontal diseases has led to poor efficacy and development of drug-resistant bacteria.7−9 One of the current treatment options for chronic periodontitis includes scaling and root planning with antibiotic adjunctive therapy using doxycycline or a combination of amoxicillin and metronidazole.10−13 Although there are antibiotics currently used to treat chronic periodontitis, it is not feasible to use these antibiotics for a prolonged period of time.14−16 Therefore, there is a growing need for novel and specific antibacterials active against periodontopathogens such as P. gingivalis to prevent/treat chronic periodontitis.
An appealing option to uncover new antibacterials with a fast and affordable strategy is the repositioning of FDA-approved drugs for new applications to treat periodontitis.17,18 Drug repositioning is simply using existing drugs approved for the treatment of one disease to treat a different disease. Screening of a drug repositioning library has recently led to the discovery that the FDA-approved drug zafirlukast (ZAF) displayed specific antibacterial activity against P. gingivalis (Figure 1).8,19,20 This is significant given the need for developing new antimicrobials with specificity for oral pathogens associated with periodontal diseases without compromising the normal “good” oral bacteria associated with periodontal health. Additionally, ZAF has been shown to be a potential treatment option for Mycobacterium tuberculosis and West Nile virus infections.21,22 Inspired by these findings, we have been developing ZAF derivatives as novel drug candidates for targeting P. gingivalis. We previously identified three ZAF derivatives with promising anti-P. gingivalis activity.8 Herein, these first generation ZAF derivatives were used as starting points for further structure optimization on the ZAF acylsulfonamide ring (e.g., addition of fluoro and nitro groups along with replacement of phenyl by a naphthyl moiety) with the goal of increasing inhibition of P. gingivalis growth and reducing cytotoxicity. The short 3–4 linear step synthesis of 15 ZAF derivatives is reported. The antibacterial activity against P. gingivalis and other oral bacterial species as well as the cytotoxic activity against human oral epithelial cells of these compounds is also presented.
Figure 1.
Chemical structure of ZAF and overview of 15 second generation derivatives synthesized herein.
Design and Synthesis of Second Generation ZAF Derivatives
In our first generation of ZAF derivatives,8 the primary focus was to optimize the positions of the substituents on the ZAF benzoyl ring. It was found that a compound with a 2-methoxy-5-indoyl organization displayed increased P. gingivalis growth inhibition compared to those with the 3-methoxy-4-indoyl from the parent ZAF (Figure 1), and this new substitution pattern was therefore kept constant on the benzoyl ring of the second generation of compounds presented herein. With the limited preliminary structure–activity relationship (SAR) that was performed in our previous study, it appeared that a methyl group on the indole nitrogen might be beneficial for P. gingivalis growth inhibition and that having a hydrogen versus a nitro group at the R1 position on the indole ring might not have any effect on activity. To corroborate these very preliminary observations and to, more importantly, investigate the potential of having different acylsulfonamides, which was not investigated previously, we synthesized compounds 12a, 12c, 12f, 13a–13f, and 14a–14f.
The preparation of compounds 12a, 12c, 12f, 13a–13f, and 14a–14f was performed by a linear 3–4 step synthesis to modify the following parts of the ZAF scaffold: its indole (at the R1 and R2 positions), benzoyl (arrangement of the three different substituents “indole, methoxy, and acylsulfonamide”), and acylsulfonamide (R3 position) rings (Scheme 1A). Briefly, the synthesis proceeded as follows. Commercially available 5-nitroindole underwent methylation to form compound 3 in 66% yield. The condensation reaction between the commercially available (indicated by a $ sign in Scheme 1) indole (1) or 1-methylindole (2), or the synthesized 1-methyl-5-nitroindole (3) with methyl-5-formyl-2-methoxybenzoate (4) first generated compounds 5, 6, and 7 in 10–93% yields. This condensation proceeds via a unique mechanism, which involves resonance stabilized carbocation formation in the presence of trifluoroacetic acid (TFA), followed by C–C bond formation, reduction, protonation of hydroxyl group, and reduction with hydride from triethylsilane (Et3SiH) to yield the methylene compounds 5, 6, and 7 (Scheme 1A and Figure S1). Hydrolysis of the ester group of 5, 6, and 7 to a carboxylic acid then afforded compounds 8, 9, and 10 in 85–96% yields. Finally, amide coupling of the carboxylic acids with various commercially available acylsulfonamides (11a–11f) yielded 15 novel ZAF derivatives 12a, 12c, 12f, 13a–13f, and 14a–14f in 16%-quantitative yields. Two of the three first generation lead ZAF derivatives, 13g and 14h (previously published as 22b and 23a),8 were also resynthesized in 38% and 24% yields (Scheme 1B) for comparison study with the new second generation ZAF derivatives 12a, 12c, 12f, 13a–13f, and 14a–14f. It is important to note that as 13g and 14h had been found to display superior growth inhibition of P. gingivalis compared to the parent ZAF from which they were derived, in this study, the second generation of compounds were mostly compared to 13g and 14h.
Scheme 1. Synthetic Scheme for the Preparation of (A) Second Generation ZAF Derivatives 12a, 12c, 12f, 13a–13f, and 14a–14f and (B) First Generation ZAF Derivatives 13g and 14h(8).
Reaction conditions: (a) Et3SiH, TFA, CH2Cl2, 0 °C to rt, 10–93%; (b) MeOH:THF:H2O/5:1:1, KOH, 65 °C, 85–96%; (c) acylsulfonamide, EDC·HCl, DMAP, CH2Cl2, rt, 16%–quantitative yields.
With these second generation ZAF derivatives aimed at increasing P. gingivalis growth inhibition and decreasing cytotoxicity, the goal was to answer the following four a priori questions in terms of SAR. The series/compound numbers used to answer these questions are provided in parentheses following the questions. The four questions are (i) is a methyl R2 group on the indole nitrogen truly enhancing bacterial growth inhibition? (compounds 12a vs 13a, 12c vs 13c, and 12f vs 13f); (ii) what is the effect of adding an R3 electron-withdrawing group or bulkiness to the acylsulfonamide ring when R1 = H and R2 = Me? (compounds 13a vs 13b vs 13c vs 13d vs 13e vs 13f); (iii) does an electron-withdrawing group (e.g., R1 = NO2) on the indole ring affect the trend established for the acylsulfonamide substituent patterns (trend for compounds 14a vs 14b vs 14c vs 14d vs 14e vs 14f compared to that of series 13)?; and (iv) is an electron-withdrawing group (e.g., R1 = NO2) on the indole ring beneficial for bacterial growth inhibition? (pairwise comparison of series 13 vs 14).
Antibacterial Activity
To answer the four a priori questions posed and to determine the potential of the second generation ZAF derivatives 12a, 12c, 12f, 13a–13f, and 14a–14f as antibacterials compared to the first generation and the parent ZAF, they were first tested against P. gingivalis 381. A colorimetric water-soluble tetrazolium-1 (WST-1) assay was used to determine the antimicrobial effect of the 15 synthesized ZAF derivatives at 1, 10, and 100 μM. Their activity was compared to commercially available positive controls tetracycline (T, at 2.81 μM, which displayed 51% P. gingivalis growth inhibition) and ZAF (Z, at 25 and 50 μM, which displayed 70% and 68% P. gingivalis growth inhibition, respectively) (Figure 2, different blue bars), as well as to the first generation ZAF derivatives 13g and 14h (at 1, 10, and 100 μM). It is to be noted that, as expected, the DMSO control did not display any bacterial growth inhibition. Additionally, it is important to note that the second generation ZAF derivatives were used as a preliminary screening of antibacterial activity in order to identify lead compounds for further structure optimization along with future studies outside the scope of this preliminary investigation. The concentrations of the ZAF control, 25 and 50 μM, were based on a previous publication, which showed that these concentrations were the minimum concentration that inhibited the growth of P. gingivalis.19 In order to test the ability of the ZAF derivatives to inhibit P. gingivalis growth, a wide range of concentrations, 1–100 μM, was used to determine their level of potency.
Figure 2.
Percent growth inhibition of P. gingivalis 381 by ZAF derivatives 12a, 12c, 12f, 13a–13f, and 14a–14f, as determined by a colorimetric WST-1 assay after 24 h. Negative control = DMSO. Positive controls = 2.81 μM of tetracycline (T) or 25 and 50 μM of ZAF (Z), and first generation ZAF derivatives 13g and 14h. For each ZAF derivative, the data represent the average from six independent replicates per condition of the growth inhibitory effect versus bacterial cultures grown in medium only. For the controls, the data represent the average from 36 independent replicates. *The first generation ZAF derivatives 13g and 14h were previously published as 22b and 23a.8
At a quick glance, when compared to the first generation compounds 13g and 14h, it was found that all second generation compounds in series 14 were generally better at inhibiting the growth of P. gingivalis at 1 μM. Overall, at 100 μM, 10 out of the 15 compounds (13b–13d, 13f, and 14a–14f) displayed 58–75% growth inhibition comparable or superior to the parent drug ZAF with 68% growth inhibition at 50 μM. In addition to these compounds, one more compound (13a) also displayed superior activity with 55% inhibition of P. gingivalis growth when compared to the effect of tetracycline with 51% growth inhibition. At 10 μM, six out of the 10 compounds (13c and 14a–14e) still exhibited 59–67% growth inhibition similar to that of the parent ZAF with 68–70% growth inhibition. Additionally, three more compounds (13b, 13d, and 14f) displayed 57%, 50%, and 57% growth inhibition, which were comparable to or better than the antibiotic tetracycline with 51% growth inhibition. At 1 μM, four of these compounds (14a, 14b, 14d, and 14e) displayed 53–62% growth inhibition, which was comparable or superior to the parent compound ZAF (68% at 50 μM), its derivatives 13g and 14h (16% and 0%, respectively, at 1 μM), and tetracycline (51% at 2.81 μM).
To establish the importance of an N-methyl R2 group on bacterial growth inhibition (question (i), three pairs of ZAF derivatives (12a and 13a, 12c and 13c, as well as 12f and 13f with 2-F-Ph, 4-F-Ph, and 2-naphthylsulfonamides, respectively) were generated (Scheme 1) and tested for growth inhibition of P. gingivalis (Figure 2). As previously observed, it was found that the compounds lacking the methyl group on the indole nitrogen (series 12) were much less efficient at inhibiting bacterial growth at all three concentrations tested when compared to those with a methyl at the R2 position (series 13). Having confirmed the importance of the N-methyl R2 group, series 12 was not further expanded/pursued.
To investigate the effect of adding an R3 electron-withdrawing group or bulkiness to the acylsulfonamide ring when R1 = H and R2 = Me (question (ii), three additional compounds were prepared in series 13: 13b with R3 = 3-F-Ph, 13d with R3 = 3-NO2-Ph, and 13e with R3 = 4-NO2-Ph. The series 13 derivatives (13a–13f) were rationally designed to investigate the effect of replacing the R3 = 2-Me on the acylsulfonamide of first generation 13g with a weak electron-withdrawing group, the bioisostere fluorine, at the ortho (13a), meta (13b), and para (13c) positions. It was postulated that due to the weak electron-withdrawing effect fluorine has on the phenyl ring, a preferred conformational change in 13a–13c could lead to increased antibacterial activity compared to the counterpart first generation 13g, which was indeed the case with compound 13c (with R3 = 4-F-Ph) at all concentrations tested. Compound 13b (with R3 = 3-F-Ph) was also found to display 14%, 57%, and 73% growth inhibition at 1, 10, and 100 μM, respectively, which were similar or superior to that of 13g (16%, 56%, and 74%) at all concentrations tested. As addition of a weak electron-withdrawing group at the meta and para positions of the acylsulfonamide proved to be beneficial for P. gingivalis growth inhibition, the effect of a strong electron-withdrawing group, nitro, at these positions was also investigated. It was found that the R3 = 3-NO2-Ph of 13d yielded 18–75% growth inhibition, which was better than the R3 = 4-NO2-Ph of 13e with 0–27% growth inhibition. Finally, the phenyl ring of the acylsulfonamide was replaced by its larger naphthyl bioisostere to yield compound 13f, which displayed intermediate activity compared to the other series 13 compounds. Overall, at all concentrations tested, it was found that compound 13c (R3 = 4-F-Ph) displayed better P. gingivalis growth inhibition than (>) 13d (R3 = 3-NO2-Ph), which was better than (>) 13b (R3 = 3-F-Ph) > 13f (R3 = 2-naphthyl) > 13a (R3 = 2-F-Ph) > 13e (R3 = 4-NO2-Ph).
Having established the trend for the acylsulfonamide substituent patterns in series 13 in terms of bacterial growth inhibition activity (question ii), the effect of an additional electron-withdrawing group (e.g., R1 = NO2) on the indole ring on that trend was investigated by generating the counterpart series 14a–14f (question iii). When comparing the compounds of series 14, it was found that 14e (R3 = 4-NO2-Ph) displayed better P. gingivalis growth inhibition than (>) 14b (R3 = 3-F-Ph), which was similar to (≈) 14a (R3 = 2-F-Ph), which was better than (>) 14d (R3 = 3-NO2-Ph) > 14c (R3 = 4-F-Ph) > 14f (R3 = 2-naphthyl). Interestingly, when compared to the trend observed with series 13, the trend with series 14 was completely opposite with the exception of compounds with R3 = 2-naphthyl (13f and 14f), which were randomly placed in these series in terms of their activity. A summary of the SAR activity discussed above is presented in Figure 3.
Figure 3.

Summary of SAR activity for the second generation of ZAF derivatives for series 12, 13, and 14.
Finally, to establish if an electron-withdrawing group (e.g., R1 = NO2) on the indole ring is beneficial for bacterial growth inhibition (question iv), a pairwise comparison of series 13 vs 14 was done. It was found that at 1 and 10 μM, the presence of the nitro group on the indole ring was generally beneficial for P. gingivalis growth inhibition, with all compounds in series 14 (with the exception of 14c) displaying comparable or superior activity to those in series 13. At 100 μM, this was also mostly true, but compounds 13b, 13d, and 13f displayed a weak improvement in activity over that of their series 14 counterparts. These novel results indicate the importance of an electron-withdrawing group on the indole, while in the first generation, because of the very limited SAR that was done, this conclusion could not be made.
These results indicated that the six most active second generation ZAF derivatives against P. gingivalis were 13b, 13c, 13d, 13f, 14b, and 14e. These compounds were then further tested against other selected oral Gram-positive and Gram-negative bacterial species to determine their antibacterial specificity profile (Figure 4). The oral bacterial species chosen were representative of those more abundant in health (Actinomyces naeslundii, Streptococcus sanguinis, and Veillonella parvula) and in periodontal diseases (Aggregatibacter actinomycetemcomitans). Overall, the compounds displayed little to no antibacterial activity against these other oral bacterial species, with the exception of 20% growth inhibition with S. sanguinis and A. naeslundii. These data suggest that the six most active second generation ZAF derivatives seem to display specificity against P. gingivalis. Future studies using an expanded number of oral bacterial species or whole subgingival plaque samples from healthy and periodontitis patients will be useful to further validate the specificity of these new ZAF compounds, but not necessary at this time.
Figure 4.
Percent growth inhibition of oral bacterial species by ZAF derivatives 13b, 13c, 13d, 13f, 14b, and 14e, as determined by a colorimetric WST-1 assay after 24 h. Negative control = DMSO. Positive controls = 25 and 50 μM of ZAF (Z) or penicillin/streptomycin −1X (P/S) (100 U/mL of penicillin and 100 μg/mL of streptomycin). These data show the average from six independent replicates per condition of the growth inhibitory effect versus bacterial cultures grown in medium only.
Further investigation into the activity of the six most active ZAF derivatives (13b, 13c, 13d, 13f, 14b, and 14e) was performed to determine if they displayed bactericidal activity by colony forming unit (CFU) assays (Figure 5). P. gingivalis 381 was incubated with the antibiotic tetracycline (T, 2.81 μM) and ZAF (Z, 25 and 50 μM) as positive controls and bacteria only with DMSO as a negative control. P. gingivalis was exposed to ZAF derivatives 13b, 13c, 13d, 13f, 14b, and 14e (1, 10, and 100 μM) for 24 h in media followed by bacterial growth in blood agar plates for 5 days under anaerobic conditions and CFUs determination. All six compounds exhibited bactericidal activity at 100 μM with 100% growth inhibition. Compounds 14b and 14e were also bactericidal at 10 μM and 1 μM; however, at 10 μM, compounds 13b, 13c, 13d, and 13f were not. Even at 1 μM, compound 13d revealed moderate antibacterial activity with 30% growth inhibition. At all three concentrations tested, the second generation compounds 14b and 14e proved to display a higher percent growth inhibition by CFU assays than their first generation counterpart 14h. At 100 μM, compounds 13b, 13c, 13d, and 13f displayed higher percent growth inhibition by CFU assays than their first generation counterpart 13g, and most of them showed better antimicrobial activity than 13g at 10 μM.
Figure 5.
Bactericidal effect of ZAF derivatives 13b, 13c, 13d, 13f, 14b, and 14e determined by colony forming unit (CFU) assays. Negative control = DMSO. Positive controls = tetracycline (T, 2.81 μM) or ZAF (Z, 25 and 50 μM). The data shows the calculated percentage of inhibitory effect from six independent replicates per condition by comparison of the treated bacteria groups (CFUs/mL) versus bacteria grown in medium only (CFUs/mL) using the formula 100 × [CFUs/mL of (control) – CFUs/mL (experimental)/CFUs/mL (control)]. For the controls, the data represent the average from 12 independent replicates per condition.
Cytotoxic Effect of ZAF Derivatives in Oral Epithelial Cells
The first line of defense against periodontopathogens is oral epithelial cells. To determine the value of drugs as potential antibacterials, their selectivity toward pathogenic bacterial species with little to no cytotoxicity toward host cells has to be determined. The cytotoxic effect of the six most active second generation ZAF derivatives 13b, 13c, 13d, 13f, 14b, and 14e was therefore evaluated against immortalized oral keratinocyte (OKF6) cells. To examine the effect of various treatments on OKF6 cell morphology and density, the cells were visualized by microscopy (Figure 6). There was a higher density of OKF6 cells in the control and DMSO wells, in contrast to the lower density in the ZAF and staurosporine (STS, a pro-apoptotic chemical) wells. Wells containing compounds 13b and 13d had the highest density of cells, followed closely by compound 13c. A decrease in cell density was seen in the presence of compounds 13f, 14b, and 14e, indicating a slight increase in cytotoxicity against OKF6 cells, nowhere near that of the ZAF and STS positive controls. Untreated OKF6 cells along with cells treated with DMSO displayed epithelial-like morphology, i.e., elongated and flat.23 OKF6 cells treated with compounds 13b, 13c, 13d, and 13f, resembled the large and flattened cells seen in the untreated OKF6 cells, which are also all in close proximity to one another. This is in direct contrast to the OKF6 cells treated with controls STS and ZAF as well as with compounds 14b and 14e that seem to be smaller in size, more round in shape, and at an increased distance from one another.
Figure 6.

Images representative of the whole well used to evaluate the effect of ZAF derivatives 13b, 13c, 13d, 13f, 14b, and 14e on cell morphology of oral epithelial cells (OKF6). Cells treated with medium only or DMSO were used as negative controls, while cells treated with 8 μM of staurosporine (STS) or 25 μM of ZAF were used as positive controls. Cells were treated with 10 μM of ZAF derivatives 13b, 13c, 13d, 13f, 14b, and 14e for 24 h.
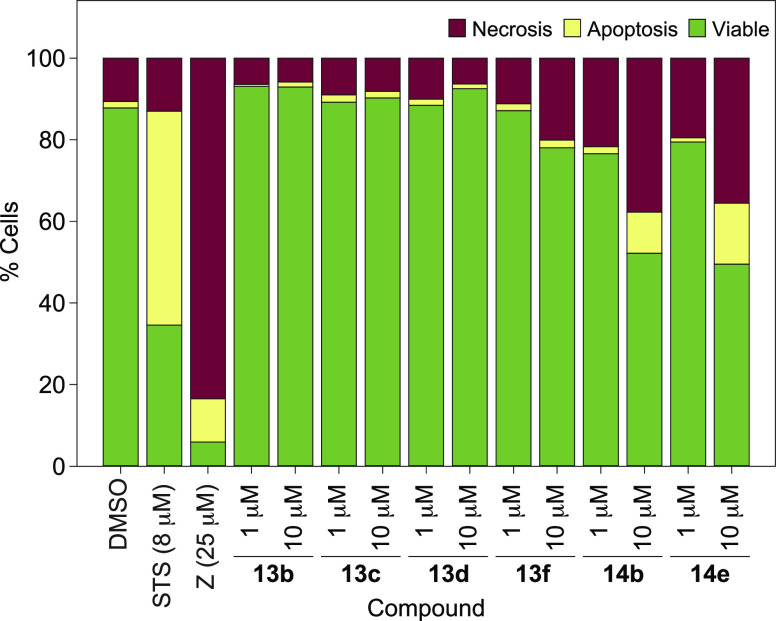
Cytotoxicity determined by flow cytometry revealed 76–93% cell viability with ZAF derivatives 13b, 13c, 13d, 13f, 14b, and 14e at the concentration of 1 μM, and 49–92% cell viability at 10 μM (Figure 7). Of note, all ZAF derivatives displayed less cytotoxicity than the parent drug ZAF. Overall, at 1 μM, it was found that compound 13b (R3 = 3-F-Ph) displayed less cytotoxicity than (<) 13c (R3 = 4-F-Ph), which was less toxic than (<) 13d (R3 = 3-NO2-Ph) < 13f (R3 = 2-naphthyl) < 14e (R3 = 4-NO2-Ph) < 14b (R3 = 3-F-Ph). At 10 μM, a similar trend was observed. As desired, these second generation of ZAF derivatives achieved the overall goal of increasing P. gingivalis growth inhibition and decreasing cytotoxicity compared to the first generation compounds and ZAF parent drug. Briefly, at all concentrations tested, compounds in series 13 all displayed less cytotoxicity when compared to their first generation counterpart 13g. Compounds in series 14 also displayed similar or decreased cytotoxicity when compared to their first generation counterpart 14h (Note: the viability for 13g and 14h used for comparison is not shown in this paper.)
Figure 7.
Cell viability assay used to evaluate the effect of ZAF derivatives 13b, 13c, 13d, 13f, 14b, and 14e on apoptosis and necrosis of oral epithelial cells (OKF6) after 24 h. Negative control = DMSO. Positive controls = 8 μM of staurosporine (STS) or 25 μM of ZAF (Z). Two independent experiments analyzing at least 10,000 events per condition in duplicate were used to generate the FACS data.
In summary, 15 novel ZAF derivatives (12a, 12c, 12f, 13a-13f, and 14a–14f) were synthesized. Six of these (13b, 13c, 13d, 13f, 14b, and 14e) displayed increased antibacterial activity against P. gingivalis and decreased cytotoxicity against OKF6 cells when compared to the parent ZAF and its first generation derivatives 13g and 14h. It was found that the most promising compounds all contained a methyl group on the nitrogen of the indole ring. It was also discovered that, at low concentrations (1 and 10 μM), the presence of an electron-withdrawing group (e.g., R1 = NO2) on the indole ring, as in series 14, was beneficial for bacterial growth inhibition and seemed to determine which acylsulfonamides imparted the best antibacterial activity (with complete opposite trends observed with series 13 (R3 = 4-F-Ph > 3-NO2-Ph > 3-F-Ph > 2-F-Ph > 4-NO2-Ph) and 14 (R3 = 4-NO2-Ph > 2-F-Ph ≈ 3-F-Ph > 3-NO2-Ph > 4-F-Ph) when comparing which sulfonamides result in better growth inhibition). These promising results pave the way for future investigations, outside the scope of this study, seeking to identify the mechanism of action as well as further optimization of these compounds for in vivo studies using animal models of P. gingivalis-induced periodontal disease. These lengthy studies are currently underway in our laboratory and will be reported in due course.
Acknowledgments
This work was supported by startup funds from the University of Kentucky (UK) (to S.G.-T. and O.A.G.) and a grant from the UK Igniting Research Collaborations Pilot Program (to S.G.-T. and O.A.G.). We thank the UK PharmNMR Center for NMR support. We thank Nishad Thamban Chandrika for his help with HPLC and MS. We thank J. G. Rheinwald (Harvard Medical School) for sharing the oral keratinocyte cell line OKF6/hTERT.
Glossary
Abbreviations
- CFU
colony forming unit
- DMSO
dimethyl sulfoxide
- FACS
flow cytometry analysis
- FITC
fluorescein isothiocyanate
- OKF6
immortalized oral keratinocyte cells
- P/S
penicillin/streptomycin
- SAR
structure–activity relationship
- STS
staurosporine
- T
tetracycline
- Et3SiH
triethylsilane
- TFA
trifluoroacetic acid
- WST-1
water-soluble tetrazolium-1
- ZAF or Z
zafirlukast
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsmedchemlett.9b00614.
(i) Materials and instrumentation for chemistry, (ii) all experimental protocols for the synthesis and characterization of compounds, (iii) experimental protocols for all biological experiments, and (iv) all 1H and 13C NMR spectra along with HPLC traces for the final compounds tested (PDF)
Author Contributions
The manuscript was written by K.C.H. and S.G.-T., and comments on the manuscript were provided by O.A.G. The figures were generated by K.C.H. and S.G.-T. All experiments were performed by K.C.H. All authors participated in the design of experiments and data interpretation and have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Hegde R.; Awan K. H. Effects of periodontal disease on systemic health. DM, Dis.-Mon. 2019, 65, 185–192. 10.1016/j.disamonth.2018.09.011. [DOI] [PubMed] [Google Scholar]
- Eke P. I.; Dye B. A.; Wei L.; Thornton-Evans G. O.; Genco R. J. CDC Periodontal Disease Surveillance workgroup: James Beck, Gordon Douglass, Roy Page. Prevalence of periodontitis in adults in the United States: 2009 and 2010. J. Dent. Res. 2012, 91, 914–920. 10.1177/0022034512457373. [DOI] [PubMed] [Google Scholar]
- Kim J.; Amar S. Periodontal disease and systemic conditions: A bidirectional relationship. Odontology 2006, 94, 10–21. 10.1007/s10266-006-0060-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Solari J.; Barrionuevo P.; Mastronardi C. A. Periodontal disease and its systemic associated diseases. Mediators Inflammation 2015, 2015, 153074. 10.1155/2015/153074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominy S. S.; Lynch C.; Ermini F.; Benedyk M.; Marczyk A.; Konradi A.; Nguyen M.; Haditsch U.; Raha D.; Griffin C.; Holsinger L. J.; Arastu-Kapur S.; Kaba S.; Lee A.; Ryder M. I.; Potempa B.; Mydel P.; Hellvard A.; Adamowicz K.; Hasturk H.; Walker G. D.; Reynolds E. C.; Faull R. L. M.; Curtis M. A.; Dragunow M.; Potempa J. Porphyromonas gingivalis in Alzheimer’s disease brains: Evidence for disease causation and treatment with small-molecule inhibitors. Sci. Adv. 2019, 5, eaau3333 10.1126/sciadv.aau3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikuls T. R.; Payne J. B.; Yu F.; Thiele G. M.; Reynolds R. J.; Cannon G. W.; Markt J.; McGowan D.; Kerr G. S.; Redman R. S.; Reimold A.; Griffiths G.; Beatty M.; Gonzalez S. M.; Bergman D. A.; Hamilton B. C. 3rd; Erickson A. R.; Sokolove J.; Robinson W. H.; Walker C.; Chandad F.; O’Dell J. R. Periodontitis and Porphyromonas gingivalis in patients with rheumatoid arthritis. Arthritis Rheumatol. 2014, 66, 1090–1100. 10.1002/art.38348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sum J.; O’Rourke V. J. Factors affecting periodontal disease referral and the adherence to guidelines among general dentists. Aust. Dent. J. 2018, 63, 394–401. 10.1111/adj.12641. [DOI] [PubMed] [Google Scholar]
- Thamban Chandrika N.; Fosso M. Y.; Alimova Y.; May A.; Gonzalez O. A.; Garneau-Tsodikova S. Novel zafirlukast derivatives exhibit selective antibacterial activity against Porphyromonas gingivalis. MedChemComm 2019, 10, 926–933. 10.1039/C9MD00074G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi P. A. Jr; Rudy R. J.; Jeong Y. N.; Coleman D. K.. Non-surgical control of periodontal diseases: A comprehensive handbook, 1st ed.; Springer, 2016; pp 163–173. [Google Scholar]
- Moreno Villagrana A. P.; Gomez Clavel J. F. Antimicrobial or subantimicrobial antibiotic therapy as an adjunct to the nonsurgical periodontal treatment: A meta-analysis. ISRN Dent. 2012, 2012, 581207. 10.5402/2012/581207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mestnik M. J.; Feres M.; Figueiredo L. C.; Soares G.; Teles R. P.; Fermiano D.; Duarte P. M.; Faveri M. The effects of adjunctive metronidazole plus amoxicillin in the treatment of generalized aggressive periodontitis: A 1-year double-blinded, placebo-controlled, randomized clinical trial. J. Clin. Periodontol. 2012, 39, 955–961. 10.1111/j.1600-051X.2012.01932.x. [DOI] [PubMed] [Google Scholar]
- Mombelli A.; Almaghlouth A.; Cionca N.; Courvoisier D. S.; Giannopoulou C. Differential benefits of amoxicillin-metronidazole in different phases of periodontal therapy in a randomized controlled crossover clinical trial. J. Periodontol. 2015, 86, 367–375. 10.1902/jop.2014.140478. [DOI] [PubMed] [Google Scholar]
- Mombelli A.; Cionca N.; Almaghlouth A. Does adjunctive antimicrobial therapy reduce the perceived need for periodontal surgery?. Periodontol. 2000 2011, 55, 205–216. 10.1111/j.1600-0757.2010.00356.x. [DOI] [PubMed] [Google Scholar]
- Shaikh S.; Fatima J.; Shakil S.; Rizvi S. M.; Kamal M. A. Antibiotic resistance and extended spectrum beta-lactamases: Types, epidemiology and treatment. Saudi J. Biol. Sci. 2015, 22, 90–101. 10.1016/j.sjbs.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diniz C. G.; Farias L. M.; Carvalho M. A.; Rocha E. R.; Smith C. J. Differential gene expression in a Bacteroides fragilis metronidazole-resistant mutant. J. Antimicrob. Chemother. 2004, 54, 100–108. 10.1093/jac/dkh256. [DOI] [PubMed] [Google Scholar]
- Katlam S.; Deshmukh Y. A.; Jadhav P. R. Comparative study of oxytetracycline and doxycycline on calcium chelation: In-vitro assay. Int. J. Basic Clin. Pharmacol. 2017, 6, 1160–1164. 10.18203/2319-2003.ijbcp20171670. [DOI] [Google Scholar]
- Chong C. R.; Sullivan D. J. Jr. New uses for old drugs. Nature 2007, 448, 645–646. 10.1038/448645a. [DOI] [PubMed] [Google Scholar]
- Ashburn T. T.; Thor K. B. Drug repositioning: Identifying and developing new uses for existing drugs. Nat. Rev. Drug Discovery 2004, 3, 673–683. 10.1038/nrd1468. [DOI] [PubMed] [Google Scholar]
- Gerits E.; Van der Massen I.; Vandamme K.; De Cremer K.; De Brucker K.; Thevissen K.; Cammue B. P. A.; Beullens S.; Fauvart M.; Verstraeten N.; Michiels J.; Roberts M. In vitro activity of the antiasthmatic drug zafirlukast against the oral pathogens Porphyromonas gingivalis and Streptococcus mutans. FEMS Microbiol. Lett. 2017, 364. 10.1093/femsle/fnx005. [DOI] [PubMed] [Google Scholar]
- Kelloway J. S. Zafirlukast: The first leukotriene-receptor antagonist approved for the treatment of asthma. Ann. Pharmacother. 1997, 31, 1012–1021. 10.1177/106002809703100912. [DOI] [PubMed] [Google Scholar]
- Pinault L.; Han J. S.; Kang C. M.; Franco J.; Ronning D. R. Zafirlukast inhibits complexation of Lsr2 with DNA and growth of Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2013, 57, 2134–2140. 10.1128/AAC.02407-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez A. A.; Espinosa B. A.; Adamek R. N.; Thomas B. A.; Chau J.; Gonzalez E.; Keppetipola N.; Salzameda N. T. Breathing new life into West Nile virus therapeutics; discovery and study of zafirlukast as an NS2B-NS3 protease inhibitor. Eur. J. Med. Chem. 2018, 157, 1202–1213. 10.1016/j.ejmech.2018.08.077. [DOI] [PubMed] [Google Scholar]
- Dickson M. A.; Hahn W. C.; Ino Y.; Ronfard V.; Wu J. Y.; Weinberg R. A.; Louis D. N.; Li F. P.; Rheinwald J. G. Human keratinocytes that express hTERT and also bypass a p16INK4a-enforced mechanism that limits life span become immortal yet retain normal growth and differentiation characteristics. Mol. Cell. Biol. 2000, 20, 1436–1447. 10.1128/MCB.20.4.1436-1447.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.