Abstract

The sirtuin enzymes are potential drug targets for intervention in a series of diseases. Efforts to inhibit enzymes of this class with thioamide- and thiourea-containing, substrate-mimicking entities have produced a number of high-affinity binders. However, less attention has been dedicated to the investigation of the stability of these inhibitors under various conditions. Here, we provide evidence of an unprecedented degree of cleavage of short-chain ε-N-thioacyllysine modifications meant to target these sirtuins and further provide insights into the serum stability of compounds containing both thioamides and thioureas. Our study questions the utility short-chain thioamide-based inhibitors of sirtuins for drug development and points to monoalkylated thiourea-based chemotypes as being more stable in human serum.
Keywords: Sirtuins, histone deacetylases, enzyme inhibitors, mechanism-based inhibition, posttranslational modifications
The sirtuin (SIRT) enzymes belong to a family of NAD+-dependent deacylases, which catalyzes the removal of acyl-based posttranslational modifications (PTMs) on lysine residues in proteins. The human enzyme family comprises seven isoforms (SIRT1–7), which differ in subcellular localization and substrate specificity.1 Sirtuins 1 and 2 are both cytosolic and nuclear,2,3 SIRT3–5 are primarily mitochondrial, and SIRT6–7 are nuclear.4 Sirtuins 1–3 and 6 have been shown to primarily target Nε-acetyllysine-containing (Kac) substrates in vivo;5 however, these enzymes also catalyze the removal of medium- and long-chain acyl groups in vitro.6−8 Sirtuins 4 and 5 have a preference for negatively charged PTMs, arising from acylation with dicarboxylic acids.9−11 The activity of SIRT7 is still not as well understood, with reports suggesting various deacylase activities.12−15 Numerous studies have suggested that modulating sirtuin activities has therapeutic potential for treating various cancers, metabolic disorders, and neurodegenerative diseases.5,11,16,17 Additionally, such compounds can be used as tools to further study the enzymes in the family and their individual roles in the cell. A number of developed inhibitors are designed to exploit the NAD+-dependent deacylation mechanism, hence the term “mechanism-based inhibitors”. The most commonly used group of compounds that fall under this category are thioamide- and thiourea-based sirtuin inhibitors. These inhibitors mimic the natural Nε-acyllysine substrates and are processed by the sirtuins in a similar manner, albeit at a significantly slower rate.18,19 Originally, Fatkins et al. showed that a thioacetylated 27-mer peptide derived from the tumor suppressor p53 was deacylated at a ∼400-fold slower rate than its acetylated analog.18 Similarly, Smith and Denu showed that the yeast sirtuin HST2 could catalyze dethioacetylation of a H3-derived 11-mer peptide, at an ∼80-fold lower rate compared to its acetylated counterpart.19 This has been attributed to the formation of so-called stalled intermediates, several of which have been cocrystallized with different sirtuins (Figure 1).16,20−22 Similarly, we have previously cocrystallized a thiourea-based SIRT5 inhibitor, which shows the same type of intermediates.23 In fact, several highly potent and selective mechanism-based inhibitors have been developed over the past decade.1,23−25
Figure 1.

(A) Sirtuin catalyzed deacylation mechanism of thioamide/thiourea based inhibitors. (B) X-ray crystal structures of intermediates I,21II,22 and III(20) of different thioamide-based inhibitors bound to sirtuins.
Based on these insights, the rate of conversion of thioamide and thiourea-based sirtuin inhibitors has been considered slow enough to be disregarded as a potential issue. Consequently, considerable efforts have been made in the development of mechanism-based sirtuin inhibitors.24,26−30 Here, we investigate a series of moderately selective SIRT1 inhibitors, based on a dipeptide scaffold that was previously developed.23 Combining this scaffold with acyl motifs, which have previously been applied to target SIRT1, furnished potent inhibitors of this isozyme.
Importantly, we present novel findings suggesting that thioamide-based inhibitors can be cleaved by SIRT1–3 to a higher extent than previously anticipated. These observations highlight the importance of in-depth compound assessment beyond measurement of potency.
In previous efforts to develop inhibitors against SIRT5, inhibitor 1 was synthesized as a control compound, which showed no inhibitory activity against SIRT5.23 In a subsequent study, we showed that compound 1 was a moderately potent inhibitor of Sir2La, a deacetylase/depropionylase from the Gram positive L. acidophilus, while compound 2 was less potent.31 Given that thioacetamide- and methylthiocarbamoyl-based compounds have also been shown to inhibit SIRT1–3,1,25,33 we measured the potencies of 1 and 2 against these enzymes together with control compounds TA,24,33TB,24 and EX-527(32) (Figure 2A). The nonmechanism-based SIRT1 inhibitor EX-527 was included because it is one of the most potent and selective SIRT1 inhibitors reported and a commonly used tool compound.32 Furthermore, EX-527 has completed phase I and II clinical trials for the treatment of Huntington’s disease.16 Indeed, both compounds 1 and 2 exhibited inhibitory activity against all three enzymes, with moderate selectivity toward SIRT1 over SIRT2 and higher selectivity for SIRT1 over SIRT3. With compounds 1 and 2 serving as lead chemotypes, a series of thioamide- and thiourea-based compounds was synthesized with varying acyl chain lengths (3–7). Furthermore, α-fluoroacetyl compounds 8 and 9 were included in the series, because these motifs have previously been shown to inhibit sirtuins. Albeit, lower potencies have generally been reported for these compared to their thioamide and thiourea-based counterparts.19,23,34 Consistent with previous studies, the thioamide- and thiourea-based inhibitors 1–7 were significantly more potent against SIRT1 than the α-fluoroacetyl inhibitors 8 and 9. Intriguingly, inhibitors 1–7 were all equipotent to EX-527 against SIRT1 and some of these inhibitors showed similar selectivity profiles compared to EX-527. While compounds 1–7 showed equipotent inhibition against SIRT1, the selectivity profiles of these inhibitors varied. The thioamide-based inhibitors 1, 3, and 4 showed a decrease in selectivity for SIRT1 over SIRT2 with increased acyl chain length, in agreement with previously reported inhibitors.24 The opposite trend was observed for SIRT3 where higher concentrations were required to record an inhibitory response upon chain elongation.
Figure 2.
Structure–activity relationship study, measuring compound potencies against recombinant SIRT1–3. (A) Previously reported compounds.23,24,31−33 (B) Inhibitors developed in this study. Potencies are given as IC50 values or %-inhibition at the indicated concentrations (see Supporting Figure S1 for dose–response curves and Figure S2 for bar graphs).
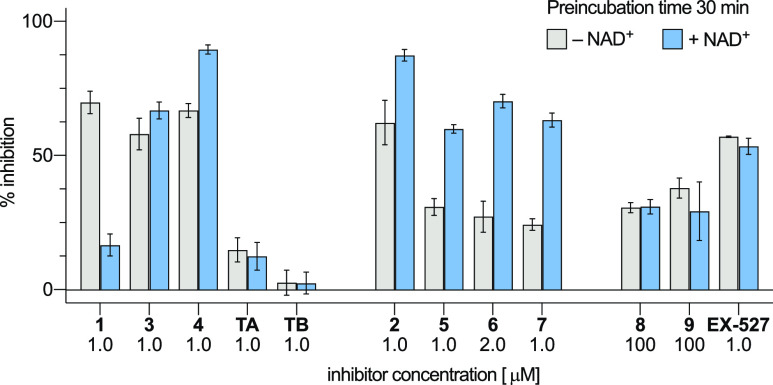
It has previously been shown that mechanism-based sirtuin inhibitors can exhibit slow and time-dependent inhibition kinetics.23,33 Thus, relying on IC50-values as the sole measure to determine and compare potencies is not sufficient. Unfortunately, the continuous fluorogenic assay format that was previously employed to analyze time-dependent inhibition of SIRT5 could not be applied for SIRT1–3, due to rapid proteolysis of the sirtuins under the assay conditions (data not shown). Instead, preincubation experiments were conducted with or without NAD+ present (Figure 3).
Figure 3.
Bar-graph showing %-inhibition from single-dose in vitro assay of compounds 1–9, TA, TB, and EX-527, following preincubation with recombinant SIRT1 (100 nM) for 30 min, with and without NAD+ (500 μM). Error bars represent the standard deviation based on two independent experiments performed in duplicate.
The thiourea-based compounds 2 and 5–7 exhibited more potent inhibition upon preincubation with NAD+, consistent with our previous study on SIRT5 inhibitors. The α-fluoroacetyl inhibitors 8 and 9 showed no significant difference upon preincubation, indicating no formation of a stalled intermediate. Similarly, the less potent mechanism-based compounds TA and TB did not show any effect of preincubation. This supports the hypothesis that an inhibitor scaffold has to contribute sufficiently to the binding affinity to increase the residence time in the binding pocket enough to enable formation of a stalled intermediate,23 such as the ones shown in Figure 1. Expectedly, the nonmechanism based inhibitor EX-527 did not show any effect upon preincubation either. Contrary to our expectations, however, the thioamide-based compounds 1, 3, and 4, which have similar IC50-values against SIRT1, exhibited a different behavior. While compound 4 showed an increase in inhibition when preincubated with enzyme and NAD+, compound 3 showed no significant difference in potency with or without NAD+. To our surprise, however, compound 1 exhibited the opposite effect when preincubated with enzyme and NAD+. This could suggest that inhibitor 1 was somehow compromised under these assay conditions, which encouraged further investigation. First, the dethioacylation activities of SIRT1–3 were addressed by monitoring the sirtuin catalyzed hydrolysis of selected inhibitors by UPLC. The oxoamide version of compound 1 (oxo-1) was synthesized and applied as a control substrate for sirtuin deacetylase activity. Sirtuin 1 furnished >80% conversion of oxo-1 to the deacylated product after 15 min, whereas SIRT3 was only able to cleave approximately 54% of oxo-1 during the 60 min incubation (Figure 4). Sirtuin 2 was unable to deacetylate oxo-1, suggesting a particularly poor affinity for this substrate. As hypothesized, SIRT1 was able to hydrolyze Nε-thioacetyllysine inhibitor 1 with ∼3% of the inhibitor cleaved after 30 min and ∼7% cleaved after 60 min. This corresponds to a just ∼33-fold lower overall degree of conversion than for deacetylation, which corresponds to a significantly higher degree of dethioacetylation than previously reported for a sirtuin.18,19 Sirtuin 1 was also able to hydrolyze the thiopropionyl-containing inhibitor 3, albeit to a lower extent. Remarkably, SIRT3 showed even higher dethioacetylation activity than SIRT1 with 10% of the inhibitor being cleaved after 60 min, corresponding to just 5.5-fold lower overall conversion compared to oxo-1. The thiourea- and α-fluoroacetyl-based analogs as well as compounds 4, TA, and TB were unaffected by SIRT1 or 3, showing no detectable degree of cleavage after 60 min in our assay (Supporting Figure S3–S10).
Figure 4.

Dethioacylation of compounds oxo-1, 1, and 3 by SIRT1 and SIRT3 (100 nM) in the presence of NAD+ (500 μM). Reactions were analyzed by UPLC (UV absorbance at 280 nm) at various time points. Red peaks represent the analyzed compounds, and blue peaks represent the deacylated product (see Supporting Figures S3–S20 for additional compounds analyzed, as well as full chromatograms).
Since we were unable to utilize our UPLC-based assay to detect if SIRT2 could catalyze dethioacylation of the inhibitors, we synthesized a series of fluorogenic thioamide-containing compounds, 10–12, based on a known scaffold with moderate to good affinity for SIRT1–3.7,8 The conversion of these substrates by SIRT1–3 was compared to the corresponding oxoamide substrates oxo-10–oxo-127,8 (Figure 5). This clearly showed that SIRT2 is also capable of cleaving thioamide substrates and even deacylates 10 to a higher extent than SIRT1. Interestingly, it appears that the dethioacylation activities of SIRT1–3 decrease with extension of the chain length of the modification; that is, 10 is deacylated to a higher extent than 11 and 12. Not too surprisingly, these experiments also clearly showed that the extent of dethioacetylation is dependent upon the affinity of the scaffold. For example, SIRT2 could not hydrolyze oxo-1, 1, or 3 but efficiently cleaved oxo-10, 10, and 11 at the applied enzyme concentrations. The TA compound was not hydrolyzed by SIRT1 or SIRT3 either, which could also be explained by poor affinity. To examine the degree of deacylation in a more complex sample, substrates 10–12 and oxo-10–oxo-12 were mixed with fresh cell lysates from HEK293 cells.
Figure 5.
(A) Chemical structures of fluorogenic substrates 10–12 and oxo-10–oxo-12. Deacylation of compounds 10–12 and oxo-10–oxo-12, by recombinant SIRT1–3 (100 nM or 250 nM) and cell lysate (HEK293, ∼65 μg). Error bars represent the standard deviation based on two independent experiments performed in duplicate.
Notably, the conversion of all substrates was increased significantly (Figure 5B). Presumably the increased deacylation of the substrates is due to the presence of multiple deacylases in the cell lysate, capable of hydrolyzing short chain acyllysines, i.e. the HDAC1–3, HDAC6, HDAC8, SIRT1–3, and SIRT6.6,7,35,36 Moreover, it has previously been shown that HDAC8 and HDAC6 can catalyze dethioacetylation, which could explain the substantial increase in conversion of substrate 10 (approximately 70–6-fold compared to SIRT1–3).18,37 Although, expression levels of the various deacylases in the cell of course have a major impact on the degree of conversion as well.
To ensure that the observed dethioacylations were not artifacts arising from inhibitor/substrate degradation or thioamide to oxoamide conversion, followed by standard sirtuin deacylation, we performed compound stability tests in assay buffer. These experiments indicated no spontaneous conversion to the free lysine product nor could thioamide to amide conversion be detected even after 24 h in buffer at 37 °C (Figures S11–S19). Thus, SIRT1–3 can indeed deacylate thioamide-based inhibitors and substrates more efficiently than previously anticipated, indicating that thiourea-based inhibitors should be more promising. To further address the stability of thioamide- and thiourea-based SIRT inhibitors beyond the deacylation, we analyzed the stability of inhibitors 1–6 in human serum, because this is an important factor to consider in drug development. Compound 1 and its thiourea analog 5 exhibited similar stabilities in serum with half-lives above 7 h. On the other hand, the dialkyl thiourea-based compounds 2 and 6 were highly labile, with half-lives of less than 1 h and full degradation observed after 4 and 6 h, respectively. In contrast, their respective thioamide analogs 2 and 3 were significantly more stable with half-lives of 6 and 7 h, respectively. The observation that the dialkyl thiourea compounds 2 and 6 are rapidly degraded in serum, whereas the monosubstituted thiourea 5 is relatively stable, led us to examine the stability of previously reported inhibitors SIRT5 as well (S5–S7).23 Although more stable than 2 and 6, the thiourea S5 was substantially less stable than the corresponding thioamide S6, consistent with the above observations. The urea analog S7 was particularly stable in human serum, showing only a slight degradation after 24 h (Figure S20). Unfortunately, we were unable to determine how the thiourea-based inhibitors were degraded as the chromatograms did not reveal any distinct byproduct peaks. Thus, it remains to be investigated in detail how the substituted thiourea-based inhibitors are degraded.
Finally, inhibitors 1, 2, 5, and 9 (25 μM) were evaluated for their ability to inhibit SIRT1 in HEK293 cells, to test their ability as tool compounds in cellular assays. Thus, acetylation levels of the tumor suppressor p53 (K382) were analyzed, which is a well-documented substrate of SIRT1.24,33,38 It has previously been shown that p53 acetylation (K382) is also affected by SIRT2 under certain assay conditions.39 Therefore, a potent and selective SIRT2 inhibitor, SirReal2, was included to ensure that the effect on p53 acetylation (K382) was SIRT1 dependent.40 Gratifyingly, compounds 1 and 2 exhibited a significant increase in the acetylation of p53 (K382) compared to vehicle-treated control (Figure 6). Interestingly, the increases in p53 acetylation by 1 and 2 were equivalent, and even higher than that of EX-527. Neither SirReal2 nor the in vitro inactive compound 9 resulted in significant upregulation of p53 acetylation (K382). However, somewhat surprisingly to us, compound 5 also had a lower effect on p53 acetylation, suggesting that this inhibitor may be less cell permeable than compounds 1 and 2. Taken together, these control experiments suggest that the effects of compounds 1 and 2 on p53 (K382) acetylation are indeed a result of inhibition of SIRT1.
Figure 6.

(A) Chemical stability of compounds 1–6 in human serum (see Figure S20 for full stability curves, including 24 h measurements). (B) Western blot analysis of whole-cell lysates (HEK293) after 6 h treatment with inhibitors EX-527, 1, and 2, 5, and 9, and Sireal2 (25 μM), cotreated with TSA (1 μM). (C) Relative levels of Ac-p53 (K382) to p53, normalized to DMSO control. The error bars represent the standard error of the mean (SEM) based on data from two independent experiments performed in duplicate (see Figure S21 for the full immunoblots and additional image quantification).
In summary, we have evaluated a series of mechanism-based inhibitors of SIRT1–3. The thioamide- and thiourea-based inhibitors exhibit potent inhibition of SIRT1, comparable to that of EX-527. While most of these inhibitors are selective toward SIRT1 over SIRT3, more moderate selectivity was generally achieved for SIRT1 over SIRT2.
More notably, we show that short-chain thioamide-based inhibitors 1 and 3 can be deacylated by SIRT1–3 to an unprecedented degree. The extent of dethioacylation appears to decrease upon extension of the acyl chain length, as SIRT1–3 showed higher activity for dethioacetylation than dethiopropionylation and dethiobutyrylation. Furthermore, thioamide-based compounds are rapidly deacylated by whole cell lysate (HEK293), suggesting that they are also likely to be short-lived in cells, though inhibitors 1 and 2 both gave rise to similar effects on p53 acetylation in HEK293 cells, presumably by inhibition of SIRT1, indicating a potential utility as tool compounds for in vitro studies.
Nevertheless, the substantial dethioacylation calls into question the utility of short chain thioamide-based sirtuin inhibitors, especially thioacetyl-containing compounds, in medicinal chemistry efforts to target sirtuins.1,25 Thiourea-based inhibitors, on the other hand, are not deacylated by SIRT1–3, suggesting that these inhibitors are more suitable for cellular studies. Furthermore, thiourea-based compounds are synthetically more readily accessible and allow for late-stage introduction of the lysine modification, which provides an additional advantage over thioamides. One major drawback is the limited stability in serum when elongating the side chain by N′-alkylation, which limits the potential utility of such thiourea-based chemotypes in drug development. Although compound 5 did not exhibit as strong an effect on p53 acetylation as 1 and 2, monoalkylated thiourea-containing compounds may offer an alternative motif for future efforts to inhibit SIRT1–3, due to their extended lifetime in serum.
The collective insight gained in this study is essential for future sirtuin inhibitor design and underscores the crucial need for comprehensive compound assessment when targeting sirtuins with mechanism-based inhibitors. We conclude that short-chain thioamide- and thiourea-based inhibitors may serve as tool compounds for cellular studies, but for drug development efforts alternative chemotypes are needed.
Acknowledgments
We thank Iacopo Galleano for donation of building blocks. We also thank Kristian Strømgaard and Christian O. R. Bartling for assistance with UPLC-assays and Kathrine Lundø for assistance with Western blotting. This work was supported by the University of Copenhagen (Ph.D. Fellowship to Nima Rajabi), the Lundbeck Foundation (Running Cost grant R289-2018-2074; CAO), the Carlsberg Foundation (2013-01-033 and CF15-0115; CAO), the Novo Nordisk Foundation (NNF17OC0029464; CAO), and the European Research Council (ERC-CoG-725172-SIRFUNCT; CAO).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsmedchemlett.9b00580.
Additional figures supporting the manuscript, experimental details, compound characterization data, as well as copies of 1H, 13C, and 19F NMR spectra (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Rajabi N.; Galleano I.; Madsen A. S.; Olsen C. A. Targeting Sirtuins: Substrate Specificity and Inhibitor Design. Prog. Mol. Biol. Transl. Sci. 2018, 154, 25–69. 10.1016/bs.pmbts.2017.11.003. [DOI] [PubMed] [Google Scholar]
- Vaquero A.; Scher M. B.; Lee D. H.; Sutton A.; Cheng H. L.; Alt F. W.; Serrano L.; Sternglanz R.; Reinberg D. SirT2 is a histone deacetylase with preference for histone H4 Lys 16 during mitosis. Genes Dev. 2006, 20, 1256–1261. 10.1101/gad.1412706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanno M.; Sakamoto J.; Miura T.; Shimamoto K.; Horio Y. Nucleocytoplasmic shuttling of the NAD+-dependent histone deacetylase SIRT1. J. Biol. Chem. 2007, 282, 6823–6832. 10.1074/jbc.M609554200. [DOI] [PubMed] [Google Scholar]
- Michishita E.; Park J. Y.; Burneskis J. M.; Barrett J. C.; Horikawa I. Evolutionarily conserved and nonconserved cellular localizations and functions of human SIRT proteins. Mol. Biol. Cell 2005, 16, 4623–4635. 10.1091/mbc.e05-01-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalkiadaki A.; Guarente L. The multifaceted functions of sirtuins in cancer. Nat. Rev. Cancer 2015, 15, 608–624. 10.1038/nrc3985. [DOI] [PubMed] [Google Scholar]
- Feldman J. L.; Baeza J.; Denu J. M. Activation of the protein deacetylase SIRT6 by long-chain fatty acids and widespread deacylation by mammalian sirtuins. J. Biol. Chem. 2013, 288, 31350–31356. 10.1074/jbc.C113.511261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen A. S.; Andersen C.; Daoud M.; Anderson K. A.; Laursen J. S.; Chakladar S.; Huynh F. K.; Colaco A. R.; Backos D. S.; Fristrup P.; Hirschey M. D.; Olsen C. A. Investigating the Sensitivity of NAD+-dependent Sirtuin Deacylation Activities to NADH. J. Biol. Chem. 2016, 291, 7128–7141. 10.1074/jbc.M115.668699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galleano I.; Schiedel M.; Jung M.; Madsen A. S.; Olsen C. A. A Continuous, Fluorogenic Sirtuin 2 Deacylase Assay: Substrate Screening and Inhibitor Evaluation. J. Med. Chem. 2016, 59, 1021–1031. 10.1021/acs.jmedchem.5b01532. [DOI] [PubMed] [Google Scholar]
- Tan M.; Peng C.; Anderson K. A.; Chhoy P.; Xie Z.; Dai L.; Park J.; Chen Y.; Huang H.; Zhang Y.; Ro J.; Wagner G. R.; Green M. F.; Madsen A. S.; Schmiesing J.; Peterson B. S.; Xu G.; Ilkayeva O. R.; Muehlbauer M. J.; Braulke T.; Muhlhausen C.; Backos D. S.; Olsen C. A.; McGuire P. J.; Pletcher S. D.; Lombard D. B.; Hirschey M. D.; Zhao Y. Lysine glutarylation is a protein posttranslational modification regulated by SIRT5. Cell Metab. 2014, 19, 605–617. 10.1016/j.cmet.2014.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson K. A.; Huynh F. K.; Fisher-Wellman K.; Stuart J. D.; Peterson B. S.; Douros J. D.; Wagner G. R.; Thompson J. W.; Madsen A. S.; Green M. F.; Sivley R. M.; Ilkayeva O. R.; Stevens R. D.; Backos D. S.; Capra J. A.; Olsen C. A.; Campbell J. E.; Muoio D. M.; Grimsrud P. A.; Hirschey M. D. SIRT4 Is a Lysine Deacylase that Controls Leucine Metabolism and Insulin Secretion. Cell Metab. 2017, 25, 838–855. 10.1016/j.cmet.2017.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrico C.; Meyer J. G.; He W.; Gibson B. W.; Verdin E. The Mitochondrial Acylome Emerges: Proteomics, Regulation by Sirtuins, and Metabolic and Disease Implications. Cell Metab. 2018, 27, 497–512. 10.1016/j.cmet.2018.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L.; Shi L.; Yang S.; Yan R.; Zhang D.; Yang J.; He L.; Li W.; Yi X.; Sun L.; Liang J.; Cheng Z.; Shi L.; Shang Y.; Yu W. SIRT7 is a histone desuccinylase that functionally links to chromatin compaction and genome stability. Nat. Commun. 2016, 7, 1–17. 10.1038/ncomms12235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong Z.; Wang M.; Wang Y.; Kim D. D.; Grenier J. K.; Cao J.; Sadhukhan S.; Hao Q.; Lin H. SIRT7 Is an RNA-Activated Protein Lysine Deacylase. ACS Chem. Biol. 2017, 12, 300–310. 10.1021/acschembio.6b00954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong Z.; Wang Y.; Zhang X.; Kim D. D.; Sadhukhan S.; Hao Q.; Lin H. SIRT7 Is Activated by DNA and Deacetylates Histone H3 in the Chromatin Context. ACS Chem. Biol. 2016, 11, 742–747. 10.1021/acschembio.5b01084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao X.; Liu Z.; Zhang W.; Gladysz K.; Fung Y. M. E.; Tian G.; Xiong Y.; Wong J. W. H.; Yuen K. W. Y.; Li X. D. Glutarylation of Histone H4 Lysine 91 Regulates Chromatin Dynamics. Mol. Cell 2019, 76, 660–675. 10.1016/j.molcel.2019.08.018. [DOI] [PubMed] [Google Scholar]
- Schiedel M.; Robaa D.; Rumpf T.; Sippl W.; Jung M. The Current State of NAD+ -Dependent Histone Deacetylases (Sirtuins) as Novel Therapeutic Targets. Med. Res. Rev. 2018, 38, 147–200. 10.1002/med.21436. [DOI] [PubMed] [Google Scholar]
- Jesko H.; Wencel P.; Strosznajder R. P.; Strosznajder J. B. Sirtuins and Their Roles in Brain Aging and Neurodegenerative Disorders. Neurochem. Res. 2017, 42, 876–890. 10.1007/s11064-016-2110-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatkins D. G.; Monnot A. D.; Zheng W. Nepsilon-thioacetyl-lysine: a multi-facet functional probe for enzymatic protein lysine Nepsilon-deacetylation. Bioorg. Med. Chem. Lett. 2006, 16, 3651–3656. 10.1016/j.bmcl.2006.04.075. [DOI] [PubMed] [Google Scholar]
- Smith B. C.; Denu J. M. Mechanism-based inhibition of Sir2 deacetylases by thioacetyl-lysine peptide. Biochemistry 2007, 46, 14478–14486. 10.1021/bi7013294. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Fung Y. M. E.; Zhang W.; He B.; Chung M. W. H.; Jin J.; Hu J.; Lin H.; Hao Q. Deacylation Mechanism by SIRT2 Revealed in the 1′-SH-2′-O-Myristoyl Intermediate Structure. Cell. Chem. Biol. 2017, 24, 339–345. 10.1016/j.chembiol.2017.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gertz M.; Fischer F.; Nguyen G. T.; Lakshminarasimhan M.; Schutkowski M.; Weyand M.; Steegborn C. Ex-527 inhibits Sirtuins by exploiting their unique NAD+-dependent deacetylation mechanism. Proc. Natl. Acad. Sci. U. S. A. 2013, 110, E2772–E2781. 10.1073/pnas.1303628110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y.; Zhang H.; He B.; Du J.; Lin H.; Cerione R. A.; Hao Q. The Bicyclic Intermediate Structure Provides Insights into the Desuccinylation Mechanism of Human Sirtuin 5 (SIRT5). J. Biol. Chem. 2012, 287, 28307–28314. 10.1074/jbc.M112.384511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajabi N.; Auth M.; Troelsen K. R.; Pannek M.; Bhatt D. P.; Fontenas M.; Hirschey M. D.; Steegborn C.; Madsen A. S.; Olsen C. A. Mechanism-Based Inhibitors of the Human Sirtuin 5 Deacylase: Structure-Activity Relationship, Biostructural, and Kinetic Insight. Angew. Chem., Int. Ed. 2017, 56, 14836–14841. 10.1002/anie.201709050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing H.; Hu J.; He B.; Negron Abril Y. L.; Stupinski J.; Weiser K.; Carbonaro M.; Chiang Y. L.; Southard T.; Giannakakou P.; Weiss R. S.; Lin H. A SIRT2-Selective Inhibitor Promotes c-Myc Oncoprotein Degradation and Exhibits Broad Anticancer Activity. Cancer Cell 2016, 29, 297–310. 10.1016/j.ccell.2016.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y.; Liu J.; Chen D.; Yan L.; Zheng W. Sirtuin Inhibition: Strategies, Inhibitors, and Therapeutic Potential. Trends Pharmacol. Sci. 2017, 38, 459–472. 10.1016/j.tips.2017.01.009. [DOI] [PubMed] [Google Scholar]
- Zheng W.; Jiang Y. (Jiangsu University; ), Selenourea Warhead and Building Method Thereof. CN 106632596 A, May 10, 2017.
- Lin H.; Cerione R. (Cornell University; ), Methods For Treatment Of Cancer By Targeting SIRT5. US 2014213530 A1, Jul 31, 2014.
- Deziel R.; Rahil J.; Wahhab A.; Allan M.; Nguyen N. (Methylgene Inc.), Sirtuin Inhibitors. WO 2009/026701 A1, Mar 5, 2009.
- Lin H. (Cornell University; ), Modulators For SIRT5 And Assays For Screening Same. US 2015057236 A1, Feb 26, 2015.
- Lin H. (Cornell University; ), Thiourea Compounds And Their Use As Inhibitors of SIRT2 or SIRT5. US 2014197775 A1, Dec 11, 2014.
- Olesen S. V.; Rajabi N.; Svensson B.; Olsen C. A.; Madsen A. S. An NAD+-Dependent Sirtuin Depropionylase and Deacetylase (Sir2La) from the Probiotic Bacterium Lactobacillus acidophilus NCFM. Biochemistry 2018, 57, 3903–3915. 10.1021/acs.biochem.8b00306. [DOI] [PubMed] [Google Scholar]
- Napper A. D.; Hixon J.; McDonagh T.; Keavey K.; Pons J. F.; Barker J.; Yau W. T.; Amouzegh P.; Flegg A.; Hamelin E.; Thomas R. J.; Kates M.; Jones S.; Navia M. A.; Saunders J. O.; DiStefano P. S.; Curtis R. Discovery of indoles as potent and selective inhibitors of the deacetylase SIRT1. J. Med. Chem. 2005, 48, 8045–8054. 10.1021/jm050522v. [DOI] [PubMed] [Google Scholar]
- Suzuki T.; Asaba T.; Imai E.; Tsumoto H.; Nakagawa H.; Miyata N. Identification of a cell-active non-peptide sirtuin inhibitor containing N-thioacetyl lysine. Bioorg. Med. Chem. Lett. 2009, 19, 5670–5672. 10.1016/j.bmcl.2009.08.028. [DOI] [PubMed] [Google Scholar]
- Smith B. C.; Denu J. M. Acetyl-lysine analog peptides as mechanistic probes of protein deacetylases. J. Biol. Chem. 2007, 282, 37256–37265. 10.1074/jbc.M707878200. [DOI] [PubMed] [Google Scholar]
- Moreno-Yruela C.; Galleano I.; Madsen A. S.; Olsen C. A. Histone Deacetylase 11 Is an 3-N-Myristoyllysine Hydrolase. Cell. Chem. Biol. 2018, 25, 849–856. 10.1016/j.chembiol.2018.04.007. [DOI] [PubMed] [Google Scholar]
- Aramsangtienchai P.; Spiegelman N. A.; He B.; Miller S. P.; Dai L.; Zhao Y.; Lin H. HDAC8 Catalyzes the Hydrolysis of Long Chain Fatty Acyl Lysine. ACS Chem. Biol. 2016, 11, 2685–2692. 10.1021/acschembio.6b00396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zessin M.; Kutil Z.; Meleshin M.; Nováková Z.; Ghazy E.; Kalbas D.; Marek M.; Romier C.; Sippl W.; Barinka C.; Schutkowski M. One-Atom Substitution Enables Direct and Continuous Monitoring of Histone Deacylase Activity. Biochemistry 2019, 58, 4777. 10.1021/acs.biochem.9b00786. [DOI] [PubMed] [Google Scholar]
- Vaziri H.; Dessain S. K.; Eaton E. N.; Imai S.-I.; Frye R. A.; Pandita T. K.; Guarente L.; Weinberg R. A. hSIR2SIRT1 Functions as an NAD-Dependent p53 Deacetylase. Cell 2001, 107, 149–159. 10.1016/S0092-8674(01)00527-X. [DOI] [PubMed] [Google Scholar]
- van Leeuwen I. M.; Higgins M.; Campbell J.; McCarthy A. R.; Sachweh M. C.; Navarro A. M.; Lain S. Modulation of p53 C-terminal acetylation by mdm2, p14ARF, and cytoplasmic SirT2. Mol. Cancer Ther. 2013, 12, 471–480. 10.1158/1535-7163.MCT-12-0904. [DOI] [PubMed] [Google Scholar]
- Rumpf T.; Schiedel M.; Karaman B.; Roessler C.; North B. J.; Lehotzky A.; Olah J.; Ladwein K. I.; Schmidtkunz K.; Gajer M.; Pannek M.; Steegborn C.; Sinclair D. A.; Gerhardt S.; Ovadi J.; Schutkowski M.; Sippl W.; Einsle O.; Jung M. Selective Sirt2 Inhibition by Ligand-Induced Rearrangement of the Active Site. Nat. Commun. 2015, 6, 1–13. 10.1038/ncomms7263. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.