ABSTRACT
Combating malnutrition is one of the greatest global health challenges. Plant-based foods offer an assortment of nutrients that are essential for adequate nutrition and can promote good health. Unfortunately, the majority of widely consumed crops are deficient in some of these nutrients. Biofortification is the umbrella term for the process by which the nutritional quality of food crops is enhanced. Traditional agricultural breeding approaches for biofortification are time consuming but can enhance the nutritional value of some foods; however, advances in molecular biology are rapidly being exploited to biofortify various crops. Globally, genetically modified organisms are a controversial topic for consumers and governmental agencies, with a vast majority of people apprehensive about the technology. Golden Rice has been genetically modified to contain elevated β-carotene concentrations and is the bellwether for both the promise and angst of agricultural biotechnology. Although there are numerous other nutritional targets of genetically biofortified crops, here I briefly summarize the work to elevate iron and folate concentrations. In addition, the possibility of using modified foods to affect the gut microbiota is examined. For several decades, plant biotechnology has measured changes in nutrient concentrations; however, the bioavailability of nutrients from many biofortified crops has not been demonstrated.
Keywords: bioavailability, biofortification, genetic engineering, golden rice, folate, iron, malnutrition, transgenics, microbiota
Introduction
Feed us first and then command us to be virtuous.
The Grand Inquisitor, Fyodor Dostoevsky
A foundation of nutrition is adequate consumption of nutritionally balanced foods. In a large part of the world this is not occurring, because malnutrition contributes to nearly half of all deaths in children under the age of 5 y (1). About 800 million people are currently suffering from hunger and some 2 billion suffer from some type of nutritional deficiency (2). Malnutrition is part of a cruel cycle of weakened immunity and recurrent infections that contribute to poor long-term health (3). Many of the world's hungry live precariously on plant-based foods and certainly a worthy life's purpose is seeking ways to improve the yield and nutritional content of crops while minimizing the environmental impacts of current agricultural practices (4). Had the Grand Inquisitor been talking about the field of nutrition, I believe he would have said something like, “Feed people first; then focus on nutrient/gene interactions, epigenetics, and food preference.”
To alleviate malnutrition, international food aid programs have developed strategies including programs that provide supplements, or fortification of processed local foods (5, 6). However, the success of these efforts has been hampered by factors such as inconsistent funding and limited access to markets and hospitals by malnourished populations (7, 8).
The engineering of crops promises a long-term sustainable solution and avoids some of the infrastructure problems that hamper the use of supplements and processing techniques (9, 10). Using breeding or molecular approaches to develop crops with higher nutrient concentrations is termed biofortification (11). Biofortified crops like rice, sorghum, corn, and banana allow consumers throughout the world more bioavailable nutrients through their daily diets (12–14). However, no single genetically modified food will be able to replace a balanced diet (15).
Conventional breeding of crops has worked for thousands of years but is limited to closely related (sexually compatible) plants, and therefore depends on the natural variation of the nutrient of interest (16, 17). For example, variation in grain zinc and iron concentrations in wheat and its closely related wild species has been exploited for improvement of modern elite cultivars with higher concentrations of these minerals (18). However, cassava varieties have inherently low protein concentrations, thus breeding cannot be used to biofortify cassava with protein (19). Even with the use of biotechnology, breeding approaches can take time, with the minimum number of generations needed for clonal propagation of crops like potatoes, banana, and cassava estimated to be 7 generations (20). For self-fertilizing crops, such as rice and sorghum, 9 generations are needed, and for cross-pollinated crops, such as corn, it increases to 17 generations (≥5 y in optimal growth conditions) (9).
For the last 30 y, the tools of molecular genetics have driven and energized numerous scientists to improve crops (21). Most consumers have little knowledge regarding the transfer of genetic information from 1 organism to another (22, 23). Understandably, terms like “genetic modification” can shake consumer confidence. About 20 y ago, genetic manipulations enabled farmers to use alternative solutions to pesticides and to delay ripening (24). Consumers were wary of these technologies; however, this lack of initial acceptance simply redirected the use of biotechnology in agriculture to less visible commercial applications (25). For instance, some products, such as chocolate, mayonnaise, tomato sauce, and bread, often contain derivates from genetically modified vegetables. The global market for genetically modified organisms (GMOs) was almost $15 billion in 2012 (10). Today this technology is resurfacing in different forms at the supermarket. Consumers may have to settle in and become more comfortable eating an apple with an added antibrowning gene or a pineapple genetically modified to contain higher concentrations of the antioxidant lycopene.
Thus far I have provided a case for GMOs and alternatives. The remainder of this article will discuss the safety of GMOs, emerging strategies to quickly generate engineered crops, and detailed examples of biofortification efforts to alter β-carotene, iron, and folate concentrations. This work concludes by focusing on emerging areas of research and the need to continue to integrate nutritional approaches with plant biotechnology.
Agricultural Biotechnology May Be Safe—But Some Impacts Are Indirect
As consumers, we face a deluge of information when visiting the grocery store. Often many products are proudly labeled “No GMOs.” The labeling for GMOs is much harder to spot, with small print on foods stating “Partially produced with genetic engineering,” a result of a 2016 federal law that requires uniform labeling of food products (26). Why do consumers tend to support organic foods but are reluctant to purchase GMOs (27)?
Since genetically modified crops reached the market several decades ago, no adverse health effects have been documented from their consumption (26, 28). This is not due to lack of testing, because millions of dollars have been spent over the last several decades addressing this issue (29). However, some of the safety issues, and consumer skepticism, can be tied to the impacts of using GMOs. The persistent use of a herbicide, glyphosate, may be associated with increased cancer rates. Glyphosate is the active ingredient in many herbicides including Roundup. Roundup Ready is the trademark for a patented line of seeds sold by Monsanto, a subsidiary of Bayer, that are resistant to the herbicide Roundup. In 2015, ∼90% of corn, soybeans, and cotton produced in the United States were Roundup Ready (30). Thus, the continued use of glyphosate is directly related to the widespread use of GMO Roundup Ready crops. Whereas the majority of scientists recognize the safety of GMO crops (31), it is this glyphosate use associated with planting these crops that has become controversial (32). In contrast to many regulatory agencies (33), a committee of scientists working for the International Agency for Research on Cancer of the WHO evaluated studies and reported that glyphosate is probably carcinogenic (34). In 2019, a couple claimed that the company's Roundup weed killer caused their cancer. A California jury agreed and awarded each of them  1 billion in punitive damages and an additional $55 million in collective compensatory damages (35). After thousands of lawsuits from cancer patients or their estates, Bayer is settling most of the current and possible future lawsuits for >$10 billion (36).
1 billion in punitive damages and an additional $55 million in collective compensatory damages (35). After thousands of lawsuits from cancer patients or their estates, Bayer is settling most of the current and possible future lawsuits for >$10 billion (36).
The Roundup Ready crops are an example where the use of GMOs must be evaluated with a complete understanding as to the altered environment they impose.
As the foregoing example clearly demonstrates, GMOs can indirectly affect consumers. But are the plants themselves safe to eat? Scientists are trained to be skeptical and argumentative; however, they generally agree that GMOs are safe to consume—a view endorsed by the American Medical Association, the National Academy of Sciences, the American Association for the Advancement of Science, and the WHO (29). Despite this near scientific consensus, only slightly more than one-third of consumers share this belief (22, 23).
Opposition to agricultural biotechnology has generated sustained impacts (37). Biotechnology companies have abandoned GMO field trials citing challenges raised by consumers (37). It typically takes more than a decade for a new modified plant to go from an idea to the field. The regulatory review process alone can take >3 y. If companies are scared that their products will not sell, they do not initiate the process (38). The potential nutritional benefits to consumers, the topic addressed here, could be astronomical (11). Change starts by educating consumers and future consumers. How many introductory nutrition classes spend a day talking about the benefits of modified foods? Does the training of clinical registered dieticians include a brief tutorial on the proper patient–provider discourse regarding GMOs? In my informal survey of college undergraduates, ∼50% of them are anti-GMO. I advocate nutrition and basic biology courses instill a small teaching module regarding the benefits and dangers of agricultural biotechnology.
The New Reality of Agricultural Biotechnology
As I discuss the different examples of nutritionally improving crops, it is important to keep in mind that altering the expression of genes in specific tissues can dramatically alter nutrient content. For example, a mineral-enhancing gene being expressed under a fruit-specific promoter will be beneficial in tomatoes, whereas a tuber-specific promoter would be desirable in potatoes. These variations require the generation of numerous different transgenic crops. In the past, this has been an arduous task and techniques to genetically modify crops required 3–5 mo of tissue culture. During this time, scientists punctiliously modulate plant hormone concentrations in the media to coax the development of roots and shoots (Figure 1) (39). This bottleneck in plant engineering often introduced unintended changes in the genome and epigenome of the regenerated plants. Recently, an alternative strategy has been developed where multipotent plant cells can be developed into seeds without externally supplied hormones (40). This time-saving technique has not yet been applied to a wide variety of crops; however, the potential for rapidly modulating plant genomes offers the promise to rapidly implement novel genetic engineering strategies into agriculturally important crops.
FIGURE 1.
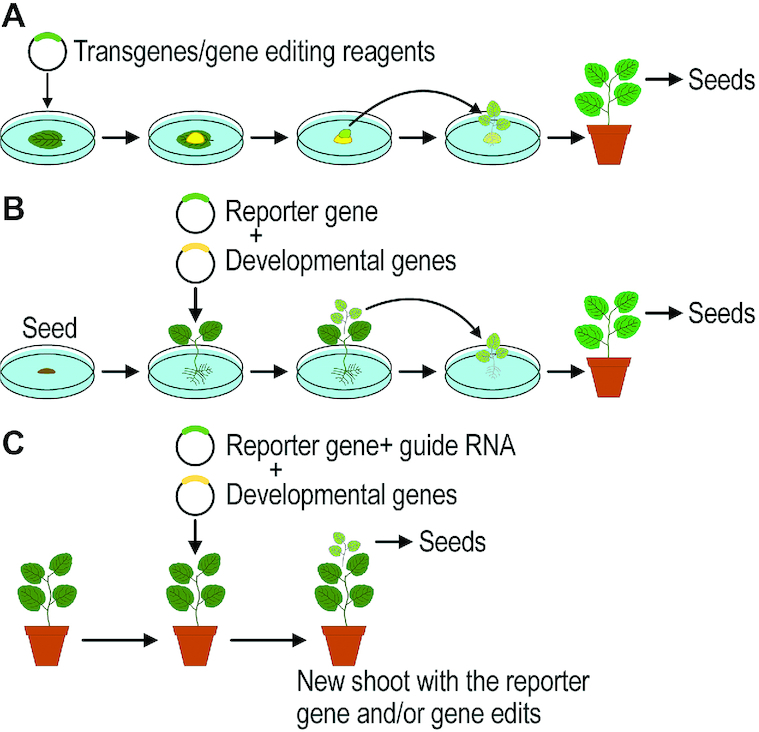
Methods to genetically engineer crops. (A) Conventional engineering requires Gram-negative bacterium Agrobacterium tumefaciens, selection for cells carrying the engineered traits (normally antibiotics), and regenerating fertile plants from cells on medium containing combinations of hormones to help make roots and shoots. Under the best of conditions this could take 4.5 mo. (B) A new method also utilizes Agrobacterium but expresses developmental genes and the transgene, where the transgenic plant grows in cell culture without using the hormone regime. This method requires ∼3 mo. (C) The most promising method uses Agrobacterium expressing developmental genes and the transgene from soil-grown plants. This method takes ∼2 mo and does not require any tissue culture (16).
β-Carotene Biofortification: Golden Rice as a Case Study
Biofortification of crops with β-carotene is intended to address vitamin A deficiency (VAD), a worldwide disease and the leading cause of preventable blindness in children; VAD has been correlated with increased risk of disease and death from severe infections (41–43). In pregnant mothers, VAD causes night blindness and renders the risk of maternal mortality higher. VAD is most prevalent in developing countries (10). Every year tens of thousands of children become blind owing to VAD, and over half of them die within 1 y of losing their sight (10). These are conservative estimates, because some sources put the incidence of VAD in the millions (44, 45).
β-Carotene is the most suitable and important precursor for vitamin A (46, 47). The edible portions of normal rice have trace amounts of β-carotene. When rice grains are engineered to express genes encoding phytoene synthase and carotene desaturase, components of the carotenoid biosynthetic pathway, the husks appear normal. Once this cover is removed, and the grains polished, they are a golden yellow—a direct product of β-carotene (Figure 2). The basic science required to engineer the carotenoid pathway was developed in the 1990s (46). The landmark achievement of 1999 demonstrated that it was possible to reconstitute the leaf-specific carotenoid pathway into rice grains (48). After a few years, it was established that the provitamin A–production trait was transferable to any rice variety, including types grown in southeast Asia, where there is widespread VAD. However, the first Golden Rice field trial in the world was harvested in September 2004 in the United States (46). This location and 5-y delay were exasperating because the target countries for Golden Rice—i.e., those who eat rice and have a high vitamin A deficit—did not have the necessary biosafety regulations in place. A condition attached to the Golden Rice licensees is that field work requires a national regulatory framework (46). This is both understandable and frustrating. Understandable because scientists and governments have to be safe, but frustrating in the face of human malnutrition, that could be alleviated with the help of this biofortified crop. This is a common theme because developing countries struggle with the pressures that make implementing this technology difficult.
FIGURE 2.

Biofortified crops. (A) Golden Rice has a yellow to orange color compared with normal rice. The more intense the color, the more β-carotene. Image courtesy of the Golden Rice Humanitarian Board and www.goldenrice.org. (B) Wheat grains treated with a histological stain that turns blue in the presence of iron. In wild-type, blue color is observed in the outer layers of grain, which do not form the flour. However, in transgenic grain, iron accumulation is observed in the central region, making it more bioavailable in wheat flour. Image courtesy of J Connorton.
Five years later, Golden Rice consumed by adult volunteers demonstrated that the engineered rice is an effective source of vitamin A (49). The trial with a limited number of participants concluded that β-carotene derived from Golden Rice was effectively converted to vitamin A in humans. Golden Rice could probably supply 50% of the RDA of vitamin A from a very modest amount—perhaps a cup of rice, if consumed daily (50, 51). This amount is within the consumption habits of most children and their mothers (42). However, this study was done in adults, and the technology was designed to help children (48).
Other work has not been published regarding the ability of Golden Rice to supply vitamin A to children in impoverished countries. These types of studies will have to overcome numerous regulatory hurdles including obtaining parents’ informed consent and conducting the proper prescreening before enrollment (45, 52). When humans are involved as research subjects, researchers must be scrupulous in their documentation and this will be an arduous task when conducting studies in countries without a strong research infrastructure.
In mid-2018 the US FDA completed its food safety evaluation for Golden Rice (42, 45, 51). Shortly before this, similar agencies in Australia, New Zealand, and Canada approved this biofortified rice. Although these countries were never the intended targets for this technology, these agencies provide a paradigm for decision-making in all countries aspiring to benefit from this rice. In late 2019, the Philippine Department of Agriculture Bureau of Plant Industry announced that Golden Rice was as safe as conventional rice (42). In the Philippines, VAD among children has increased from 15.2% in 2008 to 20.4% in 2013, despite a national supplement program (45, 51). This regulatory approval is an important step and recent work by scientists has provided further justification that Golden Rice is safe (43). Meanwhile, Philippine farmers still cannot grow Golden Rice. Regulators have to certify that the crop will not cause problems in farmers’ fields. These applications are being filed in 2020.
GMO critics are wary that for-profit corporations will have undue influence over the Golden Rice seed supply (45). However, inventors of this technology previously owned patents for Golden Rice but donated these to the Golden Rice Humanitarian Board. This rice is designed to be used only by nonprofit programs and will never cost farmers more than conventional rice.
Biotechnology boosters often present Golden Rice as the best example of the potential for agricultural biotechnology (42). Although this may be true, it is also important to think about the timeline of the various events surrounding Golden Rice. The Rockefeller Foundation first funded this project in the early 1990s (46). Today, the regulatory agencies are still working on the necessary approvals as we await the widespread use of this technology among vulnerable populations.
Iron Biofortification
Iron deficiency is the most prevalent and widespread nutrient deficiency. Without enough iron, there may be too few healthy RBCs to carry sufficient oxygen to satisfy the body's needs, resulting in anemia. This problem is magnified during pregnancy when a woman requires more iron to meet her needs. Whereas beans and millet have been successfully bred for enhanced iron content, there is too little genetic variation in iron concentrations in the endosperm of cereal grains (especially rice, wheat, and corn), therefore molecular genetic approaches are required to increase iron (53). Plant genes involved in plant iron uptake, transport, and storage have been manipulated in crops to raise iron concentrations without yield penalties (54).
Increasing iron uptake through enhanced expression of different plant transporters causes a >5-fold increase in iron concentrations in tubers (9). Iron is transported around plants in a chelated form, and increasing the amounts of these chelating agents can double iron content (55). Iron can be stored in the form of ferritin or in the plant vacuole. Plant ferritin genes have been overexpressed in cassava, rice, wheat, and maize (53, 56, 57). In rice this led to a 3-fold increase in iron concentrations in unpolished or polished grains, but the same gene was less effective in maize. A 3- to 4-fold increase in iron was demonstrated when a vacuolar localized transporter was highly expressed in cassava (53). Multigene approaches, where plant scientists manipulate several different genes or genetic pathways in the same plant (termed gene stacking), can simultaneously increase iron uptake, distribution, and storage (Figure 2) (57, 58).
As discussed in more detail at the end of this review, increased amounts of a mineral do not directly translate into improved bioavailability. The chemical form (speciation) of iron affects its bioavailability and the speciation of iron in plant foods can be altered by cooking and during digestion (59, 60). Overall, the amount of phytate in the plant, an antinutrient, is a strong indicator for mineral bioavailability. The more phytate the less bioavailable the mineral (61). However, removing the phytate can be detrimental to crop yield (62).
Folate Biofortification
Folate is a generic term for tetrahydrofolate and its derivates. Animals, unable to synthesize folates, rely primarily on dietary folates (63). The recommended daily intake of folate increases during pregnancy (64). Leafy vegetables are a rich source of folates (63, 65). However, many staple crops, such as rice, wheat, potato, and cassava, contain very low concentrations of folate. To further complicate sufficient dietary intake, folates are labile compounds, prone to (photo-)oxidative cleavage, thus many diets throughout the world are folate deficient (65).
Numerous detrimental effects arise upon folate deficiency (66). During embryogenesis, folate deficiency can cause disorders such as anencephaly and spina bifida. Together, folate deficiency–induced neural tube defects are estimated to account for >150,000 birth defects each year, predominantly in the developing world.
Boosting folate biosynthesis via metabolic engineering was the first proposed strategy to biofortify plants (65, 67). In plants, folate biosynthesis is characterized by its components occurring in 2 different subcellular compartments (65). For optimal folate biosynthesis, gene stacking is required where both the pathways are enhanced. This approach in tomato fruit appears to provide the complete adult daily requirement in <1 standard meal (68). In rice endosperm the same approach increased concentrations >100-fold and cooking experiments suggest that 100 g of this modified rice may supply the dietary allowance of folate (65). Folate stability, although often neglected, is problematic, because folate concentrations drop >50% during storage for 4 mo of engineered rice grains (69). Using a series of genetic engineering approaches focused on modulating the stability of folate in combination with enhanced biosynthesis—this new gene combination could be termed “super stacking”—results in folate concentrations that are ≤150-fold those found in normal rice (69). These super-stacked genes are next to each other on a single piece of DNA, and this genetic material, as I discussed earlier, can now be easily transferred to various crops.
The folate biofortification successes in both tomato (a dicot) and rice (a monocot) suggest that variations of gene stacking can be applied to various crops. Furthermore, it is straightforward to now augment this approach with other combinations of genes, using multiple different genes—a process that could be termed “deluxe super stacking.” Modified plants can contain multiple genes for enhancement of both iron and folate concentrations. Studying the impact of these alterations on metabolic fluxes within plants and their effects on bioavailability will require further investigation.
Plants, the Gut Microbiota, and the Potential for GMOs
The WHO recommends that all malnourished children be treated with therapeutic foods (70); however, their health-promoting components have not been identified. Microbiota-directed complementary foods (MDCFs) are a promising new strategy to use plant-based dietary components to expand the abundance of growth-discriminatory bacteria and improve growth in malnourished children (71). However, how MDCFs work remains enigmatic (72). Plant foods contain multitudes of microRNAs (miRNAs), a subset of small RNAs that are 19–24 nucleotides in length (73). In both plants and animals, an miRNA can affect gene expression by inhibiting the translation or stability of an mRNA (74). Extracellular vesicles (lipid-based nanoparticles) encapsulate miRNAs and facilitate cell-to-cell communication (75). Gut epithelial cells excrete miRNAs in vesicles and these miRNAs appear to regulate specific gut-associated bacterial gene transcripts (76). Preliminary evidence suggests that plants use extracellular vesicles to respond to and defend themselves against their own microbial pathogens, which is consistent with a potential impact on human hosts through our microbiomes (77). Edible plant nanoparticles have been previously characterized (78) and set the stage to examine if these vesicles are bioavailable to gut bacteria and whether diet-derived vesicles could be an element of MDCFs and regulate gut bacterial growth or gene expression (79). Certainly, an emerging direction in plant biotechnology is the manipulation of edible plant nanoparticles and engineering of their RNA cargos.
Measuring Nutritional Parameters in Biofortified Plants
Novel plants need to be analyzed using established and novel nutritional approaches (80). Several years ago, a high-impact review article written by a cadre of eminent plant scientists highlighted technology to improve mineral concentrations in crops and made almost no mention of the need for nutritional approaches to assess changes in bioavailability (81). Unfortunately, this oversight in nutritional assessment is the norm rather than the exception. Plant scientists tout changes in concentrations of nutrients without acknowledging the fact that increased concentrations do not always equate to increased bioavailability. Traditional nutritional tools will always be the gold standard for assessing biofortified foods (82). For example, using isotope tracers, the metabolic fate of minerals in a specific meal or a food can be distinguished from minerals from other sources and followed in the consumer (83). Efficacy studies like this are relatively expensive and not simple to perform. The dearth of these nutritional studies is probably due to lack of investment. There are certainly nutritionists who if given the opportunity and the funding would perform such studies. For now, several tools outside of the field of nutrition, although not perfect, are available to provide approximations regarding bioaccessibility. The ionome measures the mineral nutrient (dietary minerals) and trace elements found in any biological material (84, 85). Optical emission spectroscopy MS or inductively coupled plasma MS can both be utilized for ionome measurements of plant and consumer tissues. These reasonably high-throughput measurements can be used to monitor how the ionome of consumer tissues responds to modified foods.
Techniques are also available to look at the spatial distribution of minerals in biological systems. Synchrotron X-ray fluorescence (SXRF) imaging is a powerful analytical technique that collects information about the abundance and distribution of multiple elements simultaneously, in the form of a 2- or 3-dimensional image (86). Techniques such as X-ray absorption and diffraction, that accompany the SXRF experimental setup, provide information on chemical binding form and crystal structure (87). Synchrotron elemental imaging visualizes elements in situ within biological materials. Technological advances in synchrotron microscopy and sample preparation allow images of minerals in tissues to be collected from individual cells in a near-native state.
Biosensors are tools composed of a biologically active material used in close conjunction with a tool that will convert a biochemical output into a quantifiable signal (88). In the future, biosensors may be used to measure nutrient content after feeding biofortified foods to animals.
Conclusions
Genetically modified plants have the potential to boost yield, improve land use efficiency, and provide adequate nutrition for some of the world's most impoverished citizens. This review has examined need, safety, and ongoing work and explored some of the pitfalls. The most vexing issue is the lack of implementation of this technology among vulnerable populations.
Acknowledgments
I thank the editors for providing this forum and Iny Mathew and Jian Yang for editing the manuscript. Adam Gillum assisted with figure preparation. I thank A Dubock and J Connorton for the images used in Figure 2. The sole author was responsible for all aspects of this manuscript.
Notes
Supported by USDA/Agricultural Research Service grant 58-3092-9-002, National Science Foundation grant 1557890, and NIH grant R03AI149201.
Author disclosures: The author reports no conflicts of interest.
Abbreviations used: GMO, genetically modified organism; MDCF, microbiota-directed complementary food; miRNA, microRNA; SXRF, synchrotron X-ray fluorescence; VAD, vitamin A deficiency.
References
- 1. Kane AV, Dinh DM, Ward HD. Childhood malnutrition and the intestinal microbiome. Pediatr Res. 2015;77:256–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Boliko MC. FAO and the situation of food security and nutrition in the world. J Nutr Sci Vitaminol. 2019;65:S4–S8. [DOI] [PubMed] [Google Scholar]
- 3. The Lancet . A future direction for tackling malnutrition. Lancet. 2020;395:2. [DOI] [PubMed] [Google Scholar]
- 4. Dietz WH. Climate change and malnutrition: we need to act now. J Clin Invest. 2020;130:556–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sarkar SF, Poon JS, Lepage E, Bilecki L, Girard B. Enabling a sustainable and prosperous future through science and innovation in the bioeconomy at Agriculture and Agri-Food Canada. New Biotechnol. 2018;40:70–5. [DOI] [PubMed] [Google Scholar]
- 6. Ha JK. Opportunities and challenges for research on food and nutrition security and agriculture in Asia Opportunities for future research and innovation on food and nutrition security and agriculture – a global perspective. Asian-Australas J Anim Sci. 2018;31:1840–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McGuire S. International Food Policy Research Institute. 2014. Washington, DC: Global Nutrition Report 2014: actions and accountability to accelerate the world's progress on nutrition. Adv Nutr. 2015;6:278–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wright C, Garcia AL. Too much effort for too little effect: time to reconsider the merits of food supplementation programs?. J Nutr. 2020;150:190–1. [DOI] [PubMed] [Google Scholar]
- 9. Garg M, Sharma N, Sharma S, Kapoor P, Kumar A, Chunduri V, Arora P. Biofortified crops generated by breeding, agronomy, and transgenic approaches are improving lives of millions of people around the world. Front Nutr. 2018;5:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Oliver MJ. Why we need GMO crops in agriculture. Mo Med. 2014;111:492–507. [PMC free article] [PubMed] [Google Scholar]
- 11. Hirschi KD. Nutrient biofortification of food crops. In: Cousins R, Bier D, Bowman B, Dean L, editors. Annual review of nutrition. 2009/04/28 ed. Palo Alto (CA): Annual Reviews, Inc; 2009. pp. 401–21. [DOI] [PubMed] [Google Scholar]
- 12. Paul JY, Harding R, Tushemereirwe W, Dale J. Banana21: from gene discovery to deregulated golden bananas. Front Plant Sci. 2018;9:558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Che P, Zhao Z-Y, Glassman K, Dolde D, Hu TX, Jones TJ, Gruis DF, Obukosia S, Wambugu F, Albertsen MC. Elevated vitamin E content improves all-trans β-carotene accumulation and stability in biofortified sorghum. Proc Natl Acad Sci U S A. 2016;113:11040–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bouis H. Reducing mineral and vitamin deficiencies through biofortification: progress under HarvestPlus. World Rev Nutr Diet. 2018;118:112–22. [DOI] [PubMed] [Google Scholar]
- 15. Hefferon KL. Nutritionally enhanced food crops; progress and perspectives. Int J Mol Sci. 2015;16:3895–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Swamy BPM, Rahman MA, Inabangan-Asilo MA, Amparado A, Manito C, Chadha-Mohanty P, Reinke R, Slamet-Loedin IH. Advances in breeding for high grain zinc in rice. Rice. 2016;9:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. White PJ, Broadley MR. Biofortification of crops with seven mineral elements often lacking in human diets – iron, zinc, copper, calcium, magnesium, selenium and iodine. New Phytol. 2009;182:49–84. [DOI] [PubMed] [Google Scholar]
- 18. Garcia-Oliveira AL, Chander S, Ortiz R, Menkir A, Gedil M. Genetic basis and breeding perspectives of grain iron and zinc enrichment in cereals. Front Plant Sci. 2018;9:937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Leyva-Guerrero E, Narayanan NN, Ihemere U, Sayre RT. Iron and protein biofortification of cassava: lessons learned. Curr Opin Biotechnol. 2012;23:257–64. [DOI] [PubMed] [Google Scholar]
- 20. McKey D, Elias M, Pujol B, Duputie A. The evolutionary ecology of clonally propagated domesticated plants. New Phytol. 2010;186:318–32. [DOI] [PubMed] [Google Scholar]
- 21. Gao H, Gadlage MJ, Lafitte HR, Lenderts B, Yang M, Schroder M, Farrell J, Snopek K, Peterson D, Feigenbutz Let al. Superior field performance of waxy corn engineered using CRISPR-Cas9. Nat Biotechnol. 2020;38:579–81. [DOI] [PubMed] [Google Scholar]
- 22. Vecchione M, Feldman C, Wunderlich S. Consumer knowledge and attitudes about genetically modified food products and labelling policy. Int J Food Sci Nutr. 2015;66:329–35. [DOI] [PubMed] [Google Scholar]
- 23. Wunderlich S, Gatto KA. Consumer perception of genetically modified organisms and sources of information. Adv Nutr. 2015;6:842–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Constable A, Jonas D, Cockburn A, Davi A, Edwards G, Hepburn P, Herouet-Guicheney C, Knowles M, Moseley B, Oberdorfer Ret al. History of safe use as applied to the safety assessment of novel foods and foods derived from genetically modified organisms. Food Chem Toxicol. 2007;45:2513–25. [DOI] [PubMed] [Google Scholar]
- 25. Hirschi KD. New foods for thought. Trends Plant Sci. 2012;17:123–5. [DOI] [PubMed] [Google Scholar]
- 26. Giraldo PA, Shinozuka H, Spangenberg GC, Cogan NOI, Smith KF. Safety assessment of genetically modified feed: is there any difference from food?. Front Plant Sci. 2019;10:1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nguyen HV, Nguyen N, Nguyen BK, Lobo A, Vu PA. Organic food purchases in an emerging market: the influence of consumers’ personal factors and green marketing practices of food stores. Int J Environ Res Public Health. 2019;16:1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cui K, Shoemaker SP. Public perception of genetically-modified (GM) food: a nationwide Chinese consumer study. NPJ Sci Food. 2018;2:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bawa AS, Anilakumar KR. Genetically modified foods: safety, risks and public concerns—a review. J Food Sci Technol. 2013;50:1035–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Delaney B, Goodman RE, Ladics GS. Food and feed safety of genetically engineered food crops. Toxicol Sci. 2018;162:361–71. [DOI] [PubMed] [Google Scholar]
- 31. Nair RS, Fuchs RL, Schuette SA. Current methods for assessing safety of genetically modified crops as exemplified by data on Roundup Ready soybeans. Toxicol Pathol. 2002;30:117–25. [DOI] [PubMed] [Google Scholar]
- 32. Myers JP, Antoniou MN, Blumberg B, Carroll L, Colborn T, Everett LG, Hansen M, Landrigan PJ, Lanphear BP, Mesnage Ret al. Concerns over use of glyphosate-based herbicides and risks associated with exposures: a consensus statement. Environ Health. 2016;15:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tarone RE. On the International Agency for Research on Cancer classification of glyphosate as a probable human carcinogen. Eur J Cancer Prev. 2018;27:82–7. [DOI] [PubMed] [Google Scholar]
- 34. Tarone RE. Conflicts of interest, bias, and the IARC monographs program. Regul Toxicol Pharmacol. 2018;98:A1–A4. [DOI] [PubMed] [Google Scholar]
- 35. Cohen P. $2 billion verdict against Monsanto is third to find Roundup caused cancer. New York Times;2019; May 13. [Google Scholar]
- 36. Yan H. Bayer settles lawsuits from cancer patients over Roundup weed killer in $10 billion agreement. CNN;2020; June 24. [Google Scholar]
- 37. Schmidt CW. Genetically modified foods: breeding uncertainty. Environ Health Perspect. 2005;113:A526–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Weil J-H. Are genetically modified plants useful and safe?. IUBMB Life. 2005;57:311–4. [DOI] [PubMed] [Google Scholar]
- 39. Dong OX, Ronald PC. Fast-track for engineered plants. Nat Biotechnol. 2020;38:32–4. [DOI] [PubMed] [Google Scholar]
- 40. Maher MF, Nasti RA, Vollbrecht M, Starker CG, Clark MD, Voytas DF. Plant gene editing through de novo induction of meristems. Nat Biotechnol. 2020;38:84–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mason J, Rivers J, Helwig C. Recent trends in malnutrition in developing regions: vitamin A deficiency, anemia, iodine deficiency, and child underweight. Food Nutr Bull. 2005;26:57. [PubMed] [Google Scholar]
- 42. Stokstad E. After 20 years, golden rice nears approval. Science. 2019;366:934. [DOI] [PubMed] [Google Scholar]
- 43. Oliva N, Florida Cueto-Reano M, Trijatmiko KR, Samia M, Welsch R, Schaub P, Beyer P, Mackenzie D, Boncodin R, Reinke Ret al. Molecular characterization and safety assessment of biofortified provitamin A rice. Sci Rep. 2020;10:1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dubock A. The politics of golden rice. GM Crops Food. 2014;5:210–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kettenburg AJ, Hanspach J, Abson DJ, Fischer J. From disagreements to dialogue: unpacking the golden rice debate. Sustain Sci. 2018;13:1469–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Potrykus I. Lessons from the ‘Humanitarian Golden Rice’ project: regulation prevents development of public good genetically engineered crop products. New Biotechnol. 2010;27:466–72. [DOI] [PubMed] [Google Scholar]
- 47. Grune T, Lietz G, Palou A, Ross AC, Stahl W, Tang G, Thurnham D, Yin SA, Biesalski HK. β-Carotene is an important vitamin A source for humans. J Nutr. 2010;140:2268S–85S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gartland KMA, Bruschi F, Dundar M, Gahan PB, Viola Magni MP, Akbarova Y. Progress towards the ‘Golden Age’ of biotechnology. Curr Opin Biotechnol. 2013;24(Suppl 1):S6–S13. [DOI] [PubMed] [Google Scholar]
- 49. Tang G, Qin J, Dolnikowski GG, Russell RM, Grusak MA. Golden Rice is an effective source of vitamin A. Am J Clin Nutr. 2009;89:1776–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Swamy BPM, Samia M, Boncodin R, Marundan S, Rebong DB, Ordonio RL, Miranda RT, Rebong ATO, Alibuyog AY, Adeva CCet al. Compositional analysis of genetically engineered GR2E “Golden Rice” in comparison to that of conventional rice. J Agric Food Chem. 2019;67:7986–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Owens B. Golden Rice is safe to eat, says FDA. Nat Biotechnol. 2018;36:559–60. [DOI] [PubMed] [Google Scholar]
- 52. Das P, Babaei P, Nielsen J. Metagenomic analysis of microbe-mediated vitamin metabolism in the human gut microbiome. BMC Genomics. 2019;20:208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Connorton JM, Balk J, Rodríguez-Celma J. Iron homeostasis in plants – a brief overview. Metallomics. 2017;9:813–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Connorton JM, Balk J. Iron biofortification of staple crops: lessons and challenges in plant genetics. Plant Cell Physiol. 2019;60:1447–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Goto F, Yoshihara T, Shigemoto N, Toki S, Takaiwa F. Iron fortification of rice seed by the soybean ferritin gene. Nat Biotechnol. 1999;17:282–6. [DOI] [PubMed] [Google Scholar]
- 56. Narayanan N, Beyene G, Chauhan RD, Gaitán-Solís E, Gehan J, Butts P, Siritunga D, Okwuonu I, Woll A, Jiménez-Aguilar DMet al. Biofortification of field-grown cassava by engineering expression of an iron transporter and ferritin. Nat Biotechnol. 2019;37:144–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Boonyaves K, Wu T-Y, Gruissem W, Bhullar NK. Enhanced grain iron levels in rice expressing an iron-regulated metal transporter, nicotianamine synthase, and ferritin gene cassette. Front Plant Sci. 2017;8:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Connorton JM, Jones ER, Rodríguez-Ramiro I, Fairweather-Tait S, Uauy C, Balk J. Wheat vacuolar iron transporter TaVIT2 transports Fe and Mn and is effective for biofortification. Plant Physiol. 2017;174:2434–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Moore KL, Rodríguez-Ramiro I, Jones ER, Jones EJ, Rodríguez-Celma J, Halsey K, Domoney C, Shewry PR, Fairweather-Tait S, Balk J. The stage of seed development influences iron bioavailability in pea (Pisum sativum L.). Sci Rep. 2018;8:6865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Perfecto A, Rodriguez-Ramiro I, Rodriguez-Celma J, Sharp P, Balk J, Fairweather-Tait S. Pea ferritin stability under gastric pH conditions determines the mechanism of iron uptake in Caco-2 cells. J Nutr. 2018;148:1229–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Raboy V. The ABCs of low-phytate crops. Nat Biotechnol. 2007;25:874–5. [DOI] [PubMed] [Google Scholar]
- 62. Gupta RK, Gangoliya SS, Singh NK. Reduction of phytic acid and enhancement of bioavailable micronutrients in food grains. J Food Sci Technol. 2015;52:676–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Field MS, Kamynina E, Chon J, Stover PJ. Nuclear folate metabolism. Annu Rev Nutr. 2018;38:219–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Bailey RL, Dodd KW, Gahche JJ, Dwyer JT, McDowell MA, Yetley EA, Sempos CA, Burt VL, Radimer KL, Picciano MF. Total folate and folic acid intake from foods and dietary supplements in the United States: 2003–2006. Am J Clin Nutr. 2010;91:231–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Strobbe S, Van Der Straeten D. Folate biofortification in food crops. Curr Opin Biotechnol. 2017;44:202–11. [DOI] [PubMed] [Google Scholar]
- 66. Martiniova L, Field MS, Finkelstein JL, Perry CA, Stover PJ. Maternal dietary uridine causes, and deoxyuridine prevents, neural tube closure defects in a mouse model of folate-responsive neural tube defects. Am J Clin Nutr. 2015;101:860–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. DellaPenna D. Biofortification of plant-based food: enhancing folate levels by metabolic engineering. Proc Natl Acad Sci U S A. 2007;104:3675–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Díaz de la Garza RI, Gregory JF III, Hanson AD. Folate biofortification of tomato fruit. Proc Natl Acad Sci U S A. 2007;104:4218–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Blancquaert D, Van Daele J, Strobbe S, Kiekens F, Storozhenko S, De Steur H, Gellynck X, Lambert W, Stove C, Van Der Straeten D. Improving folate (vitamin B9) stability in biofortified rice through metabolic engineering. Nat Biotechnol. 2015;33:1076–8. [DOI] [PubMed] [Google Scholar]
- 70. Malik S, Mittal M, Kushwaha KP. WHO/UNICEF recommended therapeutic food versus home based therapeutic food in the management of severe acute malnutrition: a randomized controlled trial. Sudan J Paediatr. 2016;16:21–7. [PMC free article] [PubMed] [Google Scholar]
- 71. Gehrig JL, Venkatesh S, Chang HW, Hibberd MC, Kung VL, Cheng J, Chen RY, Subramanian S, Cowardin CA, Meier MFet al. Effects of microbiota-directed foods in gnotobiotic animals and undernourished children. Science. 2019;365:eaau4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Tomova A, Bukovsky I, Rembert E, Yonas W, Alwarith J, Barnard ND, Kahleova H. The effects of vegetarian and vegan diets on gut microbiota. Front Nutr. 2019;6:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Bartel B, Bartel DP. MicroRNAs: at the root of plant development?. Plant Physiol. 2003;132:709–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Pegtel DM, Cosmopoulos K, Thorley-Lawson DA, van Eijndhoven MAJ, Hopmans ES, Lindenberg JL, de Gruijl TD, Wurdinger T, Middeldorp JM. Functional delivery of viral miRNAs via exosomes. Proc Natl Acad Sci U S A. 2010;107:6328–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Liu S, da Cunha AP, Rezende RM, Cialic R, Wei Z, Bry L, Comstock LE, Gandhi R, Weiner HL. The host shapes the gut microbiota via fecal microRNA. Cell Host Microbe. 2016;19:32–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Rutter BD, Innes RW. Extracellular vesicles as key mediators of plant–microbe interactions. Curr Opin Plant Biol. 2018;44:16–22. [DOI] [PubMed] [Google Scholar]
- 78. Mu J, Zhuang X, Wang Q, Jiang H, Deng ZB, Wang B, Zhang L, Kakar S, Jun Y, Miller Det al. Interspecies communication between plant and mouse gut host cells through edible plant derived exosome-like nanoparticles. Mol Nutr Food Res. 2014;58:1561–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Teng Y, Ren Y, Sayed M, Hu X, Lei C, Kumar A, Hutchins E, Mu J, Deng Z, Luo Cet al. Plant-derived exosomal microRNAs shape the gut microbiota. Cell Host Microbe. 2018;24:637–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Tanumihardjo SA. The dawn of a new era in vitamin A assessment. J Nutr. 2020;150:185–7. [DOI] [PubMed] [Google Scholar]
- 81. Schroeder JI, Delhaize E, Frommer WB, Guerinot ML, Harrison MJ, Herrera-Estrella L, Horie T, Kochian LV, Munns R, Nishizawa NKet al. Using membrane transporters to improve crops for sustainable food production. Nature. 2013;497:60–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Morris J, Hawthorne KM, Hotze T, Abrams SA, Hirschi KD. Nutritional impact of elevated calcium transport activity in carrots. Proc Natl Acad Sci U S A. 2008;105:1431–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Turnlund JR. The use of stable isotopes in mineral nutrition research. J Nutr. 1989;119:7–14. [DOI] [PubMed] [Google Scholar]
- 84. Baxter I. Should we treat the ionome as a combination of individual elements, or should we be deriving novel combined traits?. J Exp Bot. 2015;66:2127–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Fikas AA, Dilkes BP, Baxter I. Multivariate analysis reveals environmental and genetic determinants of element covariation in the maize grain ionome. Plant Direct. 2019;3:e00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Kopittke PM, Punshon T, Paterson DJ, Tappero RV, Wang P, Blamey FPC, van der Ent A, Lombi E. Synchrotron-based X-ray fluorescence microscopy as a technique for imaging of elements in plants. Plant Physiol. 2018;178:507–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. VanderSchee CR, Kuter D, Chou H, Jackson BP, Mann KK, Bohle DS. Addressing K/L-edge overlap in elemental analysis from micro-X-ray fluorescence: bioimaging of tungsten and zinc in bone tissue using synchrotron radiation and laser ablation inductively coupled plasma mass spectrometry. Anal Bioanal Chem. 2020;412:259–65. [DOI] [PubMed] [Google Scholar]
- 88. Pirzada M, Altintas Z. Nanomaterials for healthcare biosensing applications. Sensors. 2019;19:5311. [DOI] [PMC free article] [PubMed] [Google Scholar]


