ABSTRACT
Background
Recent meta-analyses suggest that the consumption of fermented dairy products reduces type 2 diabetes and cardiovascular disease (CVD) risk, although the underlying mechanisms remain unclear.
Objective
We evaluated whether dairy protein products modulated gut microbiota and cardiometabolic features in mouse models of diet-induced obesity and CVD.
Methods
Eight-week-old C57BL/6J wild-type (WT) and LDLr−/−ApoB100/100 (LRKO) male mice were fed for 12 and 24 wk, respectively, with a high-fat/high-sucrose diet [66% kcal lipids, 22% kcal carbohydrates (100% sucrose), 12% kcal proteins]. The protein sources of the 4 diets were 100% nondairy protein (NDP), or 50% of the NDP energy replaced by milk (MP), milk fermented by Lactobacillus helveticus (FMP), or Greek-style yogurt (YP) protein. Fecal 16S rRNA gene-based amplicon sequencing, intestinal gene expression, and glucose tolerance test were conducted. Hepatic inflammation and circulating adhesion molecules were measured by multiplex assays.
Results
Feeding WT mice for 12 wk led to a 74% increase in body weight, whereas after 24 wk the LRKO mice had a 101.5% increase compared with initial body weight. Compared with NDP and MP, the consumption of FMP and YP modulated the gut microbiota composition in a similar clustering pattern, upregulating the Streptococcus genus in both genotypes. In WT mice, feeding YP compared with NDP increased the expression of genes involved in jejunal (Reg3b, 7.3-fold, P = 0.049) and ileal (Ocln, 1.7-fold, P = 0.047; Il1-β,1.7-fold, P = 0.038; Nos2, 3.8-fold, P = 0.018) immunity and integrity. In LRKO mice, feeding YP compared with MP improved insulin sensitivity by 65% (P = 0.039). In LRKO mice, feeding with FMP versus NDP attenuated hepatic inflammation (monocyte chemoattractant protein 1, 2.1-fold, P ˂ 0.0001; IL1-β, 5.7-fold, P = 0.0003; INF-γ, 1.7-fold, P = 0.002) whereas both FMP [vascular adhesion molecule 1 (VCAM1), 1.3-fold, P = 0.0003] and YP (VCAM1, 1.04-fold, P = 0.013; intracellular adhesion molecule 1, 1.4-fold, P = 0.028) decreased circulating adhesion molecules.
Conclusion
Both fermented dairy protein products reduce cardiometabolic risk factors in diet-induced obese mice, possibly by modulating the gut microbiota.
Keywords: dairy products, peptides, bacteria, fermentation, gut inflammation
Introduction
Milk and dairy products are widely consumed around the world, with over 6 billion consumers, mostly in developing countries (1). Such widespread consumption is partially explained by the heterogeneity of available products. These products may be fermented (i.e. yogurt, cheese, and fermented milk such as kefir), nonfermented (i.e. fluid milk, some forms of cheese), liquid, solid, sweet, or savory. Growing evidence of health improvements has been associated with the consumption of milk and its derived products. Indeed, meta-analyses showed that dairy consumption is associated with both reduced weight gain (2) and waist circumference (3). Furthermore, reduced risk of type 2 diabetes (T2D) (4–6), development of cardiovascular disease (CVD) (7, 8), and reduced CVD event or mortality (9) are associated with dairy consumption. In agreement, dairy consumption was also associated with a lower risk of hypertension (10). However, a healthier prognosis is not always associated with milk consumption and its derived products (11) and such divergent findings may be related to the nutritional heterogeneity of this food class.
In addition to being a source of calcium, vitamins, medium-chain and odd-chain SFAs, PUFAs and branched-chain SFAs and, occasionally, probiotics (12), dairy products provide proteins essential for human health. Indeed, milk is considered a major high-quality protein source (32–34 g/L) of which nearly 80% is casein and 20% whey protein (12). During protein fermentation, mostly from lactic acid bacteria (LAB) activity, a plethora of bioactive peptides with a wide array of biological activities are released (13). For example, casein-enriched milk fermented by Lactobacillus helveticus shows a high angiotensin-converting enzyme (ACE)-inhibitory activity (14), potentially through the ability of the peptides to compete with substrate for binding to the active site of the enzyme (15). Furthermore, dairy-derived casein and whey peptides can inhibit dipeptidyl peptidase-IV (16), an incretin-degrading protein that impairs glucose metabolism. Similarly, whey-derived branched-chain amino acids, such as leucine, activate mammalian target of rapamycin (mTOR) in β-pancreatic cells, which may improve insulin secretion dysfunction in T2D (17).
Few studies have investigated the impact of dairy protein on the gut microbiota, which is now recognized as a key player in immunometabolic regulation. Indeed, the absence of gut microbiota in germ-free mice (18) or by antibiotic treatment (19) prevented high-fat-induced intestinal inflammation and gut barrier disruption. Furthermore, it has been shown that gut-derived metabolites, such as SCFA, influence host metabolism and glucose homeostasis, as well as body weight gain (20, 21). In the current study, we evaluated the effect of a 50% replacement of nondairy protein (NDP) with protein from either fermented [i.e. fermented milk (FMP) and Greek-style yogurt (YP) protein] or nonfermented [i.e. milk protein (MP)] dairy protein, on the modulation of gut microbiota composition and cardiometabolic risk factors in 2 mouse models: diet-induced obesity and CVD.
Methods
Animals
Eight-week-old male C57BL/6J wild-type (WT) and in-house colony atherosclerotic LDLr−/- ApoB100/100 backcrossed on C57BL/6J background (LRKO) mice were housed individually on a regulated daylight cycle at the Institute of Nutraceuticals and Functional Foods and at the Quebec Heart and Lung Institute facilities, respectively. Studies were not performed concurrently. Animals had access to food and water ad libitum and were separated in diet-based experimental groups matched for body weight (n = 14–15 animals per dietary group for WT mice, n = 13–16 animals per dietary group for LRKO mice). Following 2 wk of acclimatization on a low-fat grain-based diet (Teklad 2018, Harlan), mice were fed with a high-fat/high-sucrose (HFHS) diet containing 66% kcal lipids (8% protein mix, 46% corn oil, and 46% lard), 22% kcal carbohydrates (∼100% sucrose), and 12% kcal proteins (∼100% nondairy protein mix; 10% eggs, 10% soya, 53% meat, and 27% chicken; remaining percentage from L-cystine). The NDP diet accounted for 100% of the protein mix based on the reported food sources of nutrients in the diet of Canadian adults (22). Nonhydrolyzed, high-protein content lyophilized ingredients used to make the protein mix were purchased from Envigo Teklad (for egg and soy) and Happy Yak (for beef and chicken). The protein mix was ∼81% w/w protein, 13% w/w fat, and 1% w/w carbohydrates with diets being adjusted for macronutrient content. The dairy-containing diets were generated by replacing 50% of the energetic content of the protein mix with either nonfermented milk (MP), milk fermented by Lactobacillus helveticus (FMP), or YP protein. Dairy protein products contained 57–59% protein and diets were matched for protein, fat, and carbohydrate (Table 1). Since WT mice do not develop extensive atherosclerosis spontaneously, we took advantage of the atherosclerotic-prone LRKO genotype whose diet had 0.2% of cholesterol added to accelerate atherosclerosis development. Body weight gain was assessed once a week and food intake 3 times a week, and final weight gain was estimated as the difference between final (week 12 for WT and week 24 for LRKO) and initial body weights. In order to estimate daily energy excretion, 24-h feces were collected at weeks 12 and 24 for WT and LRKO, respectively. Following 12 and 24 wk of diet, fasted WT and LRKO mice, respectively, were anesthetized with isoflurane and killed by cardiac puncture. Epididymal, retroperitoneal, and inguinal white adipose tissue (eWAT, rpWAT and iWAT, respectively), liver, gastrocnemius, and soleus muscles as well as heart were snap frozen in liquid nitrogen. Visceral adiposity was estimated by the sum of the weights of eWAT and rpWAT, and subcutaneous adiposity by iWAT. The aorta was fixed in 4% paraformaldehyde (PFA)/PBS solution and subsequently kept in 1% PBS solution for further analysis. Animal protocols were approved by the Animal Care Committee of Laval University (2014–06-25). The experimental strategy is described in Supplemental Figure 1.
TABLE 1.
Nutritional composition of NDP, MP, FMP, and YP diets
| Diet | ||||
|---|---|---|---|---|
| Macronutrients | NDP | MP | FMP | YP |
| Protein, %kcal | 12.2 | 11.9 | 11.9 | 11.8 |
| Carbohydrate, %kcal | 22.0 | 21.8 | 22.2 | 22.7 |
| Fat,1 %kcal | 65.7 | 66.2 | 65.9 | 65.5 |
| kcal/g | 5.51 | 5.47 | 5.38 | 5.34 |
| Ingredients | g/kg | g/kg | g/kg | g/kg |
| Nondairy protein mix2 | 207 | 103 | 100 | 99.0 |
| protein | 158 | 78.4 | 76.7 | 75.6 |
| carbohydrates | 0.21 | 0.10 | 0.10 | 0.10 |
| fat | 34.1 | 16.9 | 16.6 | 16.3 |
| MP | 0.00 | 133 | 0.00 | 0.00 |
| protein | — | 78.4 | — | — |
| carbohydrates | — | 36.9 | — | — |
| fat | — | 1.47 | — | — |
| FMP | 0.00 | 0.00 | 131 | 0.00 |
| protein | — | — | 76.7 | — |
| carbohydrates | — | — | 22.7 | — |
| fat | — | — | 0.91 | — |
| YP | 0.00 | 0.00 | 0.00 | 131 |
| protein | — | — | — | 75.6 |
| carbohydrates | — | — | — | 18.6 |
| fat | — | — | — | 1.51 |
| L-cystine | 1.86 | 1.85 | 1.81 | 1.78 |
| Sucrose | 278 | 238 | 254 | 262 |
| Mineral mix3 | 69.3 | 68.8 | 67.3 | 66.3 |
| Vitamin mix4 | 14.5 | 14.4 | 14.1 | 13.9 |
| Cellulose | 51.7 | 51.3 | 50.2 | 49.5 |
| Lard | 188 | 194 | 190 | 187 |
| Corn oil | 188 | 194 | 190 | 187 |
| Choline bitartrate | 2.07 | 2.05 | 2.01 | 1.98 |
| Butylhydroxytoluene | 0.31 | 0.31 | 0.30 | 0.30 |
| Total | 1000 | 1000 | 1000 | 1000 |
1An additional 0.2% cholesterol was added in diets of the LRKO model. Cholesterol content was 486 mg/100 g of NDP diet and 365 mg/100 g of MP, FMP, and YP diets.
Home-made nondairy protein mix (76.4% protein, 0.1% carbohydrates, 16.5% fat)—10% egg white and 10% soy (Teklad), 53% beef and 27% chicken (Happy Yak).
Teklad mineral mix AIN-76 (Teklad)—12% sucrose.
Vitamin mix AIN-76A (Teklad)—98% sucrose.
FMP, fermented milk product; LRKO, LDLr−/- ApoB100/100 backcrossed on C57BL/6J background; MP, milk product; NDP, nondairy protein; YP, yogurt product.
Dairy protein products
Dairy protein products' preparation
Skim milk powder (SMP) containing 36% of protein (w/w) was used for dairy protein product preparations. MP was prepared from SMP rehydrated (12% of total solids) and concentrated by ultrafiltration (UF) by a 3× volume concentration factor using a 10 kDa molecular weight cut-off (MWCO) membrane. UF-retentate was freeze-dried, generating a powder containing 59% protein (w/w). FMP was produced from MP solubilized at 10% w/w protein basis. The solution was stored (4°C, 18 h) to ensure complete solubilization and thereafter heat-treated (90°C, 5 min) before bacterial inoculation using Lactobacillus helveticus (Rosell®-52). YP was generated from liquid MP fermented with a commercial yogurt starter composed of Streptococcus thermophilus (Rosell®-83) and Lactobacillus delbrueckii subsp. bulgaricus (Rosell®-440), and YP were finally freeze-dried for diet incorporation. Dairy protein product processing is detailed in Supplemental Figure 2.
Dairy protein products' characterization
Total composition of MP, FMP, and YP was determined on final powders (Supplemental Table 1). Total solids content was estimated according to 926.08 (23) and 927.05 (24) methods proposed by the Association of Official Agricultural Chemists (AOAC). Total nitrogen (TN) and nonprotein nitrogen (NPN) were determined by the Kjeldahl method (25) according to standard procedures 20–3:2004 (26), 20–4:2001 (27), and 224:2011 (28) proposed by the International Dairy Federation (IDF). Total nitrogen protein (TPN) was calculated as TN × 6.38 and proteolysis index as NPN/TN. The molecular weight distribution profile of protein/peptide components was assessed by high-pressure size exclusion chromatography (HPSEC, Supplemental Figure 3). Fat content was analyzed using the Mojonnier method according to standard methods of IDF [5:2004 (29) and 9C:1987 (30)], whereas cholesterol was determined by reverse phase HPLC (RP-HPLC) after extraction using a Nova-Pak C18 column as previously described (31). Ash content was assessed according to the AOAC 930–30 (32) method and cations (Ca, Na, K, Mg) and anions (PO4, Cl, SO4−2) by inductive coupling plasma (ICP) spectroscopy. Carbohydrates (lactose, galactose) and organic (acetic, citric, lactic) acids were quantified by HPLC analysis using an ION-300 column (Transgenomic Inc.). An automatic titrator was used to measure pH and titratable acidity. Bacteria enumeration and viability were assessed by plate counts of cells, qPCR, and PCR coupled with a propidium monoazide DNA intercalating agent.
Glucose tolerance test
After 11 and 17 wk on the diet, WT and LRKO mice, respectively, were fasted 12 h prior to an oral-glucose-tolerance test (OGTT) as previously detailed (33). Blood samples were collected during all time points for insulin determination, measured with the Ultrasensitive mouse ELISA kit (ALPCO). The HOMA-IR was determined using the following formula: fasting insulinemia (μUI/mL) × fasting glycemia (mM)/22.5. The Matsuda insulin sensitivity index was calculated as previously described (34).
Analytical methods
In order to assess the fed-state lipid profile in circulation at week 21, LRKO mice were fasted for 12 h and then refed for 1 h followed by blood collection from the lateral saphenous vein. Standard colorimetric kits were used for triglycerides (TGs) (Thermo Scientific), cholesterol (Randox), HDL (Randox), LDL (Randox), and oxidized-LDL (MyBioSource). In LRKO mice, cytokines were measured in fasted plasma and liver lysates using a Bio-plex pro assay (Bio-Rad Laboratories), whereas circulating adhesion molecules were measured by a Milliplex map kit assay (Millipore Sigma) and vascular cell adhesion molecule 1 (VCAM1) by a quantitative ELISA test (R&D systems). LPS was measured in fasted plasma withdrawn by cardiac puncture at euthanasia of mice from both genotypes by a quantitative ELISA test (MyBioSource). Systemic (anti-IgG; 375,112, GE) and mucosal (anti-IgA; 14–10-01, KPL) immunomodulatory antiflagellin response was measured by quantitative ELISA in a 96-well plate coated with 100 μL flagellin (from Salmonella enterica subsp. enterica serovar Typhimurium str. LT2, SRP8029, Sigma). Liver TGs were analyzed by an adapted Folch method (35). To calculate energy excretion, 24-h fecal energy content was estimated by the combustion with oxygen in a sealed bomb (Parr 6100 Calorimeter Instruments). At week 24, body composition from LRKO mice was estimated by lean and fat mass content determined by ©Bruker's Minispec Analyzer based on NMR.
Histological analysis and immunohistochemistry
Jejunal and ileal tissues were fixed in 4% PFA prior to enclosement in paraffin. α-Proximal sections of both tissues (4 μm) were stained with hematoxylin and eosin (H&E) for histological analysis and with Periodic Acid Schiff for Goblet cell mucin labeling. For immunohistochemistry experiments, sections were blocked in a PBS solution with 0.1% BSA and 0.2% Triton for Ki67 staining. Sections were then incubated overnight at 4°C with rabbit anti-Ki67 (1:200, GeneTex), to label proliferative cells before labeling with EnVision+ System-HRP (Dako Canada Inc.).
Atherosclerosis quantification
Atheroma lesions were quantified in dissected and stained aorta from LRKB100 mice, according to the en face technique, as previously described (36). Briefly, excised aortas were stored at 4°C in 5% formalin. Perivascular adipose tissue was removed, and the aorta was bisected exposing the intima layer. The aorta was then pinned en face and stained with 0.5% Sudan IV. Images were taken using a camera attached to a Leica MZ6 dissecting microscope. Atherosclerosis severity was estimated as a percentage of atherosclerotic lesion area corrected by the total aortic area with the use of ImageJ software (V. 1.51j8, NIH).
Echocardiography
Transthoracic echocardiography was performed in LRKO mice at weeks 0, 12, and 24 under isoflurane anesthesia with the L15–7io (5–12 Megahertz) and S12–4 (4–12 Megahertz) probes connected to a Philips HD11XE ultrasound system (Philips Healthcare Ultrasound). In short, left ventricular (LV) dimensions: LV interior diameter at end-diastole (LVIDd), LV relative wall thickness (LVRWT), and LV outflow-tract diameter (LVOT) were acquired in M-mode imaging of parasternal short-axis view. Fractional shortening (LVFS), ejection fraction (EF), and LV mass calculations were based on LV dimensions. Transmitral (A and E wave) and LVOT flow velocity were accessed by pulsed-wave Doppler and mitral annulus motion velocity (E´ wave) by Doppler tissue imaging. Stroke volume (SV) was based on LVOT flow velocity and cardiac output (CO) was estimated by the product of SV and heart rate (HR).
16S ribosomal DNA amplification and sequencing
Fresh fecal samples collected at weeks 1, 6, and 12 (WT mice) and 12 and 24 (LRKO mice) were stored at −80°C. Bacterial genomic DNA was extracted using a DNA extraction kit (Quick-DNA fecal/soil microbe, Zymo Research). Extracted DNA was stored at −20°C for subsequent 16S amplification of the V3-V4 region as previously described (37).
Microbiota data analysis
16S rRNA gene-based amplicon sequencing was performed on fecal samples from mid- and end-protocol harvested at weeks 6 and 12 in WT mice, or weeks 12 and 24 in LRKO mice. Fecal samples from WT mice were also analyzed at week 1 to determine a possible early shift in the gut microbiota with dairy treatments. Forward and reverse primers were removed from 16S rRNA gene amplicons using Cutadapt (v1.14) (38). Sequence reads were analyzed using the DADA2 package (v1.5.0) (39) in R (http://www.R-project.org). Forward and reverse reads were first trimmed to remove low-quality regions. Sequences with an expected error threshold >2 and >4 for the forward and reverse reads, respectively, with ambiguous bases, and with a quality score ≤2 were discarded. Dereplication and denoising of filtered sequences were carried out using DADA2 default parameters. Denoised forward and reverse reads were merged (all reads with any mismatches were removed) and searched for chimeras. Taxonomic assignment of amplicon sequence variants (ASVs) was performed using the ribosomal database project (RDP) classifier algorithm (v2.2) (40) trained against the Silva database 132 (41). In order to normalize sampling effort, samples were rarefied to an even sampling depth of 10,456 sequences. At week 12, the Shannon index was calculated as a measure of α-diversity and principal coordinate analysis (PCoA) was generated using the Bray–Curtis dissimilarity metric.
Linear discriminant analysis effect size (LEfSe) was performed to identify genera differentially enriched in the between-group comparisons and to evaluate the reproducibility of these results among the 2 genotypes (42). A significant difference was inferred when P < 0.05 with a linear discriminant analysis (LDA) score threshold ≥2.5.
Gene expression analysis by real-time PCR
Total RNA was isolated from homogenized jejunum and ileum using TRIzol reagent (Sigma-Aldrich). One microgram of RNA was reverse transcribed using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems) and real-time qPCR was performed with the Quantitec SYBR Green PCR kit (Qiagen) on the Rotor-Gene 6000 system (Corbett Robotics Inc.). Gene expression was estimated by the ΔΔCt method and the ubiquitous isoform of porphobilinogen deaminase was used as the reference gene.
Statistical analyses
Data were tested for Gaussian distribution and variance homogeneity and subsequently tested using a 1-factor ANOVA or the equivalent Kruskal–Wallis nonparametric test with a Tukey or Dunn posthoc test to calculate significance levels between groups (GraphPad). Glycemia and insulinemia during OGTT data were statistically compared using 2-factor repeated measures ANOVA with a Tukey posthoc test (Sigmaplot). Means without a common letter significantly differ. All results were considered statistically significant at P < 0.05. Genotypes were not compared via 2-factor ANOVA since the 2 studies were handled in different animal facilities, by different students, and were not conducted in parallel (e.g. the WT study was conducted first and a few months apart from the LRKO study).
Results
Nutrient composition of fermented dairy products
Final powders of dairy protein products contained 55–57% protein and 1% fat. Lactose represented 28% of MP, 13% of FMP, and 3% of YP. Galactose was absent from MP and represented 4% of FMP and 11% of YP (Supplemental Table 1). The final bacterial concentration was ∼109 CFU/g for YP and 108 CFU/g for FMP (Supplemental Table 2). The peptide molecular weight distribution was characterized for each dairy protein product. YP had the highest degree of proteolysis followed by FMP. After fermentation, YP contained only 34% peptides >10 kDa (90% in MP and 52% in FMP) and 46% had a molecular weight lower than 2 kDa (6% for MP and 32% for FMP) (Supplemental Figure 3).
Fermented dairy protein consumption shifts gut microbiota profile independently of the mouse genotype
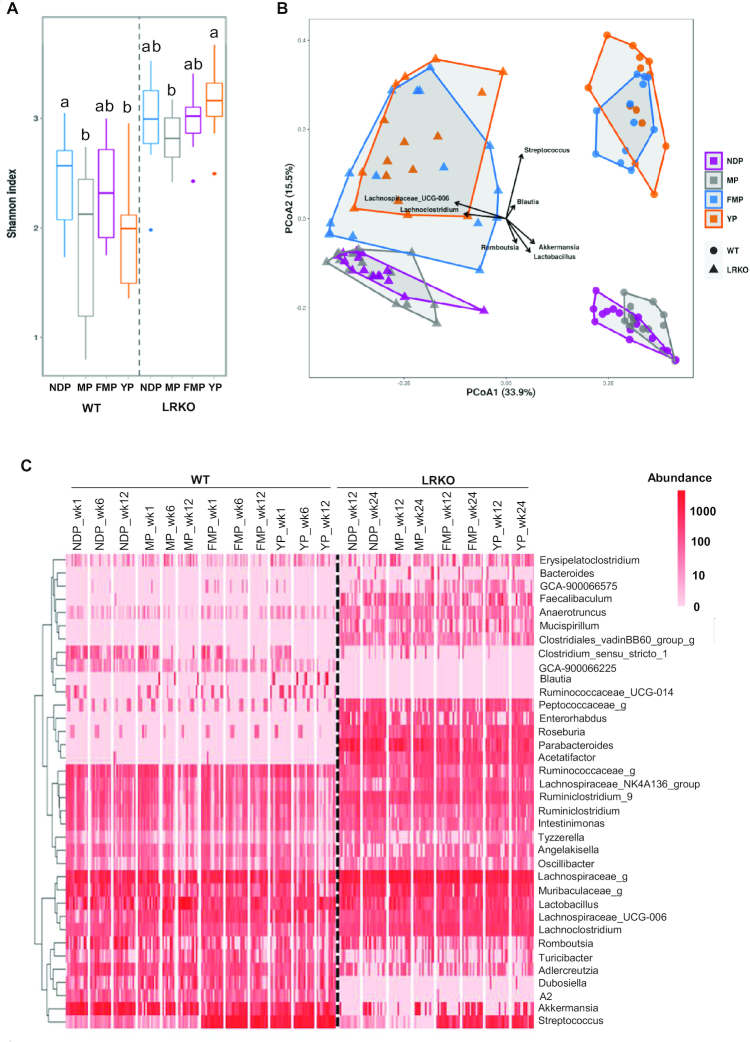
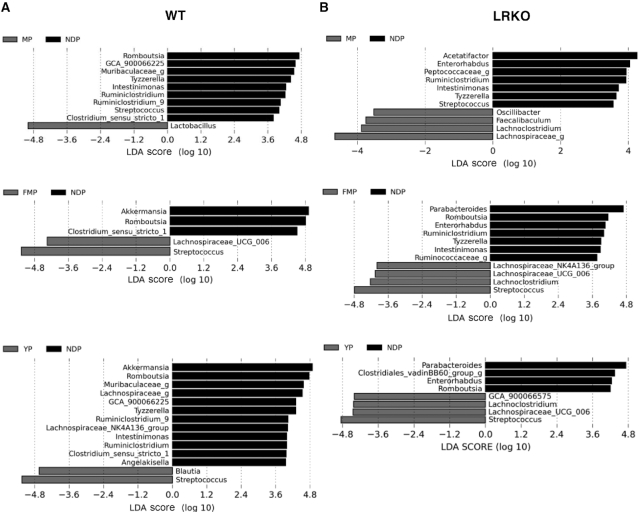
The Shannon index indicated that MP- and YP-fed WT mice had lower α-diversity than NDP-fed WT mice, whereas LRKO mice fed with a YP diet had increased α-diversity compared with mice fed MP (Figure 1A). PCoA showed a distinct genotype- and diet-related metagenomic profile, where mice fed with both fermented dairy products had the microbiota clustered apart from the nonfermented protein fed in both genotypes along the second axis explaining 15.5% of the variance. This suggests that dairy fermentation significantly modulates the gut microbiota profile (Figure 1B). Genus-level complete-linkage clustering, illustrated by a heat map, also revealed a distinct profile according to genotype (Figure 1C). Indeed, several genera were exclusively found in WT (e.g. GCA_900066225, Blautia, Ruminococcaceae_UCG-014, and A2) or LRKO (e.g. Bacteroides, Faecalibaculum,Mucispirillum, Clostridiales_vadinBB60_group_g, and Parabacteroides) mice, which was confirmed by LEfSe analysis (Figure 1C, Supplemental Figure 4). LEfSe was used to identify differentially abundant bacteria between groups after 12 wk of obesogenic diets. Comparisons of the 2 mouse models revealed that WT-derived fecal microbiota also stood out from that of LRKO by an enrichment in Akkermansia, Adlercreutzia, Dubosiella, Turicibacter, Streptococcus, and Clostridium_sensu_stricto_1 and depletion of Faecalibaculum, Tyzzerella, Oscillibacter, Anaerotruncus, Intestinimonas, GCA_900066575,Ruminiclostridium_9,Peptococcaceae_g,Roseburia,Enterorhabdus,Acetatifactor, and Lachnospiraceae_g (Supplemental Figure 4). However, regardless of the differences in the genera profile inherent to the genetic background of each mouse model, fermented dairy protein products were found to consistently modulate the relative abundance of some bacteria. As depicted in LEfSe analysis (Figure 2A, B), the taxon associated with Lachnospiraceae_UCG_006 was relatively more abundant after fermented dairy protein product intake, except for YP-fed WT mice, whereas the genus Romboutsia was underrepresented in fecal samples of mice fed the fermented protein, compared with their NDP-fed counterparts (Figure 2A, B and Supplemental Figure 5). Furthermore, LEfSe confirmed the heat map findings and revealed an enrichment of the Streptococcus genus in mice fed either FMP or YP when compared with their respective NDP-fed mice in both genotypes (Figures 1C, 2A, B, and Supplemental Figure 5). Accordingly, Streptococcus is the main genus clustering fermented dairy protein products as revealed by the PCoA (Figure 1B). Streptococcus and Lactobacillus are the most common genera of LAB present in dairy products. As the main players in milk fermentation, LAB convert lactose into lactic acid (43) and contribute to the release of many bioactive compounds such as conjugated linoleic acid, bioactive peptides, vitamins, and γ-aminobutyric acid (44). Lactobacillus delbrueckii and Streptococcus thermophilus were both used in YP production, whereas Lactobacillus helveticus fermentation produced FMP. LEfSe analyses did not identify a specific Streptococcus species involved in fermented dairy protein modulation, establish significant differences between groups in Lactobacillus genus relative abundance (Figures 2 and Supplemental Figure 6A), or detect Lactobacillus helveticus presence. However, we were able to identify with reasonable confidence 2 species comprised in the Lactobacillus genus. As expected, Lactobacillus delbrueckii, which was used to ferment the YP preparation, was exclusively enhanced in YP-fed mice feces in both WT and LRKO models (Supplemental Figure 6B). Thus, our results showed that both fermented dairy protein commonly shifted gut microbiota populations despite a specific genetic microbial signature.
FIGURE 1.
Gut microbiota profiles of WT and LRKO mice after 12 wk of NDP, MP, YP, or FMP feeding. (A) Shannon α-diversity index, (B) principal coordinate analysis of fecal samples collected at week 12, and (C) heat map representing complete-linkage clustering based on the Pearson correlation coefficients of the abundance (on a log10 scale) of ASVs at the genus level. In A, values are median, upper quartile, lower quartile, maximum, and minimum expressed as box-Whisker plot. In B, arrows represent the significant (P < 0.05) correlations between PCoA axes and abundances of bacterial genera of interest. In C, “g” at the end of taxon denotes unclassified genus. n = 14–15 for WT mice or n = 12–16 for LRKO mice. Within genotype, labeled means without a common letter differ, P < 0.05. ASV, amplicon sequence variant; FMP: fermented milk product; LRKO, LDLr−/- ApoB100/100 backcrossed on C57BL/6J background; MP: milk product; NDP: nondairy protein; PCoA, principal coordinate analysis; WT, wild type; YP: yogurt product.
FIGURE 2.
Gut microbiota signature of WT and LRKO mice fed NDP, MP, YP, or FMP diet for 12 wk. Linear discriminant analysis with effect size analysis of bacterial taxa present in fecal samples from (A) WT mice and (B) LRKO mice at week 12 comparing MP versus NDP, FMP versus NDP, and YP versus NDP. “g” at the end of taxon denotes unclassified genus. FMP: fermented milk product; LDA, linear discriminant analysis; LRKO, LDLr−/- ApoB100/100 backcrossed on C57BL/6J background; MP: milk product; NDP: nondairy protein; WT, wild type; YP: yogurt product.
Dairy protein product consumption leads to a distinct microbiota signature
We next compared the individual effect of different dairy protein products on genus abundance and found that Tyzzerella, Intestinimonas, Ruminiclostridium, and Streptococcus were underrepresented in fecal samples from MP-fed mice compared with NDP-fed mice in both genotypes (Figure 2A, B and Supplemental Figure 7). MP feeding in WT mice led to fecal overrepresentation of Lactobacillus, whereas Oscillibacter, Faecalibaculum, Lachnoclostridium, and Lachnospiraceae_g were the most relative abundant genera in MP-fed LRKO mice (Figure 2A, B and Supplemental Figure 7). Some specific effects of both fermented products were also seen on the fecal microbiota of WT and LRKO mice. In WT mice, we observed an enrichment of fecal Akkermansia and Clostridium_sensu_strico_1 versus NDP fed, whereas in LRKO mice, Parabacteroides and Enterorhabdus were underrepresented and Lachnoclostridium was overrepresented following fermented dairy consumption (Figure 2A, B and Supplemental Figure 7).
Apart from the common effects induced by the intake of both fermented products, FMP feeding of LRKO mice also exerted a distinct effect on the relative abundance of some bacteria, as revealed by the depletion of Ruminiclostridium, Tyzzerella, Intestinimonas, and Ruminococcaceae_g and the increased relative abundance of Lachnospiraceae_NK4A136_group when compared with NDP-fed mice (Figure 2B and Supplemental Figure 8). On the other hand, YP feeding selectively reduced the relative abundance of Angelakisella, Ruminiclostridium, Intestinimonas, Lachnospiraceae_NK4A136_group,Ruminiclostridium_9,Tyzzerella,Muribaculaceae_g, and Lachnospiraceae_g, while increasing the concentration of Blautia compared with NDP feeding in WT mice (Figure 2A and Supplemental Figure 9). In LRKO mice, the differential effect of YP consumption led to an overrepresentation of Clostridiales_vadinBB60_group_g (Figure 2B and Supplemental Figure 9). Thus, besides a global fermentation effect, YP and FMP distinctly modulated gut microbiota in both genotypes, leading us to further examine intestinal immunity and integrity which are intimately related to gut microbes.
YP feeding increases expression of genes involved in intestinal immunity and integrity compared with NDP in WT mice
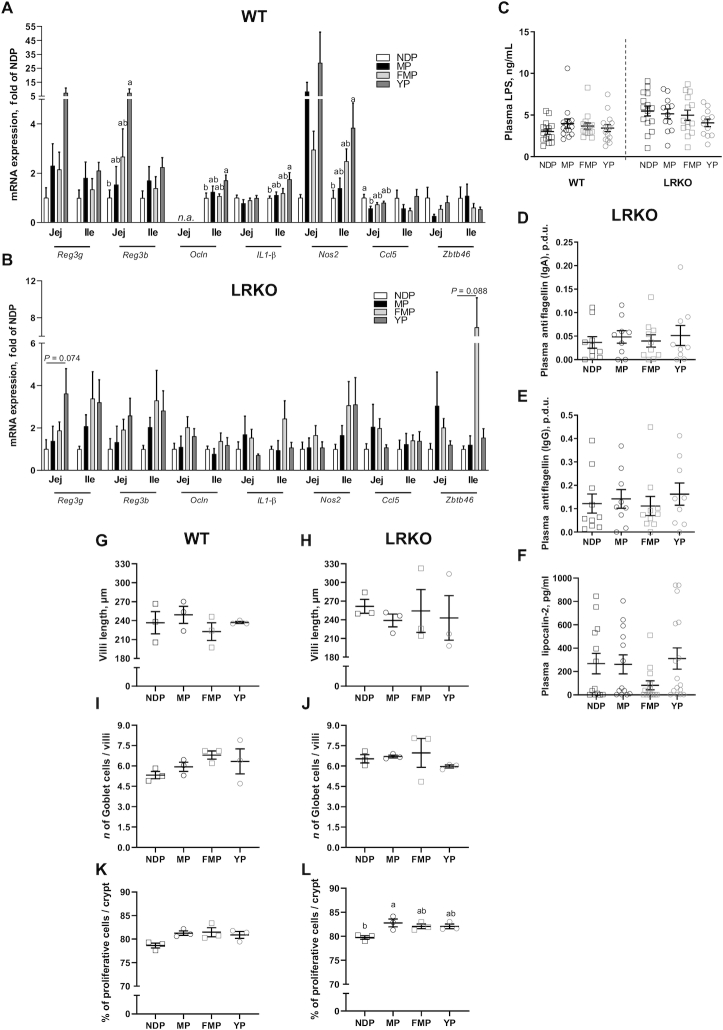
Genes involved in intestinal immunity and structure were evaluated in the jejunum and ileum of both genotypes. YP-fed WT mice showed increased Ocln,Il1-β, and Nos2 gene expression in the ileum, as well as increased Reg3b in the jejunum compared with NDP feeding (Figure 3A). Furthermore, YP feeding was shown to upregulate Reg3g gene expression in the jejunum of LRKO mice (P-trend = 0.074, Figure 3B). These data strongly suggest that YP exerts a selective small intestine immune response potentially improving intestinal integrity. In addition, MP feeding resulted in reduced Ccl5 (RANTES) gene expression in the jejunum of WT mice compared with NDP feeding (Figure 3A).
FIGURE 3.
Intestinal immunity and morphology of WT and LRKO mice fed NDP, MP, YP, or FMP diet for 12 or 24 wk, respectively. Jejunal and ileal immunoregulatory gene expression (Reg3g, Reg3b, Ocln, Il1b, Nos2, Ccl5, Zbtb46) was quantified by qPCR in (A) WT mice and (B) LRKO mice, n = 8–11 (WT and LRKO). (C) Plasma LPS of both genotypes, n = 14–15 (WT) or n = 12–15 (LRKO). (D) Mucosal (IgA) and (E) systemic (IgG) antiflagellin immune defense, n = 9–10, and (F) plasma lipocalin-2 of LRKO mice, n = 13–16. Villi length, Goblet cells, and cell proliferation rate were quantified in the ileum from WT (G, I, and K, respectively) and LRKO (H, J, and L, respectively) mice, n = 3 (WT and LRKO). Values are mean ± SEM. All P values were determined by 1-factor ANOVA or Kruskal–Wallis test followed by Tukey posthoc test. Within genotype, labeled means without a common letter differ, P < 0.05. FMP, fermented milk product; Ile, ileum; Jej, jejunum; LRKO, LDLr−/- ApoB100/100 backcrossed on C57BL/6J background; MP, milk product; NDP, nondairy protein; p.d.u., protocol data unit; WT, wild type; YP, yogurt product.
Disruption of the gut barrier increases gut permeability, thus favoring opportunistic pathogens and their fragments to leak into the circulation. Thus, a higher circulating concentration of LPSs is often used as a marker of increased intestinal permeability. Despite upregulating Ocln gene expression in the ileum of WT mice, a feature often associated with reduced intestinal permeability, YP feeding did not result in reduced circulating LPSs. In LRKO mice we also failed to detect any changes in either LPS concentrations as well as in the plasma titers of antiflagellins and lipocalin-2, which are other established readouts of intestinal integrity (Figure 3C–F).
We next evaluated the impact of dairy protein products on the intestinal epithelium. In the ileum, no dietary modulations affected villi length or Goblet cell number in either genotype (Figure 3G–J). Only MP-LRKO-fed mice showed increased proliferative cell number compared with NDP (Figure 3L), all other groups had no change (Figure 3K–L). In the jejunum, YP feeding, in LRKO mice only, reduced villi length compared with FMP-fed mice (Supplemental Figure 10A,B), but dairy protein products did not impact Goblet or proliferative cells in either genotype (Supplemental Figure 10C–F).
YP consumption ameliorates insulin sensitivity compared with nonfermented MP in LRKO mice
Dairy protein products did not affect energy intake, body weight, fat mass gain, or fasting glycemia and insulinemia (Table 2). In LRKO mice, we observed a slight increase of lean mass gain in FMP-fed mice compared with NDP-fed controls (Table 2). Interestingly, the YP-fed WT mice showed decreased energy excretion, but this did not affect total weight gain (Table 2).
TABLE 2.
Metabolic data of WT and LRKO mice fed NDP, MP, YP, or FMP diet for 12 or 24 wk, respectively
| WT | LRKO | |||||||
|---|---|---|---|---|---|---|---|---|
| NDP | MP | FMP | YP | NDP | MP | FMP | YP | |
| Initial BW, g1,2 | 21.7 ± 0.36 | 21.8 ± 0.39 | 21.6 ± 0.42 | 21.7 ± 0.36 | 22.9 ± 0.43 | 22.9 ± 0.51 | 22.7 ± 0.58 | 23.2 ± 0.64 |
| Final BW gain, g1,2 | 15.4 ± 0.98 | 16.2 ± 1.08 | 16.2 ± 0.86 | 16.8 ± 1.24 | 22.9 ± 1.25 | 24.6 ± 1.03 | 24.1 ± 1.52 | 21.4 ± 1.47 |
| Food intake, g/d1,2 | 2.12 ± 0.05 | 2.24 ± 0.05 | 2.27 ± 0.06 | 2.15 ± 0.04 | 2.25 ± 0.04 | 2.28 ± 0.04 | 2.37 ± 0.04 | 2.30 ± 0.04 |
| Energy intake, kcal/d1,2 | 11.7 ± 0.28 | 12.2 ± 0.28 | 12.2 ± 0.32 | 11.5 ± 0.24 | 13.7 ± 0.27 | 13.7 ± 0.27 | 14.4 ± 0.26 | 13.9 ± 0.26 |
| Energy excretion, kcal/d1,2,3 | 0.71 ± 0.06a | 0.79 ± 0.08a | 0.87 ± 0.07ab | 0.47 ± 0.04b | 1.18 ± 0.07 | 1.30 ± 0.09 | 1.18 ± 0.07 | 1.18 ± 0.07 |
| Final visceral adiposity, g1,2 | 3.32 ± 0.22 | 3.25 ± 0.19 | 3.48 ± 0.20 | 3.35 ± 0.22 | 3.06 ± 0.23 | 3.07 ± 0.17 | 3.18 ± 0.23 | 3.03 ± 0.15 |
| Final subcutaneous adiposity, g1,2 | 1.06 ± 0.11 | 1.15 ± 0.12 | 1.11 ± 0.09 | 1.18 ± 0.16 | 2.11 ± 0.17 | 2.23 ± 0.16 | 2.15 ± 0.20 | 1.88 ± 0.17 |
| Final fat mass gain, g1,2 | — | — | — | — | 11.8 ± 1.15 | 12.9 ± 0.9 | 14.5 ± 0.68 | 11.0 ± 1.25 |
| Final lean mass gain, g1,2,3 | — | — | — | — | 5.17 ± 0.52b | 5.85 ± 0.43ab | 7.03 ± 0.50a | 4.68 ± 0.51b |
| Fasting blood glycemia, mmol/L1,2 | 10.3 ± 0.52 | 11.1 ± 0.44 | 10.6 ± 0.45 | 11.2 ± 0.59 | 9.27 ± 0.29 | 10.2 ± 0.32 | 9.34 ± 0.42 | 9.17 ± 0.39 |
| Fasting plasma insulinemia, ng/mL1,2 | 1.14 ± 0.17 | 1.25 ± 0.22 | 1.09 ± 0.12 | 1.20 ± 0.20 | 2.22 ± 0.24 | 2.44 ± 0.25 | 2.22 ± 0.19 | 2.05 ± 0.31 |
Values are mean ± SEM, n = 8–15 (WT and LRKO).
Statistical differences were determined by 1-factor ANOVA followed by a posthoc Tukey test.
Within genotype, labeled means without a common letter differ, P < 0.05.
BW, body weight; FMP, fermented milk product; LRKO, LDLr−/- ApoB100/100 backcrossed on C57BL/6J background; MP, milk product; NDP, nondairy protein; WT, wild type; YP, yogurt product.
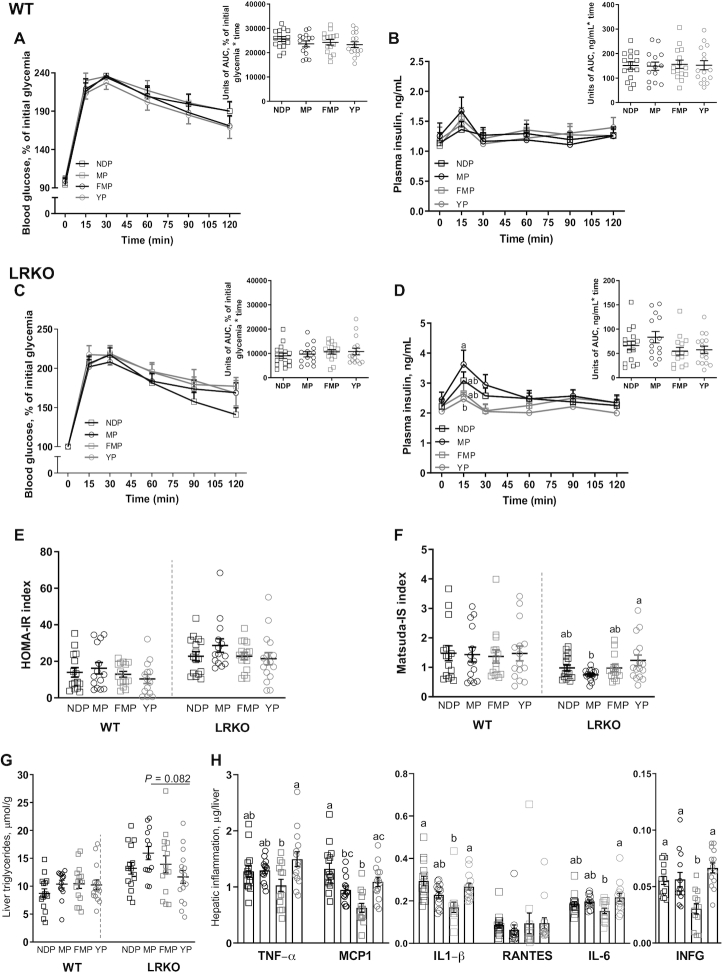
Regarding glucose homeostasis, no differences were observed among WT mice groups for both glucose and insulin concentrations during OGTT (Figure 4A, B). Even without dietary modulation of glycemia (Figure 4C), LRKO mice fed with the YP diet showed a reduced insulin peak at 15 min after glucose challenge (Figure 4D). This effect of YP on insulinemia did not affect the HOMA-IR index but resulted in a higher Matsuda IS index compared with the nonfermented MP diet (Figure 4E, F), further indicating that feeding YP improved insulin sensitivity in this genetic model of dyslipidemia.
FIGURE 4.
Insulin sensitivity and hepatic inflammation of WT and LRKO mice fed NDP, MP, YP, or FMP diet. (A) OGTT and inserted AUC, (B) insulinemia during OGTT and inserted AUC of WT mice, n = 14–15. (C) OGTT and inserted AUC, (D) insulinemia during OGTT and inserted AUC of LRKO mice, n = 13–16. (E) HOMA-IR, n = 14–15 (WT) or n = 14–16 (LRKO) and (F) Matsuda-IS, n = 14–15 (WT) or n = 14–16 (LRKO) of both genotypes. (G) Hepatic triglycerides content of both genotypes, n = 13–15 (WT) or n = 13–16 (LRKO), (H) as well as hepatic inflammatory cytokines of LRKO mice were quantified, n = 13–16. Values are mean ± SEM. Differences between groups for hepatic triglycerides and inflammation, as well as HOMA-IR and Matsuda-IS were determined by 1-factor ANOVA followed by Tukey posthoc test. Two-factor repeated measures ANOVAs were applied to test dietary effect on glycemia and insulinemia during OGTT in both genotypes. Within genotype, labeled means without a common letter differ, P < 0.05. FMP, fermented milk product; LRKO, LDLr−/- ApoB100/100 backcrossed on C57BL/6J background; MP, milk product; NDP, nondairy protein; OGTT, oral glucose tolerance test; RANTES, regulated upon activation, normal T Cell expressed and presumably secreted; WT, wild type; YP, yogurt product.
Dairy protein products modulate plasma lipids and nonalcoholic fatty liver disease development in LRKO mice
We next evaluated the impact of dairy protein products on the plasma lipid profile and nonalcoholic fatty liver disease (NAFLD) in dyslipidemic LRKO mice. Mice fed MP had lower concentrations of plasma cholesterol compared with mice fed NDP (Supplemental Figure 11B). YP-fed LRKO mouse livers tended to have a lower accumulation of TGs compared with the MP-fed group (P = 0.082; Figure 4G). Furthermore, although FMP-fed LRKO mice showed similar steatosis when compared with NDP-fed animals, we found that these mice showed reduced hepatic inflammation, as revealed by decreased expression of inflammatory markers, including monocyte chemoattractant protein 1 (MCP1), IL1-β, and IFN-γ (Figure 4H). This suggests that FMP potentially reduces NAFLD development in HFHS-fed LRKO mice.
Fermented dairy product consumption reduces circulating intracellular and vascular adhesion molecules in LRKO mice
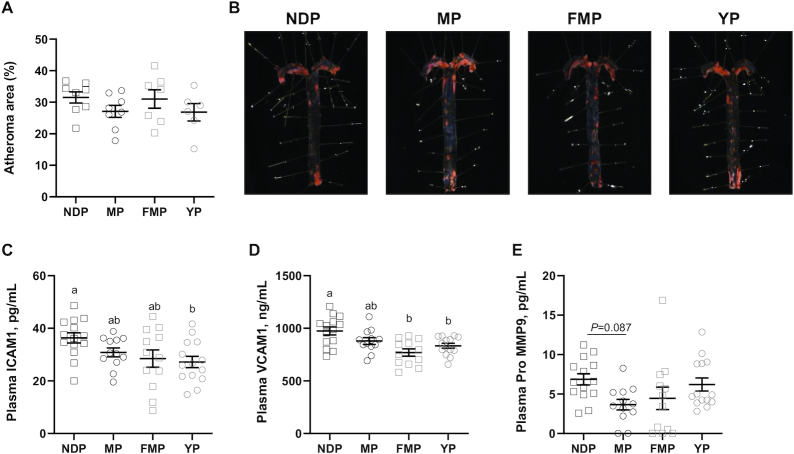
We next evaluated the impact of dairy protein products on atherosclerosis development and CVD risk markers in LRKO mice. Dairy protein products (Figure 5A, B) did not affect atherosclerotic lesions. However, both fermented dairy products significantly reduced the concentrations of adhesion molecules. Indeed, YP-fed LRKO mice showed reduced intracellular adhesion molecule 1 (ICAM1) and VCAM1 circulating concentrations (Figure 5C–D), whereas FMP feeding led to a reduced circulating amount of VCAM1 (Figure 5D). Dairy protein products had no impact on plasma cytokines (Supplemental Figure 11F–J) nor circulating promatrix metallopeptidase 9 (MMP9) (Figure 5E).
FIGURE 5.
Circulating intracellular and vascular adhesion molecules of LRKO mice fed NDP, MP, YP, or FMP diet for 24 wk. (A) Aortic atherosclerosis quantification, n = 6–8, (B) most representative en face image of atherosclerosis, (C) plasma ICAM1, (D) plasma VCAM1, and (E) plasma pro MMP9, n = 12–14. Values are mean ± SEM. All P values were determined by 1-factor ANOVA followed by Tukey's post hoc test. Within genotype, labeled means without a common letter differ, P < 0.05. FMP: fermented milk product; ICAM1, intracellular adhesion molecule 1; LRKO, LDLr−/- ApoB100/100 backcrossed on C57BL/6J background; MP: milk product; MMP9, matrix metallopeptidase 9; NDP: nondairy protein; VCAM1, vascular adhesion molecule 1; YP: yogurt product.
Finally, we assessed the potential impact of dairy protein products on cardiac function in LRKO mice (Table 3). Dairy protein products did not affect cardiac hypertrophy determined by LV mass and LVIDd. LV function as measured by EF and fractional shortening (FS) and was not changed. Dairy protein products did not affect HR, CO, or SV. Diastolic function, as determined from early (E) mitral flow velocity corrected by the early diastolic velocity (E’) of medial mitral annulus, was not changed by any treatment.
TABLE 3.
Echocardiography data of LRKO mice fed NDP, MP, YP, or FMP diet at weeks 12 and 24
| 12 wk | 24 wk | |||||||
|---|---|---|---|---|---|---|---|---|
| NDP | MP | FMP | YP | NDP | MP | FMP | YP | |
| LV mass, mg1 | 212 ± 2.21 | 214 ± 4.27 | 209 ± 3.35 | 215 ± 22.1 | 217 ± 2.5 | 220 ± 4.34 | 226 ± 4.96 | 221 ± 4.18 |
| LVIDd, mm1 | 3.68 ± 0.08 | 3.77 ± 0.11 | 3.56 ± 0.08 | 3.77 ± 0.08 | 3.75 ± 0.06 | 3.79 ± 0.11 | 3.90 ± 0.11 | 3.83 ± 0.10 |
| FS, %1 | 38.4 ± 2.20 | 40.8 ± 0.92 | 42.9 ± 1.80 | 38.1 ± 1.53 | 33.3 ± 1.28 | 34.3 ± 1.16 | 30.5 ± 1.21 | 36.6 ± 1.32 |
| EF, %1 | 61.4 ± 2.63 | 64.6 ± 1.10 | 66.8 ± 2.42 | 61.3 ± 1.88 | 68.5 ± 1.68 | 70 ± 1.49 | 64.6 ± 1.75 | 72.8 ± 1.52 |
| SV, mL1 | 0.04 ± 0.002 | 0.05 ± 0.01 | 0.05 ± 0.003 | 0.05 ± 0.004 | 0.04 ± 0.02 | 0.04 ± 0.002 | 0.05 ± 0.01 | 0.04 ± 0.02 |
| Heart rate, bpm1 | 485 ± 25.3 | 463 ± 15 | 458 ± 14.6 | 469 ± 24.6 | 486.2 ± 15.2 | 469 ± 19.8 | 476 ± 14.7 | 488 ± 21.43 |
| CO, L/min1 | 0.02 ± 0.001 | 0.02 ± 0.001 | 0.02 ± 0.001 | 0.02 ± 0.002 | 0.04 ± 0.0017 | 0.02 ± 0.001 | 0.03 ± 0.002 | 0.02 ± 0.0008 |
| Mitral E/E′ ratio1 | 27.9 ± 1.48 | 29.6 ± 1.65 | 32.4 ± 2.81 | 25.6 ± 0.85 | 35 ± 1.37 | 32.4 ± 2.81 | 29.2 ± 1.94 | 32.4 ± 2.81 |
Values are mean ± SEM, n = 8–15.
CO: cardiac output; E/E′, mitral inflow measured by pulsed-wave over tissue Doppler; EF, ejection fraction; FMP, fermented milk product; FS, fractional shortening; LRKO, LDLr−/- ApoB100/100 backcrossed on C57BL/6J background; LV, left ventricular; LVIDd, LV interior diameter in end-diastole; MP, milk product; NDP, nondairy protein; SV, stroke volume; YP, yogurt product.
Discussion
Epidemiological evidence suggests that the consumption of dairy protein products reduces the risk of cardiometabolic diseases (45), thus supporting their inclusion in dietary guidelines worldwide. Bioactive metabolites (e.g. peptides) released during dairy fermentation and gastrointestinal digestion are considered one of the potential mechanisms whereby dairy consumption may promote health benefits (46). Taking advantage of mouse models of diet-induced obesity and CVD, we found that replacing 50% of nondairy protein with dairy protein, and especially with fermented dairy protein/peptides, promotes cardiometabolic benefits potentially through their impact on the gut microbiota. Gut dysbiosis, indicated by altered microbial composition and function, is associated with many metabolic disorders, including obesity (47). It is well documented that diet-induced obesity and metabolic syndrome are not only associated with adipose tissue expansion and inflammation, but also with altered gut integrity and perturbed intestinal microbiota composition. Of the different dairy protein products, yogurt has been the most extensively studied as a gut microbiome modulator not only because of its high nutritional quality, but also because it contains LAB (classic starters) and, in some instances, probiotics (46). However, whether the purported metabolic benefits of fermented dairy are due to their bacterial content and/or their nutritional matrix remains vastly unexplored. In our study, YP production was started with Streptococcus thermophilus and Lactobacillus delbrueckii subsp. bulgaricus, whereas Lactobacillus helveticus was used for FMP fermentation. LAB (e.g. Streptococcus and Lactobacillus) are classical fermented dairy starters and are delivered to the gut lumen after dairy ingestion. Once in the gastrointestinal tract LAB, particularly Lactobacillus, are reported to upregulate tight junctions in the gut epithelium (48) as well as intestinal immunity (49). Our data show that, despite being a nonfermented product, MP feeding is associated with overrepresentation of a Lactobacillus (uncharacterized species) in fecal samples from WT mice, that may be related to lactose supply (e.g. 27.81% of lyophilized MP).
YP feeding leads to an exclusive increase in the relative abundance of only 1 of the 2 bacterial strain starters, Lactobacillus delbrueckii, previously associated with immunoregulatory potential (50). Genome sequencing of Lactobacillus delbrueckii ssp. bulgaricus has revealed high numbers of rRNA and tRNA genes together with other genomic features, supporting the hypothesis that this bacteria is in a phase of rapid evolution (51).
Fermented dairy product consumption significantly modulates the gut microbiota, in which the Streptococcus genus seemed to have a pivotal role as the main taxa enriched in fecal samples of mice fed with both FMP and YP. Similarly, a variety of studies have described the impact of fermented dairy consumption on the gut microbiome (52–54). Furthermore, the consumption of fermented dairy products (53), particularly yogurt (55), was also previously shown to increase Streptococcus abundance.
Nutrient satiation with obesogenic diets is known to decrease SCFA-producing bacteria (56) while increasing the intestinal absorptive surface by augmenting villi length (57). Our data show that both fermented dairy products increased health-promoting bacteria without affecting intestinal cell proliferation. Furthermore, YP feeding led to increased expression of intestinal immune genes compared with NDP-fed mice, agreeing with reports on yogurt consumption in mice and humans (58–63), although most of the previously tested products also contained probiotics and not only LAB starters. In addition, our study indicates that substituting nondairy protein for fermented dairy protein is sufficient to change the composition of the gut microbiota, leading to beneficial metabolic effects.
Our hypothesis was that various peptides with immunometabolic properties are released in the intestinal lumen after hydrolysis of dairy-derived proteins, and especially with fermented products, by either LAB or the process of digestion, and that this translates into cardiometabolic benefits. Accordingly, YP-fed LRKO mice showed improved insulin sensitivity compared with nonfermented dairy-fed counterparts. Furthermore, YP feeding resulted in increased expression of Reg3 genes in the small intestines of both genotypes, which might contribute to the activation of gut defense mechanisms against invading pathogens (64). On the other hand, FMP consumption decreases inflammatory markers in the liver compared with nondairy receivers. Hepatic inflammation is a key process leading to NAFLD (65) and a mechanistic link connecting liver fat accretion to insulin resistance (66). Importantly, fermented dairy protein might affect host metabolism through the activation of a gut-liver axis leading to reduced hepatic inflammation and improved liver insulin sensitivity, thus reducing the risk of T2D and NAFLD.
It is well documented that adhesion molecules are key promoters of atherosclerosis and CVD risk factors (67). They exert their proatherogenic effects through facilitating leucocyte adherence to the lesion-forming site. Our study shows that fermented dairy protein consumption was associated with reduced concentrations of circulating intracellular and vascular adhesion molecules (68). Although, in these relatively short-term investigations, this did not result in reduced aortic lesions and improved cardiac function, long-term dairy consumption may reduce the development of atherosclerosis and cardiac events.
In conclusion, our results provide new insights into the mechanisms by which fermented and nonfermented dairy protein products may reduce cardiometabolic diseases. We found that replacing NDP by various dairy and fermented dairy protein products exerted important immunometabolic effects, as revealed by the modulation of specific gut microbes and markers of intestinal immunity and integrity. The effects of fermented dairy products were associated with improved insulin sensitivity and reduced NAFLD in the more severe LRKO mouse model, as compared to nonfermented dairy product (MP) consumption. Both YP and FMP also decreased systemic concentrations of proatherosclerotic adhesion molecules in LRKO mice, suggesting a potential reduction in long-term cardiometabolic risk. We further propose that the greater immunometabolic effect of both fermented dairy products might be driven by the specific action of small peptides released during proteolysis fermentation, and this will be the focus of our future work.
Limitations of this study include the potential probiotic effect of YP and FMP's starters, which was not evaluated in this study. We believe that, although the presence of living bacteria might have played a role in gut microbiota and host metabolism changes, it is very unlikely that the consumption of only 7.6 × 106–107 CFU/g of diet (∼1.7 × 105–106 CFU per day) would confer a significant modulation expected from a probiotic strategy. Indeed, it is generally considered that probiotics should be given at much higher daily dose (∼107–109 CFU/mg) in order to provide health benefits in humans (69, 70).
Experimental groups also differed in the lactose and galactose content. Indeed, comparing MP-fed mice to those consuming fermented dairy protein products, we calculated that animals consumed only 83 mg versus 39 mg for FMP and 9 mg for YP of lactose daily, representing only 3.7, 1.7, and 0.4% of total daily intake, respectively. Moreover, MP-fed mice had a very similar clustering pattern to NDP-fed mice despite having the greatest difference in lactose intake (i.e. 83 versus 0 mg daily). Likewise, YP-fed mice, despite consuming twice as much galactose as the FMP-fed mice, showed a similar gut microbiota profile. Thus, it is unlikely that product differences in either lactose or galactose contributed to changes in gut microbiota composition. However, we cannot fully discard the potential effect of lactose on gut immunity in our study as lactose was shown to increase abdominal sensitivity while increasing colonic mast cell content and advanced glycosylation end-product-specific receptor expression, despite not altering gut microbiota composition (71).
Supplementary Material
Acknowledgments
We would like to thank Dominic Lachance, Rihab Bouchareb, and Eric Plante for technical support with echocardiography. We also thank Valérie Dumais, Christine Dion, Christine Dallaire, Joanie Dupont-Morissette, and Marion Valle for their assistance with animal protocols. We also thank Patricia Mitchell for revising the manuscript. The authors’ responsibilities were as follows—LRP, ND, GP, and AM: designed the research; YP: produced and provided the dairy protein products; LRP and ND: conducted the 24-wk LRKO and the 12-wk WT animal protocols, respectively, and subsequent laboratory and statistical analyses; MJD and JLM: provided help with laboratory experiments; TVV: conducted the fecal metataxonomic analysis and DR revised it; MBou and MBl: harvested the intestines at sacrifice and performed the qPCR in the intestinal samples; MBou: prepared and analyzed histological preparations of small intestines; CA, ML, and DR: provided guidance and helped with manuscript preparation; LRP, ND, and AM: wrote the manuscript and had primary responsibility for final content; and all authors: read and approved the final manuscript.
Notes
This research was funded by Agriculture and Agri-Food Canada, with additional contributions from Dairy Farmers of Canada, the Canadian Dairy Network, and the Canadian Dairy Commission under the Agri-Science Clusters Initiative. This work was also partly funded by a Canadian Institutes for Health Research (CIHR) (FDN#143247) grant to AM.
Author disclosures: AM holds a Pfizer/CIHR Research Chair on the pathogenesis of insulin resistance and cardiovascular diseases.
As per the research agreement, aside from providing financial support, the funders had no role in the design and conduct of the studies, data collection and analysis, or interpretation of the data. Researchers maintain independence in conducting their studies, own their data, and report the outcomes regardless of the results. The decision to publish the findings rested solely with the researchers.
Supplemental Table 1 and Supplemental Figures 1–11 are available from the ‘‘Supplementary data’’ link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com.jn/.
LRP and ND shared first authorship.
Abbreviations used: AOAC, Association of Official Agricultural Chemists; ASV, amplicon sequence variant; CO, cardiac output; CVD, cardiovascular disease; EF, ejection fraction; eWAT, epididymal white adipose tissue; FMP, fermented milk product; HFHS, high-fat high-sucrose; HR, heart rate; IDF, International Dairy Federation; iWAT, inguinal white adipose tissue; LAB, lactic acid bacteria; LEfSe, linear discriminant analysis effect size; LRKO, LDLr−/- ApoB100/100 backcrossed on C57BL/6J background; LV, left ventricular; LVIDd, left ventricular interior diameter at end-diastole; LVOT, left ventricular outflow-tract diameter; MP, milk product; NAFLD, nonalcoholic fatty liver disease; NDP, nondairy protein; NPN, nonprotein nitrogen; OGTT, oral-glucose-tolerance test; PCoA, principal coordinate analysis; PFA, paraformaldehyde; rpWAT, retroperitoneal white adipose tissue; SMP, skim milk product; SV, stroke volume; T2D, type 2 diabetes; TG, triglyceride; TN, total nitrogen; UF, ultrafiltration; VCAM1, vascular adhesion molecule 1; WT, wild type; YP, yogurt product.
Contributor Information
Laís Rossi Perazza, Faculty of Medicine, Laval University, Quebec City, Quebec, Canada; Institute of Nutrition and Functional Foods, Laval University, Quebec City, Quebec, Canada.
Noëmie Daniel, Faculty of Medicine, Laval University, Quebec City, Quebec, Canada; Institute of Nutrition and Functional Foods, Laval University, Quebec City, Quebec, Canada.
Marie-Julie Dubois, Faculty of Medicine, Laval University, Quebec City, Quebec, Canada; Institute of Nutrition and Functional Foods, Laval University, Quebec City, Quebec, Canada.
Geneviève Pilon, Faculty of Medicine, Laval University, Quebec City, Quebec, Canada; Institute of Nutrition and Functional Foods, Laval University, Quebec City, Quebec, Canada.
Thibault Vincent Varin, Institute of Nutrition and Functional Foods, Laval University, Quebec City, Quebec, Canada.
Mylène Blais, Sherbrooke R & D Center, Agriculture and Agri-Food Canada, Sherbrooke, Quebec, Canada.
José Luis Martinez Gonzales, Institute of Nutrition and Functional Foods, Laval University, Quebec City, Quebec, Canada.
Michaël Bouchard, Sherbrooke R & D Center, Agriculture and Agri-Food Canada, Sherbrooke, Quebec, Canada.
Claude Asselin, Faculty of Medicine and Health Sciences, Sherbrooke University, Sherbrooke, Quebec, Canada.
Martin Lessard, Sherbrooke R & D Center, Agriculture and Agri-Food Canada, Sherbrooke, Quebec, Canada.
Yves Pouliot, Institute of Nutrition and Functional Foods, Laval University, Quebec City, Quebec, Canada.
Denis Roy, Institute of Nutrition and Functional Foods, Laval University, Quebec City, Quebec, Canada.
André Marette, Faculty of Medicine, Laval University, Quebec City, Quebec, Canada; Institute of Nutrition and Functional Foods, Laval University, Quebec City, Quebec, Canada.
References
- 1. OECD-FAO Agricultural Outlook 2017–2026 - en - OECD. [Internet]. [cited 2019 Aug 10]. Available from: https://www.oecd.org/publications/oecd-fao-agricultural-outlook-19991142.htm. [Google Scholar]
- 2. Mozaffarian D, Hao T, Rimm EB, Willett WC, Hu FB. Changes in diet and lifestyle and long-term weight gain in women and men. N Engl J Med. 2011;364(25):2392–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Romaguera D, Ängquist L, Du H, Jakobsen MU, Forouhi NG, Halkjær J, Feskens EJM, van Der ADL, Masala G, Steffen A et al. Food composition of the diet in relation to changes in waist circumference adjusted for body mass index. PLoS One. 2011;6(8):e23384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Drouin-Chartier J-P, Brassard D, Tessier-Grenier M, Côté JA, Labonté M-È, Desroches S, Couture P, Lamarche B. Systematic review of the association between dairy product consumption and risk of cardiovascular-related clinical outcomes. Adv Nutr. 2016;7(6):1026–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Alvarez-Bueno C, Cavero-Redondo I, Martinez-Vizcaino V, Sotos-Prieto M, Ruiz JR, Gil A. Effects of milk and dairy product consumption on type 2 diabetes: overview of systematic reviews and meta-analyses. Adv Nutr. 2019;10(suppl_2):S154–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Drouin-Chartier J-P, Li Y, Ardisson Korat AV, Ding M, Lamarche B, Manson JE, Rimm EB, Willett WC, Hu FB. Changes in dairy product consumption and risk of type 2 diabetes: results from 3 large prospective cohorts of US men and women. Am J Clin Nutr. 2019;110(5):1201–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Alexander DD, Bylsma LC, Vargas AJ, Cohen SS, Doucette A, Mohamed M, Irvin SR, Miller PE, Watson H, Frysek JP. Dairy consumption and CVD: a systematic review and meta-analysis. Br J Nutr. 2016;115(4):737–50. [DOI] [PubMed] [Google Scholar]
- 8. Soedamah-Muthu SS, de Goede J. Dairy consumption and cardiometabolic diseases: systematic review and updated meta-analyses of prospective cohort studies. Curr Nutr Rep. 2018;7(4):171–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dehghan M, Mente A, Rangarajan S, Sheridan P, Mohan V, Iqbal R, Gupta R, Lear S, Wentzel-Viljoen E, Avezum A et al. Association of dairy intake with cardiovascular disease and mortality in 21 countries from five continents (PURE): a prospective cohort study. Lancet. 2018;392(10161):2288–97. [DOI] [PubMed] [Google Scholar]
- 10. Soedamah-Muthu S, Verberne LDM, Ding E L, Engberink M F, Geleijnse J M. Dairy consumption and incidence of hypertension. Hypertension. 2012;60(5):1131–7. [DOI] [PubMed] [Google Scholar]
- 11. Tognon G, Rothenberg E, Petrolo M, Sundh V, Lissner L. Dairy product intake and mortality in a cohort of 70-year-old Swedes: a contribution to the Nordic diet discussion. Eur J Nutr. 2018;57(8):2869–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mozaffarian D, Wu JHY. Flavonoids, dairy foods, and cardiovascular and metabolic health: a review of emerging biologic pathways. Circ Res. 2018;122(2):369–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sultan S, Huma N, Butt MS, Aleem M, Abbas M. Therapeutic potential of dairy bioactive peptides: a contemporary perspective. Crit Rev Food Sci Nutr. 2018;58(1):105–15. [DOI] [PubMed] [Google Scholar]
- 14. Leclerc P-L, Gauthier SF, Bachelard H, Santure M, Roy D. Antihypertensive activity of casein-enriched milk fermented by Lactobacillus helveticus. Int Dairy J. 2002;12(12):995–1004. [Google Scholar]
- 15. Jäkälä P, Vapaatalo H. Antihypertensive peptides from milk proteins. Pharmaceuticals. 2010;3(1):251–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tulipano G, Sibilia V, Caroli AM, Cocchi D. Whey proteins as source of dipeptidyl dipeptidase IV (dipeptidyl peptidase-4) inhibitors. Peptides. 2011;32(4):835–8. [DOI] [PubMed] [Google Scholar]
- 17. Yang J, Chi Y, Burkhardt BR, Guan Y, Wolf BA. Leucine metabolism in regulation of insulin secretion from pancreatic beta cells. Nutr Rev. 2010;68(5):270–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ding S, Chi MM, Scull BP, Rigby R, Schwerbrock NMJ, Magness S, Jobin C, Lund PK. High-fat diet:bacteria interactions promote intestinal inflammation which precedes and correlates with obesity and insulin resistance in mouse. PLoS One. 2010;5(8):e12191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, Burcelin R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57(6):1470–81. [DOI] [PubMed] [Google Scholar]
- 20. den Besten G, Bleeker A, Gerding A, van Eunen K, Havinga R, van Dijk TH, Oosterveer MH, Jonker JW, Groen AK, Reijngound DJ et al. Short-chain fatty acids protect against high-fat diet-induced obesity via a pparγ-dependent switch from lipogenesis to fat oxidation. Diabetes. 2015;64(7):2398–408. [DOI] [PubMed] [Google Scholar]
- 21. Canfora EE, Jocken JW, Blaak EE. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat Rev Endocrinol. 2015;11(10):577–91. [DOI] [PubMed] [Google Scholar]
- 22. Johnson-Down L, Ritter H, Starkey LJ, Gray-Donald K. Primary food sources of nutrients in the diet of Canadian adults. Can J Diet Pract Res. 2006;67(1):7–13. [DOI] [PubMed] [Google Scholar]
- 23.AOAC Official Method 926.08. JAOAC 9, 44(1926); 18, 57(1935).
- 24. AOAC Official Method 927.05. JAOAC 10, 308(1927); 11, 289(1928).
- 25. Chromý V, Vinklárková B, Šprongl L, Bittová M. The Kjeldahl method as a primary reference procedure for total protein in certified reference materials used in clinical chemistry. I. A review of Kjeldahl methods adopted by laboratory medicine. Crit Rev Anal Chem. 2015;45(2):106–11. [DOI] [PubMed] [Google Scholar]
- 26. ISO 8968-3 . IDF 20–3:2004. Milk—determination of nitrogen content—Part 3: Block digestion method (semi-micro rapid routine method). International Dairy Federation, Brussels.
- 27. ISO 8968-4:. 2001 | IDF 20–4:2001. Milk—determination of nitrogen content—4: determination of non-protein-nitrogen content.
- 28. ISO 27871 | IDF 224:2011. Cheese and processed cheese—determination of the nitrogenous fractions. International Dairy Federation, Brussels.
- 29. ISO 1735:2004. | IDF 5:2004. Cheese and processed cheese products. Determination of fat content. Gravimetric method.
- 30. FIL/IDF 9C:1987. “Determination of fat content,” Tech. Rep., International Dairy Federation, Brussels, Belgium, 1987.
- 31. Oh HI, Shin TS, Chang EJ. Determination of cholesterol in milk and dairy products by high-performance liquid chromatography. Asian Australas J Anim Sci. 2001;14(10):1465–9. [Google Scholar]
- 32. AOAC Official Method 930.30.
- 33. Le Barz M, Daniel N, Varin TV, Naimi S, Demers-Mathieu V, Pilon G, Audy J, Laurin É, Roy D, Urdaci MC et al. In vivo screening of multiple bacterial strains identifies Lactobacillus rhamnosus Lb102 and Bifidobacterium animalis ssp. lactis Bf141 as probiotics that improve metabolic disorders in a mouse model of obesity. FASEB J. 2019;33(4):4921–35. [DOI] [PubMed] [Google Scholar]
- 34. Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22(9):1462–70. [DOI] [PubMed] [Google Scholar]
- 35. Folch J, Lees M, Stanley GHS. A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem. 1957;226(1):497–509. [PubMed] [Google Scholar]
- 36. Ko KA, Fujiwara K, Krishnan S, Abe J-I. En face preparation of mouse blood vessels. J Vis Exp. 2017;(123):55460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Anhê FF, Nachbar RT, Varin TV, Trottier J, Dudonné S, Barz ML, Feutry P, Pilon G, Barbier O, Desjardins Y et al. Treatment with camu camu (Myrciaria dubia) prevents obesity by altering the gut microbiota and increasing energy expenditure in diet-induced obese mice. Gut. 2019;68(3):453–64. [DOI] [PubMed] [Google Scholar]
- 38. Martin M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 2011;17(1):10–2. [Google Scholar]
- 39. Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJA, Holmes SP. DADA2: high-resolution sample inference from Illumina amplicon data. Nat Methods. 2016;13(7):581–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wang Q, Garrity GM, Tiedje JM, Cole JR. Naïve Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol. 2007;73(16):5261–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, Peplies J, Glöckner FO. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 2013;41(Database issue):D590–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS, Huttenhower C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011;12(6):1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Quigley L, O'Sullivan O, Beresford TP, Ross RP, Fitzgerald GF, Cotter PD. Molecular approaches to analysing the microbial composition of raw milk and raw milk cheese. Int J Food Microbiol. 2011;150(2–3):81–94. [DOI] [PubMed] [Google Scholar]
- 44. Beermann C, Hartung J. Physiological properties of milk ingredients released by fermentation. Food Funct. 2013;4(2):185–99. [DOI] [PubMed] [Google Scholar]
- 45. Mozaffarian D. Dietary and policy priorities for cardiovascular disease, diabetes, and obesity. Circulation. 2016;133(2):187–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fernandez MA, Marette A. Novel perspectives on fermented milks and cardiometabolic health with a focus on type 2 diabetes. Nutr Rev. 2018;76(Supplement_1):16–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56(7):1761–72. [DOI] [PubMed] [Google Scholar]
- 48. Lim S-M, Jeong J-J, Woo KH, Han MJ, Kim D-H. Lactobacillus sakei OK67 ameliorates high-fat diet-induced blood glucose intolerance and obesity in mice by inhibiting gut microbiota lipopolysaccharide production and inducing colon tight junction protein expression. Nutr Res. 2016;36(4):337–48. [DOI] [PubMed] [Google Scholar]
- 49. Kamiya T, Watanabe Y, Makino S, Kano H, Tsuji NM. Improvement of intestinal immune cell function by lactic acid bacteria for dairy products. Microorganisms. 2016;5(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kitazawa H, Watanabe H, Shimosato T, Kawai Y, Itoh T, Saito T. Immunostimulatory oligonucleotide, CpG-like motif exists in Lactobacillus delbrueckii ssp. bulgaricus NIAI B6. Int J Food Microbiol. 2003;85(1):11–21. [DOI] [PubMed] [Google Scholar]
- 51. van de Guchte M, Penaud S, Grimaldi C, Barbe V, Bryson K, Nicolas P, Robert C, Oztas S, Mangenot S, Couloux A et al. The complete genome sequence of Lactobacillus bulgaricus reveals extensive and ongoing reductive evolution. Proc Natl Acad Sci. 2006;103(24):9274–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Alvaro E, Andrieux C, Rochet V, Rigottier-Gois L, Lepercq P, Sutren M, Galan P, Duval Y, Juste C, Doré J. Composition and metabolism of the intestinal microbiota in consumers and non-consumers of yogurt. Br J Nutr. 2007;97(1):126–33. [DOI] [PubMed] [Google Scholar]
- 53. Volokh O, Klimenko N, Berezhnaya Y, Tyakht A, Nesterova P, Popenko A, Alexeev D. Human gut microbiome response induced by fermented dairy product intake in healthy volunteers. Nutrients. 2019;11(3):547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. González S, Fernández-Navarro T, Arboleya S, Reyes-Gavilán CG, Salazar N, Gueimonde M. Fermented dairy foods: impact on intestinal microbiota and health-linked biomarkers. Front Microbiol. 2019;10:1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. García-Albiach R, José M, de Felipe P, Angulo S, Morosini M-I, Bravo D, Baquero F, del Campo R. Molecular analysis of yogurt containing Lactobacillus delbrueckii subsp. bulgaricus and Streptococcus thermophilus in human intestinal microbiota. Am J Clin Nutr. 2008;87(1):91–6. [DOI] [PubMed] [Google Scholar]
- 56. Daniel H, Gholami AM, Berry D, Desmarchelier C, Hahne H, Loh G, Mondot S, Lepage P, Rothballer M, Walker A et al. High-fat diet alters gut microbiota physiology in mice. ISME J. 2014;8(2):295–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Dailey MJ. Nutrient-induced intestinal adaption and its effect in obesity. Physiol Behav. 2014;136:74–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Puri P, Rattan A, Bijlani RL, Mahapatra SC, Nath I. Splenic and intestinal lymphocyte proliferation response in mice fed milk or yogurt and challenged with Salmonella Typhimurium. Int J Food Sci Nutr. 1996;47(5):391–8. [DOI] [PubMed] [Google Scholar]
- 59. Chaves S, Perdigon G, de Moreno de LeBlanc A. Yoghurt consumption regulates the immune cells implicated in acute intestinal inflammation and prevents the recurrence of the inflammatory process in a mouse model. J Food Prot. 2011;74(5):801–11. [DOI] [PubMed] [Google Scholar]
- 60. Kabeerdoss J, Devi RS, Mary RR, Prabhavathi D, Vidya R, Mechenro J, Mahendri NV, Pugazhendhi S, Ramakrishna BS. Effect of yoghurt containing Bifidobacterium lactis Bb12® on faecal excretion of secretory immunoglobulin A and human beta-defensin 2 in healthy adult volunteers. Nutr J. 2011;10:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Halpern GM, Vruwink KG, Van de Water JA, Keen CL, Gershwin ME. Influence of long-term yoghurt consumption in young adults. Int J Immunother. 1991;7(4):205–10. [Google Scholar]
- 62. Lv Z, Wang B, Zhou X, Wang F, Xie Y, Zheng H, Lv N. Efficacy and safety of probiotics as adjuvant agents for Helicobacter pylori infection: a meta-analysis. Exp Ther Med. 2015;9(3):707–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Link-Amster H, Rochat F, Saudan KY, Mignot O, Aeschlimann JM. Modulation of a specific humoral immune response and changes in intestinal flora mediated through fermented milk intake. FEMS Immunol Med Microbiol. 1994;10(1):55–63. [DOI] [PubMed] [Google Scholar]
- 64. Wang L, Fouts DE, Stärkel P, Hartmann P, Chen P, Llorente C, DePew J, Moncera K, Ho SB, Brenner DA et al. Intestinal REG3 lectins protect against alcoholic steatohepatitis by reducing mucosa-associated microbiota and preventing bacterial translocation. Cell Host Microbe. 2016;19(2):227–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Tarantino G, Savastano S, Colao A. Hepatic steatosis, low-grade chronic inflammation and hormone/growth factor/adipokine imbalance. WJG. 2010;16(38):4773–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Asrih M, Jornayvaz FR. Inflammation as a potential link between nonalcoholic fatty liver disease and insulin resistance. J Endocrinol. 2013;218(3):R25–36. [DOI] [PubMed] [Google Scholar]
- 67. Galkina E, Ley K. Vascular adhesion molecules in atherosclerosis. ATVB. 2007;27(11):2292–301. [DOI] [PubMed] [Google Scholar]
- 68. Blankenberg S, Barbaux S, Tiret L. Adhesion molecules and atherosclerosis. Atherosclerosis. 2003;170(2):191–203. [DOI] [PubMed] [Google Scholar]
- 69. Minelli EB, Benini A. Relationship between number of bacteria and their probiotic effects. Microb Ecol Health Dis. 2008;20(4):180–3. [Google Scholar]
- 70. Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen S et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11(8):506–14. [DOI] [PubMed] [Google Scholar]
- 71. Kamphuis JBJ, Guiard B, Leveque M, Olier M, Jouanin I, Yvon S, Tondereau V, Rivière P, Guéraud F, Chevolleau S et al. Lactose and fructo-oligosaccharides increase visceral sensitivity in mice via glycation processes, increasing mast cell density in colonic mucosa. Gastroenterology. 2020;158(3):652–63.e6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.