The type III secretion system (T3SS) of Bordetella pertussis forms a needlelike structure that protrudes from the bacterial cell surface. B. pertussis uses a T3SS to translocate virulence proteins called effectors into host cells. The culture conditions for effector production in B. pertussis have not been investigated. We attempted to optimize culture medium compositions for producing and secreting type III secreted proteins. We found that B. pertussis secretes type III secreted proteins in reducing agent-deprived liquid medium and that BteA-secreting B. pertussis provokes cytotoxicity against cultured mammalian cells. These results suggest that redox signaling is involved in the regulation of B. pertussis T3SS.
KEYWORDS: Bordetella, type III secretion system
ABSTRACT
Bordetella pertussis uses a type III secretion system (T3SS) to inject virulence proteins into host cells. Although the B. pertussis T3SS was presumed to be involved in host colonization, efficient secretion of type III secreted proteins from B. pertussis has not been observed. To investigate the roles of type III secreted proteins during infection, we attempted to optimize culture conditions for the production and secretion of a type III secreted protein, BteA, in B. pertussis. We observed that B. pertussis efficiently secretes BteA in ascorbic acid-depleted (AsA−) medium. When L2 cells, a rat lung epithelial cell line, were infected with B. pertussis cultured in the AsA− medium, BteA-dependent cytotoxicity was observed. We also performed an immunofluorescence assay of L2 cells infected with B. pertussis. Clear fluorescence signals of Bsp22, a needle structure of T3SS, were detected on the bacterial surface of B. pertussis cultured in the AsA− medium. Since ascorbic acid is known as a reducing agent, we cultured B. pertussis in liquid medium containing other reducing agents such as 2-mercaptoethanol and dithioerythritol. Under these reducing conditions, the production of type III secreted proteins was repressed. These results suggest that in B. pertussis, the production and secretion of type III secreted proteins are downregulated under reducing conditions.
IMPORTANCE The type III secretion system (T3SS) of Bordetella pertussis forms a needlelike structure that protrudes from the bacterial cell surface. B. pertussis uses a T3SS to translocate virulence proteins called effectors into host cells. The culture conditions for effector production in B. pertussis have not been investigated. We attempted to optimize culture medium compositions for producing and secreting type III secreted proteins. We found that B. pertussis secretes type III secreted proteins in reducing agent-deprived liquid medium and that BteA-secreting B. pertussis provokes cytotoxicity against cultured mammalian cells. These results suggest that redox signaling is involved in the regulation of B. pertussis T3SS.
INTRODUCTION
The genus Bordetella is made up of Gram-negative bacteria and has been subdivided into 16 subspecies to date (1). Bordetella pertussis is a causative agent of whooping cough, also known as pertussis. B. pertussis strictly adapts to humans, and it lacks an environmental reservoir (2). A variety of virulence factors have been identified in Bordetella, e.g., filamentous hemagglutinin (3), adenylate cyclase toxin (4), and pertactin (5). These virulence factors are regulated by a Bordetella two-component regulatory system, BvgAS. In addition to these virulence factors, the type III secretion system (T3SS) of B. pertussis is also regulated by BvgAS (6, 7).
A T3SS that forms a needlelike structure is conserved among many Gram-negative bacteria. To subvert hosts, bacteria use a T3SS to inject type III secreted proteins into host cells (8). Such type III secreted proteins are called effectors. Three types of effectors have been identified in Bordetella: BteA (9) (also referred to as BopC [10]), BopN (11, 12), and BspR (13) (also referred to as BtrA [14, 15]). BteA is 658 amino acids in length and is localized to lipid rafts in host cells via its N-terminal region (16). BteA induces necrotic cell death in several types of cultured mammalian cells (9, 10), and BteA translocation into host cells is promoted by the functioning of BopN (11). BspR is a negative regulator for type III secreted proteins (13) and is translocated into nuclei of cultured mammalian cells (17). BspR has been suggested to be an anti-sigma factor against BtrS that functions as a positive regulator for type III secreted proteins (15). Some type III secreted proteins are called translocators rather than effectors. Three types of translocators have been identified in Bordetella, i.e., BopB (18), BopD (19), and Bsp22 (20), and they function as the path for effectors delivered into host cells. The complex of BopB and BopD forms the pores on the host membrane (19), and Bsp22 forms a filament-like structure and is associated with the T3SS needle and with a pore-forming component, BopD (21).
The optimal culture conditions for the production and secretion of BteA have not been established. To investigate the roles of BteA and other type III secreted proteins during B. pertussis infection, we investigated culture conditions for the maximal production and secretion of type III secreted proteins in B. pertussis strain Tohama I.
RESULTS
Type III secreted proteins are secreted from B. pertussis cultured in LCA medium.
Cyclodextrin solid medium (CSM) is reported as a synthetic medium for clinical isolation of B. pertussis (22). We sought to determine whether B. pertussis grown on this agar secretes type III effectors, and we prepared a liquid medium, designated “low-Casamino Acids (LCA)” medium, by removing the agar from CSM contents (Table 1). B. pertussis was cultured in Stainer-Scholte (SS) or LCA medium, and then the whole-cell lysate (WCL) and supernatant fraction (Sup) samples were subjected to Western blotting with antibodies against BteA (an effector, a type III secreted protein) and BopB (a translocator, a type III secreted protein).
TABLE 1.
Medium compositions
| Compound | Amt (g/liter) in: |
|
|---|---|---|
| SS medium | LCA medium | |
| Basal media | ||
| Sodium glutamate | 10.7 | 10.7 |
| l-Proline | 0.24 | 0.24 |
| NaCl | 2.5 | 2.5 |
| KCl | 0.2 | 0.2 |
| KH2PO4 | 0.5 | 0.5 |
| MgCl2·6H2O | 0.1 | 0.1 |
| CaCl2 | 0.02 | 0.02 |
| Tris | 6.1 | 6.1 |
| Casamino Acids | 10 | 0.5 |
| Dimethyl-β-cyclodextrin | 1 | 1 |
| Supplements | ||
| l-Cysteine·HCl | 4 | 4 |
| FeSO4·7H2O | 1 | 1 |
| l-Ascorbic acid | 40 | 2 |
| Niacin | 0.4 | 0.4 |
| l-Glutathione, reduced | 15 | 15 |
BteA and BopB signals were detected in the WCL and Sup of the wild-type Tohama I cultured in LCA medium (Fig. 1). As reported previously, BteA forms SDS-resistant multimers, and in our present study, we also detected the signals at around 200 kDa. In contrast, the BteA and BopB signals were faintly detected in the WCL samples of SS medium, and no BteA and BopB signals were detected in the Sup samples of MGKU2 (a T3SS-inactive strain) or the wild-type cultured in SS medium (Fig. 1). These results suggest that B. pertussis secretes type III secreted proteins in LCA medium.
FIG 1.
BteA and BopB production by B. pertussis Tohama I cultured in LCA medium. Tohama I and MGKU2 (a T3SS-inactive strain) were cultured in SS or LCA medium. Whole-cell lysates (WCL) and supernatant fraction (Sup) samples were separated by SDS-PAGE and analyzed by Western blotting with antibodies against BteA (top), BopB (middle), or BtrS (bottom). BtrS, an RNA polymerase sigma factor (15), was used as the unsecreted protein control. BteA forms SDS-resistant multimers (10). NS, nonspecific signals. Loaded WCL and Sup samples were prepared from equal volumes of bacterial cultures. Experiments were performed at least three times, and representative data are shown.
Ascorbic acid downregulates the production and secretion of type III secreted proteins in B. pertussis.
Although the ingredients of the LCA and SS media are the same, the amounts of ascorbic acid and Casamino Acids in LCA medium are lower than those in SS medium (Table 1). To determine which substance is involved in the production and secretion of type III secreted protein, we cultured B. pertussis in SS medium, ascorbic acid-deprived SS medium (SS_AsA−), Casamino Acids-deprived SS medium (SS_CaA−), or both ascorbic acid- and Casamino Acids-deprived medium (SS_AsA−_CaA−). WCL and Sup samples were subjected to Western blotting with antibodies against BteA and BopB (Fig. 2).
FIG 2.
Effects of ascorbic acid and Casamino Acids on Bvg-regulated virulence factors. Tohama I was cultured in SS medium or ascorbic acid-deprived SS medium (SS_AsA–), Casamino Acids-deprived SS medium (SS_CaA–), or ascorbic acid- and Casamino Acids-deprived SS medium (SS AsA–_CaA–). WCL and Sup samples were separated by SDS-PAGE and analyzed by Western blotting with antibodies against BteA, BopB, FHA, or CyaA. NS, nonspecific signals. Loaded WCL and Sup samples were prepared from equal volumes of bacterial cultures. Experiments were performed at least three times, and representative data are shown.
The BteA multimer signal intensities in the WCL samples from the SS_AsA−, SS_CaA−, and SS_AsA−_CaA− media were stronger than those from the SS medium (Fig. 2). A faint BteA signal was detected for the Sup samples from SS medium (Fig. 2). BteA signals were evident in the Sup samples from the SS_AsA− and SS_AsA−_CaA− media (Fig. 2). Again, BopB signals were evident in both WCL and Sup samples from the SS_AsA− and SS_AsA−_CaA− media (Fig. 2).
We also investigated the potential involvement of ascorbic acid or Casamino Acids in Bvg-regulated virulence factors, e.g., filamentous hemagglutinin (FHA) and CyaA. WCL and Sup samples were subjected to Western blotting with antibodies against FHA and CyaA (Fig. 2). The results revealed that there were no significant differences in the FHA or CyaA signal intensities in the WCL and Sup samples from the media used here (Fig. 2). Collectively, these results suggest that ascorbic acid has a specific influence on the production and secretion of BteA and BopB in B. pertussis.
Standard SS medium contains ascorbic acid at a final concentration of 2,270 μM. To further explore whether ascorbic acid affects the production and secretion of type III secreted proteins, we cultured B. pertussis in SS medium in the presence of ascorbic acid at a final concentration of 91, 454, or 2,270 μM. WCL and Sup samples were prepared from each bacterial culture and analyzed by Western blotting (Fig. 3). The BteA signal was detected in both WCL and Sup samples from SS medium at 0 to 454 μM ascorbic acid, but the BteA signal from the SS medium at 2,270 μM ascorbic acid was faint or absent (Fig. 3). The BopB signals showed a similar pattern (Fig. 3). These results indicate that B. pertussis produces and secretes type III secreted proteins at low ascorbic acid concentrations.
FIG 3.
Effects of ascorbic acid on type III secreted proteins. Whole-cell lysates and secreted proteins prepared from Tohama I cultured in SS medium in the presence of ascorbic acid at the final concentration of 91, 454, or 2,270 μM were separated by SDS-PAGE and then analyzed by Western blotting with antibodies against BteA or BopB. NS, nonspecific signals. Loaded WCL and Sup samples were prepared from equal volumes of bacterial cultures. Experiments were performed at least three times, and representative data are shown.
BteA and BopB are secreted from other B. pertussis clinical isolates cultured in SS_AsA− medium.
To determine whether B. pertussis clinical isolates secrete type III secreted proteins at low ascorbic acid concentrations, we cultured four strains of B. pertussis (Table 2) in SS medium or ascorbic acid-deprived medium (SS_AsA−). The prepared WCL and Sup samples were analyzed by Western blotting (Fig. 4). BteA signals were evident in the Sup samples of clinical isolates BP350 and BP351 from the SS_AsA− medium (Fig. 4). Again, BopB signals were evident in the Sup samples of BP350 and BP351 from SS_AsA− medium. These results suggest that some clinical strains, e.g., BP350 and BP351, also secrete type III secreted proteins at low ascorbic acid concentrations.
TABLE 2.
Bacterial strains and plasmids used in the study
| Strain or plasmid | Relevant genotype or description | Reference or source |
|---|---|---|
| Strains | ||
| B. pertussis | ||
| Tohama I | Clinical isolate | 34 |
| BP344 | Clinical isolate | 35 |
| BP348 | Clinical isolate | 35 |
| BP350 | Clinical isolate | 35 |
| BP351 | Clinical isolate | 35 |
| MGKU1 | Tohama I nalidixic acid-resistant strain | This study |
| MGKU2 | T3SS-inactive strain | This study |
| MGKU3 | BteA-deficient strain | This study |
| MGKU4 | BspR-deficient strain | This study |
| E. coli | ||
| DH10B | Host strain for pDONR201 | Invitrogen |
| Sm10λpir | Host strain for pABB-CRS2 | 18 |
| Plasmids | ||
| pDONR201 | DNA cloning vector, Kmr | Invitrogen |
| pABB-CRS2 | Suicide vector for conjugation, Ampr | 38 |
| pABB-ΔbscN | pABB-CRS2, ΔbscN gene | 18 |
| pABB-ΔbopC | pABB-CRS2, ΔbopC (ΔbteA) gene | 10 |
| pMGKU401 | pDONR201, bspR gene | This study |
| pMGKU402 | pDONR201, ΔbspR gene | This study |
| pMGKU403 | pABB-CRS2, ΔbspR gene | This study |
FIG 4.
Effects of ascorbic acid on the secretion of BteA and BopB in clinical isolates cultured in SS_AsA− medium. The B. pertussis clinical isolates BP344, BP348, BP350, and BP351 were cultured in SS (+) or SS_AsA– (–) medium. WCL and Sup samples were separated by SDS-PAGE and analyzed by Western blotting with antibodies against BteA (top) or BopB (bottom). NS, nonspecific signals. Loaded WCL and Sup samples were prepared from equal volumes of bacterial cultures. Experiments were performed at least three times, and representative data are shown.
Translocator BopB is upregulated at the transcriptional level in B. pertussis cultured in SS_AsA− medium.
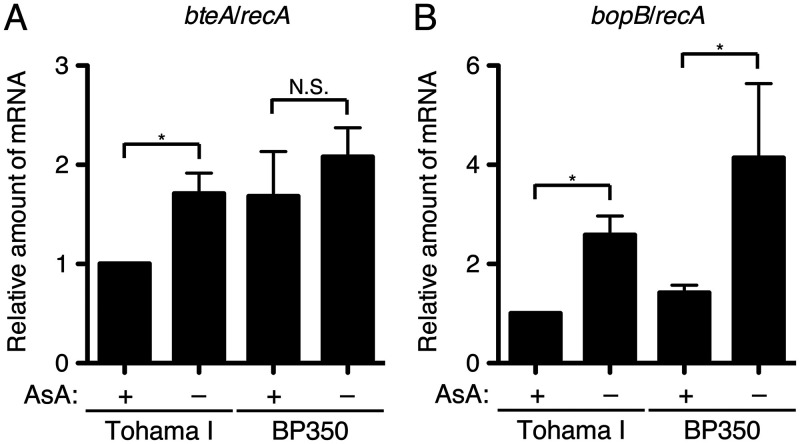
To investigate the gene expressions of type III secreted proteins under ascorbic acid-starved conditions, we prepared total RNA from B. pertussis cultured in SS or SS_AsA− medium. The cDNA samples reverse transcribed from the total RNA samples were subjected to a quantitative reverse transcription-PCR (qRT-PCR) analysis to quantify the relative amounts of bteA and bopB mRNA. The results demonstrated that the relative amount of bteA mRNA of Tohama I in SS_AsA− medium was increased compared to that in SS medium (Fig. 5A). However, the relative amount of bteA mRNA of BP350 in the SS_AsA− medium was not significantly different from that in the SS medium (Fig. 5A). The relative amounts of bopB mRNA of Tohama I and BP350 in SS_AsA− medium were increased compared to those in SS medium (Fig. 5B).
FIG 5.
Results of the RT-PCR analysis for mRNA levels of bteA and bopB from B. pertussis cultured under ascorbic acid-starved conditions. Total RNA was prepared from Tohama I or BP350 cultured in SS or SS_AsA– medium and subjected to a quantitative RT-PCR analysis. The histograms show the relative amounts of bteA (A) and bopB (B) mRNA normalized by housekeeping gene recA mRNA. Error bars are the standard errors of the means (SEMs) from triplicate experiments. *, P < 0.05; N.S., no significant difference. Experiments were performed at least three times, and representative data are shown.
These results suggest that BopB is upregulated at the transcriptional level in Tohama I and BP350 at low ascorbic acid concentrations. BteA is also upregulated at the transcriptional level in Tohama I at low ascorbic acid concentrations but not in BP350.
B. pertussis induces cytotoxicity against cultured mammalian cells.
A B. pertussis BspR-deficient strain was reported to induce cytotoxicity against cultured mammalian cells (15). In the present study, we identified better culture conditions for the production and secretion of BteA in B. pertussis than those provided with standard SS medium. We thus investigated whether wild-type B. pertussis provokes cytotoxicity against cultured mammalian cells under these conditions. L2 cells, a rat lung epithelial cell line, were infected at a multiplicity of infection (MOI) of 500 for 3 h in several modified SS media, shown in Fig. 6. The amount of Casamino Acids and/or ascorbic acid was reduced to one-half (1/2CaA) and 1/20 (1/20AsA), respectively, of that in SS medium. We also used the SS media without Casamino Acids and/or ascorbic acid. The amounts of lactate dehydrogenase (LDH) released into the extracellular medium were then measured.
FIG 6.
Cytotoxic activity of B. pertussis cultured in SS_AsA– medium. L2 cells were infected with Tohama I cultured in SS medium, SS_AsA– medium, SS medium containing 1/20 as much ascorbic acid as SS medium (SS_1/20AsA), SS medium containing 1/20 as much ascorbic acid and one-half as much Casamino Acids as SS medium (SS_1/20AsA_1/2CaA), or SS medium containing 1/20 as much ascorbic acid as SS medium and no Casamino Acids (SS_1/20AsA_CaA–) at an MOI of 500 for 3 h. +, 1/2, and 1/20 show the amounts of indicated substances that are equal to, one-half of, and 1/20 of those in SS medium, respectively. The amounts of LDH released into the extracellular medium from infected cells are shown, and the relative cytotoxicity (percent) was determined as described in Materials and Methods. Error bars are the SEMs from triplicate experiments. n.a., no available result. Experiments were performed at least three times, and representative data are shown.
The results revealed that released LDH was detected from L2 cells infected with Tohama I in the SS_1/20AsA or SS_1/20AsA_1/2CaA medium (Fig. 6). The SS_1/20AsA medium and SS_1/20AsA_1/2CaA medium showed no cytotoxicity. On the other hand, the L2 cells incubated in the SS_AsA− or SS_1/20AsA_CaA− medium were injured by the medium itself. Therefore, these media were not useful for this assay. Taken together, these results demonstrate that we successfully observed cytotoxicity of mammalian cells induced by wild-type B. pertussis.
The T3SS of B. pertussis cultured in SS_AsA− medium is active during infection against cultured mammalian cells.
As described above, we observed cytotoxicity of B. pertussis against cultured cells. To confirm that B. pertussis indeed secretes type III secreted proteins during infection, we infected L2 cells with B. pertussis in SS or SS_1/20AsA_1/2CaA medium at an MOI of 125 for 3 h. After infection, Bsp22 (a translocator and a component of the filament-like structure) and F-actin were stained with anti-Bsp22 and rhodamine-phalloidin, respectively.
The results showed that the amounts of Bsp22 signals (Fig. 7A, green) on L2 cells infected with Tohama I and BP350 cultured in SS_1/20AsA_1/2CaA medium were greater than those in SS medium (Fig. 7). Thus, we demonstrated that B. pertussis T3SS is activated during the infection of cultured mammalian cells under appropriate conditions.
FIG 7.
Immunofluorescent staining of Bsp22 on L2 cells infected with B. pertussis. (A) L2 cells were infected with Tohama I or BP350 in the indicated media at an MOI of 125 for 3 h. +, 1/2, and 1/20 indicate the amounts of indicated substances that are equal to, one-half of, and 1/20 of those in SS medium, respectively. After fixation, cells were stained with anti-Bsp22 antibody (green) and rhodamine-phalloidin (red). Arrows indicate the Bsp22 (green) signal. (B) Bsp22 signals per cell were counted under a fluorescence microscope. At least 120 cells were randomly chosen. Error bars indicate the SEMs from triplicate experiments. *, P < 0.05. Experiments were performed at least three times, and representative data are shown.
The cytotoxicity induced by wild-type B. pertussis depends on the T3SS.
It has been reported that Bordetella bronchiseptica induces BteA-dependent necrotic cell death against cultured mammalian cells (10). To investigate whether the cytotoxicity provoked by wild-type B. pertussis also depends on BteA function, we infected L2 cells with MGKU1 (a Tohama I nalidixic acid-resistant strain), MGKU2 (a T3SS-inactive strain), MGKU3 (a BteA-deficient strain), or MGKU4 (a BspR-deficient strain that allows the constitutive activation of T3SS) in SS_1/20AsA_1/2CaA medium at an MOI of 125 or 250 for 3 h. The amounts of LDH released into extracellular medium were measured.
As a result, LDH was not detected in the medium of L2 cells infected with MGKU2 (Fig. 8A and B). The amount of LDH detected in the medium of L2 cells infected with MGKU3 was significantly smaller than that in the medium of L2 cells infected with MGKU1 (Fig. 8A and B). The amount of LDH detected in the medium of L2 cells infected with MGKU4 was significantly greater than that in the medium of L2 cells infected with MGKU1 at an MOI of 125 (Fig. 8A) but not at an MOI of 250 (Fig. 8B).
FIG 8.
Cytotoxic activities of B. pertussis mutants. The results of LDH assays at an MOI of 125 (A) or 250 (B) are shown as a histograms. Error bars indicate the SEMs from triplicate experiments. *, P < 0.05. Experiments were performed at least three times, and representative data are shown. (C) MGKU1 (Tohama I nalidixic acid-resistant strain [1: WT Nalr]), MGKU2 (T3SS-inactive strain [2: ΔT3SS]), MGKU3 (BteA-deficient strain [3: ΔBteA]), or MGKU4 (BspR-deficient strain [4: ΔBspR]) was cultured in SS_1/20AsA_1/2CaA medium. WCL and Sup samples were separated by SDS-PAGE and analyzed by Western blotting with anti-BteA antibody. We loaded a 3-fold-smaller amount of MGKU4 supernatant fraction on the SDS-PAGE gel to avoid obtaining an excess signal intensity. NS, nonspecific signals. Loaded WCL and Sup samples were prepared from equal volumes of bacterial culture. Experiments were performed at least three times, and representative data are shown.
WCL and Sup samples were prepared from each strain, separated by SDS-PAGE, and then analyzed by Western blotting with anti-BteA antibody (Fig. 8C). BteA signals were detected in the WCL samples with MGKU1, MGKU2, and MGKU4 but not with MGKU3 (Fig. 8C). BteA signals were detected in the Sup samples with MGKU1 and MGKU4 but not with MGKU2 or MGKU3 (Fig. 8C). These results suggest that the cytotoxicity induced by wild-type B. pertussis (Fig. 6) is dependent on T3SS activity and is partially dependent on BteA.
The secretion of type III secreted proteins in B. pertussis is downregulated under reducing conditions.
Ascorbic acid is known as a reducing agent. To determine whether the secretion of type III secreted proteins is downregulated under reducing conditions, we cultured Tohama I in ascorbic acid-deprived SS medium (SS_AsA−) (Fig. 9, none) and in SS_AsA− medium containing the reducing agent ascorbic acid (AsA), 2-mercaptoethanol (2-ME), or dithioerythritol (DTE). The prepared WCL and Sup samples were analyzed by Western blotting with anti-BteA, anti-BopB, and anti-FHA antibodies (Fig. 9). The BteA and BopB signal intensities from both WCL and Sup samples were decreased in the presence of each of the reducing agents (Fig. 9). In contrast, the FHA signal intensities from WCL and Sup samples were not affected by the reducing agents. The growth of bacteria cultured in SS medium with reducing agents was not significantly different from that of the bacteria cultured in SS medium (data not shown). These results suggest that the production and secretion of type III secreted proteins in B. pertussis are downregulated under reducing conditions.
FIG 9.
Effects of reducing agents on the production and secretion of type III secreted proteins in B. pertussis. Tohama I was cultured in SS_AsA– medium or SS_AsA– medium containing ascorbic acid (AsA), 2-mercaptoethanol (2-ME), or dithioerythritol (DTE) at a final concentration of 2.3, 2.1, or 3.0 mM, respectively. WCL and Sup samples were separated by SDS-PAGE and then analyzed by Western blotting with antibodies against BteA, BopB, or FHA. Loaded WCL and Sup samples were prepared from equal volumes of bacterial culture. NS, nonspecific signals. Experiments were performed at least three times, and representative data are shown.
DISCUSSION
In this study, B. pertussis increased the production and secretion of type III secreted proteins, BteA and BopB, when cultured in ascorbic acid-deprived SS medium (SS_AsA−). We successfully produced cytotoxicity against mammalian cells by the infection of wild-type Tohama I cultured in SS 1/20AsA_1/2CaA medium. B. pertussis decreased the production and secretion of type III secreted proteins when cultured in SS_AsA− medium containing reducing agents such as 2-ME or dithioerythritol (DTE). These results suggest that redox signaling is involved in regulation of the T3SS in B. pertussis.
It has been reported that no secretion of the type III secreted proteins BteA and Bsp22 was detected from Tohama I (23) and that the secretion of the type III secreted proteins BteA and BopD was detected from a nonvaccine-type strain, BP157. However, BP157 showed no cytotoxicity against L2 cells, J774 mouse macrophage-like cells, and HeLa cells (24). In another report, glutamate limitation upregulated production of the type III secreted proteins (25). Thus, the requirement of a type III secretion system (T3SS) for virulence in B. pertussis remains unclear. On the other hand, a BscN-deficient mutant in which the T3SS was inactive colonized mouse lungs to a significantly lower degree than the wild type (23). In addition, cytotoxicity against HeLa cells was observed by infection with a BspR (a negative regulator for type III secreted proteins)-deficient B. pertussis strain (15). These results suggest that the T3SS has a significant role in the virulence of B. pertussis.
The production and the relative amounts of bopB mRNA of Tohama I cultured in SS_AsA− medium were significantly increased compared to those of Tohama cultured in SS medium, but this was not the case for BteA mRNA (Fig. 2 and 5). It was reported that bteA and bopB genes are upregulated by BtrS (a sigma factor for type III secreted proteins) and downregulated by BspR (13, 15). It has also been reported that the amount of bteA mRNA was increased 2-fold in a BspR-deficient strain compared to that in the wild type, whereas the amount of bopB mRNA was increased by 18-fold compared to that in the wild type by transcriptome sequencing (RNA-seq) (15). The bopB gene is localized in the T3SS apparatus locus (bsc locus) adjacent to the btrS gene. Since the bteA gene is separated from the bsc locus by >2.5 Mb (9), BtrS would preferentially regulate transcription of the bopB gene over that of the bteA gene.
The cytotoxicity against L2 cells was observed by the infection of Tohama I cultured in SS_1/20AsA_1/2CaA medium (Fig. 6). Before infection, we exchanged Ham’s F-12K medium for the SS_1/20AsA_1/2CaA medium. When L2 cells were infected with Tohama I in Ham’s F-12K medium, the cytotoxicity against L2 cells was not observed (see Fig. S1 in the supplemental material). To establish oxidizing conditions in the tissue culture media, we used several oxidizing agents, i.e., hydrogen peroxide (H2O2), potassium ferricyanide(III) {K3[Fe(CN)6]}, methyl 3-nitro-2-pyridinesulfenate (Npys-OMe), and 5,5′-dithiobis(2-nitrobenzoic acid) (DTNB). We also used another tissue culture medium, i.e., Eagle minimum essential medium (MEM), because MEM contains no reducing agents, while F-12K, which is the medium for the L2 cell culture, contains a reducing agent, i.e., cysteine. Even when L2 cells were infected in F-12K medium containing the oxidizing agent or MEM, B. pertussis showed no cytotoxicity (Fig. S1). We therefore speculate that a substance which inhibits the secretion of type III secreted proteins from Tohama I was present in the Ham’s F-12K medium or a substance that is necessary for the secretion of type III secreted proteins from Tohama I was absent in the Ham’s F-12K medium. We found that infection of the human alveolar epithelium cell line A549 with wild-type B. pertussis cultured in SS_1/20AsA_1/2CaA medium did not result in cytotoxicity (data not shown). For the observation of cytotoxicity against A549 cells, it is necessary to optimize the conditions for the production and secretion of type III secreted proteins in B. pertussis.
A previous study showed that cytotoxicity against the cell line J774A.1 was achieved by infection with wild-type B. pertussis 18323 but not by infection with a CyaA-deficient strain (26). This finding suggested that CyaA has the potential to provoke cytotoxicity. However, we confirmed herein that the production and secretion of CyaA in a BteA-deficient strain (MGKU3) were not significantly different from those of the wild type (data not shown). In our results, the amounts of LDH released from L2 cells infected with MGKU3 were greater than those of the T3SS-inactive strain (MGKU2), and no LDH release was detected from L2 cells infected with MGKU2 (Fig. 8). These results raised the possibility that an unknown type III effector other than BteA is involved in the cytotoxicity of B. pertussis.
Although we observed no significant difference in the growth rate of Tohama I between SS medium and SS_AsA− medium (data not shown), the production and secretion of type III secreted proteins of Tohama I cultured in the SS_AsA− medium were increased compared to those in the SS medium (Fig. 2). It had not been determined whether the expression of type III secreted proteins is regulated by reducing agents at the molecular level, and in this study, the production and secretion of CyaA and FHA in Tohama I cultured in SS medium were not significantly different from those in SS_AsA− medium (Fig. 2). It was reported that there is no difference in Prn production by Tohama I cultured in modified SS medium compared to that in a culture in modified SS medium containing DTT, a reducing agent (27). These results suggest that BvgAS-regulated virulence factors other than type III secreted proteins are not affected by reducing agents.
Ascorbic acid, urea, and glutathione (GSH) function as reducing agents in the epithelial lining fluid (ELF) of the human airway. The concentrations of ascorbic acid and urea in ELF in the upper respiratory tract are not significantly different from those in the lower respiratory tract. However, the concentration of GSH in the ELF in the upper respiratory tract is 100-fold lower than that in the lower respiratory tract. One of the possibilities is that GSH forms GS-SG (an oxidized from of GSH) in the ELF in the upper respiratory tract (28). It may thus be considered that the upper respiratory tract is more oxidized than the lower respiratory tract. It was reported that the transcription of genes encoding type III secreted proteins of B. pertussis recovered from mouse nasal lavage samples was higher than that from bronchoalveolar lavage samples (29). We thus speculate that the T3SS is important for B. pertussis to colonize the upper respiratory tract of the host.
In this study, the production and secretion of BopB by Tohama I cultured in the SS_AsA− medium were significantly increased compared to those in SS medium (Fig. 2). Bacteria use redox-sensing proteins to resist oxidative stress (30). For example, OxyR (a member of the LysR family of transcriptional regulators) of Escherichia coli is converted into an active form by the oxidation of intramolecular cysteine residues, which then increases the expression of antioxidants genes, e.g., hydroperoxidase I (katG [31]). Vfr (a member of the CRP/FNR superfamily) of Pseudomonas becomes active by retaining intramolecular cysteine residues as reducing states and then increases the expression of type III secreted proteins (32). Btr is a regulator protein produced by B. pertussis and is homologous to Vfr (33). A cysteine cluster is located in the N-terminal domain of Btr, and a helix-turn-helix DNA binding domain is located in the C-terminal domain of Btr. Therefore, when B. pertussis is cultured in reducing agent-deprived SS medium, Btr might be oxidized and change its conformation, thereby increasing the expression of type III secreted proteins. Although the signal pathway that B. pertussis uses to increase the production of type III secreted proteins is unclear, it is possible that Btr regulates gene expressions for type III secreted proteins.
MATERIALS AND METHODS
Bacterial strains.
The strains used in this study are listed in Table 2. B. pertussis Tohama I (34) was used as the wild-type strain. BP344, BP348, BP350, and BP351 were used as clinical isolates and were a kind gift from K. Kamachi (National Institute of Infectious Diseases) (35). Escherichia coli DH10B and Sm10λpir were used for DNA cloning and conjugation, respectively (Table 2). B. pertussis was grown on Bordet-Gengou agar plates at 37°C for 5 days. Bordet-Gengou agar plates were prepared as follows. We mixed 15 g of Bordet-Gengou agar base (BD number 248200), 5 ml of glycerol, 100 ml of defibrinated horse blood (Nippon Bio-Supp. Center), and 400 ml of distilled water. The mixture was autoclaved and poured onto plastic plates. Fresh colonies of B. pertussis on each Bordet-Gengou agar plate were suspended in Stainer-Scholte (SS) medium (Table 1) (36). In this study, we used a liquid medium described as a cyclodextrin-containing medium as the SS medium (37). Low-Casamino Acids (LCA) medium was prepared by removing the agar from cyclodextrin solid medium (CSM) contents (Table 1) (22). B. pertussis was cultured in SS medium with a starting A600 of 0.23 under static conditions at 37°C. To measure the bacterial density, a spectrophotometer (Ultrospec 2100 pro; Amersham) was used. The final A600 of B. pertussis Tohama I culture after 22 h was ca. 0.67, and the culture contained ca. 1.0 × 109 CFU/ml. The liquid cultivation period was 22 h for the protein preparation, mRNA preparation, and infection.
Generation of a Tohama I nalidixic acid-resistant mutant.
For the generation of a Tohama I nalidixic acid-resistant mutant, Tohama I cultured in SS medium for 24 h as described above was spread on 20 sets of Bordet-Gengou agar plates containing nalidixic acid at the final concentration of 30 μg/ml. After 5 days, surviving colonies were designated MGKU1. The growth rate, colony size, and hemolysis on the BG plates of the resulting nalidixic acid-resistant strain were almost equivalent to those of the wild-type strain, and we observed that the amounts of the production and secretion of type III secreted proteins in MGKU1 were not significantly different from those of Tohama I wild type (data not shown).
Cell culture.
L2 cells, a rat lung epithelial cell line (ACTT CCL-149), were maintained in F-12K (Invitrogen, Tokyo, Japan). The cell culture medium was supplemented with 10% fetal calf serum (FCS). L2 cells were grown at 37°C under a 5% CO2 atmosphere.
Construction of gene-disrupted mutants.
The primers used in this study are listed in Table 3. To construct the bspR mutant, the 4.0-kb DNA fragment harboring bspR and its flanking regions was amplified by PCR with primers B1-bspR and B2-bspR using B. pertussis Tohama I genomic DNA as the template. The resulting PCR product was cloned into pDONR201 to obtain pMGKU401 by means of adapter PCR and site-specific recombination techniques using a Gateway cloning system (Invitrogen). An inverse PCR was carried out with the set of primers R1-bspR and R2-bspR using circular pMGKU401 as the template.
TABLE 3.
Primers used in the study
| Name | Sequence (5′→3′)a |
|---|---|
| B1-bspR | AAAAAGCAGGCTCGCCGGTTTCCACCACGTAA |
| B2-bspR | AGAAAGCTGGGTGGCCAATATCCTGTCTCAGC |
| R1-bspR | CGCGGATCCCATACGGCGCAAGAATGGCT |
| R2-bspR | CGCGGATCCAAAGACATCAAGCTCCAAAG |
| 5-recA | ATGAAGATCGGCGTGATGT |
| 3-recA | TAGAACTTGAGCGCGTTGC |
| 5-bteA | AATGGCCTTGGTGGGAAC |
| 3-bteA | ATTTCAGCGCCGTGATCTT |
| 5-bopB | GTCTGCTGACAGCGTTGG |
| 3-bopB | GGGGTTCGATCAGCAAGAC |
Restriction enzyme recognitions sites are underlined.
The resulting PCR product was digested with BamHI and self-ligated to obtain pMGKU402. This plasmid contained the BamHI site in addition to a 474-bp in-frame deletion from 175 bp downstream of the 5′ end of the bspR gene to 52 bp upstream of the 3′ end of the gene. This plasmid, pMGKU402, was mixed with a positive suicide vector, pABB-CRS2 (38), to obtain pMGKU403 using the Gateway cloning system. This plasmid, pMGKU403, was introduced into E. coli Sm10λpir (18) and transconjugated into MGKU1 as described previously (39). The resulting mutant strain was designated MGKU4 (BspR-deficient strain). Similarly, pABB-ΔbscN (18) and pABB-ΔbopC (10) were separately introduced into E. coli Sm10λpir and were transconjugated into MGKU1. The resulting mutant strains were designated MGKU2 (a T3SS-inactive strain) and MGKU3 (a BteA-deficient strain), respectively.
Preparation of proteins from culture supernatants and whole bacterial cell lysates.
Secreted proteins released into bacterial culture supernatants and whole bacterial cell lysates were prepared by trichloroacetic acid precipitation. In the case of the supernatant fraction (Sup) samples, the culture supernatants were filtered with a 0.22-μm filter (Millipore number SLGVR33RB). Next, 2 μl of 5% deoxycholic acid and 100 μl of 100% trichloroacetic acid were added to the 1 ml of filtered supernatants. After being incubated on ice for 15 min, the samples were centrifuged at 21,130 × g for 5 min. The resulting precipitated proteins were neutralized with 2 μl of 2 M Tris base and dissolved in 13 μl of 2× SDS-PAGE sample buffer. In the case of the whole-cell lysate (WCL) samples, bacterial pellets collected from 1 ml of bacterial cultures were resuspended in distilled water. One hundred microliters of 100% trichloroacetic acid was added to 1 ml of the bacterial suspension. After being incubated on ice for 15 min, the samples were centrifuged at 21,130 × g for 5 min. The resulting precipitated proteins were neutralized with 5 μl of 2 M Tris base and dissolved in 95 μl of 2× SDS-PAGE sample buffer. When the A600 of bacterial culture was 0.67, 0.5 μl of WCL and 5 μl of Sup samples were loaded on the SDS-PAGE gels. The loaded sample amounts were adjusted by the A600 of each bacterial culture in order to load samples prepared from the same number of bacteria. The protein samples were separated by SDS-PAGE and analyzed by Western blotting.
Antibodies.
Anti-BteA, anti-BopB, and anti-Bsp22 antibodies were purified from rabbit serum in our previous study (10, 18, 40). To detect filamentous hemagglutinin (FHA) signals, we used mouse anti-FHA serum (41). Mouse anti-CyaA monoclonal antibody was purchased from Santa Cruz Biotechnology (Dallas, TX). To prepare the anti-BtrS antibody, the peptide corresponding to the C-terminal region of BtrS (CALREALRERGYDSVP) was conjugated to hemocyanin from keyhole limpets (Sigma) by using 3-maleimidobenzoic acid N-hydroxysuccinimide ester (Sigma). These cross-linked peptides were used to immunize rabbits, and resulting antisera were incubated with peptide immobilized on epoxy-activated Sepharose 6B (Amersham) to obtain specific Ig fractions.
Quantitative reverse transcription-PCR.
The amounts of mRNA were measured by qRT-PCR. Bacterial total RNA was prepared using a Trizol Max Bacterial RNA isolation kit (Invitrogen), an RNeasy minikit (Qiagen, Hilden, Germany), and an RNase-free DNase-free kit (Qiagen). The RNA sample was reverse transcribed with Transcriptor Universal cDNA master (Roche Diagnostics, Indianapolis, IN) and a T100 thermal cycler (Bio-Rad, Hercules, CA). The resulting cDNA was amplified by FastStart Essential DNA Probes Master (Roche) using the following primer pairs 5-recA and 3-recA for recA, 5-bteA and 3-bteA for bteA, and 5-bopB and 3-bopB for bopB (Table 3). The results were calculated as described in the Roche manual. The amount of recA mRNA was used as an internal control. The mRNA amounts are presented here as relative to the amount in the Tohama I cultured in SS medium, which was defined as 1.
LDH assays.
To examine the release of lactate dehydrogenase (LDH) from B. pertussis-infected cells, 5.0 × 104 cells/well of L2 cells seeded in 24-well plates were infected with bacteria at the multiplicity of infection (MOI) of 125, 250, or 500. The plates were centrifuged at 900 × g for 5 min and incubated for 3 h at 37°C under a 5% CO2 atmosphere. The amounts of LDH were measured spectrophotometrically using a Cyto-Tox 96 nonradioactive cytotoxicity assay kit (Promega, Madison, WI). We added 50 μl of 10% Triton X-100 to 950 μl of extracellular medium of L2 cells in a 24-well plate. We then mixed the extracellular medium by pipetting. We subtracted the LDH value obtained from extracellular medium of uninfected cells from the value obtained from the Triton X-100-treated cells and used the resulting value as 100%.
Immunofluorescent staining.
For the immunofluorescent staining assay, 2.5 × 105 cells/well of L2 cells were seeded on coverslips in six-well plates, incubated overnight, and then infected with B. pertussis at an MOI of 125. The plates were centrifuged at 900 × g for 5 min and incubated for 3 h at 37°C under a 5% CO2 atmosphere. The infected L2 cells were then immunostained as described previously (12). Briefly, the cells were treated with 4% paraformaldehyde, 50 mM NH4Cl, 0.2% Triton X-100, and 4% bovine serum albumin (BSA). After blocking, the cells were incubated with an anti-Bsp22 antibody (40). As a secondary antibody, Alexa Fluor 488–goat anti-rabbit IgG (Invitrogen) was used. F-actin was stained with rhodamine-phalloidin (Molecular Probes, Eugene, OR). Average numbers of Bsp22 signal dots on single L2 cells were measured by fluorescence microscopy.
Statistical analyses.
The statistical analyses were performed using the nonparametric unpaired t test with Prism ver. 5.0f software (GraphPad, La Jolla, CA). One-tailed P values of <0.05 were considered significant.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported in part by grants from the Ministry of Education, Culture, Sports, Sciences and Technology and the Japan Society for the Promotion of Science (KAKENHI) no. 19K07542 (to A.A.), 17K08838 (A.K.), and 19K07561 (T.H.) and by a research grant from the Takeda Science Foundation in 2016 (A.K.).
The funders had no role in the study design, data collection or analysis, the decision to publish, or the preparation of the manuscript.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Ivanov YV, Linz B, Register KB, Newman JD, Taylor DL, Boschert KR, Le Guyon S, Wilson EF, Brinkac LM, Sanka R, Greco SC, Klender PM, Losada L, Harvill ET. 2016. Identification and taxonomic characterization of Bordetella pseudohinzii sp. nov. isolated from laboratory-raised mice. Int J Syst Evol Microbiol 66:5452–5459. doi: 10.1099/ijsem.0.001540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mattoo S, Cherry JD. 2005. Molecular pathogenesis, epidemiology, and clinical manifestations of respiratory infections due to Bordetella pertussis and other Bordetella subspecies. Clin Microbiol Rev 18:326–382. doi: 10.1128/CMR.18.2.326-382.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Relman DA, Domenighini M, Tuomanen E, Rappuoli R, Falkow S. 1989. Filamentous hemagglutinin of Bordetella pertussis: nucleotide sequence and crucial role in adherence. Proc Natl Acad Sci U S A 86:2637–2641. doi: 10.1073/pnas.86.8.2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hewlett EL, Gordon VM, McCaffery JD, Sutherland WM, Gray MC. 1989. Adenylate cyclase toxin from Bordetella pertussis. Identification and purification of the holotoxin molecule. J Biol Chem 264:19379–19384. [PubMed] [Google Scholar]
- 5.Roberts M, Fairweather NF, Leininger E, Pickard D, Hewlett EL, Robinson A, Hayward C, Dougan G, Charles IG. 1991. Construction and characterization of Bordetella pertussis mutants lacking the vir-regulated P.69 outer membrane protein. Mol Microbiol 5:1393–1404. doi: 10.1111/j.1365-2958.1991.tb00786.x. [DOI] [PubMed] [Google Scholar]
- 6.Melvin JA, Scheller EV, Miller JF, Cotter PA. 2014. Bordetella pertussis pathogenesis: current and future challenges. Nat Rev Microbiol 12:274–288. doi: 10.1038/nrmicro3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cummings CA, Bootsma HJ, Relman DA, Miller JF. 2006. Species- and strain-specific control of a complex, flexible regulon by Bordetella BvgAS. J Bacteriol 188:1775–1785. doi: 10.1128/JB.188.5.1775-1785.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cornelis GR, Van Gijsegem F. 2000. Assembly and function of type III secretory systems. Annu Rev Microbiol 54:735–774. doi: 10.1146/annurev.micro.54.1.735. [DOI] [PubMed] [Google Scholar]
- 9.Panina EM, Mattoo S, Griffith N, Kozak NA, Yuk MH, Miller JF. 2005. A genome-wide screen identifies a Bordetella type III secretion effector and candidate effectors in other species. Mol Microbiol 58:267–279. doi: 10.1111/j.1365-2958.2005.04823.x. [DOI] [PubMed] [Google Scholar]
- 10.Kuwae A, Matsuzawa T, Ishikawa N, Abe H, Nonaka T, Fukuda H, Imajoh-Ohmi S, Abe A. 2006. BopC is a novel type III effector secreted by Bordetella bronchiseptica and has a critical role in type III-dependent necrotic cell death. J Biol Chem 281:6589–6600. doi: 10.1074/jbc.M512711200. [DOI] [PubMed] [Google Scholar]
- 11.Abe A, Nishimura R, Kuwae A. 2017. Bordetella effector BopN is translocated into host cells via its N-terminal residues. Microbiol Immunol 61:206–214. doi: 10.1111/1348-0421.12489. [DOI] [PubMed] [Google Scholar]
- 12.Nagamatsu K, Kuwae A, Konaka T, Nagai S, Yoshida S, Eguchi M, Watanabe M, Mimuro H, Koyasu S, Abe A. 2009. Bordetella evades the host immune system by inducing IL-10 through a type III effector, BopN. J Exp Med 206:3073–3088. doi: 10.1084/jem.20090494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kurushima J, Kuwae A, Abe A. 2012. The type III secreted protein BspR regulates the virulence genes in Bordetella bronchiseptica. PLoS One 7:e38925. doi: 10.1371/journal.pone.0038925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahuja U, Liu M, Tomida S, Park J, Souda P, Whitelegge J, Li H, Harvill ET, Parkhill J, Miller JF. 2012. Phenotypic and genomic analysis of hypervirulent human-associated Bordetella bronchiseptica. BMC Microbiol 12:167. doi: 10.1186/1471-2180-12-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahuja U, Shokeen B, Cheng N, Cho Y, Blum C, Coppola G, Miller JF. 2016. Differential regulation of type III secretion and virulence genes in Bordetella pertussis and Bordetella bronchiseptica by a secreted anti-sigma factor. Proc Natl Acad Sci U S A 113:2341–2348. doi: 10.1073/pnas.1600320113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.French CT, Panina EM, Yeh SH, Griffith N, Arambula DG, Miller JF. 2009. The Bordetella type III secretion system effector BteA contains a conserved N-terminal motif that guides bacterial virulence factors to lipid rafts. Cell Microbiol 11:1735–1749. doi: 10.1111/j.1462-5822.2009.01361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abe A, Nishimura R, Tanaka N, Kurushima J, Kuwae A. 2015. The Bordetella secreted regulator BspR is translocated into the nucleus of host cells via its N-terminal moiety: evaluation of bacterial effector translocation by the Escherichia coli type III secretion system. PLoS One 10:e0135140. doi: 10.1371/journal.pone.0135140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuwae A, Ohishi M, Watanabe M, Nagai M, Abe A. 2003. BopB is a type III secreted protein in Bordetella bronchiseptica and is required for cytotoxicity against cultured mammalian cells. Cell Microbiol 5:973–983. doi: 10.1046/j.1462-5822.2003.00341.x. [DOI] [PubMed] [Google Scholar]
- 19.Nogawa H, Kuwae A, Matsuzawa T, Abe A. 2004. The type III secreted protein BopD in Bordetella bronchiseptica is complexed with BopB for pore formation on the host plasma membrane. J Bacteriol 186:3806–3813. doi: 10.1128/JB.186.12.3806-3813.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yuk MH, Harvill ET, Cotter PA, Miller JF. 2000. Modulation of host immune responses, induction of apoptosis and inhibition of NF-kappaB activation by the Bordetella type III secretion system. Mol Microbiol 35:991–1004. doi: 10.1046/j.1365-2958.2000.01785.x. [DOI] [PubMed] [Google Scholar]
- 21.Medhekar B, Shrivastava R, Mattoo S, Gingery M, Miller JF. 2009. Bordetella Bsp22 forms a filamentous type III secretion system tip complex and is immunoprotective in vitro and in vivo. Mol Microbiol 71:492–504. doi: 10.1111/j.1365-2958.2008.06543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aoyama T, Murase Y, Iwata T, Imaizumi A, Suzuki Y, Sato Y. 1986. Comparison of blood-free medium (cyclodextrin solid medium) with Bordet-Gengou medium for clinical isolation of Bordetella pertussis. J Clin Microbiol 23:1046–1048. doi: 10.1128/JCM.23.6.1046-1048.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fennelly NK, Sisti F, Higgins SC, Ross PJ, van der Heide H, Mooi FR, Boyd A, Mills KHG. 2008. Bordetella pertussis expresses a functional type III secretion system that subverts protective innate and adaptive immune responses. Infect Immun 76:1257–1266. doi: 10.1128/IAI.00836-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Han HJ, Kuwae A, Abe A, Arakawa Y, Kamachi K. 2011. Differential expression of type III effector BteA protein due to IS481 insertion in Bordetella pertussis. PLoS One 6:e17797. doi: 10.1371/journal.pone.0017797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hanawa T, Kamachi K, Yonezawa H, Fukutomi T, Kawakami H, Kamiya S. 2016. Glutamate limitation, BvgAS activation, and (p)ppGpp regulate the expression of the Bordetella pertussis type 3 secretion system. J Bacteriol 198:343–351. doi: 10.1128/JB.00596-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khelef N, Zychlinsky A, Guiso N. 1993. Bordetella pertussis induces apoptosis in macrophages: role of adenylate cyclase-hemolysin. Infect Immun 61:4064–4071. doi: 10.1128/IAI.61.10.4064-4071.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stenson TH, Patton AK, Weiss AA. 2003. Reduced glutathione is required for pertussis toxin secretion by Bordetella pertussis. Infect Immun 71:1316–1320. doi: 10.1128/iai.71.3.1316-1320.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van der Vliet A, O’Neill CA, Cross CE, Koostra JM, Volz WG, Halliwell B, Louie S. 1999. Determination of low-molecular-mass antioxidant concentrations in human respiratory tract lining fluids. Am J Physiol 276:L289–L296. doi: 10.1152/ajplung.1999.276.2.L289. [DOI] [PubMed] [Google Scholar]
- 29.van Beek LF, de Gouw D, Eleveld MJ, Bootsma HJ, de Jonge MI, Mooi FR, Zomer A, Diavatopoulos DA. 2018. Adaptation of Bordetella pertussis to the respiratory tract. J Infect Dis 217:1987–1996. doi: 10.1093/infdis/jiy125. [DOI] [PubMed] [Google Scholar]
- 30.Lee SJ, Kim DG, Lee KY, Koo JS, Lee BJ. 2018. Regulatory mechanisms of thiol-based redox sensors: lessons learned from structural studies on prokaryotic redox sensors. Arch Pharm Res 41:583–593. doi: 10.1007/s12272-018-1036-0. [DOI] [PubMed] [Google Scholar]
- 31.Aslund F, Zheng M, Beckwith J, Storz G. 1999. Regulation of the OxyR transcription factor by hydrogen peroxide and the cellular thiol-disulfide status. Proc Natl Acad Sci U S A 96:6161–6165. doi: 10.1073/pnas.96.11.6161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang Y, Zhang C, Du X, Zhou Y, Kong W, Lau GW, Chen G, Kohli GS, Yang L, Wang T, Liang H. 2019. Glutathione activates type III secretion system through Vfr in Pseudomonas aeruginosa. Front Cell Infect Microbiol 9:164. doi: 10.3389/fcimb.2019.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bannan JD, Moran MJ, MacInnes JI, Soltes GA, Friedman RL. 1993. Cloning and characterization of btr, a Bordetella pertussis gene encoding an FNR-like transcriptional regulator. J Bacteriol 175:7228–7235. doi: 10.1128/jb.175.22.7228-7235.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kasuga T, Nakase Y, Ukishima K, Takatsu K. 1954. Studies on Haemophilus pertussis. V. Relation between the phase of bacilli and the progress of the whooping-cough. Kitasato Arch Exp Med 27:57–62. [PubMed] [Google Scholar]
- 35.Otsuka N, Han HJ, Toyoizumi-Ajisaka H, Nakamura Y, Arakawa Y, Shibayama K, Kamachi K. 2012. Prevalence and genetic characterization of pertactin-deficient Bordetella pertussis in Japan. PLoS One 7:e31985. doi: 10.1371/journal.pone.0031985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stainer DW, Scholte MJ. 1970. A simple chemically defined medium for the production of phase I Bordetella pertussis. J Gen Microbiol 63:211–220. doi: 10.1099/00221287-63-2-211. [DOI] [PubMed] [Google Scholar]
- 37.Imaizumi A, Suzuki Y, Ono S, Sato H, Sato Y. 1983. Effect of heptakis(2,6-O-dimethyl)beta-cyclodextrin on the production of pertussis toxin by Bordetella pertussis. Infect Immun 41:1138–1143. doi: 10.1128/IAI.41.3.1138-1143.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sekiya K, Ohishi M, Ogino T, Tamano K, Sasakawa C, Abe A. 2001. Supermolecular structure of the enteropathogenic Escherichia coli type III secretion system and its direct interaction with the EspA-sheath-like structure. Proc Natl Acad Sci U S A 98:11638–11643. doi: 10.1073/pnas.191378598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Donnenberg MS, Kaper JB. 1991. Construction of an eae deletion mutant of enteropathogenic Escherichia coli by using a positive-selection suicide vector. Infect Immun 59:4310–4317. doi: 10.1128/IAI.59.12.4310-4317.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kurushima J, Kuwae A, Abe A. 2012. Btc22 chaperone is required for secretion and stability of the type III secreted protein Bsp22 in Bordetella bronchiseptica. FEMS Microbiol Lett 331:144–151. doi: 10.1111/j.1574-6968.2012.02561.x. [DOI] [PubMed] [Google Scholar]
- 41.Watanabe M, Nagai M, Funaishi K, Endoh M. 2000. Efficacy of chemically cross-linked antigens for acellular pertussis vaccine. Vaccine 19:1199–1203. doi: 10.1016/S0264-410X(00)00308-X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.