Abstract
Ceramides are products of metabolism that accumulate in individuals with obesity or dyslipidaemia and alter cellular processes in response to fuel surplus. Their actions, when prolonged, elicit the tissue dysfunction that underlies diabetes and heart disease. Here, we review the history of research on these enigmatic molecules, exploring their discovery and mechanisms of action, the evolutionary pressures that have given them their unique attributes and the potential of ceramide-reduction therapies as treatments for cardiometabolic disease.
The accumulation of ectopic lipid metabolites in tissues not suited for fat storage drives the cellular dysfunction that underlies diabetes and heart disease. Of the numerous types of lipids that accumulate, sphingolipids such as ceramides are amongst the most deleterious, because they modulate signalling and metabolic pathways driving insulin resistance, triglyceride production, apoptosis and fibrosis. Inhibition of ceramide biosynthesis in rodents ameliorates insulin resistance1,2, hepatic steatosis1–7, hypertriglyceridemia1,2,8, atherosclerosis9–14, type 2 diabetes1 and heart failure15,16. Moreover, ceramide degradation is a primary means by which adiponectin receptors, which are ligand-activated ceramidases, exert their anti-diabetic, cardioprotective and insulin-sensitizing actions17,18. Owing to a strong association between serum ceramides and both insulin resistance and major adverse cardiac events, the Mayo Clinic is now marketing tests to measure circulating ceramides as markers of cardiovascular mortality19,20. Here, we review the history of research on these important molecules and discuss the evolutionary pressures that may have endowed them with such important roles as signals of lipid overload.
Discovery of ceramides as signalling molecules
Within the past 30 years, researchers have elucidated the molecular composition of sphingolipids and the biochemical pathways that control their production in great molecular detail. Owing largely to the work of Alfred Merrill, an exceptional analytical chemist and mass spectroscopist, great progress has been made in discerning the sphingolipidome. The LIPID MAPS Lipidomics Gateway (http://www.lipidmaps.org/) now lists 4,000 distinct chemical entities within this lipid class. Researchers have also cloned and characterized most enzymes involved in sphingolipid synthesis and degradation21.
Ceramides, which are the precursors of most sphingolipids, are produced through a four-step biosynthetic cascade initiated by the condensation of palmitoyl-CoA and serine21. This first reaction, catalysed by serine palmitoyltransferase (SPT), generates ketosphinganine, which is rapidly converted by 3-ketosphinganine reductase into sphinganine. This sphingoid scaffold subsequently acquires additional fatty acids, thus producing dihydroceramides. The Futerman laboratory has characterized the (dihydro)ceramide synthase (CERS) enzymes that add this second acyl chain, and has rigorously examined the substrate specificity and tissue distribution of the six CERS isoforms22. Dihydroceramide desaturase isoforms 1 and 2 (DES1 and DES2) insert an essential double bond into the sphingoid base of dihydroceramides, thus producing ceramides. Various head groups can be added to ceramides to produce the predominant complex sphingolipids, including sphingomyelin and gangliosides. Ceramides are the first point of substantial sphingolipid accumulation.
In the late 1980s and early 1990s, pioneering studies in the Hannun, Obeid and Kolesnick laboratories revealed that stress stimuli such as tumour necrosis factor activate sphingomyelinases, thereby acutely liberating ceramides. The subsequent paradigm-shifting work of these researchers established that ceramides function as intracellular messengers23–25. Initially, much of the effort was devoted to understanding the ability of ceramides to induce apoptosis25. Building on these discoveries, the Unger laboratory found that ceramides produced by de novo synthesis are obligate intermediates linking saturated fatty acids to pancreatic beta-cell apoptosis, thus suggesting for the first time that ceramides play a key role in the beta-cell failure that underlies diabetes26,27.
In the late 1990s, studies by our group and others demonstrated that ceramides also inhibit insulin-stimulated glucose uptake28,29. In particular, ceramide analogues block activation of Akt (PKB), an insulin-responsive serine/threonine kinase essential for GLUT4 glucose-transporter translocation, cell survival (that is, anti-apoptosis) and the synthesis of glycogen, fatty acids and protein28,30. These studies, which suggested roles for ceramides in the development of insulin resistance, laid the foundation for the topic discussed here: ceramides as metabolic messengers (Fig. 1).
Fig. 1 |. Discovery of ceramides as metabolic messengers.
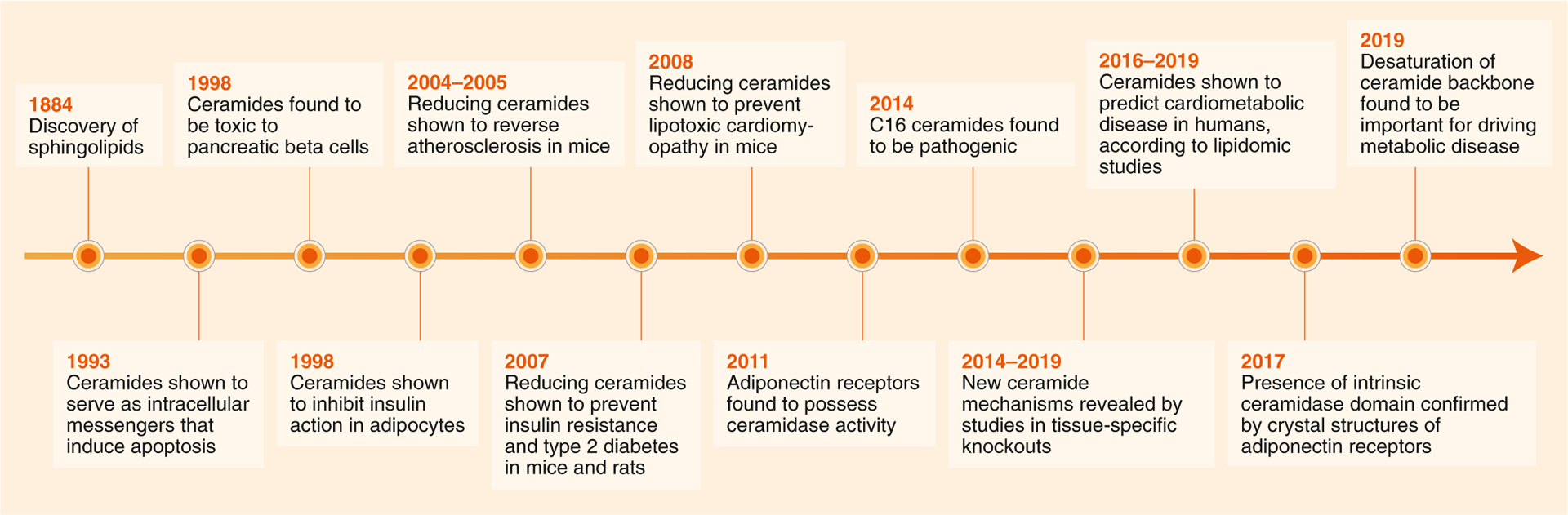
Nearly 100 years passed between the discovery of sphingolipids (1884) and the determination that sphingolipids such as ceramides have biological activities as intracellular messengers that alter cell survival and metabolism (1990s). In the mid- to late 2000s, a series of studies using pharmacological reagents (for example, myriocin) or genetically engineered mice revealed that blocking ceramide synthesis ameliorates atherosclerosis, diabetes and heart disease. Subsequently, adiponectin receptors were identified as ceramides, and the fields of research on these two metabolic messengers converged (2011–2017). As lipidomic technologies improved, large-scale lipidomic profiling studies became possible, revealing tight associations between serum and tissue ceramides and cardiometabolic disease (2014–2019). Finally, research in mice has revealed that making subtle modifications to the ceramide molecules (for example, by altering their acylation patterns or the desaturation status of the sphingoid backbone) is sufficient to ameliorate cardiometabolic disease, thus revealing new therapeutic strategies for combating these disorders (2014–2019).
Theory: an evolutionarily conserved role of ceramides as signals of lipid overload
Free fatty acids (FFA) are energy-dense molecules with amphipathic, detergent-like properties. Evolutionary pressures have equipped cells with means to rapidly incorporate FFA into macromolecules, thus ensuring that their intracellular concentrations remain very low. Immediately after entering cells, FFA couple to CoA, which prepares them for subsequent enzymatic modification. When energy needs are high, the resultant acyl-CoAs couple to carnitine, which allows them to be shuttled into mitochondria for beta-oxidation. When energy needs are low, these acyl-CoAs couple to glycerol and produce triglycerides—the major fuel store in the body—and the other glycerophospholipids that make lipid bilayers. When a cell’s energy needs are met and its triglyceride stores are saturated, acyl-CoAs enter the ceramide-biosynthesis pathway described above. Incorporation of FFA into these complex structures (for example, acyl-CoAs, glycerolipids and sphingolipids) prevents them from concentrating in bilayers; otherwise, they would aberrantly disrupt the bilayer structure.
We speculate that the sphingolipids, and particularly the ceramides, that accumulate under conditions of nutritional overload initiate actions that help cells handle the excessive incoming fatty acids (Fig. 2, right). The following activities are consistent with this role:
Although FFA can diffuse through cellular membranes, their uptake can be facilitated by translocases such as CD36, which also accelerate their esterification31,32. Ceramides promote the translocation of CD36 to plasma membranes2,17, thus supporting the safe transport of FFA across membranes and enhancing their rapid conversion into acyl-CoAs.
Ceramides induce and/or activate genes (for example, SREBP genes) that promote the incorporation of FFA into triglycerides and facilitate their storage in lipid droplets2,33.
Ceramides inhibit the uptake of glucose28,29 and amino acids34–36, thus leading to the preferential utilization of fatty acids for energy. This conserved attribute of sphingolipids is present in single-cell organisms, such as yeast36–39, as well as being an underlying cause of mammalian insulin resistance1.
Ceramides decrease mitochondrial efficiency, inhibiting oxygen consumption through the complexes of the electron-transport chain and decreasing mitochondrial membrane potential, thereby leading to a decrease in the amount of ATP produced by a given fatty acid molecule3,40–42. Lower mitochondrial efficiency ultimately allows the cell to burn more fatty acids, because it decreases the effect of reducing equivalents produced from fat metabolism on the electrochemical gradient across the inner mitochondrial membrane.
Ceramides slow lipolysis by blocking activation of hormone-sensitive lipase (HSL)2.
Fig. 2 |. Ceramides as signals of lipid excess.

Left, simplified schematic of the ceramide-biosynthesis pathway, highlighting enzymes discussed in this review. Pharmacological inhibition or genetic depletion of SPT, CERS1, CERS6 and DES1 lowers ceramides and ameliorates insulin resistance and cardiometabolic disorders. Moreover, transgenic overexpression of ASAH1, ADIPOR1 or ADIPOR2 produces the same beneficial effects. Right, simplified schematic depicting the central hypothesis that ceramides are gauges of FFA excess. The mechanisms that ceramides invoke are as follows: (1) redistribution of CD36 toward the plasma membrane, thus facilitating the safe uptake of FFA and enhancing the conversion of fatty acids into acyl-CoA; (2) induction of Srebf1, thus stimulating the synthesis of cholesterol, a partner of ceramide and sphingomyelin in lipid rafts, and inducing genes that facilitate the conversion of FFA into inert triglycerides; (3) inhibition of Akt (PKB), which slows glucose utilization in adipose tissue and muscle, thus facilitating the usage of lipids for energy, while inhibiting hepatic gluconeogenesis; (4) inhibition of HSL, which prevents liberation of additional FFA from lipid droplest; (5) inhibition of mitochondrial complexes, thus calibrating the metabolic efficiency in situations with energy surplus; (6) repression of lipase expression by ceramide synthases. KDSR, 3-ketodihydrosphingosine reductase; ASAH1, acid ceramidase; MFF, mitochondrial fission factor.
Each of these mechanisms lowers levels of FFA, by promoting either their storage as triglycerides or their utilization for energy. Most of these actions have been shown by using both short-chain ceramide analogues (for example, C2 ceramide) and by specific experimental manipulations to modulate the endogenous ceramide pool. The exceptions are the activation of HSL and the inhibition of mammalian amino acid transporters, which, to our knowledge, have been shown only by using C2 ceramide.
Many of these actions result from ceramide activation of two downstream effectors: protein kinase Cζ (PKCζ) and protein phosphatase 2A (PP2A). PKCζ mediates ceramide effects on Akt (PKB)43–45, which regulates glucose uptake, as well as CD36 (ref.46) and SREBP7,47. PP2A regulates Akt (PKB)48–50 and HSL51. Of note, Akt (PKB) is modulated by both PKCζ and PP2A through distinct and separable mechanisms48,49. Inhibition of Akt (PKB) is likely to inhibit synthesis of new fatty acids, although this has never been shown experimentally.
The mechanisms by which ceramides inhibit mitochondrial efficiency have, until recently, been elusive. Large, extended mitochondria produced by fusion display enhanced mitochondrial efficiency; they are often elevated in cells with high respiratory activity52. On the opposite end of the spectrum, mitochondria can be fragmented through fission, which renders them less efficient and more susceptible to mitophagy52. Mitochondrial C16 ceramides have been found to promote mitochondrial fragmentation by interacting with mitochondrial fission factor53. Remarkably, this mechanism involving ceramide and mitochondrial fission factor fully accounts for the increased mitochondrial fragmentation that occurs in mice fed an obesogenic diet53. These results are consistent with prior findings by Bikman and colleagues54, who have similarly concluded that fission is requisite for the impairment in oxygen consumption caused by short-chain ceramide analogues in vitro. Ceramides have also been proposed to alter mitochondrial membrane potential by forming channels in mitochondrial membranes.
Ceramides can also be converted to sphingomyelins, which are binding partners of sterols such as cholesterol. Sphingomyelin and cholesterol are components of caveolae, which are particularly abundant in adipocytes and serve as scaffolds that support fatty acid diffusion and uptake55–57. Thus, sphingomyelins may also have a role in supporting the safe passage of detergent-like fatty acids across cellular membranes.
Some of the enzymes in the biosynthetic pathway serve dual functions beyond their role as enzymes that produce ceramides. For example, the Drosophila ceramide synthase (Schlank) and its mammalian isoform CERS2 have been shown to respond to fatty acids by translocating to the nucleus, where they repress transcription of lipases58,59. This dual action of the enzymes that produce ceramides further prevents liberation of FFA from the lipid droplet, thus reinforcing the evolutionarily conserved role of the sphingolipid pathway as a means of protecting cells in situations of lipid surplus.
Collectively, these sphingolipid actions promote the utilization and storage of fatty acids (Fig. 2). Such cellular capabilities would be beneficial in instances of lipid surplus. We further predict that the well-known effects of ceramide on apoptosis60–62 and fibrosis63,64 may have evolved as an extension of this response, helping organisms rid themselves of compromised cells when FFA levels are particularly high. Such protective mechanisms would limit the damage resulting from the spilling of cytosolic components after aberrant FFA-driven membrane lysis.
Inhibition of ceramide biosynthesis ameliorates cardiometabolic diseases in rodents
Although the ceramide actions described above protect cells and/or organisms in times of acute nutritional overload, they are deleterious in the context of obesity. In particular, these ceramide actions explain the ‘selective’ insulin resistance65 that characterizes the pre-diabetic condition. This state, which is exemplified by defective insulin action towards glucose but enhanced insulin-stimulated triglyceride production, increases the risk of diabetes, atherosclerosis, hypertension and heart failure. Indeed, ceramide-reduction interventions reverse selective insulin resistance51 and prevent the development of future cardiometabolic disorders1,14,15. A summary of tissue-specific actions of ceramides in cardiometabolic disease is provided in Fig. 3.
Fig. 3 |. Target tissues and metabolic activities of ceramides.
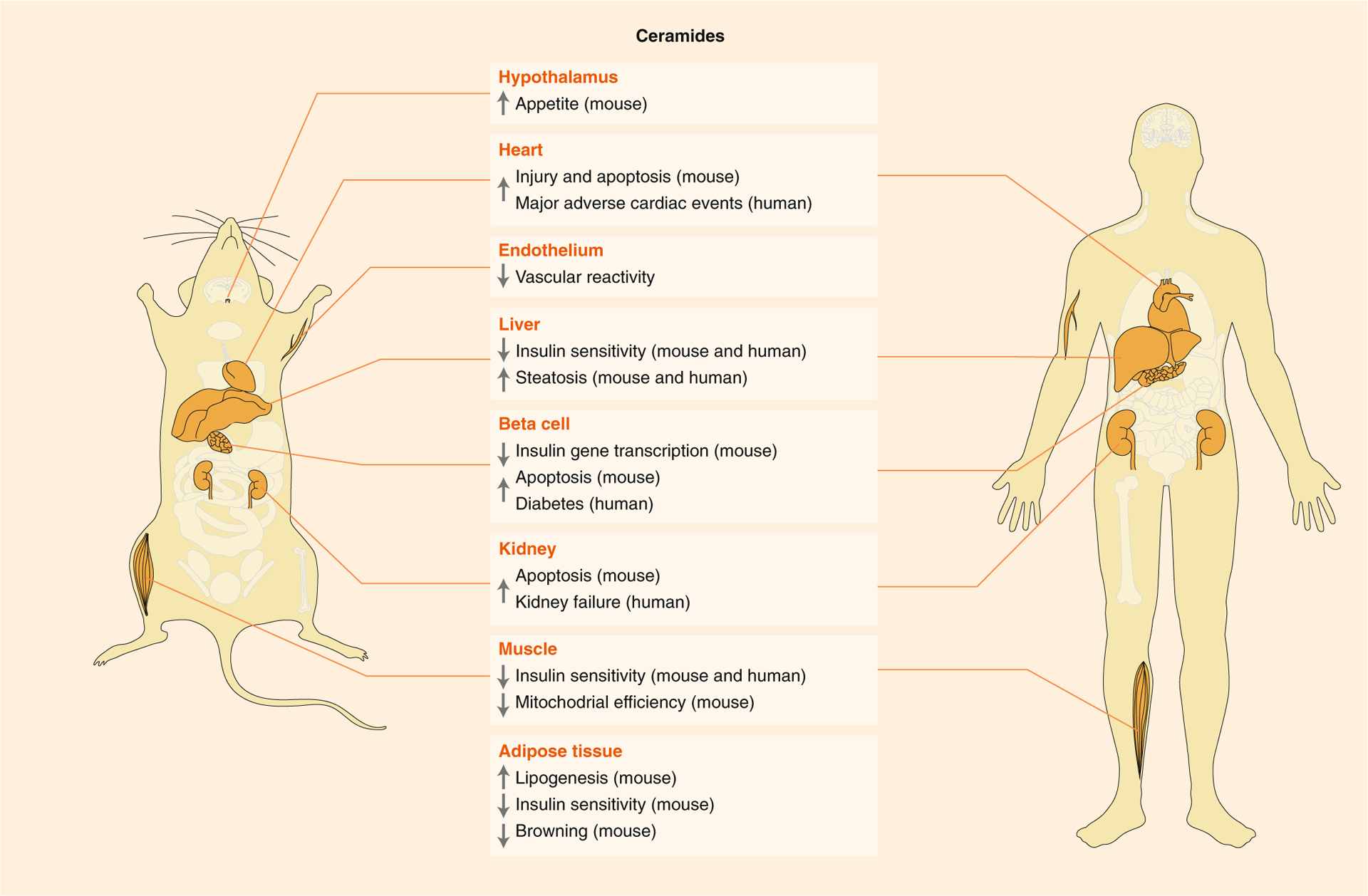
Ceramides influence cardiometabolic disease in mice and humans. Most observations have been conclusively demonstrated in rodents and are supported by strong correlational data from humans. The physiological effects of ceramide are therefore strongly preserved between the two organisms. Ceramide effects include induction of insulin resistance, impairment in vascular reactivity, enhancement of triglyceride synthesis, impairment in lipid oxidation, inhibition of insulin gene transcription and stimulation of apoptosis and fibrosis.
The first studies to convincingly demonstrate the therapeutic potential of ceramide-reduction interventions were conducted with myriocin, an irreversible and high-affinity inhibitor of SPT. The compound was first used in ApoE-knockout mice, in which it prevented or reversed atherosclerosis9–14. Shortly thereafter, studies in various rodent models (mice, rats and hamsters) revealed that SPT inhibition prevents or reverses insulin resistance1,3,8,66–68, hepatic steatosis1,3–7, type 2 diabetes1, hypertension69 and/or cardiomyopathy15,16. Deletion of essential SPT subunits from mouse tissues recapitulates many of these myriocin actions7,15,70.
We have recently found that excising the Degs1 gene (encoding dihydroceramide desaturase-1 (DES1), the enzyme that inserts an important double bond in the ceramide backbone) from adult mice fully reverses the pre-diabetic condition2. In this study, total sphingolipid levels were unchanged, but the proportion of ceramides lacking the double bond (that is, dihydrosphingolipids) greatly increased. This double bond was found to be essential for all the mechanisms described above. Excising Degs1 from the whole body, liver or adipose tissue resolved hepatic steatosis and insulin resistance in leptin-deficient and high-fat-diet-fed mice. In contrast, ablation of Degs1 from intestinal or myeloid cells had no effect. These studies reinforce the importance of ceramides as regulators of glucose homeostasis and identify a tractable therapeutic target for reversing the pre-diabetic condition. These findings suggest that the formation of ceramide platforms, which is dependent on the double bond, is essential for the signalling mechanisms described herein.
The nature of the acyl chain attached to the sphingoid backbone also influences the ability of ceramides to initiate the actions described above. Specifically, ceramides containing C16:0 or C18:0 side chains serve as nutritional signals, whereas those containing much longer side chains such as C24:0 or C24:1 do not. For example, ablation of the Cers6 gene, which encodes the enzyme that makes C16:0 ceramides and is upregulated in obesity, from the whole body, brown adipose tissue or the liver, is sufficient to resolve insulin resistance and hepatic steatosis42. Excision of Cers5, which encodes the enzyme that makes C16:0 ceramides, has protective actions in the heart51, and excision of Cers1, which encodes the dominant isoform in skeletal muscle and the enzyme that makes C18 ceramides, improves insulin sensitivity71. In comparison, haploinsufficiency for Cers2, which encodes the major synthase isoform in the liver that makes very long-chain ceramides (for example, C24:0 or C24:1 ceramides), induces compensatory increases in Cers6 and C16:0 ceramides, thereby worsening the metabolic dysfunction caused by high-fat diets3,42.
These data are consistent with the idea that the long-chain ceramides (for example, C16 ceramides) are uniquely capable of initiating the signalling pathways that influence cellular metabolism. Kolesnick and colleagues have reported that C16:0 ceramides, but not forms with longer acyl chains (that is, very long-chain ceramides), self-associate in non-bilayer macrodomains termed ceramide-rich platforms72. The authors have identified these membrane microdomains as sites that initiate downstream signalling and disrupt membrane structure. These biophysical attributes may account for the ability of C16 ceramides to serve as signalling nodes that alter the metabolic program of lipid-laden cells.
Serum and tissue ceramide predict the severity of cardiometabolic disease
Advances in mass spectroscopy and lipidomics have enabled researchers to measure sphingolipids in large tissue biobanks, revealing strong relationships between serum and tissue ceramides and insulin resistance, diabetes and major adverse cardiac events. Because of these associations, the Mayo Clinic now offers diagnostic tests to measure circulating ceramides as indices of cardiovascular risk19,20. Herein, we briefly summarize the larger studies.
Serum profiling studies have revealed particularly strong relationships between certain ceramide species and major adverse cardiac events, insulin resistance and diabetes. (1) In studies of patients with or without coronary artery disease, ceramides containing the C16:0, C18:0 and C24:1 acyl chains have been found to display independent predictive value for future fatality and/or major adverse cardiac events exceeding those of conventional risk measures, including LDL cholesterol73–81. Most of these studies were performed by Zora Biosciences, profiling individuals in Europe and North America. The total number of individuals screened in these studies now exceeds 70,000 (R. Laaksonen, personal communication). (2) Numerous additional groups have reported associations between serum ceramides and insulin resistance. In particular, Lemaitre and colleagues have profiled several thousand Native Americans (that is, the Strong Family Heart Study) at high risk for diabetes82–84. Several ceramide species, including C16:0 and C18:0 ceramides, correlated with hyperinsulinemia and the homeostatic model of insulin resistance (HOMA-IR) in this at-risk population. These studies are consistent with earlier, smaller studies evaluating relationships between serum ceramides and insulin resistance in both humans and non-human primates85–90. (3) Wigger and colleagues have identified relationships between dihydroceramides and ceramides and the development of diabetes91. The dihydroceramides, which are much less abundant and serve as good readouts of changes in ceramide flux, have been found to predict diabetes development up to 9 years in advance of disease onset.
Within tissues, the largest profiling study to date was performed by Yki-Jarvinen and colleagues, who characterized 125 liver biopsies. These authors have determined that C16:0 and other ‘saturated’ ceramides correlate strongly with insulin resistance, independently of steatosis92. In a follow-up study, these authors have found that overfeeding saturated fat leads to increases in harmful C16:0 ceramides in plasma and worsened insulin resistance93.
The Goodpaster laboratory has found positive relationships between muscle ceramide levels and insulin resistance in several study populations94–98. Similar findings have been reported by Mandarino and colleagues99. Insulin-sensitizing treatments (such as metformin, pioglitazone, exercise and bariatric surgery) have all been found to decrease muscle ceramides94–98.
Some recent studies have drawn attention to less abundant sphingolipids, such as deoxysphingolipids or other sphingolipids with a shorter sphingosine backbone. These are produced because of substrate promiscuity in serine palmitoyltransferase, which can occasionally use either alanine/glycine or myristoyl-CoA, instead of serine and palmitoyl-CoA, as substrates. Serum levels of these less abundant sphingolipids have also been found to be associated with cardiometabolic disease in humans and/or animal models89,100–105.
Although the preponderance of profiling studies describes strong relationships between sphingolipids and indices of metabolic dysfunction, we acknowledge come controversy in the literature, because some earlier studies have reported that no such relationship exists between muscle or liver ceramides and insulin resistance106,107. Some of these studies have involved infusion of a lipid emulsion comprising unsaturated fats108–110, which induce insulin resistance via a ceramide-independent mechanism1. For a longer discussion of this issue and the apparent discrepancy, we refer readers to a prior debate111,112.
Regulation of ceramide synthesis and degradation
The concentration of precursor substrates (for example, palmitoyl-CoA and serine) is an important determinant of ceramide levels50. Indeed, the aforementioned dietary studies have indicated that consumption of saturated fat increases levels of ceramides93. Beyond that, a wealth of evidence has emerged demonstrating that rates of ceramide production and degradation can be further modified by other factors associated with obesity and cardiometabolic health and disease.
Adiponectin.
Adiponectin is an anti-diabetic and cardioprotective molecule released during fasting by small metabolically active adipocytes. During these periods, which are characterized by enhanced lipolysis, adiponectin migrates to other peripheral tissues, where it is insulin sensitizing and anti-apoptotic. Noting that adiponectin and ceramides had opposing effects, Holland and Scherer have hypothesized that the adipokine elicits its broad swath of actions by degrading ceramides. In particular, the authors have observed that adiponectin receptors (ADIPOR1 and ADIPOR2), as well as other members of the progesterone and adipoQ-receptor superfamily, show strong homology to intracellular ceramidases113. The authors subsequently determined that adiponectin promotes ceramide degradation and that deletion of an essential residue in the receptors’ ceramidase motif renders them ineffectual17. Moreover, they have found that overexpression of a ceramidase in adipose tissue or the liver is sufficient to mimic adiponectin actions46. Granier and colleagues later solved the crystal structure of the receptor, revealing that it bears a strong resemblance to those of ceramidases18. Moreover, they have demonstrated that the purified receptor has ceramidase activity18. These data strongly suggest that adiponectin prevents ceramide accumulation when FFA are needed for energy production. Evidence for the clinical relevance of these findings is starting to emerge: adiponectin levels have been found to inversely correlate with serum and tissue ceramides in insulin resistant individuals88,114.
β-adrenergic agonists.
We predicted that factors that liberate fatty acids from adipocytes, such as β-adrenergic agonists, would decrease ceramide levels, thus relieving their inhibition of HSL, releasing FFA and enhancing mitochondrial efficiency. Using a novel flux assay to monitor rates of ceramide production, we have found that β-adrenergic agonists rapidly and completely shut down ceramide biosynthesis in adipocytes2.
Inflammatory agonists.
Inflammatory agonists (for example, Toll-like receptor-4 agonists (TLR4), tumour necrosis factor and interleukins) selectively increase levels of sphingolipids by upregulating enzymes in the de novo ceramide biosynthesis at pathway and by activating sphingomyelinases17,23–25,115. Indeed, TLR4 signalling is required for palmitate induction of ceramides17,116,117 and for lipid-induced insulin resistance17,118–120. Of note, correlational studies have shown particularly tight relationships between plasma ceramides and insulin resistance in concert with levels of circulating inflammatory cytokines115,121.
Farnesoid X receptors (FXR).
FXR are bile-acid-responsive nuclear receptors that modify bile acid, lipid and glucose metabolism. Gonzalez and colleagues have found that intestinal FXR is a strong inducer of non-alcoholic fatty liver disease, thus identifying it as an important link between changes in the microbiome and the disruption of liver metabolism33,122–125. These authors have also found that FXR activation leads to the production of intestinal ceramides. Moreover, they have determined that these ceramides transmigrate to the liver, where they stimulate hepatic lipid deposition and gluconeogenesis. Thus, increased lipid-driven bile release in the gut activates a compensatory feedback loop that increases ceramides and signals the organism in times of lipid excess.
Determination of the precise means through which these factors influence ceramide levels may reveal exciting opportunities for therapeutic intervention to improve metabolic health.
Critical questions for future research
The data described above unequivocally identify ceramides as important nutrient signals that contribute to cardiometabolic disease. These transformative discoveries, although exciting, have led to several unresolved questions.
First, what is the importance of the double bond or the difference in acyl-chain lengths in conferring biological activity to ceramides? How do these small modifications influence the downstream signalling mechanisms that translate minor changes in ceramides into profound alterations in cellular metabolism and cardiometabolic health?
Second, what are the genetic determinants of ceramide accumulation in the population? Numerous single-nucleotide polymorphisms have been identified in ceramide-modifying enzymes. For example, Curran, Blangero and colleagues have identified an inactivating missense mutation in Degs1 that associates with increased dihydroceramides, and decreased ceramides, 2-h glucose, cholesterol esters and diabetes risk scores126. However, many other rare variants have not been characterized. Will research reveal that these mutations have functional effects on sphingolipid profiles or disease risk? If so, can this information be used to develop personalized interventions to lower ceramides and improve health?
Third, will any of the enzymes that control ceramide production or degradation emerge as tractable therapeutic targets? Our research has focused on dihydroceramide desaturase-1, which expresses a favourable combination of druggability, biomarker availability and safety. Will small-molecule inhibitors of the enzyme show clinical utility? Or will other enzymes in the pathway (for example, one of the ceramide synthases or ceramidases) prove to be better targets for lowering ceramides to improve cardiometabolic health?
Fourth, can specific dietary or exercise regimens be developed to lower ceramides and improve patient health? What should we tell patients today with high ceramide scores?
Research addressing these important queries holds great promise for combating the metabolic disease epidemic.
Acknowledgements
The authors receive research support from the National Institutes of Health (DK112826 and DK108833 to W.L.H. and DK115824 and DK116450 to S.A.S.), the Juvenile Diabetes Research Foundation (JDRF 3-SRA-2019-768-A-B to W.L.H.), the American Diabetes Association (to S.A.S.), the American Heart Association (to S.A.S.), the Margolis Foundation (to S.A.S.) and the USDA (2019-67018-29250 to B.C.). B.C. received a pilot grant from the Diabetes Research Center at Washington University in St. Louis from the NIH under award number P30DK020579.
Footnotes
Competing interests
S.A.S. is a co-founder and consultant for Centaurus Therapeutics.
Peer review information Primary Handling Editor: Pooja Jha.
References
- 1.Holland WL et al. Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metab. 5, 167–179 (2007). [DOI] [PubMed] [Google Scholar]
- 2.Chaurasia B et al. Targeting a ceramide double bond improves insulin resistance and hepatic steatosis. Science 365, 386–392 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raichur S et al. CerS2 haploinsufficiency inhibits β-oxidation and confers susceptibility to diet-induced steatohepatitis and insulin resistance. Cell Metab. 20, 687–695 (2014). [DOI] [PubMed] [Google Scholar]
- 4.Kurek K et al. Inhibition of ceramide de novo synthesis reduces liver lipid accumulation in rats with nonalcoholic fatty liver disease. Liver Int. 34, 1074–1083 (2014). [DOI] [PubMed] [Google Scholar]
- 5.Correnti JM, Juskeviciute E, Swarup A & Hoek JB Pharmacological ceramide reduction alleviates alcohol-induced steatosis and hepatomegaly in adiponectin knockout mice. Am. J. Physiol. Gastrointest. Liver Physiol 306, G959–G973 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kasumov T et al. Ceramide as a mediator of non-alcoholic fatty liver disease and associated atherosclerosis. PLoS One 10, e0126910 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen TC et al. An ANGPTL4-ceramide-PKCzeta axis mediates chronic glucocorticoid exposure-induced hepatic steatosis and hypertriglyceridemia in mice. J. Biol. Chem 294, 9213–9224 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dekker MJ et al. Inhibition of sphingolipid synthesis improves dyslipidemia in the diet-induced hamster model of insulin resistance: evidence for the role of sphingosine and sphinganine in hepatic VLDL-apoB100 overproduction. Atherosclerosis 228, 98–109 (2013). [DOI] [PubMed] [Google Scholar]
- 9.Park TS, Rosebury W, Kindt EK, Kowala MC & Panek RL Serine palmitoyltransferase inhibitor myriocin induces the regression of atherosclerotic plaques in hyperlipidemic ApoE-deficient mice. Pharmacol. Res 58, 45–51 (2008). [DOI] [PubMed] [Google Scholar]
- 10.Glaros EN et al. Myriocin slows the progression of established atherosclerotic lesions in apolipoprotein E gene knockout mice. J. Lipid Res 49, 324–331 (2008). [DOI] [PubMed] [Google Scholar]
- 11.Glaros EN et al. Inhibition of atherosclerosis by the serine palmitoyl transferase inhibitor myriocin is associated with reduced plasma glycosphingolipid concentration. Biochem. Pharmacol 73, 1340–1346 (2007). [DOI] [PubMed] [Google Scholar]
- 12.Park TS et al. Modulation of lipoprotein metabolism by inhibition of sphingomyelin synthesis in ApoE knockout mice. Atherosclerosis 189, 264–272 (2006). [DOI] [PubMed] [Google Scholar]
- 13.Hojjati MR et al. Effect of myriocin on plasma sphingolipid metabolism and atherosclerosis in apoE-deficient mice. J. Biol. Chem 280, 10284–10289 (2005). [DOI] [PubMed] [Google Scholar]
- 14.Park TS et al. Inhibition of sphingomyelin synthesis reduces atherogenesis in apolipoprotein E-knockout mice. Circulation 110, 3465–3471 (2004). [DOI] [PubMed] [Google Scholar]
- 15.Park TS et al. Ceramide is a cardiotoxin in lipotoxic cardiomyopathy. J. Lipid Res 49, 2101–2112 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ji R et al. Increased de novo ceramide synthesis and accumulation in failing myocardium. JCI Insight 2, 82922 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holland WL et al. Lipid-induced insulin resistance mediated by the proinflammatory receptor TLR4 requires saturated fatty acid-induced ceramide biosynthesis in mice. J. Clin. Invest 121, 1858–1870 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vasiliauskaité-Brooks I et al. Structural insights into adiponectin receptors suggest ceramidase activity. Nature 544, 120–123 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Westra B Ceramides, Plasma [A Test in Focus]. Mayo Clinic Laboratories https://news.mayomedicallaboratories.com/2016/07/28/ceramides-plasma-a-test-in-focus/ (2016).
- 20.Summers SA Could ceramides become the new cholesterol? Cell Metab. 27, 276–280 (2018). [DOI] [PubMed] [Google Scholar]
- 21.Merrill AH Jr. De novo sphingolipid biosynthesis: a necessary, but dangerous, pathway. J. Biol. Chem 277, 25843–25846 (2002). [DOI] [PubMed] [Google Scholar]
- 22.Park JW, Park WJ & Futerman AH Ceramide synthases as potential targets for therapeutic intervention in human diseases. Biochim. Biophys. Acta 1841, 671–681 (2014). [DOI] [PubMed] [Google Scholar]
- 23.Hannun YA & Obeid LM Ceramide: an intracellular signal for apoptosis. Trends Biochem. Sci 20, 73–77 (1995). [DOI] [PubMed] [Google Scholar]
- 24.Kolesnick R Ceramide: a novel second messenger. Trends Cell Biol. 2, 232–236 (1992). [DOI] [PubMed] [Google Scholar]
- 25.Obeid LM, Linardic CM, Karolak LA & Hannun YA Programmed cell death induced by ceramide. Science 259, 1769–1771 (1993). [DOI] [PubMed] [Google Scholar]
- 26.Shimabukuro M et al. Lipoapoptosis in beta-cells of obese prediabetic fa/fa rats: role of serine palmitoyltransferase overexpression. J. Biol. Chem 273, 32487–32490 (1998). [DOI] [PubMed] [Google Scholar]
- 27.Shimabukuro M, Zhou YT, Levi M & Unger RH Fatty acid-induced beta cell apoptosis: a link between obesity and diabetes. Proc. Natl Acad. Sci. USA 95, 2498–2502 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Summers SA, Garza LA, Zhou H & Birnbaum MJ Regulation of insulin-stimulated glucose transporter GLUT4 translocation and Akt kinase activity by ceramide. Mol. Cell. Biol 18, 5457–5464 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang CN, O’Brien L & Brindley DN Effects of cell-permeable ceramides and tumor necrosis factor-alpha on insulin signaling and glucose uptake in 3T3-L1 adipocytes. Diabetes 47, 24–31 (1998). [DOI] [PubMed] [Google Scholar]
- 30.Zhou H, Summers SA, Birnbaum MJ & Pittman RN Inhibition of Akt kinase by cell-permeable ceramide and its implications for ceramide-induced apoptosis. J. Biol. Chem 273, 16568–16575 (1998). [DOI] [PubMed] [Google Scholar]
- 31.Jay AG & Hamilton JA The enigmatic membrane fatty acid transporter CD36: new insights into fatty acid binding and their effects on uptake of oxidized LDL. Prostaglandins Leukot. Essent. Fat. Acids 138, 64–70 (2018). [DOI] [PubMed] [Google Scholar]
- 32.Xu S, Jay A, Brunaldi K, Huang N & Hamilton JA CD36 enhances fatty acid uptake by increasing the rate of intracellular esterification but not transport across the plasma membrane. Biochemistry 52, 7254–7261 (2013). [DOI] [PubMed] [Google Scholar]
- 33.Jiang C et al. Intestinal farnesoid X receptor signaling promotes nonalcoholic fatty liver disease. J. Clin. Invest 125, 386–402 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hyde R, Hajduch E, Powell DJ, Taylor PM & Hundal HS Ceramide down-regulates System A amino acid transport and protein synthesis in rat skeletal muscle cells. FASEB J. 19, 461–463 (2005). [DOI] [PubMed] [Google Scholar]
- 35.Finicle BT et al. Sphingolipids inhibit endosomal recycling of nutrient transporters by inactivating ARF6. J. Cell Sci 131, jcs213314 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guenther GG et al. Ceramide starves cells to death by downregulating nutrient transporter proteins. Proc. Natl Acad. Sci. USA 105, 17402–17407 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Edinger AL Starvation in the midst of plenty: making sense of ceramide-induced autophagy by analysing nutrient transporter expression. Biochem. Soc. Trans 37, 253–258 (2009). [DOI] [PubMed] [Google Scholar]
- 38.Cowart LA & Obeid LM Yeast sphingolipids: recent developments in understanding biosynthesis, regulation, and function. Biochim. Biophys. Acta 1771, 421–431 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chung N, Mao C, Heitman J, Hannun YA & Obeid LM Phytosphingosine as a specific inhibitor of growth and nutrient import in Saccharomyces cerevisiae. J. Biol. Chem 276, 35614–35621 (2001). [DOI] [PubMed] [Google Scholar]
- 40.Kogot-Levin A & Saada A Ceramide and the mitochondrial respiratory chain. Biochimie 100, 88–94 (2014). [DOI] [PubMed] [Google Scholar]
- 41.Zigdon H et al. Ablation of ceramide synthase 2 causes chronic oxidative stress due to disruption of the mitochondrial respiratory chain. J. Biol. Chem 288, 4947–4956 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Turpin SM et al. Obesity-induced CerS6-dependent C16:0 ceramide production promotes weight gain and glucose intolerance. Cell Metab. 20, 678–686 (2014). [DOI] [PubMed] [Google Scholar]
- 43.Hajduch E et al. Targeting of PKCzeta and PKB to caveolin-enriched microdomains represents a crucial step underpinning the disruption in PKB-directed signalling by ceramide. Biochem. J 410, 369–379 (2008). [DOI] [PubMed] [Google Scholar]
- 44.Powell DJ, Hajduch E, Kular G & Hundal HS Ceramide disables 3-phosphoinositide binding to the pleckstrin homology domain of protein kinase B (PKB)/Akt by a PKCzeta-dependent mechanism. Mol. Cell. Biol 23, 7794–7808 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bourbon NA, Sandirasegarane L & Kester M Ceramide-induced inhibition of Akt is mediated through protein kinase Czeta: implications for growth arrest. J. Biol. Chem 277, 3286–3292 (2002). [DOI] [PubMed] [Google Scholar]
- 46.Xia JY et al. Targeted induction of ceramide degradation leads to improved systemic metabolism and reduced hepatic steatosis. Cell Metab. 22, 266–278 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Taniguchi CM et al. Divergent regulation of hepatic glucose and lipid metabolism by phosphoinositide 3-kinase via Akt and PKClambda/zeta. Cell Metab. 3, 343–353 (2006). [DOI] [PubMed] [Google Scholar]
- 48.Blouin CM et al. Plasma membrane subdomain compartmentalization contributes to distinct mechanisms of ceramide action on insulin signaling. Diabetes 59, 600–610 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stratford S, Hoehn KL, Liu F & Summers SA Regulation of insulin action by ceramide: dual mechanisms linking ceramide accumulation to the inhibition of Akt/protein kinase B. J. Biol. Chem 279, 36608–36615 (2004). [DOI] [PubMed] [Google Scholar]
- 50.Chavez JA & Summers SA Characterizing the effects of saturated fatty acids on insulin signaling and ceramide and diacylglycerol accumulation in 3T3-L1 adipocytes and C2C12 myotubes. Arch. Biochem. Biophys 419, 101–109 (2003). [DOI] [PubMed] [Google Scholar]
- 51.Russo SB et al. Ceramide synthase 5 mediates lipid-induced autophagy and hypertrophy in cardiomyocytes. J. Clin. Invest 122, 3919–3930 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Westermann B Bioenergetic role of mitochondrial fusion and fission. Biochim. Biophys. Acta 1817, 1833–1838 (2012). [DOI] [PubMed] [Google Scholar]
- 53.Hammerschmidt P et al. CerS6-derived sphingolipids interact with MFF and promote mitochondrial fragmentation in obesity. Cell 177, 1536–1552. e1523 (2019). [DOI] [PubMed] [Google Scholar]
- 54.Smith ME et al. Mitochondrial fission mediates ceramide-induced metabolic disruption in skeletal muscle. Biochem. J 456, 427–439 (2013). [DOI] [PubMed] [Google Scholar]
- 55.Ehehalt R et al. Uptake of long chain fatty acids is regulated by dynamic interaction of FAT/CD36 with cholesterol/sphingolipid enriched microdomains (lipid rafts). BMC Cell Biol. 9, 45 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pohl J et al. Long-chain fatty acid uptake into adipocytes depends on lipid raft function. Biochemistry 43, 4179–4187 (2004). [DOI] [PubMed] [Google Scholar]
- 57.Covey SD et al. Cholesterol depletion inhibits fatty acid uptake without affecting CD36 or caveolin-1 distribution in adipocytes. Biochem. Biophys. Res. Commun 355, 67–71 (2007). [DOI] [PubMed] [Google Scholar]
- 58.Chaurasia B, Holland WL & Summers SA Does this Schlank make me look fat? Trends Endocrinol. Metab 29, 597–599 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sociale M et al. Ceramide synthase Schlank is a transcriptional regulator adapting gene expression to energy requirements. Cell Rep. 22, 967–978 (2018). [DOI] [PubMed] [Google Scholar]
- 60.Marra F & Svegliati-Baroni G Lipotoxicity and the gut-liver axis in NASH pathogenesis. J. Hepatol 68, 280–295 (2018). [DOI] [PubMed] [Google Scholar]
- 61.Alkhouri N, Dixon LJ & Feldstein AE Lipotoxicity in nonalcoholic fatty liver disease: not all lipids are created equal. Expert Rev. Gastroenterol. Hepatol 3, 445–451 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hoekstra D Ceramide-mediated apoptosis of hepatocytes in vivo: a matter of the nucleus? J. Hepatol 31, 161–164 (1999). [DOI] [PubMed] [Google Scholar]
- 63.Nikolova-Karakashian M Sphingolipids at the crossroads of NAFLD and senescence. Adv. Cancer Res 140, 155–190 (2018). [DOI] [PubMed] [Google Scholar]
- 64.Garcia-Ruiz C, Marí M, Colell A, Morales A & Fernandez-Checa JC Metabolic therapy: lessons from liver diseases. Curr. Pharm. Des 17, 3933–3944 (2011). [DOI] [PubMed] [Google Scholar]
- 65.Brown MS & Goldstein JL Selective versus total insulin resistance: a pathogenic paradox. Cell Metab. 7, 95–96 (2008). [DOI] [PubMed] [Google Scholar]
- 66.Ussher JR et al. Inhibition of de novo ceramide synthesis reverses diet-induced insulin resistance and enhances whole-body oxygen consumption. Diabetes 59, 2453–2464 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yang G et al. Central role of ceramide biosynthesis in body weight regulation, energy metabolism, and the metabolic syndrome. Am. J. Physiol. Endocrinol. Metab 297, E211–E224 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Blachnio-Zabielska AU et al. Inhibition of ceramide de novo synthesis affects adipocytokine secretion and improves systemic and adipose tissue insulin sensitivity. Int. J. Mol. Sci 19, E3995 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang QJ et al. Ceramide mediates vascular dysfunction in diet-induced obesity by PP2A-mediated dephosphorylation of the eNOS-Akt complex. Diabetes 61, 1848–1859 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chaurasia B et al. Adipocyte ceramides regulate subcutaneous adipose browning, inflammation, and metabolism. Cell Metab. 24, 820–834 (2016). [DOI] [PubMed] [Google Scholar]
- 71.Turpin-Nolan SM et al. CerS1-derived C18:0 ceramide in skeletal muscle promotes obesity-induced insulin resistance. Cell Rep. 26, 1–10.e17 (2019). [DOI] [PubMed] [Google Scholar]
- 72.Stancevic B & Kolesnick R Ceramide-rich platforms in transmembrane signaling. FEBS Lett. 584, 1728–1740 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mantovani A et al. Association of plasma ceramides with myocardial perfusion in patients with coronary artery disease undergoing stress myocardial perfusion scintigraphy. Arterioscler. Thromb. Vasc. Biol 38, 2854–2861 (2018). [DOI] [PubMed] [Google Scholar]
- 74.Anroedh S et al. Plasma concentrations of molecular lipid species predict long-term clinical outcome in coronary artery disease patients. J. Lipid Res 59, 1729–1737 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hilvo M et al. PCSK9 inhibition alters the lipidome of plasma and lipoprotein fractions. Atherosclerosis 269, 159–165 (2018). [DOI] [PubMed] [Google Scholar]
- 76.Havulinna AS et al. Circulating ceramides predict cardiovascular outcomes in the population-based FINRISK 2002 Cohort. Arterioscler. Thromb. Vasc. Biol 36, 2424–2430 (2016). [DOI] [PubMed] [Google Scholar]
- 77.Laaksonen R et al. Plasma ceramides predict cardiovascular death in patients with stable coronary artery disease and acute coronary syndromes beyond LDL-cholesterol. Eur. Heart J 37, 1967–1976 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tarasov K et al. Molecular lipids identify cardiovascular risk and are efficiently lowered by simvastatin and PCSK9 deficiency. J. Clin. Endocrinol. Metab 99, E45–E52 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cheng JM et al. Plasma concentrations of molecular lipid species in relation to coronary plaque characteristics and cardiovascular outcome: results of the ATHEROREMO-IVUS study. Atherosclerosis 243, 560–566 (2015). [DOI] [PubMed] [Google Scholar]
- 80.Yu J et al. Ceramide is upregulated and associated with mortality in patients with chronic heart failure. Can. J. Cardiol 31, 357–363 (2015). [DOI] [PubMed] [Google Scholar]
- 81.Pan W et al. Elevation of ceramide and activation of secretory acid sphingomyelinase in patients with acute coronary syndromes. Coron. Artery Dis 25, 230–235 (2014). [DOI] [PubMed] [Google Scholar]
- 82.Lemaitre RN et al. Circulating sphingolipids, insulin, HOMA-IR and HOMA-B: the Strong Heart Family Study. Diabetes 67, 1663–1672 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jensen PN et al. Circulating sphingolipids, fasting glucose, and impaired fasting glucose: the Strong Heart Family Study. EBioMedicine 41, 44–49 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lemaitre RN et al. Circulating sphingolipids, insulin, HOMA-IR, and HOMA-b: the Strong Heart Family Study. Diabetes 67, 1663–1672 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bergman BC et al. Serum sphingolipids: relationships to insulin sensitivity and changes with exercise in humans. Am. J. Physiol. Endocrinol. Metab 309, E398–E408 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Haus JM et al. Plasma ceramides are elevated in obese subjects with type 2 diabetes and correlate with the severity of insulin resistance. Diabetes 58, 337–343 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Boon J et al. Ceramides contained in LDL are elevated in type 2 diabetes and promote inflammation and skeletal muscle insulin resistance. Diabetes 62, 401–410 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lopez X, Goldfine AB, Holland WL, Gordillo R & Scherer PE Plasma ceramides are elevated in female children and adolescents with type 2 diabetes. J. Pediatr. Endocrinol. Metab 26, 995–998 (2013). [DOI] [PubMed] [Google Scholar]
- 89.Brozinick JT et al. Plasma sphingolipids are biomarkers of metabolic syndrome in non-human primates maintained on a Western-style diet. Int. J. Obes. (Lond.) 37, 1064–1070 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Warshauer JT et al. Effect of pioglitazone on plasma ceramides in adults with metabolic syndrome. Diabetes Metab. Res. Rev 31, 734–744 (2015). [DOI] [PubMed] [Google Scholar]
- 91.Wigger L et al. Plasma dihydroceramides are diabetes susceptibility biomarker candidates in mice and humans. Cell Rep. 18, 2269–2279 (2017). [DOI] [PubMed] [Google Scholar]
- 92.Luukkonen PK et al. Hepatic ceramides dissociate steatosis and insulin resistance in patients with non-alcoholic fatty liver disease. J. Hepatol 64, 1167–1175 (2016). [DOI] [PubMed] [Google Scholar]
- 93.Luukkonen PK et al. Saturated fat is more metabolically harmful for the human liver than unsaturated fat or simple sugars. Diabetes Care 41, 1732–1739 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Amati F et al. Skeletal muscle triglycerides, diacylglycerols, and ceramides in insulin resistance: another paradox in endurance-trained athletes? Diabetes 60, 2588–2597 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Coen PM et al. Insulin resistance is associated with higher intramyocellular triglycerides in type I but not type II myocytes concomitant with higher ceramide content. Diabetes 59, 80–88 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Coen PM & Goodpaster BH Role of intramyocelluar lipids in human health. Trends Endocrinol. Metab 23, 391–398 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Coen PM et al. Reduced skeletal muscle oxidative capacity and elevated ceramide but not diacylglycerol content in severeobesity. Obes. (Silver Spring) 21, 2362–2371 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dubé JJ et al. Effects of weight loss and exercise on insulin resistance, and intramyocellular triacylglycerol, diacylglycerol and ceramide. Diabetologia 54, 1147–1156 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Adams JM II et al. Ceramide content is increased in skeletal muscle from obese insulin-resistant humans. Diabetes 53, 25–31 (2004). [DOI] [PubMed] [Google Scholar]
- 100.Othman A et al. Plasma 1-deoxysphingolipids are predictive biomarkers for type 2 diabetes mellitus. BMJ Open Diabetes Res. Care 3, e000073 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Othman A et al. Plasma deoxysphingolipids: a novel class of biomarkers for the metabolic syndrome? Diabetologia 55, 421–431 (2012). [DOI] [PubMed] [Google Scholar]
- 102.Mwinyi J et al. Plasma 1-deoxysphingolipids are early predictors of incident type 2 diabetes mellitus. PLoS One 12, e0175776 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Khan A & Hornemann T Correlation of the plasma sphingoid base profile with results from oral glucose tolerance tests in gestational diabetes mellitus. EXCLI J. 16, 497–509 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bertea M et al. Deoxysphingoid bases as plasma markers in diabetes mellitus. Lipids Health Dis. 9, 84 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Russo SB, Tidhar R, Futerman AH & Cowart LA Myristate-derived d16:0 sphingolipids constitute a cardiac sphingolipid pool with distinct synthetic routes and functional properties. J. Biol. Chem 288, 13397–13409 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Skovbro M et al. Human skeletal muscle ceramide content is not a major factor in muscle insulin sensitivity. Diabetologia 51, 1253–1260 (2008). [DOI] [PubMed] [Google Scholar]
- 107.Petersen MC & Shulman GI Roles of diacylglycerols and ceramides in hepatic insulin resistance. Trends Pharmacol. Sci 38, 649–665 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Itani SI, Ruderman NB, Schmieder F & Boden G Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C, and IκB-alpha. Diabetes 51, 2005–2011 (2002). [DOI] [PubMed] [Google Scholar]
- 109.Szendroedi J et al. Role of diacylglycerol activation of PKCθ in lipid-induced muscle insulin resistance in humans. Proc. Natl Acad. Sci. USA 111, 9597–9602 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nowotny B et al. Mechanisms underlying the onset of oral lipid-induced skeletal muscle insulin resistance in humans. Diabetes 62, 2240–2248 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Petersen MC & Jurczak MJ CrossTalk opposing view: intramyocellular ceramide accumulation does not modulate insulin resistance. J. Physiol. (Lond.) 594, 3171–3174 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Summers SA & Goodpaster BH CrossTalk proposal: intramyocellular ceramide accumulation does modulate insulin resistance. J. Physiol. (Lond.) 594, 3167–3170 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Holland WL & Scherer PE PAQRs: a counteracting force to ceramides? Mol. Pharmacol 75, 740–743 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Blachnio-Zabielska AU, Koutsari C, Tchkonia T & Jensen MD Sphingolipid content of human adipose tissue: relationship to adiponectin and insulin resistance. Obes. (Silver Spring) 20, 2341–2347 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.de Mello VD et al. Link between plasma ceramides, inflammation and insulin resistance: association with serum IL-6 concentration in patients with coronary heart disease. Diabetologia 52, 2612–2615 (2009). [DOI] [PubMed] [Google Scholar]
- 116.Sims K et al. Kdo2-lipid A, a TLR4-specific agonist, induces de novo sphingolipid biosynthesis in RAW264.7 macrophages, which is essential for induction of autophagy. J. Biol. Chem 285, 38568–38579 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Schilling JD et al. Palmitate and lipopolysaccharide trigger synergistic ceramide production in primary macrophages. J. Biol. Chem 288, 2923–2932 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Shi H et al. TLR4 links innate immunity and fatty acid-induced insulin resistance. J. Clin. Invest 116, 3015–3025 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kim JK et al. Prevention of fat-induced insulin resistance by salicylate. J. Clin. Invest 108, 437–446 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Cai D et al. Local and systemic insulin resistance resulting from hepatic activation of IKK-β and NF-κB. Nat. Med 11, 183–190 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Majumdar I & Mastrandrea LD Serum sphingolipids and inflammatory mediators in adolescents at risk for metabolic syndrome. Endocrine 41, 442–449 (2012). [DOI] [PubMed] [Google Scholar]
- 122.Gonzalez FJ, Jiang C, Xie C & Patterson AD Intestinal farnesoid X receptor signalling modulates metabolic disease. Dig. Dis 35, 178–184 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Xie C et al. An intestinal farnesoid X receptor-ceramide signaling axis modulates hepatic gluconeogenesis in mice. Diabetes 66, 613–626 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gonzalez FJ, Jiang C & Patterson AD An intestinal microbiota-farnesoid X receptor axis modulates metabolic disease. Gastroenterology 151, 845–859 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Jiang C et al. Intestine-selective farnesoid X receptor inhibition improves obesity-related metabolic dysfunction. Nat. Commun 6, 10166 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Curran JE et al. 63rd Annual Meeting Am. Soc. Hum. Genet https://www.ashg.org/2013meeting/abstracts/fulltext/f130122450.htm (2013).


