Abstract
Background:
Venetoclax with hypomethylating agents is a new standard of care for newly diagnosed (ND) patients with acute myeloid leukemia (AML) who are 75 years or older, or unfit for intensive chemotherapy. Pharmacodynamic studies have suggested superiority of the longer 10-day regimen of decitabine which has shown promising results in high-risk AML in phase 2 trials. We hypothesized that venetoclax with 10-day decitabine may be effective in ND and relapsed/refractory (R/R) AML, particularly for high-risk subgroups.
Methods:
This phase II study enrolled 168 patients with ND AML older than 60 years, secondary AML (sAML), and R/R AML. Patients were required to have an ECOG performance status of 3 or less, WBC less than 10×109/L, and adequate end-organ function. Ineligibility for intensive chemotherapy was based on the treating physicians’ assessment. Patients with favorable-risk cytogenetics, e.g., t(15;17) or core-binding factor AML, or prior BCL2-inhibitor therapy were excluded. Patients received decitabine 20mg/m2 IV for 10-days with oral venetoclax 400mg daily (DEC10-VEN) for induction, followed by decitabine 5-days with venetoclax for consolidation. The primary efficacy endpoint was overall response rate (ORR). The trial was registered on Clinicaltrials.gov, number NCT03404193 and continues to accrue patients.
Findings:
Between January 19, 2018 and December 16, 2019, we enrolled 70 patients; (42%) had ND AML, 15 patients (9%) had untreated sAML, 28 patients (17%) had treated sAML, and 55 patients (33%) had R/R AML. The median age was 71 years (IQR, 65–76) and 30% patients had ECOG performance status of 2 or higher. The ORR among all patients was 74% (95% CI 67, 80) and in disease subgroups were: ND AML, 89% (95% CI 79, 94); untreated sAML, 80% (95% CI 55, 93); treated sAML, 61% (95% CI 42, 76); and R/R AML, 62% (95% CI 49, 74). The most common treatment-emergent adverse events included infections with grade 3/4 neutropenia (n=84, 50%) and febrile neutropenia (n=49, 29%). There were six grade 5 adverse events including five infections with therapy-related grade 3/4 neutropenia, and one case of renal failure unrelated to study regimen.
Interpretation:
DEC10-VEN is safe and has high activity in ND AML and molecularly defined R/R AML subsets.
Funding:
NIH/NCI R01CA235622
Keywords: venetoclax, decitabine, 10 day, acute myeloid leukemia
INTRODUCTION
Acute myeloid leukemia (AML) is the most common acute leukemia in adults with a median age of over 65 years at presentation.1 In addition to an increased frequency of adverse-risk features conferring diminished benefit from standard induction chemotherapy, older patients with AML often have comorbidities rendering them unfit for intensive chemotherapy.2,3 As older patients may experience high early mortality of 5–40% with induction therapy, many older patients are under-treated, contributing to poor overall survival of only 10–30% at 3-years.2,3 Patients with relapsed and/or refractory (R/R) AML similarly have dismal outcomes, with less than 10% survival at 3-years.4
Decitabine is a cytosine analogue which incorporates into DNA and acts epigenetically by reducing aberrant DNA-methylation. In newly diagnosed (ND) AML, single-agent decitabine 20 mg/m2 for 5-days induces complete remission or complete remission with incomplete hematologic recovery (CR/CRi) in 25–28%, has low 30-day mortality of 7–9% with median OS of 7·7 months.5,6 Decitabine has a short half-life and pharmacodynamic studies suggest that a longer, 10-day exposure may be more efficacious.7 Ten-day decitabine has shown objective response rates of 40–64%, with high response rate (67%) in patients with unfavorable risk cytogenetics, and a remarkable 100% response in 21 patients with mutations in tumor protein 53 (TP53).8–10 Hence, we postulated that the 10-day decitabine may yield a better backbone for high-risk patients compared to the 5-day decitabine schedule used in the phase 1/2 trial of venetoclax.
Venetoclax is a selective inhibitor of the anti-apoptotic protein B-cell leukemia/lymphoma-2 (BCL2), overexpressed in leukemic stem cells (LSC) leading to LSC survival advantage and chemoresistance.11 Preclinical studies demonstrated synergism between HMA and venetoclax with capability to eradicate LSCs.12,13 A phase I/II study with 145 reported patients confirmed effectiveness of venetoclax with 5-day decitabine with CR/CRi in 60–73% of treatment-naïve elderly AML patients, with OS extending beyond 14·2 months, and the phase III randomized clinical trial has reported a positive OS endpoint.14,15 One retrospective study of venetoclax with decitabine in R/R AML showed a CR/CRi rate of 51% and 1-year survival of 53%.16
Therefore, we hypothesized that 10-day decitabine with venetoclax (DEC10-VEN) might offer superior activity in high-risk ND and R/R patients with AML.
METHODS
Study design and participants
This was a single institution non-randomized phase II trial conducted at the University of Texas MD Anderson Cancer Center. The primary objective was to determine activity of this regimen as measured by overall response rate (ORR), including CR, CRi, partial response (PR) and morphologic leukemia-free state (MLFS).17 Secondary objectives included safety, OS and duration of response (DOR) of CR/CRi.
The four study arms included (1) ND AML patients older than 60 years and ineligible for intensive chemotherapy; (2) secondary AML (sAML) patients with history of antecedent hematological disorder (AHD), MDS or chronic myelomonocytic leukemia (CMML); (3) R/R AML; (4) patients with high-risk MDS/CMML with 10–20% bone marrow blasts or R/R disease after prior HMA; and (5) younger patients with ND AML with poor risk complex karyotype and/or TP53mut. Results in the fourth and fifth cohort of patients will be reportedly separately. We defined treated sAML, a historically very poor-risk group, as patients with prior treatment for MDS/CMML, but not for AML.18 Eligibility required an ECOG performance status (PS) of 3 or less, WBC less than 10×109/L, and adequate end-organ function (appendix p 1). Ineligibility for intensive chemotherapy was based on the treating physicians’ assessment. Patients with favorable-risk cytogenetics, e.g., t(15;17) or core-binding factor AML, or prior BCL2-inhibitor therapy were excluded (appendix p 2). The treatment-naïve AML group included ND AML and untreated sAML with AHD. The previously treated AML group included sAML with prior therapy for MDS/CMML, and R/R AML.18 Between January 2018 and December 2019, we enrolled 168 patients including ND AML (n=70), untreated sAML (n=15), treated sAML (n=28), and R/R AML (n=55, Fig. 1). The database-lock date was February 6, 2020.
Fig. 1.
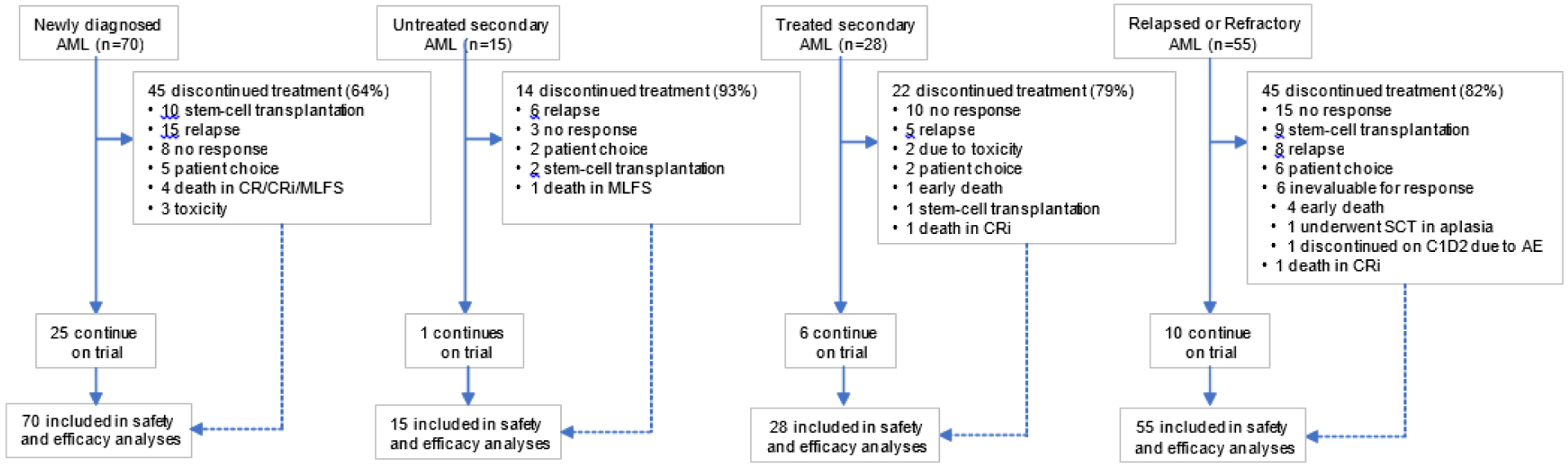
Trial profile.
Sixteen patients with high risk or relapsed/refractory myelodysplastic syndrome were enrolled on this trial and their outcomes will be reported separately. CR = complete remission, CRi = CR with incomplete hematologic recovery, MLFS = morphologic leukemia-free state.
Procedures
Patients received decitabine 20 mg/m2 IV on days 1–10 of induction. After achievement of CR/CRi patients received maintenance decitabine 20 mg/m2 for 5-days per cycle. Venetoclax was recommended on days 1–28 during cycle 1 but was held starting on day-21 if a day-21 bone marrow demonstrated aplasia or remission with ≤5% blasts. Once in remission, venetoclax was given on days 1–21 during cycle 2 onward, with further reductions in venetoclax duration (to 14, 10, or 7 days) as indicated in the setting of persistent cytopenias. Venetoclax was dosed at 100 mg on day 1, 200 mg on day 2, and 400 mg on days 3–28 of cycle 1. All patients were hospitalized for initiation of treatment and received tumor lysis syndrome (TLS) prophylaxis during ramp-up (appendix p 3), and received antibiotic, antifungal, and antiviral prophylaxis while neutropenic. Venetoclax was dose reduced by at least 50% in patients receiving concomitant moderate or strong CYP3A4-inhibitors e.g., azole antifungals. FLT3, IDH1/2, and BCR-ABL1-inhibitors, were allowed in genotype-selected patients. Targeted therapies were administered at the recommended doses. FLT3 inhibitors were administered on days 1–14 of cycle 1, and then continuously cycle 2 onward. IDH inhibitors were used continuously starting cycle 1. Targeted therapies overlapped with venetoclax during the initial period of each cycle. Hydroxyurea and/or cytarabine (one dose up to 2 g/m2) was allowed for cytoreduction prior to starting therapy. Intrathecal chemoprophylaxis with cytarabine was allowed for patients at high-risk for leukemic involvement of CNS (appendix p 4).19 Patients were eligible to receive up to a total of 24 courses of therapy, for an estimated two to three years. Dose reductions, interruption or discontinuation were allowed in the best interest of the patient and were based on the grade of toxicity (protocol in appendix p 33)
Outcomes
Bone marrow was evaluated on day 21±3, and again 1–2 weeks after count recovery if the day-21 marrow was aplastic. Subsequent bone marrow evaluations were performed after cycle 2, 4, and then as clinically needed. Morphologic, cytogenetic and MRD assessment were performed during each bone marrow evaluation. Primary efficacy endpoints for the ORR included CR, CRi, PR and MLFS per the modified IWG Criteria (appendix p 5).17 The second endpoint of OS was measured from start of treatment until death, or censored at last follow-up; and the DOR was defined from date of morphologic response until relapse, or censored at last follow-up. Survival endpoints were not censored at stem-cell transplantation. The safety endpoints included adverse events per CTCAE v4·0.20 Another secondary end-point was to determine the number of patient who transition towards stem cell transplantation after achieving a response with DEC10-VEN. Mutation profile was assessed using an 81-gene NGS panel, and BCL2 expression was quantified using flow cytometry (appendix p 6, 7).21–23 Minimal residual disease (MRD) was assessed using flow cytometry validated to a sensitivity level of 0·01–0·1%.24 Exploratory objectives included analysis of relationship between mutations, BCL2 expression, and MRD analyses with outcomes. We had initially planned on reporting disease-free survival, rates of hematologic improvement and number of patients who achieve more than 50% reduction in blasts on therapy. However, we did not pursue this analysis in favor of more widely reported response and survival outcomes as recommended by the ELN 2017 guidelines.25
Statistical Analysis
The study was not designed for comparison; we focused on the estimation of activity endpoints for the different treatment arms. For the cohort of older patients with ND AML (>60-year-old) ineligible for intensive chemotherapy, we assumed a target response rate of 60%. For the cohorts of patients with sAML with a history of MDS/CMML who received therapy for the AHD and progressed to AML or patients with R/R AML, we assumed a target response rate of 25%, and a response rate of 15% (historical rate) or lower will be considered undesirable. The futility monitoring rules were applied based on the hypothesized/targeted response rates, respectively (appendix p 8). If there was small chance (e.g., less than 2.5%) that the response rate improved over historical rate or reached the target response rate, the enrollment for that cohort would be stopped. The method of Thall, Simon and Estey was used for futility and toxicity monitoring (Multc Lean v2.1, Dept. of Biostatistics, UT MDACC; appendix p 8).26 The response rates including the primary endpoint overall response rate (ORR) along with 95% CI were estimated for each disease arm. Up to 360 patients have been planned for enrollment in the 5 previously cohorts including 120 patients in ND older AML cohort, 80 patients with sAML with history of AHD, 80 patients with R/R AML, 40 patients with high-risk MDS/CMML R/R to prior HMA, and 40 younger patients with adverse risk AML. The sample sizes were based on estimated precision of the ORR (appendix p 8). All patients receiving at least one dose of decitabine and venetoclax were eligible for safety and response assessments regardless of duration of treatment.
The association between response and patient’s clinical characteristics were examined by Wilcoxon’s rank sum test or Fisher’s exact test, as appropriate. Toxicity type, severity and attribution were summarized. The distributions of time-to-event endpoints including OS and DOR were estimated using the method of Kaplan-Meier and compared by subgroups using the log-rank tests. The univariate and multivariable logistic and Cox models were used to evaluate associations between risk factors and clinical outcomes. Risk factors significant in the univariate models at significance level 0·05 were included in multivariable model. A backward model selection was implemented until all remaining predictors had a p-value ≤0·05. The protocol was amended on February 26, 2018, after the initiation of trial to require cytoreduction to WBC count less than 10×109/L prior to enrollment to reduce the risk of tumor lysis syndrome. Hence, baseline WBC count level of 10×109/L was used as an indicator variable in the univariate analyses to evaluate the impact of this change on clinical outcomes. All analyses were conducted using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA) and Prism v8.4 (GraphPad Software Inc., SanDiego CA, USA).
Role of funding source
This study was supported in part by the MD Anderson Cancer Center Support Grant CA016672 from the National Cancer Institute and the Research Project Grant Program (R01CA235622) from the National Institutes of Health. The funder of the study (NCI/NIH) had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The first and corresponding author had full access to all of the data and the final responsibility to submit for publication. The trial was registered on Clinicaltrials.gov, number NCT03404193.
RESULTS
Between January 19, 2018 and December 16, 2019, we enrolled 168 patients including ND AML (n=70), untreated sAML (n=15), treated sAML (n=28), and R/R AML (n=55, Fig. 1). The median age was 71 years (interquartile range [IQR], 65–76), 97 patients (58%) were 70 years or older and 53 patients (32%) were 75 years or older. Fifty-one patients (30%) had ECOG PS of ≥2, and 111 patients (66%) had ELN adverse-risk disease. Previously treated patients had received a median of two prior therapies and of those, 26 patients (31%) had received prior hematopoietic stem-cell transplantation (HSCT). The median number of DEC10-VEN cycles administered was 3 (IQR, 2–4, Table 1). Additional therapies included FLT3-inhibitors (n= 22, 16%), enasidenib (n=2, 1%), and ponatinib (n=1, 1%). FLT3-inhibitors included sorafenib (n=9), gilteritinib (n=8) and midostaurin (n=5).
Table 1.
Baseline characteristics of patients with AML treated with 10-day decitabine and venetoclax
| Patient characteristics | Treatment-naïve patients | Previously treated patients | ||
|---|---|---|---|---|
| Newly Diagnosed AML (N=70) | Untreated Secondary AML (N=15)1 | Treated Secondary AML (N=28)2 | Relapsed or Refractory AML (N=55) | |
| Age, years | 72 [70–78] | 71 [68–76] | 70 [65–76] | 62 [43–73] |
| Age ≥ 70 years | 53 (76) | 10 (67) | 15 (54) | 19 (35) |
| Male sex | 35 (50) | 10 (67) | 19 (68) | 31 (56) |
| ECOG Performance Status | ||||
| 0–1 | 46 (66) | 9 (60) | 20 (71) | 42 (76) |
| ≥2 | 24 (34) | 6 (40) | 8 (29) | 13 (24) |
| WBC count, x109/L | 2∙9 [1∙8–4∙8] | 4∙3 [2∙0–6∙7] | 2∙0 [1∙0–5∙4] | 3∙6 [1∙6–6∙0] |
| Peripheral blood blasts, % | 10 [1–30] | 4 [0–27] | 8 [1–27] | 24 [3–58] |
| Bone marrow blasts, % | 45 [23–62] | 36 [18–61] | 32 [25–54] | 34 [22–64] |
| Diagnosis | ||||
| De novo | 55 (79) | 0 (0) | 0 (0) | 51 (93) |
| Therapy-related | 15 (21) | 1 (7) | 3 (11) | 4 (7) |
| ELN 2017 risk group | ||||
| Favorable | 18 (26) | 1 (7) | 4 (14) | 8 (15) |
| Intermediate | 8 (11) | 3 (20) | 3 (11) | 12 (22) |
| Adverse | 44 (63) | 11 (73) | 21 (75) | 35 (64) |
| ELN 2017 cytogenetic risk | ||||
| Favorable | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Intermediate | 36 (51) | 8 (53) | 14 (50) | 32 (58) |
| Adverse | 33 (47) | 7 (47) | 14 (50) | 23 (42) |
| Mutations | ||||
| NPM1 | 19 (27) | 1 (7) | 4 (14) | 12 (22) |
| FLT3-ITD/TKD | 14 (20) | 0 (0) | 2 (7) | 10 (18) |
| TP53 | 21 (30) | 5 (33) | 8 (29) | 16 (29) |
| RUNX1 | 9 (13) | 1 (7) | 10 (36) | 9 (16) |
| ASXL1 | 9 (13) | 3 (20) | 9 (32) | 5 (9) |
| IDH1/2 | 16 (23) | 2 (13) | 3 (11) | 11 (20) |
| K/NRAS | 14 (20) | 5 (33) | 7 (25) | 13 (24) |
| Prior therapies | 0 [0] | 0 [0] | 2 [1–2] | 2 [1–3] |
| Hypomethylator | 25 (89) | 25 (45) | ||
| (HMA)3 | 4 [4–12] | 3 [2–6] | ||
| No. of cycles | 20 (71) | 10 (18) | ||
| ≥4 cycles | 5 (18) | 42 (76) | ||
| Intensive | 3 (11) | 12 (22) | ||
| chemotherapy (IC) | 8 (29) | 18 (33) | ||
| HMA and IC | ||||
| Stem-cell transplantation | ||||
| Cytoreduction prior to start | 27 (39) | 4 (27) | 4 (14) | 12 (22) |
| Hydroxyurea only | 0 (0) | 1 (7) | 0 (0) | 3 (5) |
| Cytarabine only | 11 (16) | 2 (13) | 1 (4) | 4 (7) |
| Hydroxyurea and cytarabine | ||||
All results expressed as no. (%) or median [interquartile range], unless specified. ECOG = Eastern Cooperative Oncology Group; AML = acute myeloid leukemia. ELN = European LeukemiaNet; N/A = not applicable.
Prior disorder in the untreated secondary AML group included MDS (n=7) and myeloproliferative neoplasms (n=2);
Treated secondary AML patients had received prior therapy for preceding MDS/CMML but not for AML.
Prior HMA included azacitidine in 31 patients, decitabine in 24 patients, and guadecitabine in 7 patients.
All enrolled patients received 10 days of decitabine during induction except for three patients with infections. Re-induction with a second cycle of 10-day decitabine was administered in 10 treatment-naïve patients and 24 previously treated patients. Patients who achieved a response were transitioned to 5 days of decitabine for consolidation starting with cycle 2 per protocol. An additional 10-day course of decitabine was allowed if there was an increase in bone marrow blasts to more than 5% and was administered during cycle 3 to 4 in four patients. Reduction in decitabine dose during subsequent cycles was allowed if deemed in the patients’ best interest. Dose reduction for decitabine was done in 9 patients with ND AML (13%), 2 patients with untreated sAML (13%), 1 patient with treated sAML (4%), and 3 patients with R/R AML (6%, appendix p 18). Venetoclax dose was reduced for concomitant CYP3A4 inhibitors per protocol. Venetoclax duration was reduced once patients achieved CR/CRi to improve time to count recovery beginning with course 1. Venetoclax duration was reduced to 21 days or less in 65 patients with ND AML (93%), 14 patients with untreated sAML (93%), 20 patients with treated sAML (71%), and 44 patients with R/R AML (80%, appendix p 18).
The ORR among all patients was 74% (95% CI 67, 80) with CR/CRi in 61% patients (95% CI 54, 68). The ORRs in ND AML was 89% (95% CI 79, 94), in untreated sAML was 80% (95% CI 55, 93), in treated sAML was 61% (95% CI 42, 76) and in R/R AML was 62% (95% CI 49, 74, Table 2). Out of 119 patients with an evaluable cycle 1 day-21 bone marrow, 65 patients (55%) achieved ≤5% blasts (95% CI 46, 63), including 67% of ND AML. Flow-based MRD negativity was achieved in 60 (58%) of responding patients at best response (95% CI 49, 67). Flow cytometry for BCL2 expression in CD34+ blasts showed an association between higher baseline BCL2 expression and achievement of negative MRD (appendix p 19). The median follow-up duration was 16 months (95% CI 12, 18, actual follow-up 6·5 months, IQR 3·4–12·4 for all patients, and 9·3 months, IQR 5·2–17·6 for patients who are alive).
Table 2.
Outcomes with 10-day decitabine and venetoclax in AML
| Outcomes | Treatment-naïve patients | Previously treated patients | ||
|---|---|---|---|---|
| Newly Diagnosed AML (N=70) | Untreated Secondary AML (N=15) | Treated Secondary AML (N=28) | Relapsed or Refractory AML (N=55) | |
| Overall response rate (ORR) | 62 (89 [79, 94]) | 12 (80 [55, 93]) | 17 (61 [42, 76]) | 34 (62 [49, 74]) |
| CR | 46 (66 [54, 76]) | 6 (40 [20, 64]) | 6 (21 [10, 40]) | 13 (24 [14, 36]) |
| CRi | 13 (19 [11, 29]) | 4 (27 [11, 52]) | 5 (18 [8, 36]) | 10 (18 [10, 30]) |
| CR/CRi | 59 (84 [74, 91]) | 10 (67 [42, 85]) | 11 (39 [24, 56]) | 23 (42 [30, 55]) |
| MRD negative1,2 | 35/52 (67 [54, 79]) | 4/10 (40 [17, 69]) | 7/15 (47 [25, 70]) | 14/26 (54 [36, 71]) |
| Morphologic leukemia-free state | 3 (4 [2, 12]) | 2 (13 [4, 39]) | 6 (21 [10, 40]) | 10 (18 [10, 30) |
| No response | 8 (11 [6, 21]) | 3 (20 [7, 45]) | 10 (36 [21, 54]) | 15 (27 [17, 40]) |
| Inevaluable | 0 (0) | 0 (0) | 1 (4) | 6 (11) |
| Death prior to first evaluation | 0 (0) | 0 (0) | 1 (4) | 4 (7) |
| Complete cytogenetic response2,3 | 17/30 (57 [39, 73]) | 5/11 (46 [21, 72]) | 1/13 (8 [1, 33]) | 8/29 (28 [15, 46]) |
| Cycle 1 day 21 (±3) BM blasts ≤5%4 | 33/49 (67 [53, 79]) | 8/10 (80 [49, 94]) | 8/21 (38 [21, 59]) | 14/32 (44 [28, 61]) |
| Time to response, months | 1∙5 (1∙3, 1∙7) | 1∙3 (0∙9, 2∙2) | 1∙7 (1∙1, 2∙6) | 1∙8 (1∙4, 2∙0) |
| No∙ of cycles to response | 1 [1–2] | 1 [1–2] | 1 [1–2] | 1[1–2] |
| No∙ of cycles | 3 [2–7] | 3 [2–4] | 2 [1–3] | 2 [1–3] |
| administered Second cycle of 10-day decitabine | 8 (11) | 2 (13) | 8 (29) | 16 (29) |
All percentages are based on total number of patients in each cohort (N), unless specified. Results are reported as n (% [95% CI]) or median [interquartile range] or median (95% confidence interval). CR = complete remission, CRi = CR with incomplete hematologic recover, MRD = minimal residual disease, BM = bone marrow
Measured by multicolor flow cytometry validated to a sensitivity level of 0·01 to 0·1% (1 cell in 1,000 to 10,000),
The discrepancy in the numerators and denominators of patients undergoing MRD testing and conventional cytogenetics was related to the number of bone marrow samples with adequate specimen required for acquisition of 200,000 events needed for valid negative result, and the yield of sufficient number of metaphases for karyotyping.
Calculated at the time of best response, and only for patients with abnormal karyotype at screening and evaluable karyotype at response,
Includes patients with evaluable cycle 1 day 21 bone marrow sample.
Among all patients, achievement of CR conferred improved survival vs CRi vs MLFS vs no response (22·1 [95% CI 13·4, not reached [NR], vs 8·0 [95% CI 5·8, 12·4] vs 8·2 [95% CI 5·6, 14·1] vs 3·3 months [95% CI 2·1, 4·9], p<0·0001; CR vs CRi HR 0·35, 95% CI 0·16, 0·75, p<0·0005, appendix p 20). Achievement of CR conferred better DOR compared to CRi and MLFS (median DOR NR [95% CI 9·7, NR] vs NR [95% CI 3·3, NR] vs 1·5 months [95% CI 1·1, 6·3], p<0·0001; CR vs CRi HR 0·46, 95% CI 0·20, 1·07, p=0·03, appendix p 21).
The 30-day and 60-day mortality for all patients was 3·6% (n=6, 95%CI 1·7, 7·8) and 10·7% (n=18, 95%CI 6·9, 16·9), respectively. The 30-day and 60-day mortality for ND AML patients were 1·4% (n=1, 95% CI 0·3, 7·7) and 7·1% (n=5, 95% CI 3·1, 15·7). There were 261 treatment-emergent adverse events (TEAE) in 134 patients, with 193 grade 3/4 AEs and six grade 5 AEs (Table 3). The most common grade 3/4 TEAEs were infections with grade 3/4 neutropenia in 79 patients (47%), and febrile neutropenia in 49 patients (29%). Among infections, the most common were pneumonia (n=34), sepsis/bacteremia (n=23), and cellulitis (n=14). Overall, 139 out of 168 patients (83%) had 290 serious AEs (SAE) with most common SAEs being neutropenic fever (n=63, 38%), followed by pneumonia (n=17, 10%) and sepsis (n=16, 10%). Five of the deaths were related to neutropenic infections, and one was related to renal failure from acute tubular necrosis.
Table 3.
Treatment-emergent adverse events reported with 10-day decitabine and venetoclax, regardless of attribution.
| Treatment-emergent adverse events | Grade 1–2 | Grade 3 | Grade 4 | Grade 5 |
|---|---|---|---|---|
| Infection with ANC <1∙0 ×109/L | 0 (0) | 77 (46) | 2 (1) | 5 (3) |
| Renal Failure | 0 (0) | 0 (0) | 2 (1) | 1 (1) |
| Tumor lysis syndrome | 0 (0) | 3 (2) | 1 (1) | 0 (0) |
| Fever (ANC >1.0 × 109/L) | 0 (0) | 0 (0) | 1 (1) | 0 (0) |
| Hemorrhage, CNS | 0 (0) | 0 (0) | 1 (1) | 0 (0) |
| Febrile neutropenia (ANC <1∙0 ×109/L) | 0 (0) | 49 (2) | 0 (0) | 0 (0) |
| Infection with ANC >1∙0 ×109/L | 2 (1) | 11 (7) | 0 (0) | 0 (0) |
| Hyperbilirubinemia | 0 (0) | 3 (2) | 0 (0) | 0 (0) |
| Diarrhea | 8 (5) | 2 (1) | 0 (0) | 0 (0) |
| Colitis | 0 (0) | 2 (1) | 0 (0) | 0 (0) |
| Mucositis | 11 (7) | 1 (1) | 0 (0) | 0 (0) |
| ALT elevation | 1 (1) | 1 (1) | 0 (0) | 0 (0) |
| Fatigue | 1 (1) | 1 (1) | 0 (0) | 0 (0) |
| Cardiac ischemia | 0 (0) | 1 (1) | 0 (0) | 0 (0) |
| Cough | 0 (0) | 1 (1) | 0 (0) | 0 (0) |
| Esophagitis | 0 (0) | 1 (1) | 0 (0) | 0 (0) |
| Fracture | 0 (0) | 1 (1) | 0 (0) | 0 (0) |
| Muscle weakness | 0 (0) | 1 (1) | 0 (0) | 0 (0) |
| Nausea | 10 (6) | 0 (0) | 0 (0) | 0 (0) |
Results reported as n (%). Reported are adverse events which occurred in at least 10% of the patients or with at least 1 grade 3/4 event; ANC = absolute neutrophil count. ALT = alanine aminotransferase. There were 6 grade 5 adverse events including 5 infections with grade 3/4 neutropenia which were treatment related, and 1 grade 5 renal failure due to acute tubular necrosis which was unrelated to study regimen.
Four patients (2%) developed TLS; all patients recovered baseline renal function. The median baseline WBC count in these patients was 7·1×109/L (IQR 2–16) with median peripheral blasts of 64% (IQR 39–83), and median peak creatinine of 1·7 mg/dL (IQR 1·2–3·6). TLS occurred on cycle 1 day 1 (n=2), cycle 1 day 10 (n=1), and cycle 2 day 1 (n=1). Two patients had mutations in fms related receptor tyrosine kinase 3 (FLT3), and one patient with ND de novo AML with WBC count of 28×109/L and mutations in nucleophosmin 1 (NPM1), isocitrate dehydrogenase 2 (IDH2) required transient hemodialysis for grade 4 TLS and recovered in CR. The protocol was subsequently amended to require WBC less than 10×109/L prior to starting treatment.
In the treatment-naïve group, high responses were noted across several different subgroups of treatment-naïve AML (appendix p 10). The CR/CRi rates in the ELN favorable-risk group were 90% (9% CI 67, 97), in ELN intermediate-risk group 100% (95% CI 74, 100), in ELN adverse-risk group 75% (95% CI 62, 84).25 The CR/CRi rate in sAML patients with AHD was 67% (95% CI 42, 85) and in t-AML patients was 81% (95% CI 57, 93).
Among ND patients, some experienced a delay in obtaining venetoclax for treatment initiation. Among 13 patients with delay of 7 days or more in starting venetoclax, 12 patients (92%) achieved CR/CRi and their DOR was comparable to patients who started venetoclax within 7 days of starting decitabine (n=57, HR for death for delayed group vs. on-time group 1·06, 95% CI 0·41, 2·76, p=0·90).
Among patients with treatment-naïve AML, 12 patients had acute myelomonocytic leukemia (FAB M4) and four patients had acute monocytic leukemia (FAB M5), recently reported to experience inferior response to hypomethylating agents with venetoclax.27 ORR in these 16 patients was 88% (95% CI 64, 97) including CR (n=9), CRi (n=4) and MLFS (n=1), with MRD negativity by flow cytometry in 60% patients (95% CI 31, 83) and median OS of 12·1 months (95% CI 2·5, NR). The three patients who were primary refractory had M4 AML, TP53mut, and complex cytogenetics. Sixty-eight percent patients sustained CR/CRi/MLFS at 1 year and three patients who achieved CRi relapsed including two patients with M4 AML and one patient with M5 AML.
The median OS in the ND AML group was 18·1 months (95% CI 10, NR), with an estimated 1-yr OS rate of 61% (95% CI 73, 47; Fig. 2A). The median OS in ND de novo AML was not reached (NR, 95% CI 15·2, NR), with a 1-yr OS of 74% (95% CI 84, 58) and 23-mo OS of 56% (95% CI 71, 37). In untreated sAML patients, the mOS was 7·8 months (95% CI 2·9, 10·7) with a 1-yr OS of 16% (95% CI 3, 40). Patients with ND de novo AML had significantly longer OS with mOS NR (95% CI 12·6, NR) compared to patients with t-AML or untreated sAML (with AHD) with mOS 7·8 months (95% CI 5·2, 9·5) with HR 0·25, 95% CI 0·12, 0·52, p<0·0001. The median OS for patients with ELN adverse-risk AML was 9·3 months (95% CI 6·9, 12·1) and adverse-risk cytogenetics was 8·0 months (95% CI 5·7, 9·6; appendix p 10). The median DOR (CR + CRi) in patients with ND AML was NR (95% CI 9·0, NR) and in the untreated sAML group was 5·1 months (95% CI 0·9, NR; Fig. 2b).
Figure 2.

a. Overall survival (OS) in all patients and b. duration of CR/CRi in responding patients with ten-day decitabine and venetoclax. ND = newly diagnosed, sAML = secondary AML, R/R = relapsed or refractory, NR = not reached, CI = confidence interval, mo = months.
Detailed mutational landscapes are provided in Fig. 3 and appendix p 22. Among patients with treatment-naïve AML the CR/CRi rate in NPM1mut patients was 95% (95% CI 77, 99), in IDH1/2mutpatients was 84% (95% CI 61, 94), in N/KRASmut patients was 74% (95% CI 51, 88), and in TP53mut patients was 69% (95% CI 50, 84). The median OS among NPM1mut patients was NR (95% CI 12·4, NR), for IDH1/2mut patients was NR (95% CI 12·4, NR), for patients with mutations in ASXL transcriptional regulator 1 (ASXL1) was 12·1 months (95% CI 2·4, 18·1), for patients with mutations in RUNX family transcription factor 1 (RUNX1) was 8·3 (95% CI 2·8, 19·1), for TP53mut patients was 6·9 months (95% CI 3·4, 9·3), and for N/KRASmut patients was 15·2 months (95% CI 6·6, 22·7; appendix p 10, 23). The median DOR for patients with NPM1mut was NR (95% CI 9·0, NR), IDH1/2mut was NR (95% CI 6·4, NR), ASXL1mut was NR (95% CI 1·5, NR), RUNX1mut was NR (95% CI 1·1, NR), and for TP53mut patients was 5·7 months (95% CI 2·5, 7·5; appendix p 10, 24).
Figure 3.

Mutational landscape of 113 patients with newly diagnosed (ND) AML, untreated secondary AML (sAML) and treated sAML.
Fourteen patients had ND FLT3mut AML and 10 patients (71%) received FLT3 inhibitors including sorafenib (n=5), gilteritinib (n=4) and midostaurin (n-1). The CR/CRi rate was 86% (95% CI 60, 96), with MRD negativity by flow cytometry in 80% (95% CI 49, 94), and MRD negativity by FLT3 PCR in 86% (95% CI 60, 96). Median OS was NR (95% CI 6·6, NR), the median DOR was NR (95% CI 6·4, NR), and the 1-year OS was 78% (95% CI 37, 94; appendix p 10, 23, 24). Three patients transitioned to HSCT.
Among previously treated patients the CR/CRi rate in patients having received ≥4 prior cycles of HMA was 37% (95% CI 22, 55), in patients with prior intensive chemotherapy (IC) was 36% (95% CI 24, 51), and in patients with prior HSCT was 27% (95% CI 14, 46). The CR/CRi rate for patients receiving first salvage therapy for AML was 48% (95% CI 30, 67) and for those receiving second salvage therapy 40% (95% CI 20, 64; appendix p 11). As discussed in more detail below, CR/CRi rates above 50% in this high-risk population was noted in patients with diploid cytogenetics (75%, 95% CI 57, 87), NPM1mut (69%, 95% CI 44, 86) and IDH1/2mut (50%, 95% CI 27, 73).
The median OS in patients with treated sAML was 6·0 months (95% CI 3·4, 13·7), with a 1-yr OS of 32% (95% CI 14, 51) and median DOR of NR (95% CI 2·5, NR). The mOS in R/R AML patients was 7·8 months (95% CI 5·4, 13·3; Fig. 2a), with a 1-year OS of 38% (95% CI 23, 53) and median DOR of 16·8 months (95% CI 6·6, NR; Fig. 2b). The median DOR in patients with ≥4 prior cycles of HMA was 9·8 months (95% CI 3·2, NR) and those with prior IC was 12·9 months (95% CI 6·6, NR; appendix p 11).
Among previously treated patients the CR/CRi rates in NPM1mut patients was 69% (95% CI 44, 86), although fewer true CRs were noted than in the treatment-naive group (38% vs 81%, p<0·01 appendix p 11). The median OS for NPM1mut patients was 12·4 months (95% CI 5·7, NR). Among IDH1/2mut patients, the CR/CRi rate was 50% (95% CI 27, 73), with MRD negativity by flow cytometry in 60% patients (95% CI 31, 83), MRD negativity by NGS in 25% patients (95% CI 9, 53), with median OS of 7·8 months (95% CI 5·8, 22·1) and median DOR of 17·5 months (95% CI 16·7, NR). CR/CRi rate in TP53mut patients was 30% (95% CI 15, 49) with mOS of 4·9 months (95% CI 2·6, 6·7; appendix p 11, 25). The median DOR for patients with NPM1mut was NR (95% CI 2·5, NR), for ASXL1mut was NR (95% CI NR, NR) and for N/KRASmut was NR (95% CI NR, NR), for RUNX1mut patients was 15·3 months (95% CI 9·8, NR), and for TP53mut patients was 16·8 months (95% CI 2·1, 16·8; appendix p 11, 26).
Twelve patients had previously treated FLT3mut AML, including R/R AML (n=10) and treated sAML (n=2), and eight patients had received prior FLT3 inhibitors. All patients received FLT3 inhibitors with DEC10-VEN, and four patients underwent SCT. The CR/CRi rate was 42% (95% CI 19, 68), MRD negativity was 50% by flow cytometry (95% CI 19, 81), MRD negativity by FLT3 PCR was 27% (95% CI 10, 57), with a mOS of 12·4 months (95% CI 3·7, NR) and median DOR of 6·6 months (95% CI 2·5, 6·6; appendix p 11, 25, 26). Additionally, one patient with multiply R/R AML with t(9;22) achieved MLFS with addition of ponatinib.
Among ND AML patients achieving CR/CRi/MLFS, the median time to absolute neutrophil count (ANC) recovery to 0·5×109/L or higher after the first cycle was 40 days (95% CI 37, 44) and the median time for platelet recovery to 50×109/L or higher after first cycle was 28 days (95% CI 25, 30; appendix p 27). ANC recovery after cycle 1 was comparable among responding patients with ND AML, sAML and R/R AML, however R/R AML patients had significant delay in platelet recovery (appendix p 28). No remarkable differences were noted in time to count recovery based on venetoclax dose, age or AHD (appendix p 29), Cycle length tended to be longest in the early cycles, likely related to reduced decitabine and venetoclax durations with subsequent cycles (appendix p 30).
Concomitant azoles did not impact outcomes or time to count recovery. There were no differences in CR vs CRi rates in patients receiving different anticipated venetoclax levels (i.e. 400 mg daily or equivalent, [n=47, CR n=34, CRi n=13] vs. more than 400 mg daily or equivalent, [n=22, CR n=17, CRi n=5]) with concomitant strong or moderate CYP3A4-inhibitors, p=0·22).
One-hundred of 126 patients (75%) have discontinued treatment, and 77 patients (46%) are alive (Fig. 1). Five patients (3%) discontinued treatment due to toxicity including acute kidney injury (n=2), intracranial hemorrhage (n=1), worsening fatigue (n=1), and nausea/vomiting (n=1). There were five (3%) treatment-related deaths due to infection with grade 3/4 neutropenia. The most common reasons for treatment discontinuation were no response (n=36, 21%), relapse (n=34, 20%), and HSCT (n=23, 14%).
After a median follow-up of 14 months after HSCT (95% CI 10, 15), six patients have relapsed and 15 patients are alive with a 100-day post-transplant mortality of 4% (95% CI 1, 21; appendix p 31). The most common causes of death regardless of attribution in ND AML cohort were pneumonia (unknown pathogen, n=8), unknown reason (n=6), death in hospice (n=6), bacteremia (n=3), pneumonia (bacterial, viral or fungal, n=2), liver failure (n=1) and renal failure (n=1); in untreated sAML cohort were pneumonia (unknown pathogen, n=4), unknown reason (n=2), death in hospice (n=5), pneumonia (bacterial, viral or fungal, n=1) and intracranial hemorrhage (n=1); in treated sAML cohort were pneumonia (unknown pathogen, n=3), unknown reason (n=9), death in hospice (n=5), pneumonia (bacterial, viral or fungal, n=1) cardiac arrest (n=1), and malignant pleural effusion (n=1); and in in R/R AML cohort were pneumonia (unknown pathogen, n=9), unknown reason (n=7), death in hospice (n=7), bacteremia (n=3), pneumonia (bacterial, viral or fungal, n=2), intracranial hemorrhage (n=2), and pericardial effusion (n-1; appendix p 12).
Among 23 patients undergoing HSCT, the treatment-naïve group was an older population with median age of 69 years (IQR, 68–71) and the previously treated group was younger with median age of 46 years (IQR, 33–61). These patients underwent HSCT after a median of 3 cycles of treatment (IQR, 2–3), 16 out of 22 patients (73%) achieved negative MRD by flow cytometry. Conditioning regimens included fludarabine/melphalan-based regimens with or without anti-thymocyte globulin or total body irradiation (n=18) and fludarabine/busulfan-based regimens with or without cladribine (n=4), and unknown (n=1). Transplant sources included matched unrelated donors (n=10), matched sibling donors (n=5), haploidentical donors (n=4), matched related donors (n=2) and unknown (n=1). These patients did not experience any unexpected complications after HSCT during the protocol mandated 30-day follow up after treatment discontinuation.
A multivariable analysis for OS was performed to determine factors associated with inferior OS, and included non-achievement of CR, receipt of prior HMA, prior intensive chemotherapy, TP53mut and diagnosis of sAML (appendix p 13). Similarly, an MVA for DOR identified sAML and TP53mut associated with inferior DOR (appendix p 13). Univariate analysis for all factors considered are shown in appendix p 14–17. Cytoreduction to WBC count <10×109/L was later instituted as an inclusion criterion. We incorporated this change in our statistical analyses and found no influence of starting WBC count on OS or DOR (p>0.05) and hence this factor was not included in the multivariable analyses.
DISCUSSION
Treatment of AML has advanced rapidly, with venetoclax in combination with either azacitidine or 5-day decitabine emerging as an approved new standard of care for older patients with ND AML, leading to improved responses and overall survival.14 This trial sought to evaluate the safety and activity of venetoclax with a 10-day decitabine regimen, in older patients with ND high-risk AML and R/R AML.
DEC10-VEN had acceptable tolerability in this population, with expected TEAEs including infections with grade 3/4 neutropenia (50%) and febrile neutropenia (29%), and low 30- and 60-day mortality of 3·6% and 10·7% respectively. With necessary caution for cross-trial comparisons, this was comparable to neutropenic fever and infection rates of 33–66% and 30-day mortality of 2–9% with 10-day decitabine in ND and R/R AML/MDS, and febrile neutropenia rate of 61% and 30-day mortality of 7% in treatment-naïve AML with 5-day decitabine and venetoclax.8–10,14,28 While infrequent, TLS occurred in four ND patients which prompted a protocol amendment to require WBC less than 10×109/L prior to start, with no cases of TLS since that time. Careful monitoring for TLS in venetoclax-sensitive disease (i.e. NPM1mut or IDH1/2mut) or proliferative AML (i.e. leukocytosis, high circulating blasts, and/or FLT3-ITD) is warranted. As perhaps expected with the increased myelosuppressive nature of 10-day decitabine, a longer ANC recovery time (median 40 days) was seen in cycle one of therapy, a finding which did not persist with subsequent cycles. In responding patients, venetoclax duration during maintenance was often reduced to 21, 14, or less than 14 days and the comparable outcomes with reported 5-day decitabine and venetoclax regimen indicate that intermittent administration of venetoclax with HMAs aiming for synergy may avoid prolonged myelosuppression, potentially reduce infections, without impacting outcomes.
Response rates were impressive, with a CR/CRi rate of 84% in ND AML, 67% in untreated sAML, 39% in treated sAML, and 42% in R/R AML. ND de novo AML, in particular, had excellent outcomes with CR/CRi rate of 84% and 23-month OS of 56%. Importantly, the cycle 1 day 21 bone marrow was useful and identified marrow blast clearance (<5%) in most evaluable patients, which allowed early discontinuation of venetoclax in cycle 1 to improve count recovery. As a background, the phase I/II trial of venetoclax with HMA in patients with treatment-naïve AML showed a CR/CRi rate of 73%, MRD negativity by flow cytometry of 29%, and median OS extending beyond 14·2 months.14 In a similar treatment-naïve population, DEC10-VEN offered a CR/CRi in 81%, MRD negativity in 63%, and median OS of 12·4 months.
We identified an unexpected dichotomy in overall survival in newly diagnosed AML patients based on de novo vs therapy-related or AHD status; 10-day decitabine did not appear to improve outcomes to the same degree in patients with the latter diagnoses. While median OS was not reached for patients with ND de novo AML, patients with therapy-related AML or with AHD had median OS of 6·9 and 7·8 months, respectively. This may be because the 10-day decitabine regimen was too myelosuppressive for patients with underlying myelodysplasia. Our findings further support the value of achieving full CR that translates into longer OS, with these responses being rare in patients with preceding bone marrow dysfunction.
High response rates were noted in high-risk subgroups of treatment-naïve AML, including a CR/CRi rate of 90% in RUNX1mut AML, 90% in FLT3mut AML receiving FLT3 inhibitors, and 70% in TP53mut patients, comparing favorably to venetoclax and HMA or low-dose cytarabine which offered CR/CRi rate of 50% in RUNX1mut AML, 43–50% in FLT3mut AML, and 44% in TP53mut AML.29 While responses were durable for certain subgroups including NPM1mut, IDH1/2mut, and FLT3mut, patients with TP53mut unfortunately experienced short remissions with a DOR of only 5·7 months, and similar to 5-day decitabine with venetoclax. Patients without durable responses after DEC10-VEN were enriched for TP53mut as well as K/NRASmut. Although monocytic clones have been reported to confer primary resistance to venetoclax and HMA,27 FAB M4/M5 patients receiving frontline DEC10-VEN had favorable ORR of 88% with 68% sustaining response at 1-year.
DEC10-VEN was moderately effective in the R/R cohort with ORR of 62%, median DOR of 16·8 months, and 1-year OS of 62% in R/R de novo AML. These outcomes compare favorably with expectations with intensive chemotherapy or HMA therapy alone in the relapsed setting, and are consistent with a recent report of venetoclax and HMA for R/R AML (ORR of 64% and 1-year OS of 73%).16,30 DEC10-VEN trial outcomes were better than our retrospective experience of venetoclax with HMA or low-dose cytarabine which showed ORR of 21% and median OS of 3 months in R/R myeloid neoplasms.31 Patients in the R/R group who had particularly favorable outcomes included patients with diploid cytogenetics, NPM1mut, IDH1/2mut, and FLT3mut. Responding patients transitioning to HSCT had the most durable responses with median OS NR for treatment-naïve patients and 22·1 months in previously treated group.
Limitations of our study that may limit generalisability and cross-trial comparisons include the single-centre nature of our trial, increased heterogeneity in the patient population, delay in starting venetoclax in a minority of patients, the use of cytarabine cytoreduction to mitigate TLS, the incorporation of targeted therapies to improve outcomes in some high-risk populations e.g. FLT3mut AML, and limited sample size in some subpopulations. Future larger and randomized studies will be needed to clarify the activity within small subsets, also generalize the safety in more diverse healthcare systems, and incorporate patient-reported quality of life outcome measures to better understand the risk-vs-benefit profile of these treatments.
In summary, DEC10-VEN has a manageable safety profile and offers effective therapy for older patients with ND AML, many R/R patients with venetoclax-sensitive genomics, and can be a potential salvage approach prior to HSCT. Subgroups including ELN favorable and intermediate-risk AML, and IDH1/2mut AML have excellent responses, although responses were not substantially improved in TP53mut patients. Importantly, the addition of FLT3-inhibitors to DEC10-VEN for FLT3mut AML was safe and may lead to improved durability of responses in this high-risk population.
Supplementary Material
RESEARCH IN CONTEXT.
Evidence Before This Study
Older patients with acute myeloid leukemia, and patients with relapsed or refractory disease have historically had poor outcomes. We queried PubMed and MEDLINE with the search terms “acute myeloid leukemia”, “acute myelogenous leukemia”, for clinical trials published during the ten years between June 1st, 2009, and June 1st, 2019. The published literature demonstrates that several new combination strategies have emerged for acute myeloid leukemia including venetoclax with 5-day decitabine or azacitidine, venetoclax with low-dose cytarabine, low-dose cytarabine and glasdegib, gemtuzumab ozogamicin, as well as targeted therapeutics for patients with IDH1, IDH2, or FLT3 mutations, all of which have shown promising activity. One randomized phase II trial of 5-day vs 10-day decitabine suggested comparable efficacy of these two regimens but noted potentially different activity of these regimens when combined with other anti-leukemia agents such as venetoclax.
Added Value of This Study
Our results show that combining 10-day decitabine with venetoclax is an effective and safe strategy for patients older than 60 years with untreated acute myeloid leukemia (AML), as well as those with previously treated disease. To our knowledge, this is the first report of a prospective trial venetoclax with a hypomethylating agent in relapsed or refractory acute myeloid leukemia, as well as ‘triplet therapy’ with FLT3 inhibitors in the frontline and salvage setting, with detailed outcomes in molecular subgroups. This regimen demonstrates high rates of response and favorable overall survival for these high-risk AML treatment groups.
Implications of All the Available Evidence
This data supports the utility of this regimen in older patients with newly diagnosed acute myeloid leukemia, patients with relapsed/refractory disease, and those with adverse-risk features including in combination with FLT3 inhibitors in FLT3-mutant AML.
Acknowledgements:
This study was supported in part by the MD Anderson Cancer Center Support Grant CA016672 from the National Cancer Institute and the Research Project Grant Program (R01CA235622) from the National Institutes of Health.
We thank the patients, their families, and their caregivers; co-investigators, collaborators, and members of the study team involved in the trial.
Prior Presentation:
This data was presented in part as at the 60th Annual Meeting of the American Society of Hematology, December 1–4, 2018, San Diego, CA, and the 61st Annual Meeting of the American Society of Hematology, December 7–10, 2019, Oralndo, FL, USA.
Data sharing:
The protocol is available in the supplemental appendix. At this time, we will not be able to share individual patient level data outside of our institution.
Conflict of Interests
CDD: Personal fees from Abbvie, personal fees from Agios, personal fees from Novartis, personal fees from ImmuneOnc, personal fees from Daiichi Sankyo, personal fees from Celgene, personal fees from Jazz, personal fees from Notable Labs, outside the submitted work; .
AM: Research funding from Celgene Corporation
CRR: None
NP: Dr. Pemmaraju reports personal fees from Pacylex Pharmaceuticals, grants and other from Affymetrix, grants from SagerStrong Foundation, personal fees from Incyte, personal fees and other from Novartis, personal fees from LFB Biotechnologies, personal fees, non-financial support and other from Stemline Therapeutics, personal fees and non-financial support from Celgene, personal fees, non-financial support and other from AbbVie, personal fees and non-financial support from MustangBio, personal fees from Roche Diagnostics, personal fees from Blueprint Medicines, personal fees and non-financial support from DAVA Oncology, other from Samus Therapeutics, other from Cellectis, other from Daiichi Sankyo, other from Plexxikon, outside the submitted work; .
NGD: reports grants from Abbvie, Genentech, Astellas, Daiichi-Sankyo, Pfizer, BMS, Immunogen, Novimmune, Forty-seven; personal fees from Abbvie, Genentech, Astellas, Daiichi-Sankyo, Pfizer, BMS, Immunogen, Jazz pharmaceuticals, Trillium, Forty-seven, Gilead, Kite, Novartis
FR: Personal fees and research grants from Abbvie
GGM: None
GB: AbbVie: Research Funding; Incyte: Research Funding; Janssen: Research Funding; GSK: Research Funding; Cyclacel: Research Funding; BioLine Rx: Consultancy and Research Funding; NKarta: Consultancy; PTC Therapeutics: Consultancy; Oncoceutics, Inc.: Research Funding.
KN: None
MO: None
GMB: none
NJS: reports grants from Takeda Oncology, Astellas; Personal fees from Takeda Oncology, AstraZeneca, Amgen
YA: none
TMK: None
KT: has received personal fees for service on advisory boards of Symbio Pharmaceuticals, GSK, Celgene.
MY: None
NJ: AbbVie: Consultancy, Honoraria, Membership on an entity’s Board of Directors or advisory committees and Research Funding; Pharmacyclics, an AbbVie company: Consultancy, Honoraria, Membership on an entity’s Board of Directors or advisory committees and Research Funding; Janssen Pharmaceuticals, Inc.: Consultancy, Honoraria and Membership on an entity’s Board of Directors or advisory committees; Genentech: Consultancy, Honoraria, Membership on an entity’s Board of Directors or advisory committees and Research Funding; BMS: Research Funding; Pfizer: Consultancy, Honoraria, Membership on an entity’s Board of Directors or advisory committees and Research Funding; ADC Therapeutics: Consultancy, Honoraria, Membership on an entity’s Board of Directors or advisory committees and Research Funding; Incyte: Research Funding; AstraZeneca: Consultancy, Honoraria, Membership on an entity’s Board of Directors or advisory committees and Research Funding; Servier: Consultancy, Honoraria, Membership on an entity’s Board of Directors or advisory committees and Research Funding; Cellectis: Research Funding; Verastem: Consultancy, Honoraria, Membership on an entity’s Board of Directors or advisory committees and Research Funding; Precision Biosciences: Consultancy, Honoraria, Membership on an entity’s Board of Directors or advisory committees and Research Funding; Adaptive Biotechnologies: Consultancy, Honoraria, Membership on an entity’s Board of Directors or advisory committees and Research Funding
SMK: None
KS: Otsuka: Honoraria, Daiichi-Sankyo: Advisory Board, Pfizer Japan: Advisory Board, Novartis: Advisory Board, Research Funding.
MA: Dr. Andreeff reports grants, personal fees and other from Daiichi Sankyo, personal fees and other from Jazz Pharmaceuticals, grants from NIH/NCI, other from Celgene, grants from Breast Cancer Research Foundation, other from Amgen, other from Astra Zeneca, other from Reata, other from Aptose, other from Eutropics, other from Senti Bio, other from Oncoceutics, other from Oncolyze, grants from CPRIT, other from Center for Drug Research and Development, other from Cancer UK, other from NCI CTEP, other from Leukemia Lymphoma Society, other from Bioline, outside the submitted work; .
PB: reports research grant and personal fees from Incyte, Celgene, CTI Biopharma, Kartos Therapeutics, BluePrint Medicines, and research grants from Constellation Pharmaceuticals, NS Pharma, Promedior, Pfizer, Astellas
AF: None
GCI: received research funding from Celgene and served on an advisory board for Novartis.
EJJ: Consultancy Research funding from Takeda, BMS, Adaptive, Amgen, AbbVie, Pfizer, Cyclacel LTD, Research grants with Amgen, Abbvie, Spectrum, BMS, Takeda, Pfizer and Adaptive.
LM: None
PAT: reports research funding to institution and consultancy/honorarium paid to institution from Pharmacyclics, Abbvie, Amgen; consultancy/honorarium paid to institution from Genentech, Gilead Scienes, Janssen-Cilag; research funding to institution from Pfizer
ZE: none
SAW: None
SK: None
ZC: None
SAP: None
JN: None
WQ: None
JSW: Janssen and Notable provide clinical trial support. I participated in an advisory board for ArcherDx.
WGW: None
JB: reports grants from Pharmacyclics, Gilead, BeiGene; personal fees from Janssen and Astra Zeneca
SV: None
HMK: grants and other from AbbVie, grants and other from Agios, grants and other from Amgen, grants from Ariad, grants from Astex, grants from BMS, from Cyclacel, grants from Daiichi-Sankyo, grants and other from Immunogen, grants from Jazz Pharma, grants from Novartis, grants and other from Pfizer, other from Actinium, other from Takeda, outside the submitted work.
MYK: has received grants from NIH, NCI, Abbvie, Genentech, Stemline Therapeutics, Forty-Seven, Eli Lilly, Cellectis, Calithera, Ablynx, Astra Zeneca; Consulting/honorarium from AbbVie, Genentech, F. Hoffman La-Roche, Stemline Therapeutics, Amgen, Forty-Seven, Kisoji; clinical trial support from Ascentage; stocks/royalties in Reata Pharmaceutical
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Clinical trial registration information:
Contributor Information
Courtney D. DiNardo, Department of Leukemia, University of Texas MD Anderson Cancer Center.
Abhishek Maiti, Department of Leukemia, University of Texas MD Anderson Cancer Center; Division of Cancer Medicine, University of Texas MD Anderson Cancer Center.
Caitlin R. Rausch, Division of Pharmacy, University of Texas MD Anderson Cancer Center.
Sherry A. Pierce, Department of Leukemia, University of Texas MD Anderson Cancer Center.
Prof. Michael Andreeff, Department of Leukemia, University of Texas MD Anderson Cancer Center.
John S. Welch, Division of Hematology & Oncology, Washington University School of Medicine in St. Louis.
REFERENCES
- 1.Deschler B, Lübbert M. Acute myeloid leukemia: Epidemiology and etiology. Cancer 2006;107(9):2099–107. [DOI] [PubMed] [Google Scholar]
- 2.Appelbaum FR, Gundacker H, Head DR, et al. Age and acute myeloid leukemia. Blood 2006;107(9):3481–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Juliusson G, Antunovic P, Derolf A, et al. Age and acute myeloid leukemia: real world data on decision to treat and outcomes from the Swedish Acute Leukemia Registry. Blood 2009;113(18):4179–87. [DOI] [PubMed] [Google Scholar]
- 4.Rowe JM, Tallman MS. How I treat acute myeloid leukemia. Blood 2010;116(17):3147–56. [DOI] [PubMed] [Google Scholar]
- 5.Cashen AF, Schiller GJ, O’Donnell MR, DiPersio JF. Multicenter, Phase II Study of Decitabine for the First-Line Treatment of Older Patients With Acute Myeloid Leukemia. J Clin Oncol 2009;28(4):556–61. [DOI] [PubMed] [Google Scholar]
- 6.Kantarjian HM, Thomas XG, Dmoszynska A, et al. Multicenter, Randomized, Open-Label, Phase III Trial of Decitabine Versus Patient Choice, With Physician Advice, of Either Supportive Care or Low-Dose Cytarabine for the Treatment of Older Patients With Newly Diagnosed Acute Myeloid Leukemia. J Clin Oncol 2012;30(21):2670–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Issa J-PJ, Garcia-Manero G, Giles FJ, et al. Phase 1 study of low-dose prolonged exposure schedules of the hypomethylating agent 5-aza-2′-deoxycytidine (decitabine) in hematopoietic malignancies. Blood 2004;103(5):1635–40. [DOI] [PubMed] [Google Scholar]
- 8.Blum W, Garzon R, Klisovic RB, et al. Clinical response and miR-29b predictive significance in older AML patients treated with a 10-day schedule of decitabine. Proc Natl Acad Sci 2010;107(16):7473–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ritchie EK, Feldman EJ, Christos PJ, et al. Decitabine in patients with newly diagnosed and relapsed acute myeloid leukemia. Leuk Lymphoma 2013;54(9):2003–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Welch JS, Petti AA, Miller CA, et al. TP53 and Decitabine in Acute Myeloid Leukemia and Myelodysplastic Syndromes. N Engl J Med 2016;375(21):2023–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adams JM, Cory S. The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene 2007;26(9):1324–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsao T, Shi Y, Kornblau S, et al. Concomitant inhibition of DNA methyltransferase and BCL-2 protein function synergistically induce mitochondrial apoptosis in acute myelogenous leukemia cells. Ann Hematol 2012;91(12):1861–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bogenberger JM, Kornblau SM, Pierceall WE, et al. BCL-2 family proteins as 5-Azacytidine-sensitizing targets and determinants of response in myeloid malignancies. Leukemia 2014;28(8):1657–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DiNardo CD, Pratz K, Pullarkat V, et al. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood 2019;133(1):7–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.AbbVie Announces Positive Topline Results from Phase 3 Trial of VENCLEXTA® (venetoclax) in Combination with Azacitidine in Patients with Acute Myeloid Leukemia (AML) | AbbVie News Center [Internet]. [cited 2020 Mar 30];Available from: https://news.abbvie.com/news/press-releases/abbvie-announces-positive-topline-results-from-phase-3-trial-venclexta-venetoclax-in-combination-with-azacitidine-in-patients-with-acute-myeloid-leukemia-aml.htm
- 16.Aldoss I, Yang D, Aribi A, et al. Efficacy of the combination of venetoclax and hypomethylating agents in relapsed/refractory acute myeloid leukemia. Haematologica 2018;103(9):e404–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheson BD, Bennett JM, Kopecky KJ, et al. Revised Recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol 2003;21(24):4642–9. [DOI] [PubMed] [Google Scholar]
- 18.Boddu P, Kantarjian HM, Garcia-Manero G, et al. Treated secondary acute myeloid leukemia: a distinct high-risk subset of AML with adverse prognosis. Blood Adv 2017;1(17):1312–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rozovski U, Ohanian M, Ravandi F, et al. Incidence of and risk factors for involvement of the central nervous system in acute myeloid leukemia. Leuk Lymphoma 2015;56(5):1392–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Common Terminology Criteria for Adverse Events (CTCAE) [Internet]. 2009;Available from: https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/Archive/CTCAE_4.0_2009-05-29_QuickReference_8.5×11.pdf
- 21.Luthra R, Patel KP, Reddy NG, et al. Next-generation sequencing-based multigene mutational screening for acute myeloid leukemia using MiSeq: applicability for diagnostics and disease monitoring. Haematologica 2014;99(3):465–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patel KP, Ravandi F, Ma D, et al. Acute Myeloid Leukemia With IDH1 or IDH2 Mutation Frequency and Clinicopathologic Features. Am J Clin Pathol 2011;135(1):35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.da Costa ES, Peres RT, Almeida J, et al. Harmonization of light scatter and fluorescence flow cytometry profiles obtained after staining peripheral blood leucocytes for cell surface-only versus intracellular antigens with the Fix & Perm reagent. Cytometry B Clin Cytom 2010;78(1):11–20. [DOI] [PubMed] [Google Scholar]
- 24.Xu J, Jorgensen JL, Wang SA. How Do We Use Multicolor Flow Cytometry to Detect Minimal Residual Disease in Acute Myeloid Leukemia? Clin Lab Med 2017;37(4):787–802. [DOI] [PubMed] [Google Scholar]
- 25.Döhner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 2017;129(4):424–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thall PF, Simon RM, Estey EH. Bayesian sequential monitoring designs for single-arm clinical trials with multiple outcomes. Stat Med 1995;14(4):357–79. [DOI] [PubMed] [Google Scholar]
- 27.Pei S, Pollyea DA, Gustafson A, et al. Monocytic Subclones Confer Resistance to Venetoclax-Based Therapy in Acute Myeloid Leukemia Patients. Cancer Discov 2020;CD-19–0710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Short NJ, Ravandi F. 10-day vs 5-day decitabine: equivalence cannot be concluded – Authors’ reply. Lancet Haematol 2019;6(4):e178. [DOI] [PubMed] [Google Scholar]
- 29.DiNardo CD, Tiong IS, Quaglieri A, et al. Molecular patterns of response and treatment failure after frontline venetoclax combinations in older patients with AML. Blood [Internet] 2020. [cited 2020 Jan 23];[Epub ahead of print]. Available from: https://www.ncbi.nlm.nih.gov/pubmed/31932844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roboz GJ, Rosenblat T, Arellano M, et al. International randomized phase III study of elacytarabine versus investigator choice in patients with relapsed/refractory acute myeloid leukemia. J Clin Oncol 2014;32(18):1919–26. [DOI] [PubMed] [Google Scholar]
- 31.DiNardo CD, Rausch CR, Benton C, et al. Clinical experience with the BCL2-inhibitor venetoclax in combination therapy for relapsed and refractory acute myeloid leukemia and related myeloid malignancies. Am J Hematol 2018;93(3):401–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


