Abstract
Imaging plays a key role is assessment of myeloma. Lytic bone lesions are optimally assessed using structural imaging however the structural changes lag in assessment of functional changes in the assessment of disease. Functional imaging with fluoro deoxy glucose positron emission tomography (PET) computerized tomography is useful in assessment of high-risk myeloma, provides prognostic information and is helpful in monitoring therapy response. However, it is nonspecific and may not be optimal in assessing treatment response to immunotherapeutic agents. Specific targeted imaging may allow for better assessment of changes from therapy, that is, based on specific mechanism. ImmunoPET imaging is a novel method to assess targeting of specific antigen by therapeutic antibodies. This review summarizes the role of functional imaging and development of novel immunoPET agents for assessment of treatment response and residual disease.
Introduction
Imaging plays an inherent role in diagnosis and management of multiple myeloma. Structural changes in the bones include lytic lesions, which is one of the features that characterize multiple myeloma. Various imaging tools have been used for assessing the disease extent. Although whole body skeletal survey has been most extensively used and has been the standard of assessment for many years, more recently, other modalities including magnetic resonance imaging (MRI) and positron emission tomography (PET) have emerged as superior methods of assessing both skeletal and extramedullary disease. Both MRI and PET imaging are sensitive methods that allow for superior detection of bone lesions. Although MRI is generally performed in smaller scanning fields, whole body MRI is possible and offers a more comprehensive assessment of disease extent. PET imaging allows for survey of the whole body in a convenient single imaging session with an added advantage of combined functional assessment of disease with computed tomography (CT) assessment of lytic lesions in bones. Its use in characterizing the high-risk disease is well recognized [1]. For patients undergoing or completing treatment, assessment of residual disease is critical for further management. A minimal residual disease (MRD) status after treatment is associated with superior survival outcomes [2]. Recent NCCN guidelines have [3] have included PET-CT imaging as an assessment for MRD. Under this, “Imaging plus MRD—negative” criteria requires a complete resolution or disappearance of uptake in lesions or decreased uptake in lesions to lower than mediastinal blood pool or adjacent normal tissue uptake on PET-CT is required along with a negative next generation flow or sequencing assessment. Physiologic changes in tumor burden precede structural changes in lesions; lytic bone lesions may persist and appear unchanged for long period on CT or MR, reported up to 9–12 months with MRI for reversal of abnormalities after therapy [1,4]. Functional assessment is therefore critical in those receiving or completing treatment and imaging modalities that would allow for early and accurate assessment of response and minimal residual disease are needed.
Role of Fluorodeoxyglucose PET-CT
Fluorodeoxyglucose (FDG) PET-CT is an established and invaluable tool in evaluation of malignancies and is a standard of care for management of a number of cancers. High uptake is seen in active and high-risk myeloma and is related to glucose transporters (GLUT) proteins, mostly related to GLUT-1 and 3. FDG is useful in assessment of newly diagnosed myeloma for risk stratification [5]. It is a convenient single imaging modality that allows for detection of systemic intramedullary and extramedullary disease in whole body and is superior to whole body skeletal survey [6] (Fig. 1). Focal lesional uptake, as against presence of lytic lesions only, is suggestive of active myeloma [7] (Fig. 2); the number of lesions detected on FDG-PET scans is found to be an independent prognostic factor [6]. Serial imaging to follow a change in metabolic uptake in lesions is useful for treatment response assessment (Fig. 3); the changes may be seen as early as within first few hours after effective therapy and sustained decreased values are indicative of an ongoing response. On the other hand, persistent uptake in lesion on FDG-PET scan correlates with an earlier relapse and is a poor prognostic factor (Fig. 4); in the post-transplant setting is associated with relapse within 6 months [1]. Several studies have shown usefulness of FDG imaging for response assessment in initial and pretransplant setting [8–10]. However, the data are difficult to interpret as there is large variation in methodology across the studies including the response criteria used, limiting its application for universal use. Although uptake value in lesions as a semiquantitative measure has been used, variation is seen how this is measured in different studies such as maximum value versus mean, body weight normalized vs body surface area. It has been suggested that a cutoff value of 3.9–4.2 distinguishes active from inactive disease and may be prognostic [10,11], however, these remain to be confirmed in larger studies. Other semiquantitative measures variably used include lesion to background ratios using either liver, normal L1-L2 vertebra or mediastinal blood pool as comparative background. Some have also evaluated total metabolic tumor volume (total volume of PET active disease in the body) and total lesions glycolysis to assess disease burden [12]. Interpretation of response is a bigger challenge in cases where there are mixed changes in the metabolic activity of the disease (Fig. 5). Currently, no standardized method is recommended and it is unclear, which response criteria should be used. Some studies have used European criteria [13] and while PET response assessment criteria (PERCIST) have been developed for assessment of response for solid tumors has been developed, and Deauville criteria are recommended for FDG response assessment of lymphoma [14,15], criteria specific to myeloma are lacking.
Fig. 1.
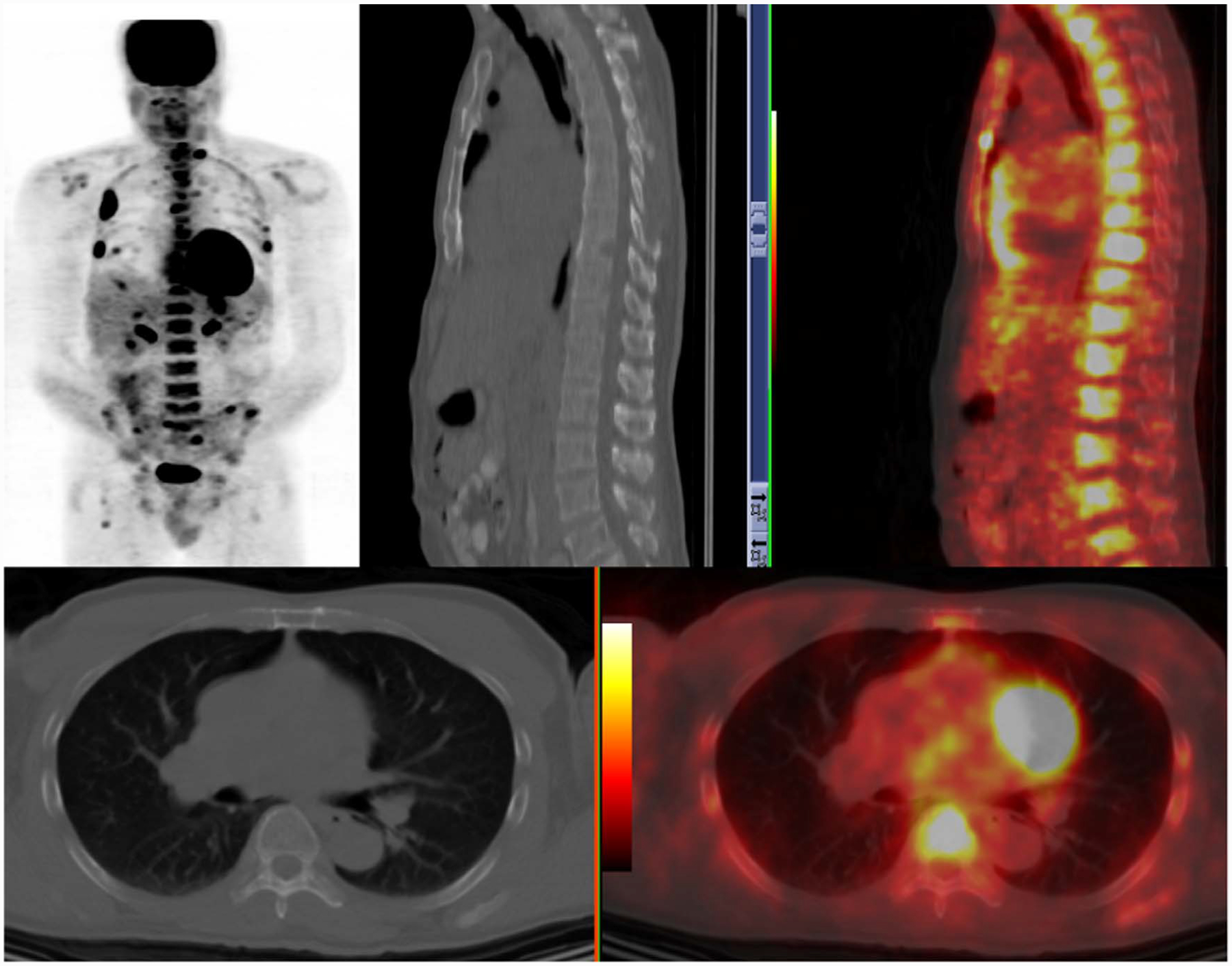
FDG-PET uptake in marrow disease and multiple lytic lesions in ribs with soft tissue disease.
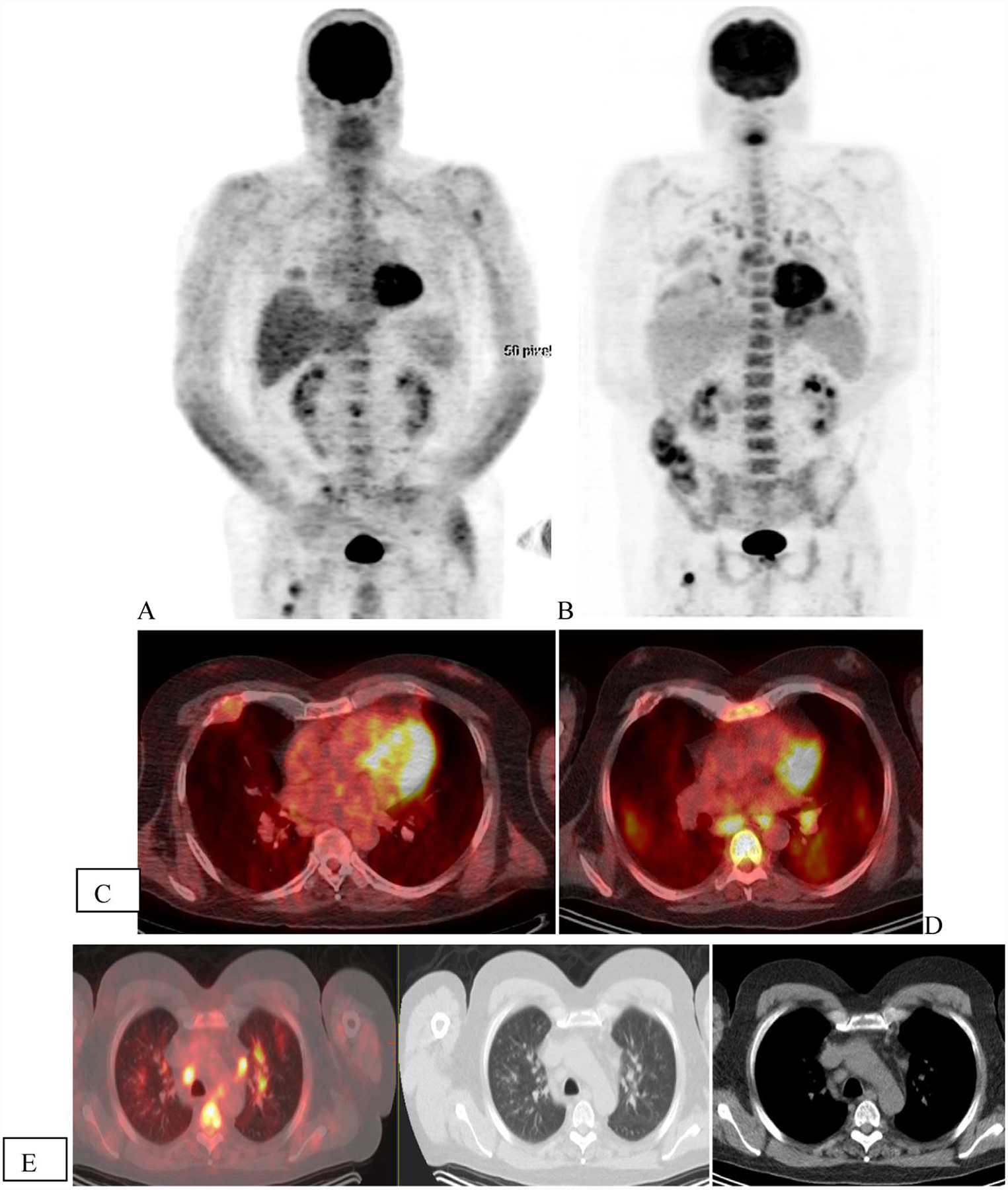
Fig. 2.
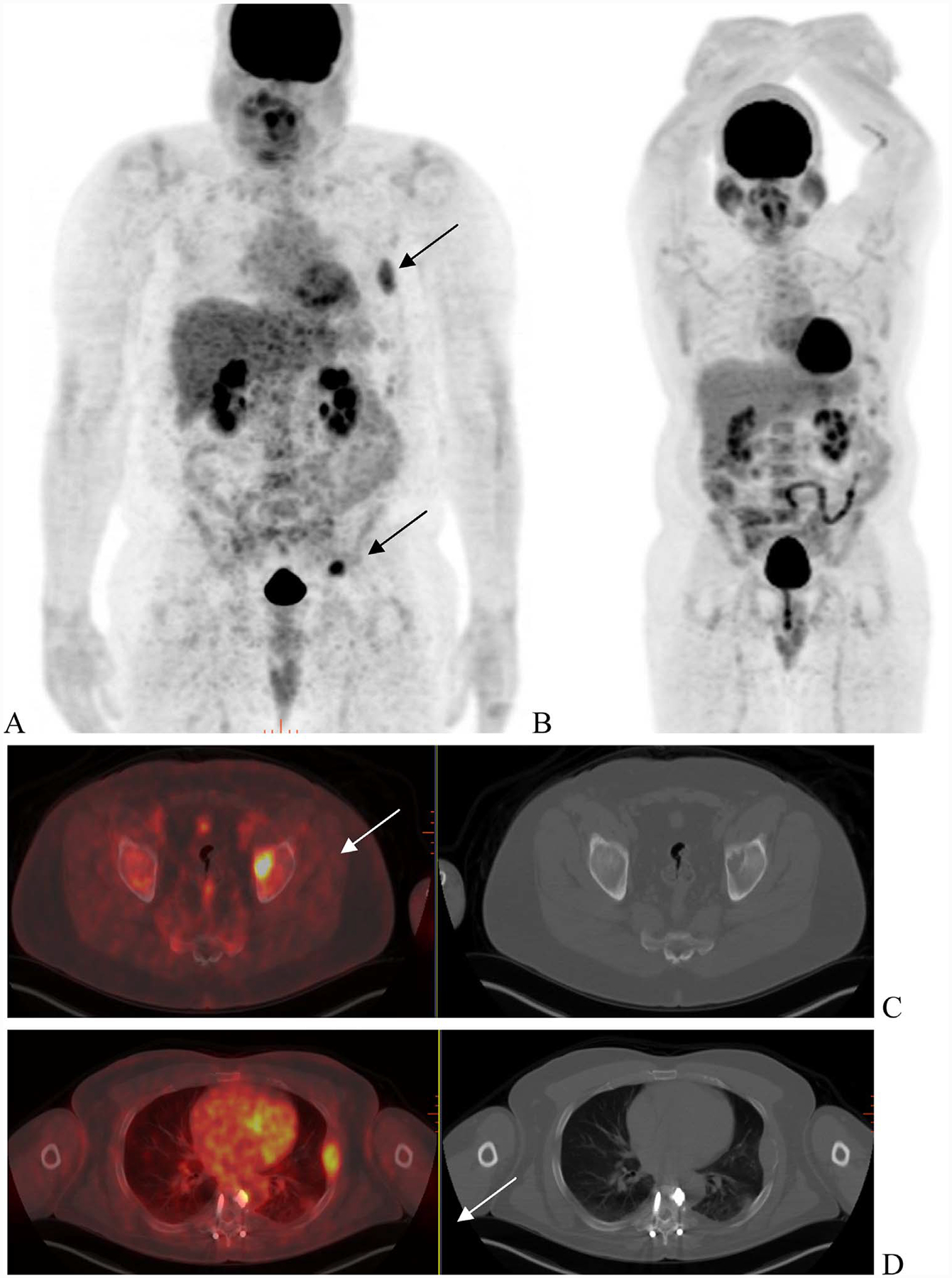
Treatment response. A 54 year with ISS-1 Ig G lambda multiple myeloma. FDG-PET scan (A) shows multiple site of uptake corresponding to lytic lesions in left acetabulum (C—arrow), left rib with soft tissue(D—arrow) and in pelvis (E). A follow-up scan 6-month later (B) was performed following interim treatment with standard combination therapy. Resolution of uptake was seen in acetabular and rib lesion (F and G) as well pelvic lesions (not shown).
Fig. 3.
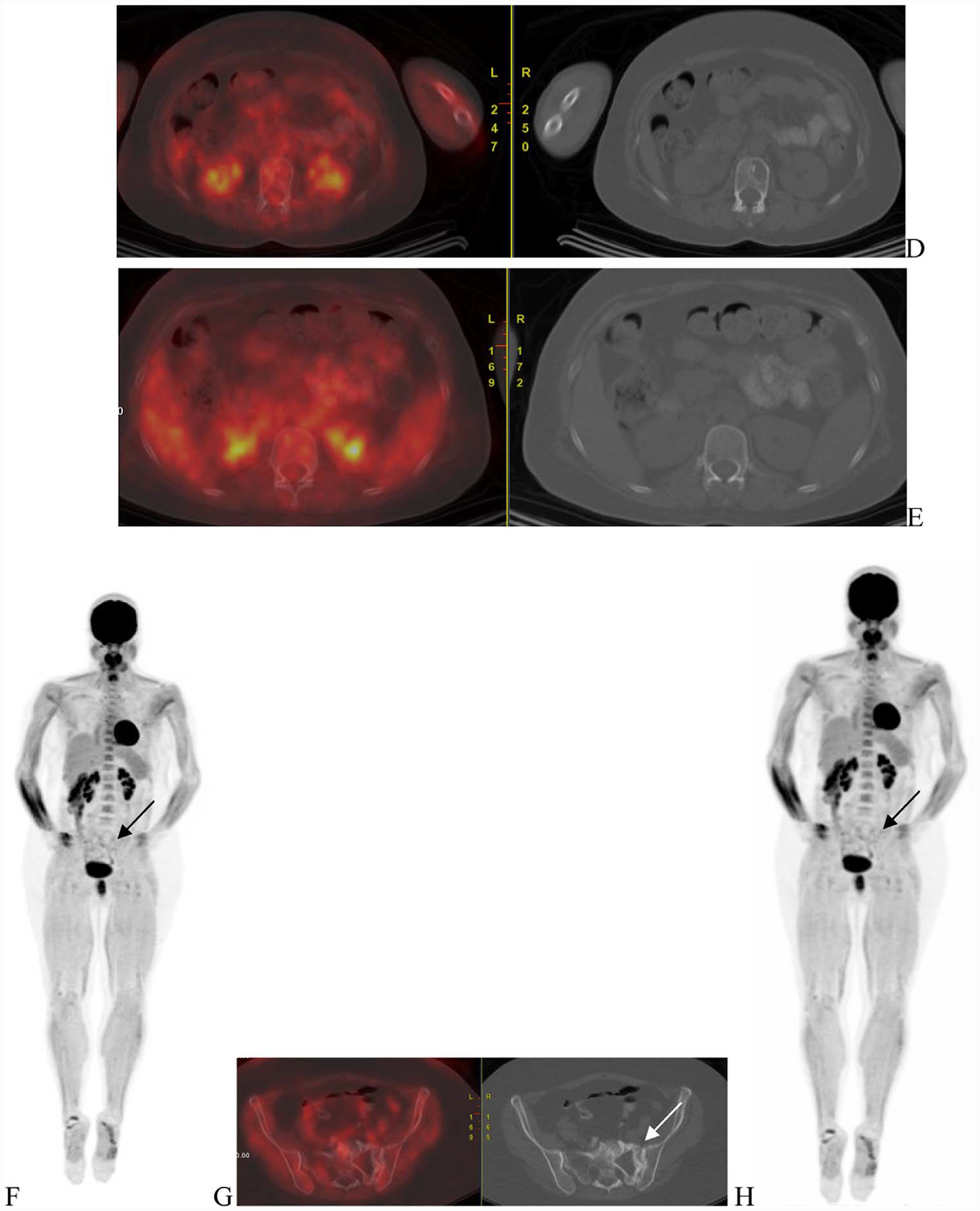
A 48-year-old woman with ISS-1 disease. FGD PET scan was done at baseline that showed uptake in the sacrum (A—arrow) corresponding to a large lytic lesion in noted on the CT with soft tissue component (B—arrow). Mild uptake was nted in the sternal lesion (C—arrows). Other lytic lesions were noted on the CT (D and E—arrows) but did not have corresponding increased uptake.
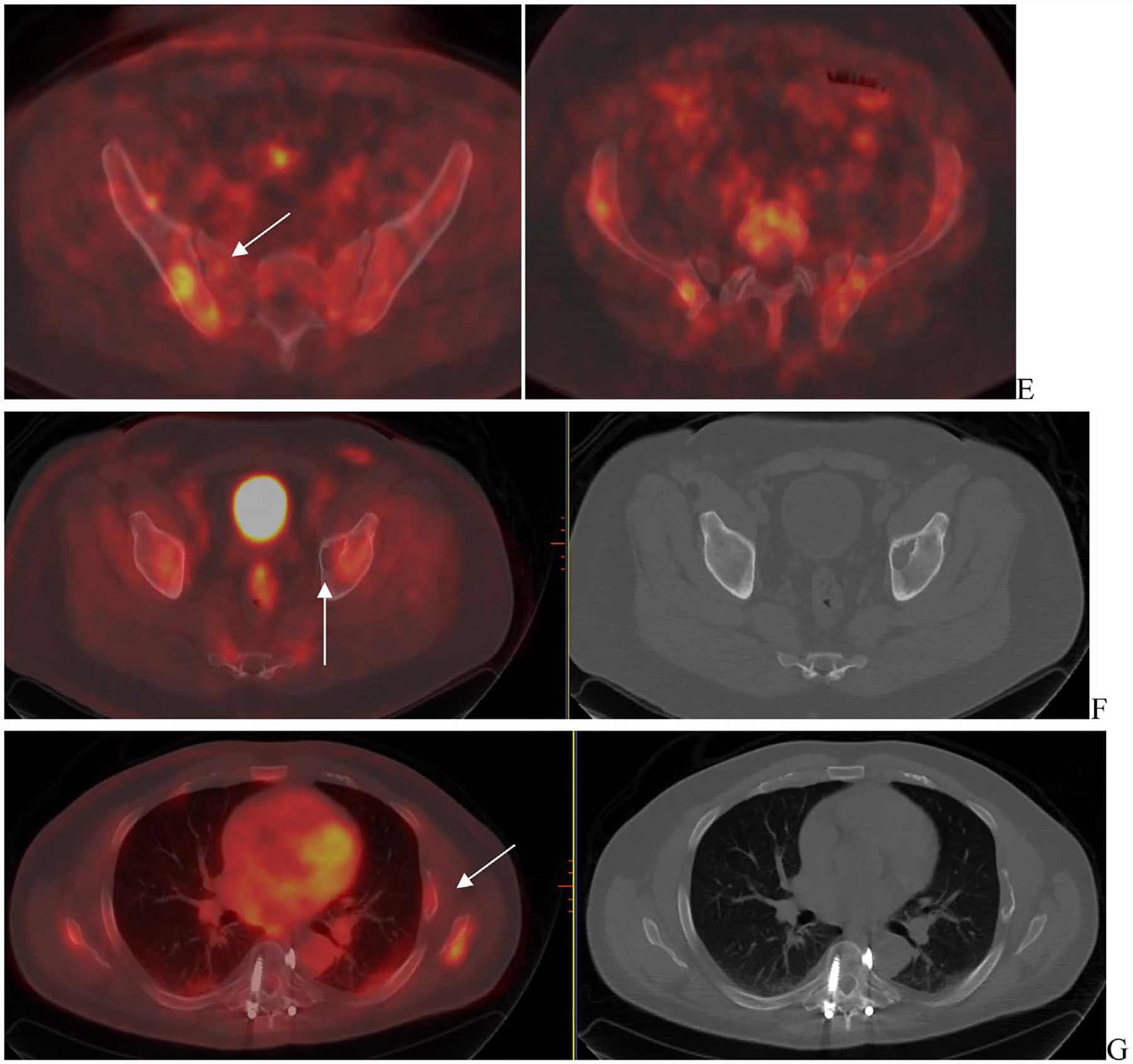
Fig. 4.
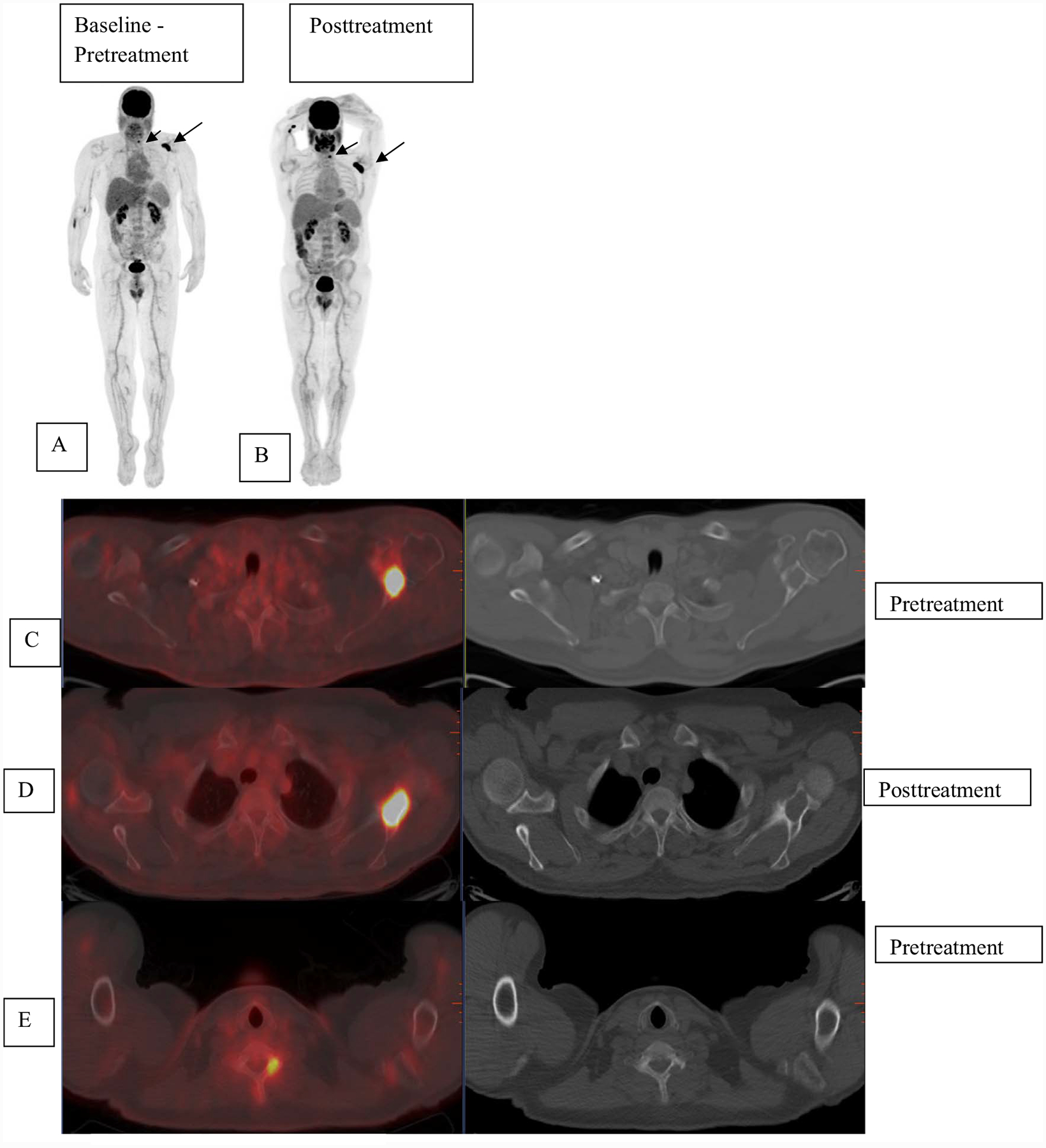
Patient with multiple myeloma was referred for evaluation with FDG-PET imaging. Pretherapy baseline scan (A) shows uptake in the left scapula SUV 16.1 (long arrow) and cervical vertebra (short arrow) that are also seen on fused images C, E localizing to lytic lesions on CT (arrows C and E). Post-therapy scan (B) shows increased extent of uptake in left scapula SUV 21 (D) and left cervical vertebra SUV 6.2 (F) as compared to prior. New uptake was seen in right sacrum (SUV 4.9), not visualized as compared to prior. SUV = standardized uptake values.
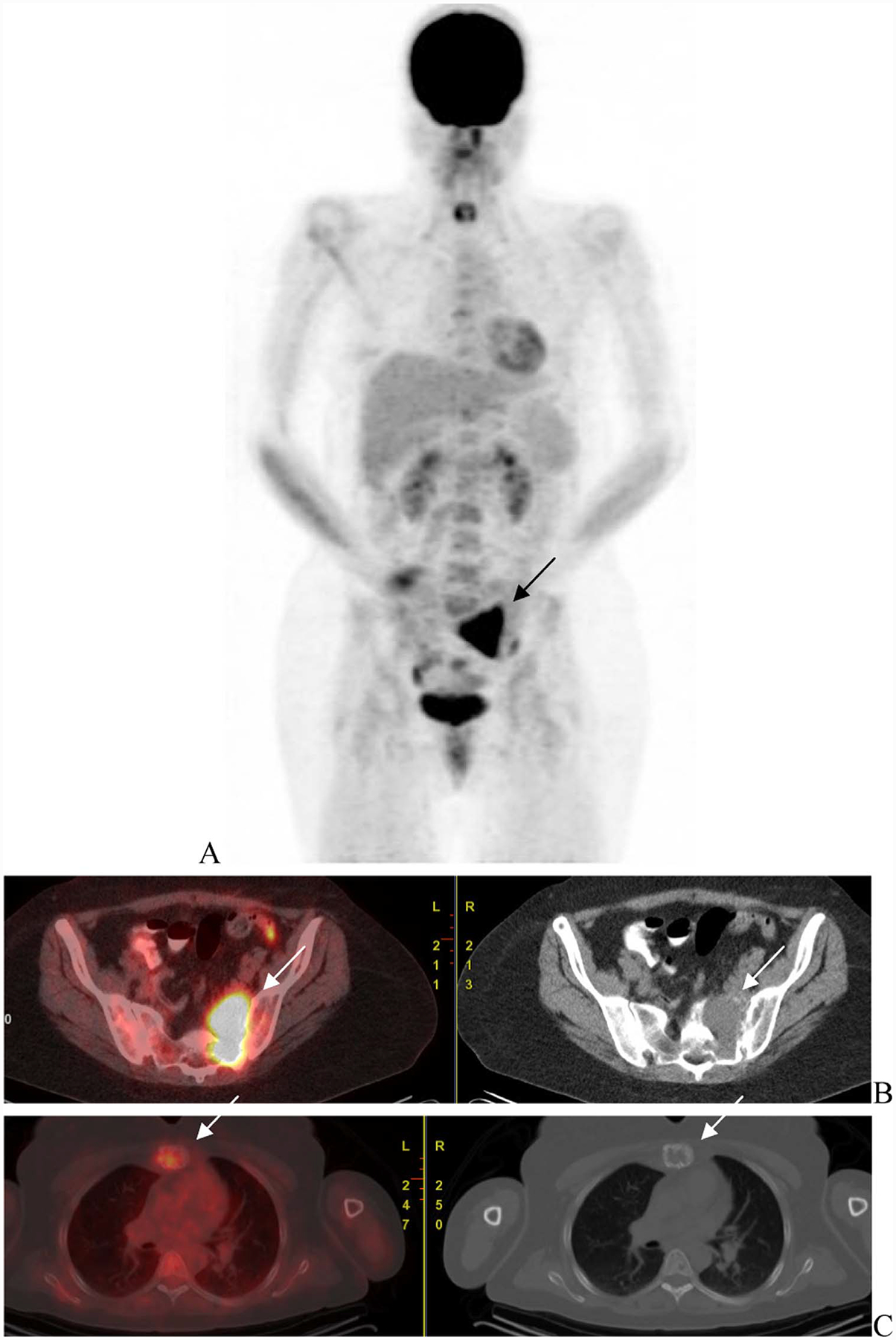
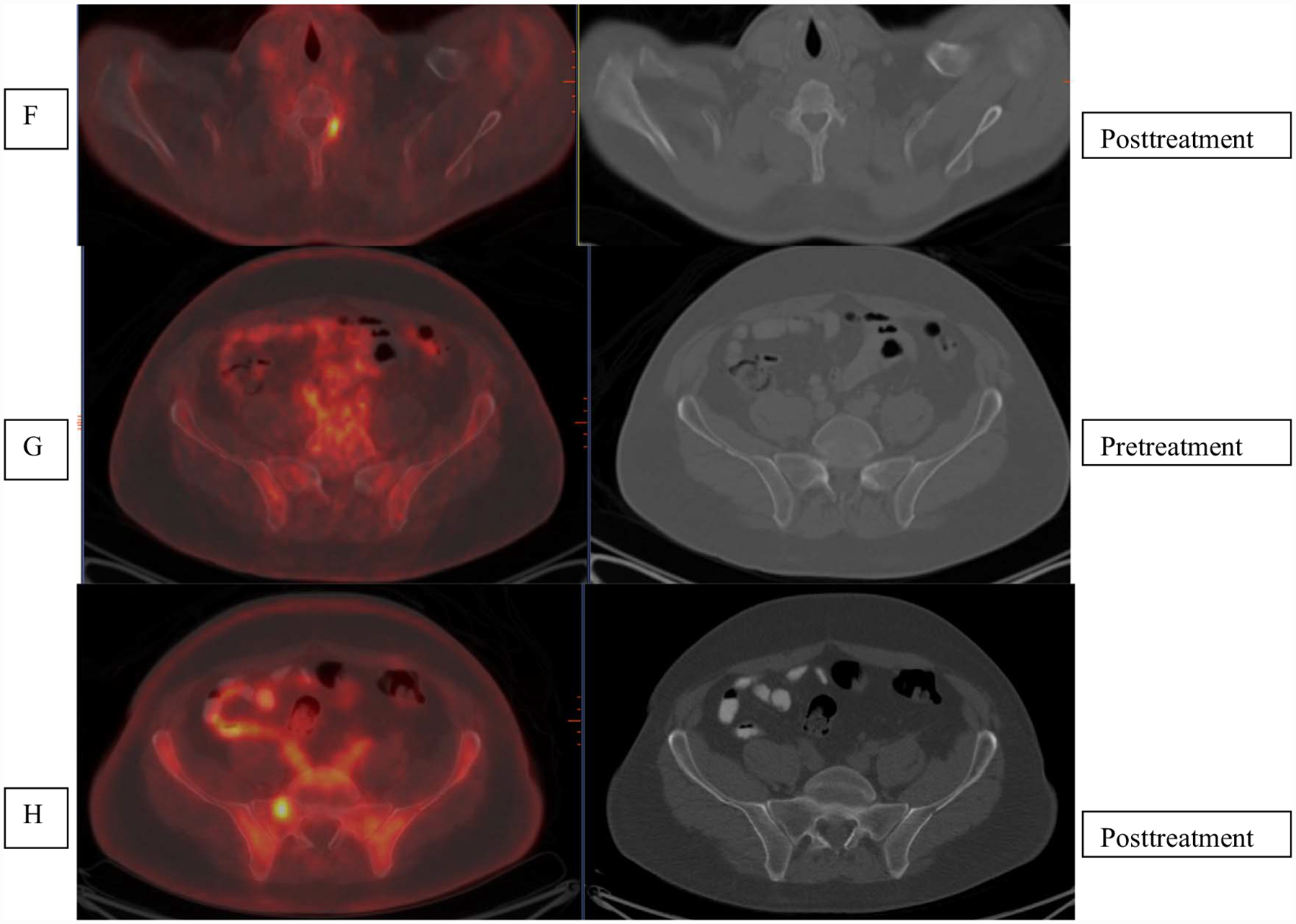
Fig. 5.
Patient with ISS2 IgA kappa multiple myeloma with high-risk disease. Initial scan (A) shows uptake in the lytic lesion in rib, vertebra and femur (arrows) that show mixed changes in FDG uptake with less uptke in the rib abd vertebra but higher uptake in the femur. Increased FDG uptake was seen in bilateral lung with infiltrative changes and mediastinal and hilar nodes (arrows) that was attributed to infectious, inflammatory change.
Although overall data support use of FDG-PET in disease evaluation, there are some limitations that must be considered. It is known that FDG can give false positive results in certain benign conditions such as infection, postsurgical and postbiopsy changes, fractures and associated reparative process and some benign bone lesions (Fig. 5). Additionally, in myeloma, fractures occur frequently and the inflammatory or reparative changes in bones may cause uptake at the site, limiting the assessment of active disease based FDG avidity alone. Certain therapies may cause inflammatory changes, again leading to false positives. In a recent analysis of patients who underwent upfront therapy including proteasome inhibitor, no significant association was noted between clinical response and FDG findings for MRD detection [16]. Also it is being realized that FDG-PET may not be optimal in assessing response to some of the immune targeted therapies [17,18]. Also, certain conditions may render false-negative results on FDG-PET imaging such as hyperglycemia and use of high-dose steroids. The assessment of marrow disease has lower sensitivity as compared to MRI, especially for diffuse disease and low grade disease with low GLUT-1expression [19]. A recent study in 227 newly diagnosed MM showed that about 11% of patients had false-negative FDG scan, related to the low hexokinase-2 expression [20]. Overall, the lack of standardized imaging parameters that would allow for reproducibility of data and uniform criteria for interpretation of response limits FDG-PET in evaluation of MRD. There is critical need for developing such criteria for assessment of MRD with FDG. Greater understanding is also needed regarding the clinical implications of a negative FDG scan for MRD in comparison with flow cytometric and DNA sequencing methods that can detect very small quantities of disease. Metabolically, negative disease with evidence of continued structural disease on imaging would generally mean lack of active disease. However there is limitation in detection of very small tumor volumes with FDG-PET for which comparative evaluation with more sensitive techniques and relation to clinical outcomes need to be better understood though focused clinical studies in MRD.
Other Tracers
Beyond targeting the glucose metabolism, other physiologic processes can also be targeted for imaging. These include targeting amino acid or fatty acid metabolism, cell proliferation mechanisms, and targeting of surface receptors or antigens or tumor vasculature (Table). A number of these tracers have been developed and tested clinically or in preclinical setting.
Table.
Radiotracers for targeted imaging.
| Radiolabeled tracer | Target/mechanism |
|---|---|
| 11C-choline | Cell membrane synthesis |
| 11C-methionine | Amino acid metabolism |
| 11C-acetate | Fatty acid metabolism |
| 18F-FDG | Glucose metabolism |
| 18F-sodium fluoride | Bone matrix |
| 18F-fluorothymidine | Thymidine kinase activity |
| 11C-4-thiothymidine | Thymidine kinase activity |
| F-18 fluoroethyldimethyl-2-hydroxyethylammonium (FECH) | Choline analog and cell membrane |
| 68Ga-pentixafor | Chemokine receptor—CXCR-4 |
| 64Cu-CB-TE1A1P-LLP2 | VLA-4 receptor targeting |
| 89Zr-bevacizumab | VEGF receptor targeting and angiogenesis |
| 111In-pentetreotide | Somatostatin receptor targeting |
| 68Ga-PSMA | Tumor vasculature |
| 89Zr-daratumomab | Anti CD-38 antibody |
| 64Cu-DOTA-Dara | Daratumomab-anti CD-38 antibody |
11C-Methionine is especially attractive as a biomarker. A possible mechanism of uptake is incorporation into the immunoglobulins and a high retention in myeloma cell lines as well as patient-derived CD138+ plasma cells has been observed [21,22]. Small studies have shown higher sensitivity in detection of focal lesions, bone marrow and extramedullary disease, a more accurate assessment of tumor burden and disease activity, superior correlation with bone marrow, beta2m and FLC levels in patient with 11C-methionine imaging as compared to FDG [23,24]. Similarly, in limited data from a single study, 11C-acetate imaging was superior to FDG for detection of marrow and extramedullary disease [25]. Choline is a substrate for cell membrane synthesis and 11C-choline has been used for imaging malignancies. Case reports and small studies have shown the ability to target myeloma lesions [26].
Another target is the l-type amino acid transporter-1 (LAT-1) that mediates sodium independent cell transport of amino acids and high levels of LAT-1 correlate with proliferation of tumors. Approximately, 56% myeloma patients may show LAT-1 expression in cells [27]. Targeted imaging of LAT-1 using 18-fluoro-l-phenyl-alanine PET is being tested in preclinical models to assess potential to stratify response [28]. Although all these alternate agents appear to have potential, there is no established data for superiority over FDG and conclusive data in evaluation of treatment response, especially for MRD assessment, are lacking. Additionally given tumor heterogeneity and inter patient variations, false-negative results are likely. More clinical studies are needed to understand potential clinical applications. Also, the availability and short half life of 11C (20.3 minute) make these agents less practical for universal application.
Strategies for targeted imaging of vasculature have also been explored for imaging myeloma. There is uniform expression of VEGF 1 receptors in myeloma leading to tumor angiogenesis and may be linked to increased microvascular density and tumor progression [29]. Bevacizumab is a humanized antibody directed to the VEGF receptor. The feasibility of imaging with PET tracer 89Zr-Bevacizumab in vascular tumors has been shown [30] and is being evaluated in myeloma patients with relapse (Clinicaltrials.gov; NCT01859234). Tumor vasculature also expresses prostate specific membrane antigen (PSMA), which can be targeted for imaging of vascular tumors [31]. Ability to image metastatic myeloma with 68Ga-PSMA has been reported and is interesting given possible use as a theranostic pair with 177Lu-PSMA, however, this needs further evaluation as a potential biomarker [32]. Also the relative utility in relation to other, more promising targets is to be considered.
Receptor Targeting Agents
More recently, novel agents targeting various receptors are being developed. One such agent is 68Ga-pentixafor that targets the chemokine (C-X-C motif) receptor 4 (CXCR4), which has been shown to target myeloma lesions [33]. Initial studies have shown good targeting of disease with overall disease detection in 66% (23/35) patients. A higher number of patients, approximately 21% (4/19), were positive for disease on 68Ga-pentixafor as compared to FDG. However, a few patients also showed reverse pattern with better assessment with FDG. In this study, CXCR4 PET positivity was not associated with myeloma subtypes, cytogenetics or any serological parameters and was found to be a negative prognostic factor. However, given the variable patterns and heterogenous expression of CXCR2 seen in some patients, the use may be limited to a subset of patients. Larger data are needed to understand the appropriate clinical application as an imaging agent, especially to delineate an incremental value to FDG imaging. Data for assessment of MRD is lacking and remains unclear. The tracer is being developed more as a theranostic imaging agent for CXCR4 directed radiotargeted therapy with 177Lu-pentixafor.
Integrins are glycoprotein receptors that signal across the plasma membrane bi-directionally in response to intracellular or extracellular stimuli. It is found to play a role in pathogenesis of myeloma including cell adhesion to marrow stroma, proliferation and trafficking. Activated form of this receptor is α4β1 or very late antigen 4 (VLA-4), transmembrane receptor that recognizes motifs on the vascular cell adhesion molecule-1 (VCAM-1), and fibronection ligands. 64Cu-CB-TE1A1P-LLP2A is radiolabeled peptide mimetic ligand PET tracer which has shown targeting of myeloma murine cell lines [34,35]. An alternate PET tracer, 68Ga-LLP2A, radiolabeled with short lived isotope 68Ga (half life = 68 minute) is being developed. However, no clinical data are available for treatment response and MRD assessment.
Radiolabeled Antibodies
Targeting of cluster of differentiation (CD-38) has emerged as an important therapeutic approach, with FDA approval of immunotherapy with daratumumab, a human IgG1K monoclonal antibody for treatment of myeloma. CD-38 receptor targeting is also attractive for imaging as it is uniformly and highly expressed on myeloma cells as against lower expression on normal hematopoietic cells. Specifically, for immune directed therapies where FDG may fall short due to associated inflammatory changes leading to false positives and underestimation of response, imaging with specific targeting agent such as antibody could prove to be highly advantageous. Radiolabeled antibody imaging allows for imaging tumor cells with antigenic expression and is independent of metabolic processes making is highly specific for tumor detection. This may allow for early and more specific assessment of response and may help predict responders vs nonresponders to enable early change in management. Daratumumab has been radiolabeled with a positron emitter Zr-89 (half life = 3.3 days) that allows for radio-immuno PET imaging or immunoPET imaging (Fig. 6A). In preclinical evaluation of myeloma cell lines, 89Zr-daratumumab showed high uptake in the tumors as against control with average concentration of 27.7% ID/g[36]. Due to the kinetics of antibodies and long half life of clearance, later time points up to 7-day postinjection appear to show highest concentration. The findings have been corroborated by Sobol et al of the Lewis Lab at our center, in preclinical mouse models where excellent targeting of myeloma was seen in mice (Fig. 6). 89Zr-daratumumab has potential to serve as a biomarker for response and possible theranostic agent in combination with therapeutic isotope labels such as alpha emitting 213bismuth (213Bi) or 225actinium(225Ac), which are under development [36]. The role in imaging specifically related to response to treatment and MRD assessment needs to be evaluated in clinical trials. An alternate tracer labeled with Copper-64 (64Cu-half life = 12.7 hour) 64Cu-daratumumab has also been developed and has shown good results in preclinical studies with efficient binding of CD-38 on the surface of multiple myeloma cells, primarily associated with tumor in bones [37]. In comparison, imaging with 64Cu-elozumab against receptor CS-1 did not identify bone engraftment of the CS-1 positive myeloma cells in vivo, making it less favorable [37]. There was a higher resolution and specificity to detect myeloma cell dissemination compared to FDG. The high sensitivity of detection of myeloma cells suggests possible use in MRD.
Fig. 6.
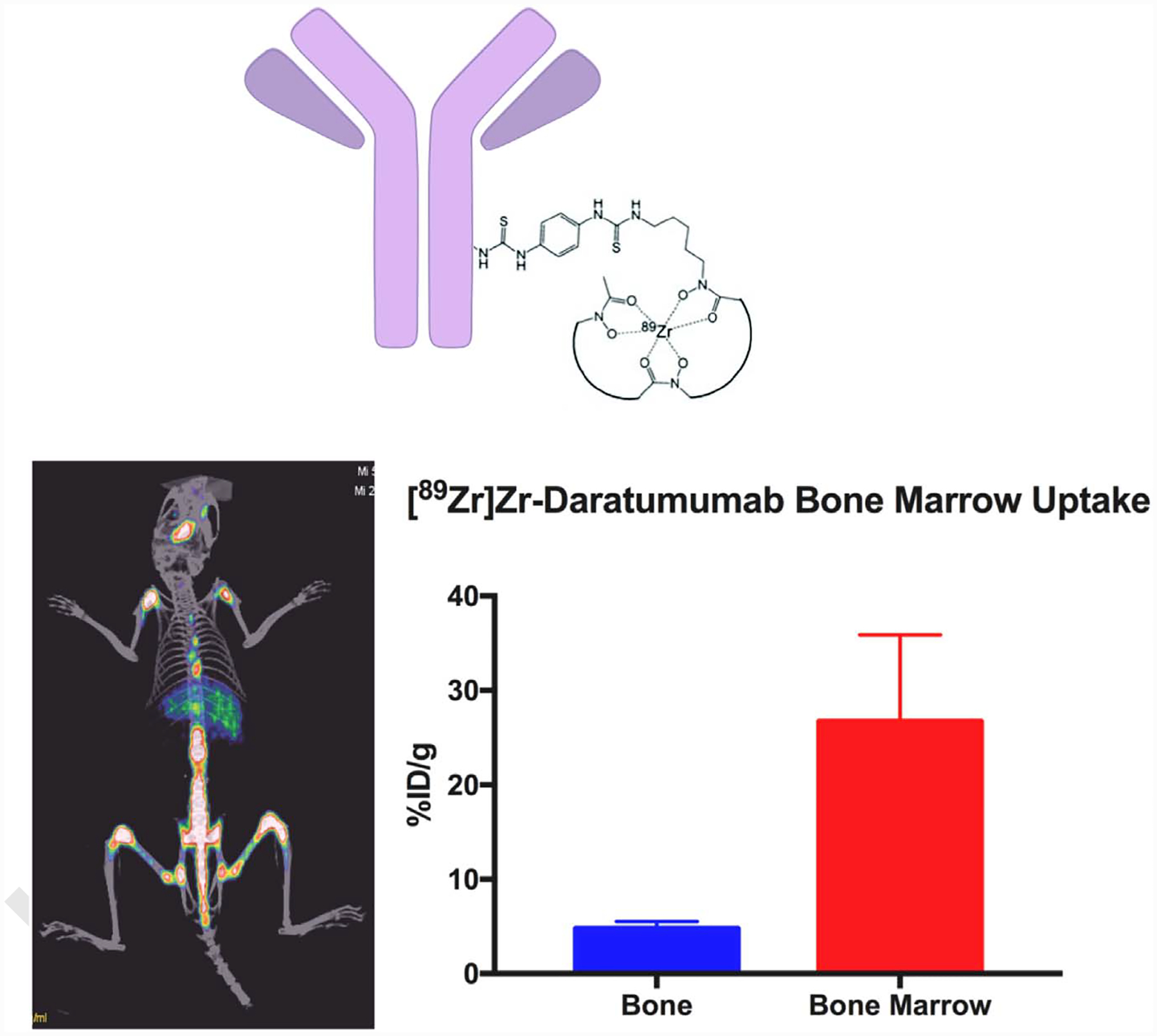
(A) Structure [89Zr]Zr-DFO-daratumumab. (B) Imaging with [89Zr]Zr-daratumumab in a disseminated mouse model (OPM-2 cells in NSG mice) of multiple myeloma (Sobol et al., unpublished data). There is prominent uptake in bones as seen along the spine and limbs. The uptake localized to the marrow and bone disease induced in the mice. This data corroborate the work done by the Shokeen group at Washington University (Ghai et al., JNM 2017) in a different MM model, showing that [89Zr]Zr-daratumumab has the ability to noninvasively identify MM in mouse models. This technology is currently being translated to the clinic at MSK. (Courtesy: Nicholas Sobol, PhD and Jason Lewis, PhD, Radiochemistry and Molecular Imaging Probes Core, MSK).
Other novel strategies also include targeted imaging of inter-leukin IL-6 [38] and targeting with anti-idiotypic nano bodies [39]. Cytokine interleukin-6 (IL-6) has pathogenic role in myeloma and is linked to cell proliferation and survival as well as interactions between the myeloma cells and stroma mediating the homing of myeloma cells in the marrow. Potential use of imaging with 99mtechnetium labeled tocilizumab, a humanized antibody against IL-6, has recently been tested preclinically in MM cell lines and mice that showed a high tumor uptake at 24 hours. These agents are also in very early preclinical development and clinical studies are needed to understand applicability and use for MRD assessment.
Summary
Assessment of MRD is increasingly becoming useful in management of myeloma and accurate assessment is critical. Imaging plays a key role and allows for noninvasive assessment of disease in the entire body, overcoming issues of sampling errors that limit other methods of testing. Functional imaging with FDG-PET has been especially valuable in assessment of extramedullary disease. For MRD, FDG imaging may be useful however some limitations are recognized that have prevented uniform application in standard of care. Proper response and evaluation criteria need to be developed MRD assessment with FDG-PET. Novel methods of receptor targeting with PET imaging, including ImmunoPET may allow for assessment of disease with high specificity and have potential for MRD assessment. Clinical studies specially focused on MRD assessment are needed to evaluate and validate the use of these novel agents.
Funding:
MSK Radiochemistry and Molecular Imaging Probes Core, Supported in part by NIH, United States/NCI, United States Cancer Center Support Grant P30 CA008748.
References
- [1].Durie BG, Waxman AD, D’Agnolo A, Williams CM. Whole-body (18)F-FDG PET identifies high-risk myeloma. J Nucl Med 2002;43(11):1457–63 [PubMed] [Google Scholar]
- [2].Munshi NC, Avet-Loiseau H, Rawstron AC, et al. Association of minimal residual disease with superior survival outcomes in patients with multiple myeloma: a meta-analysis. JAMA Oncol 2017;3(1):28–35, 10.1001/jamaoncol.2016.3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kumar SK, Callander NS, Alsina M, et al. Multiple myeloma, Version 3.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 2017;15(2):230–69. [DOI] [PubMed] [Google Scholar]
- [4].Walker R, Jones-Jackson L, Rasmussen E, et al. Diagnostic imaging of multiple myeloma—FDG PET and MRI complementary for tracking short vs. long term tumor response. Blood 2004;104(11):217a–217aa; [Google Scholar]
- [5].Lutje S, de Rooy JW, Croockewit S, Koedam E, Oyen WJ, Raymakers RA. Role of radiography, MRI and FDG-PET/CT in diagnosing, staging and therapeutical evaluation of patients with multiple myeloma. Ann Hematol 2009;88(12):1161–8, 10.1007/s00277-009-0829-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].van Lammeren-Venema D, Regelink JC, Riphagen II, Zweegman S, Hoekstra OS, Zijlstra JM. (1)(8)F-fluoro-deoxyglucose positron emission tomography in assessment of myeloma-related bone disease: a systematic review. Cancer 2012;118(8):1971–81, 10.1002/cncr.26467. [DOI] [PubMed] [Google Scholar]
- [7].Zamagni E, Nanni C, Gay F, et al. 18F-FDG PET/CT focal, but not osteolytic, lesions predict the progression of smoldering myeloma to active disease. Leukemia 2016;30(2):417–22, 10.1038/leu.2015.291. [DOI] [PubMed] [Google Scholar]
- [8].Cavo M, Terpos E, Nanni C, et al. Role of 18F-FDG PET/CT in the diagnosis and management of multiple myeloma and other plasma cell disorders: a consensus statement by the International Myeloma Working Group. Lancet Oncol 2017;18(4):e206–17, 10.1016/S1470-2045(17)30189-4. [DOI] [PubMed] [Google Scholar]
- [9].Nanni C, Zamagni E, Celli M, et al. The value of 18F-FDG PET/CT after autologous stem cell transplantation (ASCT) in patients affected by multiple myeloma (MM): experience with 77 patients. Clin Nucl Med 2013;38(2): e74–9, 10.1097/RLU.0b013e318266cee2. [DOI] [PubMed] [Google Scholar]
- [10].Bartel TB, Haessler J, Brown TL, et al. F18-fluorodeoxyglucose positron emission tomography in the context of other imaging techniques and prognostic factors in multiple myeloma. Blood 2009;114(10):2068–76, 10.1182/blood-2009-03-213280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Zamagni E, Patriarca F, Nanni C, et al. Prognostic relevance of 18-F FDG PET/CT in newly diagnosed multiple myeloma patients treated with up-front autologous transplantation. Blood 2011;118(23):5989–95, 10.1182/blood-2011-06-361386. [DOI] [PubMed] [Google Scholar]
- [12].McDonald JE, Kessler MM, Gardner MW, et al. Assessment of total lesion glycolysis by 18F FDG PET/CT significantly improves prognostic value of GEP and ISS in myeloma. Clin Cancer Res 2017;23(8):1981–7, 10.1158/1078-0432.CCR-16-0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Sachpekidis C, Hillengass J, Goldschmidt H, et al. Treatment response evaluation with 18F-FDG PET/CT and 18F-NaF PET/CT in multiple myeloma patients undergoing high-dose chemotherapy and autologous stem cell transplantation. Eur J Nucl Med Mol Imaging 2017;44(1):50–62, 10.1007/s00259-016-3502-6. [DOI] [PubMed] [Google Scholar]
- [14].Awan UE, Siddiqui N, SaadUllah M, et al. FDG-PET scan in assessing lymphomas and the application of Deauville criteria. J Pak Med Assoc 2013;63(6):725–30. [PubMed] [Google Scholar]
- [15].Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med 2009;50 (suppl 1):122S–150SS, 10.2967/jnumed.108.057307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Korde N, Roschewski M, Zingone A, et al. Treatment with carfilzomiblenalidomide-dexamethasone with lenalidomide extension in patients with smoldering or newly diagnosed multiple myeloma. JAMA Oncol 2015;1(6):746–54, 10.1001/jamaoncol.2015.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Gilles R, de Geus-Oei LF, Mulders PF, Oyen WJ. Immunotherapy response evaluation with (18)F-FDG-PET in patients with advanced stage renal cell carcinoma. World J Urol 2013;31(4):841–6, 10.1007/s00345-011-0723-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Wong ANM, McArthur GA, Hofman MS, Hicks RJ. The advantages and challenges of using FDG PET/CT for response assessment in melanoma in the era of targeted agents and immunotherapy. Eur J Nucl Med Mol Imaging 2017;44(suppl 1):S67–77, 10.1007/s00259-017-3691-7. [DOI] [PubMed] [Google Scholar]
- [19].Zamagni E, Nanni C, Patriarca F, et al. A prospective comparison of 18F-fluorodeoxyglucose positron emission tomography-computed tomography, magnetic resonance imaging and whole-body planar radiographs in the assessment of bone disease in newly diagnosed multiple myeloma. Haematologica 2007;92(1):50–5. [DOI] [PubMed] [Google Scholar]
- [20].Rasche L, Angtuaco E, McDonald JE, et al. Low expression of hexokinase-2 is associated with false-negative FDG-positron emission tomography in multiple myeloma. Blood 2017. 10.1182/blood-2017-03-774422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Dankerl A, Liebisch P, Glatting G, et al. Multiple myeloma: molecular imaging with 11C-methionine PET/CT—initial experience. Radiology 2007;242(2):498–508, 10.1148/radiol.2422051980. [DOI] [PubMed] [Google Scholar]
- [22].Luckerath K, Lapa C, Spahmann A, et al. Targeting paraprotein biosynthesis for non-invasive characterization of myeloma biology. PLoS One 2013;8(12):e84840, 10.1371/journal.pone.0084840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Lapa C, Schreder M, Luckerath K, et al. [11C]Methionine emerges as a new biomarker for tracking active myeloma lesions. Br J Haematol 2017. 10.1111/bjh.14696. [DOI] [PubMed] [Google Scholar]
- [24].Lapa C, Knop S, Schreder M, et al. 11C-methionine-PET in multiple myeloma: correlation with clinical parameters and bone marrow involvement. Theranostics 2016;6(2):254–61, 10.7150/thno.13921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Lin C, Ho CL, Ng SH, et al. (11)C-acetate as a new biomarker for PET/CT in patients with multiple myeloma: initial staging and postinduction response assessment. Eur J Nucl Med Mol Imaging 2014;41(1):41–9, 10.1007/s00259-013-2520-x. [DOI] [PubMed] [Google Scholar]
- [26].Nanni C, Zamagni E, Cavo M, et al. 11C-choline vs. 18F-FDG PET/CT in assessing bone involvement in patients with multiple myeloma. World J Surg Oncol 2007;5:68, 10.1186/1477-7819-5-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Isoda A, Kaira K, Iwashina M, et al. Expression of L-type amino acid transporter 1 (LAT1) as a prognostic and therapeutic indicator in multiple myeloma. Cancer Sci 2014;105(11):1496–502, 10.1111/cas.12529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Vij R, Fowler KJ, Shokeen M. New approaches to molecular imaging of multiple myeloma. J Nucl Med 2016;57(1):1–4, 10.2967/jnumed.115.163808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kumar S, Witzig TE, Timm M, et al. Expression of VEGF and its receptors by myeloma cells. Leukemia 2003;17(10):2025–31, 10.1038/sj.leu.2403084. [DOI] [PubMed] [Google Scholar]
- [30].van Es SC, Brouwers AH, Mahesh SVK, et al. (89)Zr-bevacizumab PET: potential early indicator of everolimus efficacy in patients with metastatic renal cell carcinoma. J Nucl Med 2017;58(6):905–10, 10.2967/jnumed.116.183475. [DOI] [PubMed] [Google Scholar]
- [31].Pandit-Taskar N, O’Donoghue JA, Divgi CR, et al. Indium 111-labeled J591 anti-PSMA antibody for vascular targeted imaging in progressive solid tumors. EJNMMI Res 2015;5:28, 10.1186/s13550-015-0104-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Sasikumar A, Joy A, Pillai MR, Nanabala R, Thomas B. 68Ga-PSMA PET/CT imaging in multiple myeloma. Clin Nucl Med 2017;42(2):e126–7, 10.1097/RLU.0000000000001479. [DOI] [PubMed] [Google Scholar]
- [33].Lapa C, Schreder M, Schirbel A, et al. [68Ga]Pentixafor-PET/CT for imaging of chemokine receptor CXCR4 expression in multiple myeloma—comparison to [18F]FDG and laboratory values. Theranostics 2017;7(1):205–12, 10.7150/thno.16576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Soodgupta D, Hurchla MA, Jiang M, et al. Very late antigen-4 (alpha(4)beta (1) Integrin) targeted PET imaging of multiple myeloma. PLoS One 2013;8(2): e55841, 10.1371/journal.pone.0055841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Soodgupta D, Zhou H, Beaino W, et al. Ex vivo and in vivo evaluation of overexpressed VLA-4 in multiple myeloma using LLP2A imaging agents. J Nucl Med 2016;57(4):640–5, 10.2967/jnumed.115.164624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Ghai A, Maji D, Cho N, et al. Preclinical development of CD38-targeted [(89)Zr] Zr-DFO-daratumumab for imaging multiple myeloma. J Nucl Med 2017. 10.2967/jnumed.117.196063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Caserta E, Chea J, Minnix M, et al. Copper-64-labeled daratumumab as a PET/CT imaging tracer for multiple myeloma. Blood 2018. 10.1182/blood-2017-09-807263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Camacho X, Machado CL, Garcia MF, et al. Tocilizumab labeling with 99mTechnetium via HYNIC as a molecular diagnostic agent for multiple myeloma. Anticancer Agents Med Chem 2017;17(9):1267–77, 10.2174/1871520617666170213144917. [DOI] [PubMed] [Google Scholar]
- [39].Lemaire M, D’Huyvetter M, Lahoutte T, et al. Imaging and radioimmunotherapy of multiple myeloma with anti-idiotypic nanobodies. Leukemia 2014;28(2):444–7, 10.1038/leu.2013.292. [DOI] [PubMed] [Google Scholar]


