Abstract
Introduction
Shared decision-making (SDM) is not yet widely used when making decisions in German hospitals. Making SDM a reality is a complex task. It involves training healthcare professionals in SDM communication and enabling patients to actively participate in communication, in addition to providing sound, easy to understand information on treatment alternatives in the form of evidence-based patient decision aids (EbPDAs). This project funded by the German Innovation Fund aims at designing, implementing and evaluating a multicomponent, large-scale and integrative SDM programme—called SHARE TO CARE (S2C)—at all clinical departments of a University Hospital Campus in Northern Germany within a 4-year time period.
Methods and analysis
S2C tackles the aforementioned components of SDM: (1) training physicians in SDM communication, (2) activating and empowering patients, (3) developing EbPDAs in the most common/relevant diseases and (4) training other healthcare professionals in SDM coaching. S2C is designed together with patients and providers. The physicians’ training programme entails an online and an in situ training module. The decision coach training is based on a similar but less comprehensive approach. The development of online EbPDAs follows the International Patient Decision Aid Standards and includes written, graphical and video-based information. Validated outcomes of SDM implementation are measured in a preintervention and postintervention evaluation design. Process evaluation accompanies programme implementation. Health economic impact of the intervention is investigated using a propensity-score-matched approach based on potentially preference-sensitive hospital decisions.
Ethics and dissemination
Ethics committee review approval has been obtained from Medical Ethics Committee of the Medical Faculty of the Christian-Albrechts-University Kiel. Project information and results will be disseminated at conferences, on project-hosted websites at University Hospital Medical Center Schleswig Holstein and by S2C as well as in peer-reviewed and professional journals.
Keywords: change management, organisational development, organisation of health services, quality in health care
Strengths and limitations of this study.
This study is the first large-scale long-term implementation of share decision-making (SDM) in an entire University Hospital involving all stakeholders in patient care in a multicomponent intervention.
Due to the size of our target intervention unit, a comparative study randomising comparable hospitals was neither feasible nor affordable.
This study aims to detect important SDM implementation barriers and supporting factors in a busy and profit-oriented hospital setting.
One limitation might be that there are no strong incentives for healthcare professionals and patients to contribute to the implementation of SDM.
Another limitation is that no patients were involved in the design of this study.
Introduction
Shared decision-making (SDM) between healthcare professionals like physicians or nurses and patients is currently not a standard in German hospitals.1 2 SDM has rather been implemented sporadically in individual indications and healthcare settings.3 4 This lack of SDM in routine settings might be due to a range of provider, patient, organisational, economic and contextual factors.1 2 5 On the other hand, German legislation with the Patients’ Rights Act gave SDM a more prominent role in German healthcare in 2013.6 The act implies that healthcare professionals and patients follow SDM communication rules. For example, physicians have to comprehensively inform their patients about relevant treatment alternatives (§630e).6 In this context, the law points out that written material like patient decision aids may support professionals in meeting these legal requirements. While legislation in Germany hence seems to be ready for SDM and supporting instruments such as evidence-based patient decision aids (EbPDAs), stakeholders in daily practice are not yet routinely implementing it.
For SDM to be effective, the patients’ and the healthcare providers’ ability and willingness to participate in SDM are crucial.2 7 To make SDM a reality in any healthcare setting is an ambitious endeavour and a complex multilevel task.5 It involves training physicians and other healthcare professionals in SDM communication skills as well as encourage patients to actively participate in communication, in addition to providing evidence-based, easy to understand information on treatment alternatives to patients and their physicians.8 To be effective in daily practice, SDM should be codesigned with involved stakeholders to gain acceptance and recognition.3 In addition, it needs an inner (ie, within the institution that wants to do SDM) and an outer (concerning the external conditions in which the institution works) settings, in which programme implementation is possible, as defined, for example, by the Consolidated Framework for Implementation Research (CFIR) (see table 1 for this project).9 The Norwegian ‘Decision Aid (DA) Factory’ approach of the University Hospital North Norway, in which researchers and developers of SDM components—so-called ‘knowledge producers’—work in close cooperation with the physicians and patients—so-called ‘knowledge users’—inspired implementation processes in this project.10
Table 1.
Consolidated Framework for Implementation Research (CFIR), S2C project-specific information for evidence-based decision aids and SDM training for physicians/training programme for decision coaching for other healthcare professionals*
| Construct | EbPDAs | SDM training for physicians/training programme for ‘decision coaching’ |
| I. Intervention characteristics | ||
| Intervention source | EbPDAs are developed internally by the S2C team. Topics for new EbPDAs are generated together with the physicians based on the DA factory approach. Patients are involved early on via needs assessments to inform EbPDA development. Evidence syntheses are conducted by well-known best-in class external consultant groups (EBSCO, USA (producers of the DynaMed point in care services, well-known to UKSH physicians, and Kleijnen Systematic Reviews (KSR), UK). | The training for physicians was developed and validated by members of the S2C team.17 18 26 The decision coaching training is developed by the S2C team in line with the physician training and based on existing decision coaching programmes.27 52 With a group of psychologists/coaching specialists, the trainer team combines scientific and practical expertise to train different types of healthcare providers in SDM communication/decision coaching skills. |
| Evidence strength and quality | A current systematic review demonstrates that decision aids improve decision and indication quality.14 Our EbPDAs follow the International Patient Decision Aid Standards (IPDAS)37 38 and provide balanced and easy to understand information on the pros and cons of treatment alternatives to patients. With the Patients’ Rights Law in place in Germany since 2013, the S2C project with its EbPDAs also puts the patients’ rights into practice. | The training programme for physicians was developed/validated by Geiger et al.17 18 26 This programme guided the development of decision coach training for other healthcare professionals. Further evidence supported the refinement and adaptation of the coaching programme to meet the specific demands in difficult indications/target populations in a hospital setting.27 52 |
| Relative advantage | The format of online EbPDAs with easy to understand written/graphical information, videos with patient narratives, and videos with physicians from the UKSH explaining disease or treatment concepts are likely attractive to patients and physicians. While physicians are involved in development and invest time into it, EbPDAs will facilitate better informed dialogue with patients. By providing more structure to the dialogue, EbPDAs are expected to make communication more efficient.48 | The online SDM training is a relatively quick and easy-to-do training programme teaching SDM basics to physicians. The video-feed-back-based training is highly individualised and based on real patient–physician communication allowing a thorough SDM learning experience. The training for decision coaching focuses on providing support with EbPDA use to patient, especially if patients are emotionally or physically not able to effectively use EbPDAs without support. |
| Adaptability | The format of the EbPDAs follows a standard structure. However, this structure is flexible. It allows for topic-specific or clinic-specific adaptations. Online decision aids will be administered to patients via printed access codes that patients receive in an envelope. Each EbPDA will contain a printable summary sheet on all relevant aspects of alternatives (questions and answers sheet). This paper-based version can be used in communication with patients not willing or able to use the online EbPDAs. | Training units are flexible and adaptable to specific demands. The online training can be easily integrated into a busy physician schedule. If physicians do not want to do personal training in a group setting, it can be done with physicians individually. If healthcare providers are not willing to video-tape interactions with their patients, trainers may offer participating observation instead and rate ‘live’ patient–physician interactions. Other adaptations might be needed throughout. |
| Trialability | Each clinic starts with one or two EbPDAs. If a clinic is interested to support further topics, additional EbPDAs may be developed. If a clinic is rather unwilling to support the project, no pressure will be exerted but the clinic may rejoin EbPDA development at a later point in time. Also, since the clinical departments are approached in a stepwise approach, learnings from one clinic might be transferred. Features of the EbPDAs may be adapted according to specific clinic or patient needs (length of texts, number of films, graphics, description of clinical studies, strength of evidence, etc) | It is not an imperative for UKSH staff to undergo SDM training sessions, but clinic directors are asked to motivate their staff to take part in these. Also, clinic directors are asked to make sure that training sessions can be done within working hours. If a clinic is unwilling to support the project at a specific point in time or to provide the time to their physicians to undergo training, no further pressure will be exerted. In selected instances (eg, if physicians of a department are under extreme time pressure), SDM training might remain limited to online sessions. |
| Complexity | Patients will have to invest at least 30–60 min to go through one EbPDA. This might be tiring to some patients. The patient-friendly and flexible format of EbPDAs addresses this issue in parts. Availability via the bedside—‘Infotainment’-system at UKSH and via portable tablets will make access to EbPDAs easy for patients. Physicians have to invest time for EbPDAs. They might not initially appreciate that EbPDAs can help save time in patient communication. Also, the departments/physicians will have to integrate the EbPDAs into patient pathways. This might not always be easy in a busy hospital setting and make pathway adaptations necessary. | Training sessions for physicians and decision coaches are time-consuming, between 1 and 2 full days for physicians and for those who undergo training as decision coaches. This time needs to be provided by clinic directors but it might still be difficult to integrate training sessions into a busy clinic schedule. Healthcare staff might refuse videotaping themselves in patient interaction for various reasons (eg, worries about an external rating of their performance). Even for well-trained physicians or decision coaches, it might sometimes be difficult to integrate SDM and decision coaching into interactions/treatment pathways. Adaptations of treatment pathways might be needed. |
| Design quality and packaging | The EbPDA is developed by a highly professional S2C team of medical writers working according to the standards of evidence-based patient information and a professional film team with wide experience in patient filming. Evidence syntheses are done by best-in class external consultants together with the S2C evidence team. All EbPDAs strictly adhere to the IPDAS criteria.37 38 | All training sessions were developed and are conducted by a group of trained and experienced psychologists/coaches. The online training was developed and realised by the S2C trainer team in cooperation with the S2C film team. All training evaluations strictly adhere to the rating criteria of the MAPPIN’SDM instrument18 26 |
| Cost | Costs of the intervention and costs associated with implementation are covered by a grant of the German Innovation Fonds (IF). The IF is hosted at the Federal Joint Committee. Opportunity costs will occur since patients and physicians have to invest time in decision aid production and/or use. Research indicates that using EbPDAs in patient–physician interaction can make communication and decisions more effective and more efficient.14 48 | As for the EbPDAs, all costs related to the development of training sessions are covered by the IF. Furthermore, physicians have to invest time into the different training sessions. Ideally, these can be done within their working hours. Physicians are rewarded by continued medical education credits by the German Medical Associations. Health care staff undergoing training as decision coaches will need to invest 2 full days. |
| II. Outer setting | ||
| Patient needs and resources | As primary cooperation partner in the S2C project, the administration of UKSH acknowledges the need for better patient participation and the resulting need for change. While it puts no formal pressure on its physicians to cooperate in the project, the directors of each department are encouraged to provide support by signing specific SDM goal attainment contracts. In these, they agree to have their staff undergo training sessions within working hours and to motivate their physicians/other staff to support the S2C programme. | |
| Cosmo-politanism | UKSH and the S2C project team work in close cooperation with other (inter) national players in the field of evidence-based Medicine and SDM. Cooperation is initiated or ongoing with, for example, the German Institute for Quality and Efficiency in Health Care (IQWiG, gesundheitsinformation.de) and the evidence-based guideline developers within the German Association of the Scientific Medical Societies (AWMF), primarily trying to avoid the redundant production of patient content or evidence reviews. At the International level, UKSH and the project team get engaged for example, in the International Shared Decision Making Society. | |
| Peer pressure | This is the first full implementation of SDM at a University Hospital in Germany. Nevertheless SDM is becoming increasingly demanded, that is, it is on the German political agenda. For example, the AWMF established a committee to add EbPDAs to its evidence-based clinical guidelines. The German branch of Choosing Wisely claims to carry forward SDM. The National Cancer Plan and the National Plan for Health Literacy demand for SDM. Also, patient organisations and the German Independent Patient Council stipulate SDM in healthcare. | |
| External policy and incentives | The objective of IF-funded projects in Germany is to test new forms of healthcare provision, to scale them up and to finally transfer these into general statutory health insurance funding (in case of successful implementation). Therefore, the S2C project can be considered a ‘lighthouse’ project, gaining a lot of attention in the media already. In the context of the Patients’ Rights Law and with SDM being a generally approved concept in German politics, this project aims to serve as a role model for other hospitals and settings. Cooperation with other National players (eg, AWMF, IQWiG, German Society of Evidence Based Medicine, German Society for Health Literacy) aims to support this development towards more SDM-based patient care. | |
| III. Inner setting | ||
| Structural characteristics | The UKSH is a tertiary care hospital with 27 clinical departments. Each of these departments and all physicians will be involved. Since the UKSH is very hierarchically structured, our approach is to get departments involved in the project in a top-down approach. Clinic directors get involved first, followed by the physicians at the next-lower levels in the hierarchy. One physician in each clinic will be chosen together with the director to be the designated ‘SDM responsible’ who oversees activities in the respective clinic (eg, training activities, EbPDA development, patient activation activities). Other physicians will be responsible for individual EbPDA topics and it is assumed that early involvement of physicians in EbPDA development will increase their acceptance and support. At the same time, the UKSH Employee Committee and individual multipliers (‘clinical champions’) will be involved early in the project. | |
| Networks and communications | At the level of physicians, the hierarchical structures need to be respected and taken into account. If the director supports SDM, it is assumed to be more likely that the entire clinic supports SDM. At the patient level, the UKSH offers the Infotainment system which can be used to make EbPDAs available to patients at the bedside. | |
| Implementation climate | Our objective in this project is nothing less than to initiate a paradigm shift towards more SDM-based healthcare in an entire hospital setting. While the UKSH is open for change at an administrative level, time and economic constraints might limit the physicians’ willingness and perceived liberty to support the project. Implementation climate will be assessed using summative (Patient questionnaire; MAPPIN’SDM evaluation) and process evaluation components (based on the CFIR constructs and NPT) as described. | |
| Readiness for change | While the UKSH is open for change at an administrative level, time and economic constraints might limit the physicians’ willingness and perceived liberty to support the project. | |
| IV. Characteristics of individuals | ||
| Knowledge and beliefs about the intervention | Preliminary research indicates that many patients in the UKSH setting might not yet be regularly involved in decisions, but are open to more information and more involvement. Individuals’ attitudes toward the SDM interventions and their role in it will be measured in the pre–post evaluation by (1)using a range of patient-based instruments to assess patient-physician interaction and the perceived role of the patient before and after the interventions, and (2) using the MAPPIN’SDM instrument to get a reviewer perspective on whether interventions/trainings influence/improve patient–physician interaction. Physicians might often rather focus on the demands placed on them by the S2C project team and less on the potential advantages/time savings in patient communication. NPT-based questionnaires/interviews to assess key stakeholder/physician perceptions of the intervention throughout implementation will be used. | |
| V. Process | ||
| Planning | The individual components of the S2C programme have been tested/validated previously in other contexts and will be implemented by a team of implementation experts. | |
| Engaging | The S2C team consists of four teams: the evidence team, the decision aid team (working closely together on decision aids), the trainer team (physician training, training for ‘decision coaching’), and the implementation team (engaging at all levels in implementation-related activities in the hospital, for example, recruiting patients for needs assessments, reminding physicians or other healthcare professionals to undergo trainings etc). Besides, the latter will realise patient activation and other marketing/exchange initiatives to foster engagement and identification with the S2C concept among patients and healthcare staff. | |
| Opinion leaders | The directors of each clinic and other ‘SDM champions’ are important to actively support the S2C intervention and engage their physicians to follow them. Also, the ‘SDM physician’ at each clinic plays a crucial role in this context. | |
| Internal implementation leaders | One physician in each clinic will be the designated ‘SDM physician’ who oversees activities in the respective clinic. For each EbPDA topic, one physician or a group of physicians will be nominated to carry primary responsibility from a clinical point of few. These physicians are expected to support the S2C team and drive project activities forward in the respective department. | |
| Champions | The ‘personal flagship’ of the project, Dr Eckhart von Hirschhausen, is a very prominent TV physician, comedian and moderator. He will play a very active role in project marketing. He will be present in videos and on posters and demonstrate his support of the S2C programme at all levels and in all its components. Dr von Hirschhausen is also an official cooperation partner in the project. | |
| Executing | The German Innovation Fund as national sponsor requires regular milestone reports on project success every 6 months. | |
| Reflecting and evaluating | All S2C teams will continuously report on the progress of implementing S2C in their respective domain and document issues, problems or highlights throughout the course of project time (field notes/documentation) | |
*The intervention component ‘patient activation programme’ is not separately described in the CFIR table but in the publication text only, given that this programme is limited to accompanying marketing and information strategies within each clinic using postcards, posters and stand-up boards.
CFIR, Consolidated Framework for Implementation Research; EbPDAs, evidence-based patient decision aids; MAPPIN'SDM, multifocal approach to sharing in shared decision-making; NPT, normalisation process theory; S2C, SHARE TO CARE; SDM, shared decision-making.
Individual components of SDM such as SDM training for healthcare professionals, patient activation/empowerment programmes or decision aids have all been previously tested in specific indications, populations and using different study designs.11–15 Their effectiveness and impact on decision processes have been assessed. For example, according to a recent systematic review of 115 randomised controlled trials (RCTs) with about 35 000 patients altogether, the use of only EbPDAs to inform patients in specific indications led to improved health education/literacy, more active participation and value congruent choices, more accurate expectations regarding course of disease and risk perceptions, more treatment satisfaction and better adherence to treatment.14 This finding has been reinforced by reviews in other specific populations.16 However, while most of the EbPDAs were previously tested in RCTs, they were often not subsequently used in the settings they were developed in.3 A recent study by Stacey et al3 concluded that ‘To improve subsequent use, researchers should codesign EbPDAs with end users to ensure fit with clinical practice and develop an implementation plan’. That study surveyed EbPDA developers who reported that the lack of physicians supporting and agreeing with the EbPDAs often hindered successful implementation. Training physicians in SDM in theory and practice has equally demonstrated to be effective, but the certainty of this evidence is low and limited to specific treatment settings.15 17–19 While there may still be a lack of evidence regarding the effectiveness of SDM on patient-relevant clinical endpoints, there is growing agreement and consensus that SDM is a necessity, a patients’ and a citizen’s right, and an ethical imperative.7
It has also become clear that effectiveness to a large extent will depend on effective implementation strategies and consistent stakeholder involvement.3 5 Hence, given a growing body of evidence supporting the effectiveness of individual SDM interventions, the next step on the ‘continuum of increasing evidence’ according to Campbell et al20 21 would be to roll out the combined implementation of SDM interventions on a larger-scale in a long-term implementation study. Few programmes until now have addressed the simultaneous implementation of a range of SDM components at the same time (see eg, Sondergaard et al,22 Steffensen et al23), some are currently ongoing (see eg, Scholl24), but none have yet introduced a multicomponent SDM programme at all departments of a hospital at a time. Therefore, in this publicly funded project, the objective was to design, implement and evaluate a multicomponent, large-scale and integrative SDM programme—called SHARE TO CARE (S2C)—at the University Hospital Medical Center Schleswig Holstein (UKSH), Campus Kiel, within a 4-year time period—from October 2017 until 30 September 2021. The project is designed and implemented in cooperation between the UKSH, Kiel, Germany and the University Hospital of Northern Norway, Tromsø, Norway.
Methods and analysis
Study design
This study implies the large-scale implementation of SDM at the University Hospital Campus Kiel within a 4-year time period based on the S2C intervention programme. It includes comprehensive outcome evaluation with measurement of (1) SDM level in patient–physician interactions based on patients’ and external observers’ perceptions before and after S2C implementation and (2) measuring the impact of the S2C intervention on healthcare use and costs in comparison to a propensity-score-matched comparison population not exposed to S2C. The programme will be accompanied by a process evaluation based on the recommendations of the Medical Research Council Guidance and using the CFIR to guide development and implementation activities.9 25
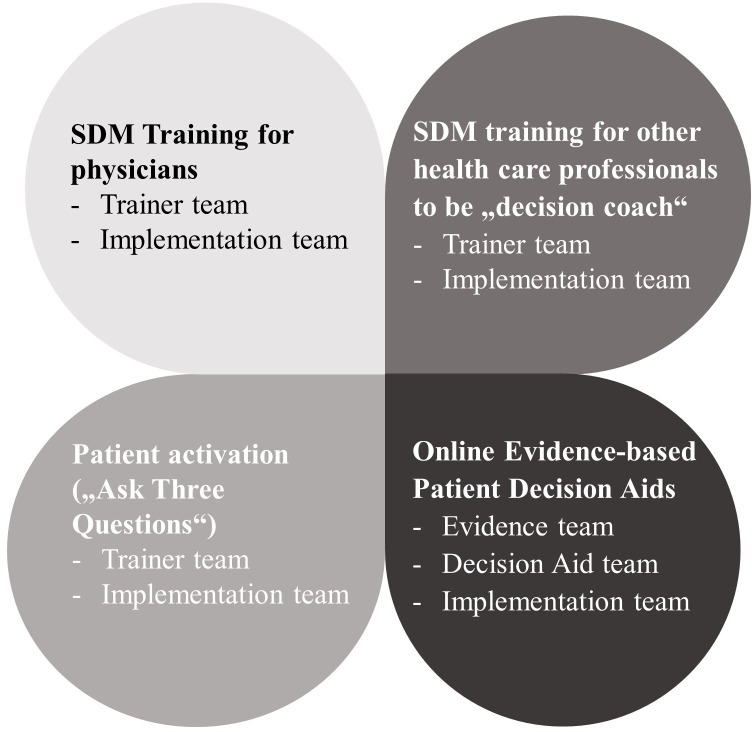
The term ‘multicomponent’ in the S2C programme refers to four different interventions (components) designed and implemented simultaneously in several clinical departments. This includes (1) SDM training for physicians,17–19 26 (2) SDM qualification as ‘decision coach’ for other healthcare professionals like nurses or physiotherapists,18 27 (3) the Ask Three Questions programme that aims at patient activation and empowerment and28 29 (4) development of online EbPDAs.14 These components and the respective responsible S2C project teams are depicted in figure 1.
Figure 1.
Project components and respective S2C project teams. S2C, SHARETO CARE, SDM, shared decision-making.
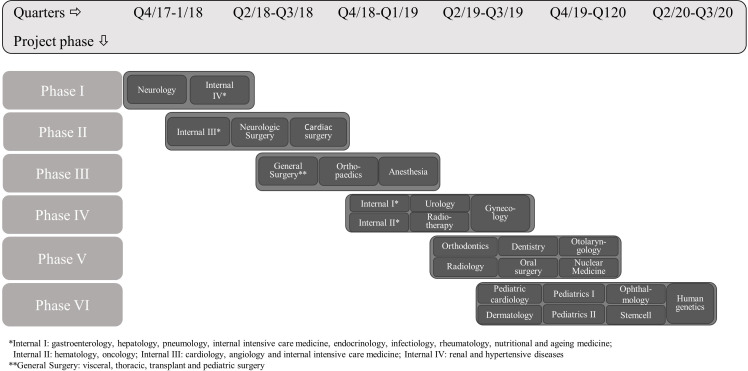
The term ‘large-scale’ means that the programme will sequentially be implemented at the University Hospital Campus Kiel involving 27 clinical departments with more than 650 physicians. The aim is to develop 83 EbPDAs enrolling new clinical departments into the programme every 6 months and identifying EbPDA topics at each clinic (figure 2). At the same time, each physician in the respective clinic undergoes SDM training. The Ask Three Questions patient activation is implemented simultaneously. In addition, in selected departments, a total of 150 other healthcare professionals will be trained as decision coaches to facilitate EbPDA use in specific patient target groups.
Figure 2.
Sequential quarterly enrolment of new clinical departments.
The term ‘integrative’ in S2C means that patients and healthcare professionals will be actively involved from the very beginning and throughout implementation, most actively not only in EbPDA development but also, for example, in training evaluation and in the patient activation programme.10 The integrative approach begins with identifying new topics together with physicians and conducting needs assessments with patients. It ends with having physicians distribute EbPDAs to patients in their clinical departments. Sample patients will also user test the EbPDAs before these will be administered to patients in daily practice.
Patient and public involvement
No patient was involved in the development or design of this study.
Theoretical framework
At the microlevel (level of healthcare professionals or patients), the S2C programme is designed and implemented on the grounds of the Theory of Planned Behavior suggesting behaviour is a result of motivation (intention) and ability (perceived behaviour control).30 31 Accordingly, the S2C programme aims to induce attitude and perception changes by training physicians and other healthcare professionals in SDM and by informing patients to enable simultaneous behaviour change at the level of patients and healthcare providers. The interactive process of EbPDA-development also aims at changing attitudes at the individual physician level. The implementation of the S2C programme is at the microlevel guided by the concept of normalisation process theory (NPT). The four components of the NPT are coherence (does the programme make sense to those who are involved?), participation (how do relevant stakeholders participate in implementation?), collective action (what to do to make implementation successful?) and reflexive monitoring (how do the involved individuals judge implementation processes?).32 As part of a process evaluation, these questions/constructs will be addressed with key stakeholders at specific points in time throughout the 4-year project time to continuously monitor implementation processes at the level of all involved stakeholders at the University Hospital Campus Kiel.
The complexity of this project taking into account context and processes of project implementation is depicted in table 1 following the CFIR (https://cfirguide.org/). This framework comprises five domains (intervention characteristics, outer setting, inner setting, characteristics of individuals and process) and 39 related constructs.9 33 34 The constructs of the CFIR were used to describe the status quo of relevant project characteristics, project settings and potential interactions between these at project initiation. CFIR will also guide our implementation processes as described later.
Setting and study population
Campus Kiel as part of the UKSH Medical Center is a tertiary care hospital with more than 200 000 cases treated each year. Twenty-seven clinical departments with more than 650 physicians and more than 150 other healthcare professionals and their patients will be part of either training modules or development and use of decision aids or both. New clinical departments and their patients will be sequentially enrolled in the study (figure 2).
S2C intervention components
Intervention ‘SDM training for physicians’
This module aims at providing structured SDM training in three steps to a minimum of 80% of physicians working at the UKSH (ie, at least 520 physicians should receive training). The module is based on the pretested and validated training approach that has demonstrated to be effective and lead to an increased patient, physician and observer perception of involvement in decision-making.17 18 Preceding training, each physician has to take a baseline video of him or herself with a patient in a real decision-making interaction. The physician then undergoes an online video tutorial that contains general information on SDM and its application in clinical practice. It also contains fictional interactions between physician and patient actors teaching physicians to differentiate ‘good’ from SDM communication ‘in need of improvement’. For the subsequent video-based small group training sessions, the baseline video recording of a patient–physician interaction and a second recording (following online training) are rated by the S2C trainer team (see table 2 for additional information). In the subsequent group training, each physician receives video-based trainer and group feedback. The aim is to provide an interactive and common SDM learning experience to physician. To increase their motivation, training participation is rewarded by continued medical education credits by the German Medical Associations.
Table 2.
Details on outcome measurement
| Outcome, instrument |
Outcome definition | Target population | Measurement scale | Reasons for choice of instrument | Assessment schedule/mode (time points, T0, T1, T2) | Planned number of interviewed individuals |
| Outcomes elicited from patient perspective via patient questionnaire | ||||||
| Primary Outcome Measure1: Perceived Involvement in Care Scales (PICS) (third subscale used as primary outcome measure)41 45 | Perceived involvement in patient–physician interaction from patient perspective | Sample of UKSH patients receiving patient questionnaires (all clinical departments or specific departments) | Three subscales:
Individual scores range from 1 (no agreement) to 4 (total agreement) |
Measures patient perception of involvement in decision-making with physician in general, not restricted to or focused on one specific decision situation; takes the perspective of a patient and is not limited to assessing the patient perceived degree of physician’s endeavour | T0: before programme starts (baseline) T1: after completion of programme in each department; immediate effect T2: 6 months before the end of the project: sustainability of ‘effect’ |
1.600 at T0 and T2, respectively; a minimum of 40 per clinic at T1; Different samples are taken at each measurement |
| Secondary outcome measure: Preparation for Decision Making Scale (PREP-DM-Scale)42 |
Perceived level of individual preparation for decision situation | same as for PICS | ten items Individual scores in patient questionnaire range from 1 (no agreement) to 5 (total agreement) |
Measures patient perception of involvement in decision-making going beyond patient–physician communication, for example, brochures, decision aids, information provided via other healthcare professionals. | T0, T1, T2 | Same as for PICS |
| Secondary outcome measure: collabo-RATE43 |
Perceived level of attempts being made by physicians to actively involve patients in decision-making | Same as for PICS | three items Individual scores in patient questionnaire range from 1 (received no attention) to 5 (received much attention) |
Allows comparison with other studies, since this questionnaire is widely used internationally. | T0, T1, T2 | Same as for PICS |
| Outcomes elicited from observer perspective* | ||||||
| Primary outcome measure2: MAPPIN’ SDM-O-dyad17–19 | Observer-based assessment of how well the physician–patient interaction is performed with respect to the MAPPIN’SDM criteria Conducted by trained raters based on videos of specific interactions. |
Patients and physicians in a personal decision-related interaction | Based on a MAPPIN’SDM rater manual. Six items reflecting the six steps in shared decision making Item 1: problem definition Item 2: key SDM message Item 3a: options (structure) Item 3b: options (content) Item 3c: options (quality of information) Item 4: patient expectations and worries Item 5: decision making Item 6: further steps |
Provides an ‘objective’ assessment of the patient–physician interaction by an independent rater, with respect to both interaction participants, the patient and the physician (‘dyad’) | T0, T1 Individual patient–physician encounters at clinical departments* Rater is blinded to the timing of the video taken. |
200–220 patient–physician interactions (all physicians at seven involved clinical departments will submit one video at each measurement time point)* Physicians are mostly the same at each measurement but patients in interaction are different. |
*Evaluated clinical departments at the University Hospital Campus Kiel are: general surgery, internal medicine I (gastroenterology, hepatology, pneumology, internal intensive care medicine, endocrinology, infectiology, rheumatology, nutritional and ageing medicine), radiotherapy, internal medicine II (haematology, oncology), trauma surgery and orthopaedics, gynaecology and urology.
MAPPIN'SDM, Multifocal approach to sharing in shared decision-making.
Intervention ‘activation of patients’
The ‘Ask Three Questions’ programme has originally been developed in Australia and tested in European countries.28 29 Patients are instructed and motivated to actively participate in communication by asking their doctors questions regarding their specific (treatment) situation. Our patient activation concept communicates the message ‘Ask Three questions—decide together’ in a unique design at all departments using various distribution channels: paper postcards, posters/stand-up displays and screen-based messages inside UKSH. It will be embedded in several other interventions, like a patient homepage within the UKSH-homepage, the bedside infotainment system, information screens and special SDM-days in the central lobby.
Intervention ‘(online) EbPDAs’
Eigthy-three online EbPDAs will be developed, at least 1 in each department. The number is arbitrary, as there is no recommended number per department. We calculated the maximum possible number given the resources and the time frame of our grant. Consistent with the DA (decision aid) factory approach implementation starts with the identification of EbPDA topics together with physicians. Topics should be important for physicians, involve at least two preference-sensitive treatment alternatives and occur frequently. Topic specification with respect to target patient population, relevant treatment options and patient-relevant outcomes/issues of treatment is done based on a literature/guideline review and in exchange with physicians and patients. Needs assessments are conducted with about four to eight patients per topic to guide and structure EbPDAs as closely to patient needs as possible. Development of EbPDAs involves a systematic search and assessment of best available evidence for all relevant interventions, focusing on systematic reviews and evidence-based guidelines. Methods are based on the German standards of evidence-based patient information and the methods of evidence generation in patient information.35 36 Text information on disease and treatment will be accompanied by video sequences with UKSH physicians and patients. In these sequences, physicians explain treatments and patients share their experience in decision-making. The latter is to motivate users of the online DAs to actively participate in decision-making. To avoid bias by testimonials, patients do not rate the different interventions in their video sequences but limit themselves to talking about their experience with the disease and their individual decision process. The process of DA development follows the International Patient Decision Aids Standards criteria (www.ipdas.ohri.ca37 38). Each EbPDA undergoes external review.
Intervention ‘SDM Training for other healthcare professionals to be decision coach’
This qualification module provides SDM training to about 150 nurses or other healthcare professionals in specific indications, where patients most likely will need support in using EbPDAs. Training principles are based on the physician training and decision coaching application in specific settings.12 13 27 39 The goal is to train healthcare staff like nurses or physiotherapists to act as ‘decision coaches’ for their patients when using EbPDAs, that is, to simultaneously provide emotional, psychological and technical support. The qualification consists of 2 workshop days communicating the principles of SDM and EbPDAs and including two individual decision coaching sessions for each participant. In addition, each decision coach will be asked to videotape coaching communications with a patient two times and receive individual SDM trainer feedback. The communication between decision coaches and patients will always centre around a specific EbPDA.
Study outcome and outcome measures
The primary intervention outcome is whether and to what degree SDM-based interaction is provided to patients at UKSH. To cover different perspectives, we focus on two types of outcome measures, one providing the patient perspective and one providing an observer-based perspective (table 2). The primary outcome is based on a validated SDM measurement instrument, the Perceived Involvement in Care Scales (PICS).40 41 It is a patient-reported outcome instrument translated and validated in Germany and consists of three subscales with 4–5 items each. The subscales are (1) patient activation by doctors (five items) (2) active information-seeking behaviour (four items) and (3) perceived patient participation in decision-making (five items). Each item is measured on a scale from 1=‘do not agree at all’ to 4=‘totally agree’. The second primary outcome consists in an observer rating of patient-physician interaction before and after the intervention using the MAPPIN’SDM (multifocal approach to sharing in shared decision making)-O(Observer)-dyad instrument.17 18 26 MAPPIN’SDM-O-dyad measures the degree of SDM performance realised by the doctor–patient dyad (ie, by the unit made up of patient and physician) as rated by independent observers. The instrument consists of nine items assessing the process and quality of SDM. Each item is scored from ‘0’ (‘the indicator is not present’) to ‘4’ (‘the indicator is present at an excellent standard’). The observer ratings are provided by independent but trained raters who rate video recordings of patient–physician interactions before and after the intervention (see ‘data collection and analyses’). All observers are blinded to the measurement objects and time points of video recordings.
Additional secondary outcomes included in the patient questionnaire are two validated and widely used questionnaires, the Preparation for Decision Making Scale (PrepDM: 10 items; 5-point scale)42 and collaboRATE43 (three items; 5-point scale). All outcome measures are detailed in table 2.
Data collection
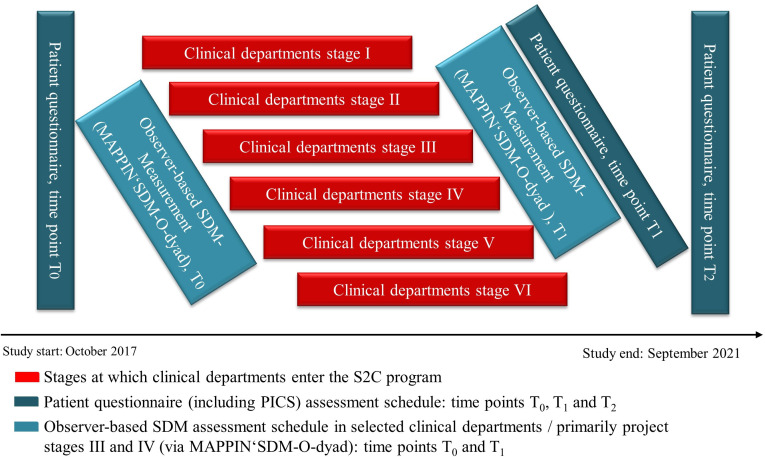
Primary outcome data collection is conducted via patient questionnaire (including the PICS instrument) before (T0) and two times after the intervention (T1, T2). The data collection and evaluation schedule are depicted in figure 3.
Figure 3.
Project stages and data collection schedule for SDM evaluation. PICS, Perceived Involvement in Care Scales; S2C SHARE TO CARE; SDM, shared decision-making; MAPPIN'SDM, multifocal approach to sharing in shared decision-making.
The first patient questionnaire/PICS measurement (T0) is scheduled at study initiation. The second (T1) is taken after completion of the S2C intervention at each department to assess immediate intervention effect. The intervention is considered complete at the department level when at least 80% of physicians have undergone training, EbPDAs are developed and in use, and the patient activation programme is in place. The last measurement (T2) is scheduled 6 months before study completion. It aims to appraise the sustainability of the S2C intervention. At T0 and T2, the patient questionnaire is mailed to a consecutive sample of patients who were hospitalised at the UKSH Kiel campus within the preceding weeks with a return envelope included in each mailing. At T1, the questionnaire is sent to a respective sample of patients from a clinical department who completed the intervention. Patients who do not return the questionnaire within a 2-week or 4-week time frame, respectively, will get a reminder either one or two times. Based on the Total Design Method approach by Dillmann et al,44 final response rates of at least 60%–70% are expected.
The observer-based outcome measurement via MAPPIN’SDM-O-dyad is performed two times throughout the 4-year study period, at T0 and T1. To minimise workload for physicians, who must videotape encounters with patients to facilitate the MAPPIN’SDM-O-dyad evaluation, these assessments focus on central domains of hospital medicine (internal medicine, oncology, gynaecology, surgery, orthopaedics) being covered by specific clinical departments (departments of general surgery, internal medicine, radiotherapy, oncology & haematology, gynaecology, trauma surgery & orthopaedics, urology, gynaecology).
Sample size calculation and data analyses
Sample size calculation for the patient-based primary outcome is based on published PICS data.41 45 An assumed difference of 0.4 in the PICS outcome at T1 versus T0 and a SD of 0.7 yields a sample size of about 40 for each clinical department at each measurement, using an independent sample t test and assuming a power of 80% and a level of significance of 5% (one-sided, assuming a positive effect of the SDM intervention). This yields a campus-wide sample of 1080 patients (27 clinics, 40 patients per clinic). A difference in PICS scores of 0.4 comparing before and after measurement is considered relevant (Hedges g>0.5, which corresponds to a medium size effect). If the distribution does not allow the assumption of normality, appropriate non-parametric tests will be applied in data analyses.
A presumed response rate of 60%–70% to the patient questionnaire mailings leads us to target about 1600 patients at measurement point T0 and T2 the campus level to finally achieve at least about 1000 patient questionnaires returned, yielding on average between 30 and 60 returned questionnaires per clinical department. These numbers will allow to measure significant differences in the primary endpoint not only at the campus but also at the individual department level (at least in the larger ones). At T1, a minimum of 65–70 patients has to be contacted to have at least 40 questionnaires returned.
Sample size for the second primary outcome assessment (MAPPIN’SDM observer assessment) is given by the number of physicians at the involved clinical departments. Seven of the 27 UKSH departments will be part of the MAPPIN’SDM assessment. Physicians in these departments sum up to 200–220 in total. Each physician will deliver a patient–physician interaction video for outcome measurement at each measurement point. This analysis includes general surgery (n=30–40 physicians), internal medicine (n=62), radiotherapy (n=16), oncology/haematology (n=21), orthopaedics (n=27), gynaecology (n=34) and urology (n=10–20). Based on a previous study including training of physicians only,18 we aim at an effect size of d=0.5 (Hedges g). To yield a power of 80% (alpha=5%), minimal sample size should be n=51. Assuming a response rate above 60% (n≥120), the sampling strategy leads to a sufficient sample size. It is hypothesised that in 80% of patient–physician interactions, patients will receive satisfactory SDM-based treatment at the second department-wide measurement (T1) compared with less than 80% before the intervention (T0). To answer the latter study hypothesis, a MAPPIN-SDM-O-dyad mean value of greater or equal to 1.5 was defined as satisfactory basic patient involvement in decision-making based on previous validation research.18
Health economic evaluation
In addition to the pre SDM post SDM evaluation, an economic evaluation will be conducted. This analysis will be based on insurance claims data provided by the largest German Health Insurance provider (Techniker Krankenkasse (TK)). In Germany, approximately 88% of the population (72.8 million) is covered under the comprehensive statutory health insurance system. The TK provides health insurance for approximately 9.8 million people (13% of the statutory contributors) and routinely collects data for reimbursement purposes on hospital stays, physician visits, medical procedures, medication and medical diagnoses. In the economic evaluation, incremental costs and use of specific services of patients admitted to the UKSH with preference-sensitive conditions in specific clinical departments (intervention group) will be compared with a matched population (control group) drawn from the administrative dataset from the TK. The control group includes patients with a hospital admission to another German University or Educational hospital (tertiary medical centre). From this sample population, patients will be matched to the intervention group using exact matching, propensity score matching or a combination of exact and propensity score matching.46 47 Matching criteria will include patient characteristics like age and sex, the main diagnosis of the hospital admission as well as measures of morbidity within 12 months preceding hospital admission. In line with previous research,48 variables that are compared across groups include preference-sensitive surgery rates, imaging rates, inpatient costs, total medical costs and hospital and emergency department admissions within 12 months after the admission to the hospital. To account for systematic differences between intervention and control group, the analysis will focus on the comparison of the difference in outcomes measured at two points in time, before and after the implementation of the SDM intervention. The analysis will be limited to about 10–15 frequently occurring and preference-sensitive conditions. These conditions will include but are not limited to cardiologic diseases, benign prostatic hyperplasia and other urologic diseases, benign uterine diseases and obstetrics, neurosurgery/back pain and orthopaedic diseases such as knee or hip replacement.
Process evaluation
Starting point for our evaluation is that the CFIR constructs as depicted in table 1. They summarise each study component, involved stakeholders, context (inner/outer setting) and processes at study initiation. Each construct is followed up throughout the course of the study aiming to (1) identify areas where adaptations to initially planned implementation might be needed and2 (2) better understand which clinical departments might be more/less accessible to the SDM interventions and why. Process evaluation is done by using (a) documentation (eg, documentation of decision aid use by simply counting click/user numbers and times or documentation of number of physician trainings performed per clinical department) and (b) interview or structured questionnaire data. Interviews and structured questionnaires with stakeholders regarding implementation processes will be developed based on the described four concepts of the NPT theory.49–51 In addition, field notes are used by the respective project teams (figure 1) to adapt implementation strategies and processes to the specific demands of individual department’s circumstances during the intervention phase.
Ethics and dissemination
The Medical Ethics Committee of the Medical Faculty of the Christian-Albrechts-University (CAU) Kiel has provided ethics approval to this study (reference number A111/18). This study will be conducted in accordance with German laws and regulations of the Medical Ethics Committee of the CAU, Kiel, Germany. Eligible patients or healthcare providers will be fully informed about the study and asked to participate in each part of the study: conducting personal interviews with patients (needs assessment), or video sequences with physicians/patients or involving physicians in training sessions. Patients/providers will receive a respective information letter and will be informed about the implications of participation. They will have sufficient opportunity to ask questions and to consider the implications of the study before deciding to participate. Before participation, all individuals will provide written informed consent, compliant with the local and ethical data regulations. Patients and clinical staff will be allowed to withdraw from the study without giving a reason, at any time. The results arising from this implementation study will be presented at scientific meetings, on project-hosted websites at UKSH and by S2C as well as published in peer-reviewed journals. There is no intention to use professional writers and authorship will be based on the International Committee of Medical Journal Editors Guidelines.
Supplementary Material
Acknowledgments
The authors acknowledge Juergen Kasper and Katrin Liethmann for their tremendous contributions to the development of the study concept and intervention program.
Footnotes
Collaborators: Corinna Knauff, Divna Tafelski, Johanna Gärtner, Stefanie Mevis, Heike Klein, Lea Kruse, Salim Greven, Gesine Steinbock, Kristina Blankenburg, Gerhard Koch, Claudia Hacke, Olga Kopeleva, Carmen Wiencke, Anja Schuldt, Christina Gesine Sommer, Barbara Kreidler, Constanze Stolz, Christine Wagner-Ullrich, Thorsten Duit, Michael Schipper, Lars Jacobsen, Christian Weymayr, Svenja Ludwig, Roya Shar-Yazdi, Ryan Naglatzki, Julia Bossert, Karoline Weik
Contributors: FG, FS, KW and JUR developed the study concept and methods, designed the intervention program and are responsible for its implementation. LS and AN developed the evaluation concept and are responsible for its realisation. TS and CK provided substantial scientific and methodological contribution. AR and MDe provided methodological input and critically revised the manuscript. MD drafted the manuscript and provided scientific and methodological input to the study concept.
Funding: This work was supported by a grant of the German Innovation Fonds (hosted by the Federal Joint Committee), grant number 01NVF17009.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Wehkamp KH, Naegler H. The commercialization of patient-related decision-making in hospital - a qualitative study of the perceptions of doctors and chief executive officers. Dtsch Ärztebl Int 2017;114:797–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gemeinsam entscheiden im Klinikalltag: Ergebnisse von Fokusgruppengesprächen mit jungen Ärzten. Bertelsmann Stiftung, 2018. Available: file:///C:/SDM%20Transfer/Publikation%20SDM%20Projekt/Literatur/Bittner_2018_VV_Studie_Gemeinsam_entscheiden_final_online.pdf [Accessed 22 Jul 2020].
- 3.Stacey D, Suwalska V, Boland L, et al. . Are patient decision AIDS used in clinical practice after rigorous evaluation? A survey of trial authors. Med Decis Making 2019;39:805–15. 10.1177/0272989X19868193 [DOI] [PubMed] [Google Scholar]
- 4.Härter M, Dirmaier J, Scholl I, et al. . The long way of implementing patient-centered care and shared decision making in Germany. Z Evid Fortbild Qual Gesundhwes 2017;123-124:46–51. 10.1016/j.zefq.2017.05.006 [DOI] [PubMed] [Google Scholar]
- 5.Tan ASL, Mazor KM, McDonald D, et al. . Designing shared decision-making interventions for dissemination and Sustainment: can implementation science help translate shared decision making into routine practice? MDM Policy Pract 2018;3:2381468318808503. 10.1177/2381468318808503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gesetz zur Verbesserung der Rechte von Patientinnen und Patienten (Law for the Improvement of Patients' Rights), 2013. Available: https://www.bundesaerztekammer.de/fileadmin/user_upload/downloads/Patientenrechtegesetz_BGBl.pdf [Accessed 22 Jul 2020].
- 7.Coulter A. Shared decision making: everyone wants it, so why isn't it happening? World Psychiatry 2017;16:117–8. 10.1002/wps.20407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maskrey N. Shared decision making: why the slow progress? An essay by Neal Maskrey. BMJ 2019;367:l6762. 10.1136/bmj.l6762 [DOI] [PubMed] [Google Scholar]
- 9.Damschroder LJ, Aron DC, Keith RE, et al. . Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci 2009;4:50. 10.1186/1748-5908-4-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kasper J, Lager AR, Rumpsfeld M, et al. . Status report from Norway: implementation of patient involvement in Norwegian health care. Z Evid Fortbild Qual Gesundhwes 2017;123-124:75–80. 10.1016/j.zefq.2017.05.015 [DOI] [PubMed] [Google Scholar]
- 11.Müller E, Strukava A, Scholl I, et al. . Strategies to evaluate healthcare provider trainings in shared decision-making (SDM): a systematic review of evaluation studies. BMJ Open 2019;9:e026488. 10.1136/bmjopen-2018-026488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stacey D, Kryworuchko J, Bennett C, et al. . Decision coaching to prepare patients for making health decisions: a systematic review of decision coaching in trials of patient decision AIDS. Med Decis Making 2012;32:E22–33. 10.1177/0272989X12443311 [DOI] [PubMed] [Google Scholar]
- 13.Stacey D, Kryworuchko J, Belkora J, et al. . Coaching and guidance with patient decision AIDS: a review of theoretical and empirical evidence. BMC Med Inform Decis Mak 2013;13:S11. 10.1186/1472-6947-13-S2-S11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stacey D, Légaré F, Lewis K, et al. . Decision AIDS for people facing health treatment or screening decisions. Cochrane Database Syst Rev 2017;4:CD001431. 10.1002/14651858.CD001431.pub5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Légaré F, Adekpedjou R, Stacey D, et al. . Interventions for increasing the use of shared decision making by healthcare professionals. Cochrane Database Syst Rev 2018;7:CD006732. 10.1002/14651858.CD006732.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Weert JCM, van Munster BC, Sanders R, et al. . Decision AIDS to help older people make health decisions: a systematic review and meta-analysis. BMC Med Inform Decis Mak 2016;16:45. 10.1186/s12911-016-0281-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kasper J, Liethmann K, Heesen C, et al. . Training doctors briefly and in situ to involve their patients in making medical decisions-Preliminary testing of a newly developed module. Health Expect 2017;20:1254–63. 10.1111/hex.12565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geiger F, Liethmann K, Reitz D, et al. . Efficacy of the doktormitSDM training module in supporting shared decision making - Results from a multicenter double-blind randomized controlled trial. Patient Educ Couns 2017;100:2331–8. 10.1016/j.pec.2017.06.022 [DOI] [PubMed] [Google Scholar]
- 19.Kienlin S, Kristiansen M, Ofstad E, et al. . Validation of the Norwegian version of MAPPIN'SDM, an observation-based instrument to measure shared decision-making in clinical encounters. Patient Educ Couns 2017;100:534–41. 10.1016/j.pec.2016.10.023 [DOI] [PubMed] [Google Scholar]
- 20.Campbell M, Fitzpatrick R, Haines A, et al. . Framework for design and evaluation of complex interventions to improve health. BMJ 2000;321:694–6. 10.1136/bmj.321.7262.694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Campbell NC, Murray E, Darbyshire J, et al. . Designing and evaluating complex interventions to improve health care. BMJ 2007;334:455–9. 10.1136/bmj.39108.379965.BE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Søndergaard SR, Madsen PH, Hilberg O, et al. . A prospective cohort study of shared decision making in lung cancer diagnostics: impact of using a patient decision aid. Patient Educ Couns 2019;102:1961–8. 10.1016/j.pec.2019.05.018 [DOI] [PubMed] [Google Scholar]
- 23.Steffensen KD, Vinter M, Crüger D, et al. . Lessons in integrating shared decision-making into cancer care. J Oncol Pract 2018;14:229–35. 10.1200/JOP.18.00019 [DOI] [PubMed] [Google Scholar]
- 24.Scholl I, Hahlweg P, Lindig A, et al. . Evaluation of a program for routine implementation of shared decision-making in cancer care: study protocol of a stepped wedge cluster randomized trial. Implement Sci 2018;13:51. 10.1186/s13012-018-0740-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moore GF, Audrey S, Barker M, et al. . Process evaluation of complex interventions: medical Research Council guidance. BMJ 2015;350:h1258. 10.1136/bmj.h1258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Geiger F, Liethmann K, Hoffmann F, et al. . Investigating a training supporting shared decision making (IT'S SDM 2011): study protocol for a randomized controlled trial. Trials 2011;12:232. 10.1186/1745-6215-12-232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berger-Höger B, Liethmann K, Mühlhauser I, et al. . Informed shared decision-making supported by decision coaches for women with ductal carcinoma in situ: study protocol for a cluster randomized controlled trial. Trials 2015;16:452. 10.1186/s13063-015-0991-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shepherd HL, Barratt A, Jones A, et al. . Can consumers learn to ask three questions to improve shared decision making? A feasibility study of the ASK (AskShareKnow) Patient-Clinician Communication Model(®) intervention in a primary health-care setting. Health Expect 2016;19:1160–8. 10.1111/hex.12409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shepherd HL, Barratt A, Trevena LJ, et al. . Three questions that patients can ask to improve the quality of information physicians give about treatment options: a cross-over trial. Patient Educ Couns 2011;84:379–85. 10.1016/j.pec.2011.07.022 [DOI] [PubMed] [Google Scholar]
- 30.Ajzen I. The theory of planned behavior. Organ Behav Hum Decis Process 1991;50:179–211. 10.1016/0749-5978(91)90020-T [DOI] [Google Scholar]
- 31.Fishbein M. A reasoned action approach to health promotion. Med Decis Making 2008;28:834–44. 10.1177/0272989X08326092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.May CR, Finch T, Ballini L, et al. . Evaluating complex interventions and health technologies using normalization process theory: development of a simplified approach and web-enabled toolkit. BMC Health Serv Res 2011;11:245. 10.1186/1472-6963-11-245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scalia P, Durand M-A, Forcino RC, et al. . Implementation of the uterine fibroids option grid patient decision AIDS across five organizational settings: a randomized stepped-wedge study protocol. Implement Sci 2019;14:88. 10.1186/s13012-019-0933-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kirk MA, Kelley C, Yankey N, et al. . A systematic review of the use of the consolidated framework for implementation research. Implement Sci 2016;11:72. 10.1186/s13012-016-0437-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leitlinie evidenzbasierte Gesundheitsinformation, 2017. Available: http://www.leitliniegesundheitsinformation.de/ [Accessed 22 Jul 2020].
- 36.General methods 5.0. IQWiG, 2017. Available: file:///C:/Users/DANNER~1.TAK/AppData/Local/Temp/General-Methods_Version-5-0.pdf [Accessed 24 May 2020].
- 37.Elwyn G, O'Connor A, Stacey D, et al. . Developing a quality criteria framework for patient decision AIDS: online international Delphi consensus process. BMJ 2006;333:417. 10.1136/bmj.38926.629329.AE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Holmes-Rovner M. International Patient Decision Aid standards (IPDAS): beyond decision AIDS to usual design of patient education materials. Health Expect 2007;10:103–7. 10.1111/j.1369-7625.2007.00445.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stacey D, Murray MA, Légaré F, et al. . Decision coaching to support shared decision making: a framework, evidence, and implications for nursing practice, education, and policy. Worldviews Evid Based Nurs 2008;5:25–35. 10.1111/j.1741-6787.2007.00108.x [DOI] [PubMed] [Google Scholar]
- 40.Lerman CE, Brody DS, Caputo GC, et al. . Patients' perceived involvement in care scale: relationship to attitudes about illness and medical care. J Gen Intern Med 1990;5:29–33. 10.1007/BF02602306 [DOI] [PubMed] [Google Scholar]
- 41.Scheibler F, Freise D, Pfaff H. Die Einbeziehung von Patienten in die Behandlung - Validierung der deutschen PICS Skalen. Journal of Public Health 2004;12:199–209. [Google Scholar]
- 42.Bennett C, Graham ID, Kristjansson E, et al. . Validation of a preparation for decision making scale. Patient Educ Couns 2010;78:130–3. 10.1016/j.pec.2009.05.012 [DOI] [PubMed] [Google Scholar]
- 43.Forcino RC, Barr PJ, O'Malley AJ, et al. . Using collaborate, a brief patient-reported measure of shared decision making: results from three clinical settings in the United States. Health Expect 2018;21:82–9. 10.1111/hex.12588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dillmann DA. Mail and Internet surveys. The tailored design method. New York: Wiley, 2007. [Google Scholar]
- 45.Scheibler F, Pfaff H, Kowalski C, et al. . Shared decision making in breast care centers in North Rhine-Westphalia: results of a 10-year trend analysis. Z Evid Fortb Qual Gesundhwes 2019;147-148:97–102. [DOI] [PubMed] [Google Scholar]
- 46.Herold R, van den Berg N, Dörr M, et al. . Telemedical care and monitoring for patients with chronic heart failure has a positive effect on survival. Health Serv Res 2018;53:532–55. 10.1111/1475-6773.12661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ho DE, Imai K, King G, et al. . Matching as nonparametric preprocessing for reducing model dependence in parametric causal inference. Political Analysis 2007;15:199–236. 10.1093/pan/mpl013 [DOI] [Google Scholar]
- 48.Veroff D, Marr A, Wennberg DE. Enhanced support for shared decision making reduced costs of care for patients with preference-sensitive conditions. Health Aff 2013;32:285–93. 10.1377/hlthaff.2011.0941 [DOI] [PubMed] [Google Scholar]
- 49.Rapley T, Girling M, Mair FS, et al. . Improving the normalization of complex interventions: part 1 - development of the NoMAD instrument for assessing implementation work based on normalization process theory (NPT). BMC Med Res Methodol 2018;18:133. 10.1186/s12874-018-0590-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Finch TL, Girling M, May CR, et al. . Improving the normalization of complex interventions: part 2 - validation of the NoMAD instrument for assessing implementation work based on normalization process theory (NPT). BMC Med Res Methodol 2018;18:135. 10.1186/s12874-018-0591-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Finch TL, Rapley T, Girling M, et al. . Improving the normalization of complex interventions: measure development based on normalization process theory (NoMAD): study protocol. Implement Sci 2013;8:43. 10.1186/1748-5908-8-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rahn AC, Köpke S, Kasper J, et al. . Evaluator-blinded trial evaluating nurse-led immunotherapy Decision Coaching In persons with relapsing-remitting Multiple Sclerosis (DECIMS) and accompanying process evaluation: study protocol for a cluster randomised controlled trial. Trials 2015;16:106. 10.1186/s13063-015-0611-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.